Abstract
A 75-year-old man with stage IV lung adenocarcinoma was treated with osimertinib due to disease progression despite having been administered erlotinib. Both an epidermal growth factor receptor (EGFR) L858R mutation on exon 21 and a T790M mutation on exon 20 were detected in a specimen from a recurrent primary tumor. Five weeks after osimertinib initiation, he developed general fatigue and dyspnea. Chest computed tomography scan revealed diffuse ground glass opacities and consolidation on both lungs. An analysis of the bronchoalveolar lavage fluid revealed marked lymphocytosis, and a transbronchial lung biopsy specimen showed a thickened interstitium with fibrosis and prominent lymphocytic infiltration. We diagnosed the patient to have interstitial lung disease induced by osimertinib.
Keywords: epidermal growth factor receptor, T790M resistance mutation, osimertinib, non-small cell lung cancer, interstitial lung disease, transbronchial lung biopsy
Introduction
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) provides dramatic antitumor activity in patients with advanced non-small cell lung cancer with active EGFR mutations. However, the majority of patients treated with EGFR-TKI frequently acquire resistance within 1 to 2 years (1-4). The most common mechanism for EGFR-TKI resistance is a T790M mutation, which has been reported to be present in approximately 50-60% (5,6). Osimertinib was developed to overcome resistance due to a T790M mutation, with clinical response rates ranging from 59-71% (7-10). Based on these results, osimertinib has been approved for use in Japan since May 25, 2016. We herein report a case of interstitial lung disease (ILD) induced by osimertinib, which was given to treat disease progression of EGFR T790M-positive, erlotinib-resistant non-small cell lung cancer.
Case Report
A 75-year-old Japanese man, never-smoker, was diagnosed to have stage IV lung adenocarcinoma (cT3N3M1b) with brain metastasis in March 2015. A genomic analysis of the left lower lung tumor revealed an EGFR L858R mutation on exon 21. He was given erlotinib as the first-line treatment, which achieved a partial response on both the lungs and brain. There were no major toxicities during the course erlotinib therapy, except for minor rashes. After 7 months, a follow-up computed tomography (CT) scan showed the regrowth of the primary lung tumor; in this case, erlotinib was continued beyond disease progression (11).
In May 2016, re-biopsy of the primary lesion revealed both an EGFR L858R mutation on exon 21 and T790M mutation on exon 20, for which osimertinib was started as a second-line treatment. During a routine visit at 20 days after the start of osimertinib administration, lactate dehydrogenase (160 U/L) was within normal limits, but C-reactive protein (10.1 mg/dL) and white blood cell count (9,000 /μL: 77.9% neutrophils, 8.4% lymphocytes, 6.3% eosinophils) were elevated on laboratory examinations. The white blood cell count (4,200 /μL: 60.5% neutrophils, 24.5% lymphocytes, and 3.8% eosinophils) was normal before using the drug. At this time, he was asymptomatic and his chest X-ray and CT findings showed no evidence of pneumonitis. With a suspicion of adverse drug reaction, we discontinued osimertinib treatment and monitored his condition carefully. Two weeks later, he developed generalized weakness and dyspnea, and was then admitted to our hospital.
On examination, his body temperature was 36.0℃, but the respiratory rate was 24/min, oxygen saturation was 90% at room air; there were inspiratory fine crackles on both lung fields. A chest CT scan revealed diffuse ground glass opacities and areas of patchy consolidation on both upper lobes, as well as widespread peribronchial consolidation on the right middle lobe; there were no remarkable changes in the primary tumor (Fig. 1A and B). The results of laboratory examination on admission are listed in Table.
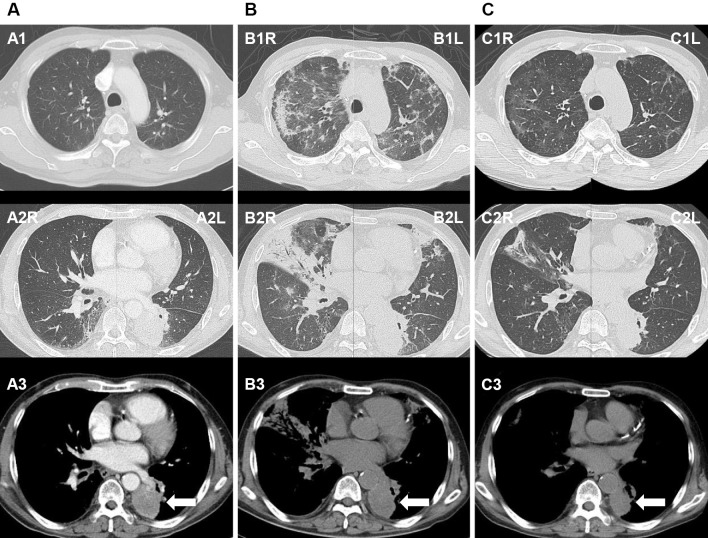
Figure 1.
A: Before osimertinib administration, a solid tumor is observed in the left lower lung (white arrow) with no evidence of interstitial pneumonia. B: After admission, at 34 days after osimertinib initiation, there are diffuse ground glass opacities, areas of patchy consolidation observed in both lungs, and peribronchial consolidation on the right middle lobe. There is no remarkable change in the size of the primary tumor (white arrow). C: Follow-up on the 16th hospital day, after steroid therapy, reveals the resolution of the bilateral ground glass opacities and areas of consolidation, as well as a decrease in the size of the primary tumor (white arrow).
Table.
Laboratory Data on Admission.
| Complete Blood Count | Biochemical Examination | ||||
| WBC | 9,300 | /μL | T-Bil | 0.4 | mg/dL |
| Neut | 69.8 | % | AST | 25 | U/L |
| Ly | 14.6 | % | ALT | 9 | U/L |
| Mo | 9.0 | % | TP | 6.4 | g/dL |
| Eo | 6.0 | % | Alb | 2.5 | g/dL |
| Baso | 0.6 | % | UN | 11 | mg/dL |
| RBC | 394×104 | /μL | Cr | 0.74 | mg/dL |
| Hb | 11.6 | g/dL | Na | 132 | mEq/L |
| Ht | 34.7 | % | K | 4.6 | mEq/L |
| Plt | 32.7×104 | /μL | Cl | 100 | mEq/L |
| Coagulation | Ca | 8.3 | mg/dL | ||
| PT | 13.8 | sec | LDH | 269 | U/L |
| APTT | 35.9 | sec | CRP | 11.48 | mg/dL |
| Arterial Blood Gas (O2 2L) | BNP | 36.8 | pg/mL | ||
| pH | 7.442 | KL-6 | 283 | U/mL | |
| PaO2 | 74 | Torr | β-D-glucan | 9.4 | pg/mL |
| PaCO2 | 37.8 | Torr | Tumor marker | ||
| BE | 1.8 | mEq/L | CEA | 5.6 | ng/mL |
| SCC | 1.4 | ng/mL | |||
We performed bronchoalveolar lavage (BAL) through the medial right middle lobe bronchus (B5) and transbronchial lung biopsy (TBLB) through the anterior right upper lobe bronchus (B3a). A BAL fluid (97 mL/ 150 mL) analysis showed pronounced lymphocytosis (total cell count of 538 cells/μL: 0.8% neutrophils, 92.2% lymphocytes, 3.6% eosinophils, 1.4% monocytes, 1% basophils, and 1% alveolar macrophages). Among the lymphocytes, CD3+ cells and CD4+ cells accounted for 86.9% and 21.5%, respectively, and CD8+ cells could not detected. Bacterial cultures in BAL fluid were negative. TBLB revealed a thickened interstitium with fibrosis, prominent lymphocytic infiltration, and pneumocyte type II hyperplasia. However, no malignant cells were detected (Fig. 2). The patient was not taking any other drugs after osimertinib initiation. Drug lymphocyte stimulation test (DLST) of peripheral blood for osimertinib was negative (stimulation index, 170%).
Figure 2.
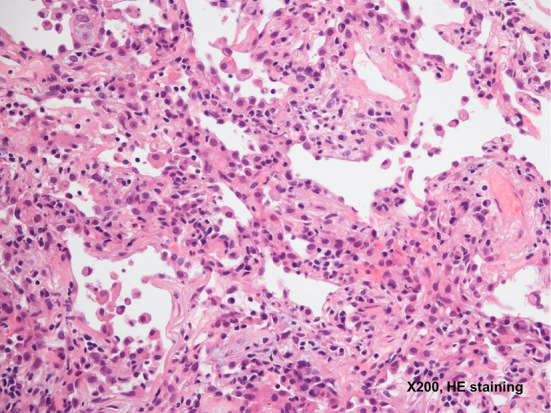
A thickened interstitium with fibrosis, prominent lymphocytic infiltration, and pneumocyte type II hyperplasia are observed (Hematoxylin and Eosin staining, ×200).
Based on the clinical and histologic findings, we diagnosed him to have osimertinib-induced ILD. Methylprednisolone pulse therapy (500 mg/day for 3 days) was initiated. After 3 days, the chest X-ray findings improved. Oral prednisolone was then administered at a dose of 40 mg/day. After obtaining an improvement of his symptoms and oxygenation, oxygen administration was discontinued after 8 days of admission. A follow-up CT scan on day 16 revealed a resolution of the bilateral ground glass opacities and consolidation, as well as a decrease in the size of the primary tumor (Fig. 1C). Oral steroid therapy was thereafter gradually reduced. He was discharged after 38 days of admission. His ILD had not recurred at 2 months after discharge.
Discussion
Osimertinib is an oral, potent, irreversible EGFR-TKI that is selective for both EGFR-TKI-sensitizing mutations and T790M resistance mutation (12). Compared with previous EGFR-TKIs, osimertinib shows significantly less in vitro activity against wild-type EGFR (12), and it is supposed to be less toxic than the first- and second-generation EGFR-TKIs. However, in clinical trials of osimertinib, which excluded patients with a past history of ILD, pneumonitis/ILD occurred in 2-4% of patients (grade 1-2: 1.0-3.2%, grade 3-4: 0-1.5%, fatal: 0.4-0.5%) (8-10), an incidence that was not much different from that of the previous EGFR-TKIs. In this case, grade 3 ILD developed about 5 weeks after osimertinib administration, although the patient had experienced no pulmonary side effects during the prior erlotinib treatment.
In our case, DLST for osimertinib was negative. The test, which can only detect the presence of sensitization, had been widely used in Japan for the diagnosis of drug-induced ILD. DLST has been reported to only be slightly helpful in detecting the causative agents (13), and a negative test result does not rule out causation between the suspected drug and ILD. The mechanism of osimertinib-induced ILD remains unclear. Given the fact that osimertinib induced ILD in patients who had no pulmonary toxicities during a prior treatment with first- or second-generation EGFR-TKI, the mechanism of ILD may be different for third-generation EGFR-TKIs.
There have been several studies that focused on the CT patterns of drug-induced ILD. According to a retrospective analysis on gefitinib-induced ILD by the West Japan Thoracic Oncology Group, the CT findings were classified into four patterns: (A) a nonspecific area with ground glass attenuation; (B) multifocal areas of airspace consolidation; (C) patchy distribution of ground glass attenuation accompanied by interlobar septal thickening; and (D) extensive bilateral ground glass attenuation or airspace consolidation with traction bronchiectasis (14). Of 51 ILD cases in this report, 24, 7, 1, and 12 cases had CT patterns A, B, C, and D, and the mortality rate in each pattern was 31.0%, 28.6%, 0%, and 75.0%, respectively. Based on this classification, our case presented with predominantly pattern A and partially pattern B, which was confirmed by the histology of the TBLB specimen as non-specific interstitial pneumonia. Pronounced lymphocytosis on a BAL fluid analysis was likewise supportive of this diagnosis, as well as indicative of a good response to steroid therapy.
During the publication process of this case report, there have been a few reports published on ILD associated with osimertinib. Mamesaya et al. reported a case of osimertinib-induced mild symptomatic ILD after treatment with anti-PD1 antibody, in which the CT pattern was compatible with hypersensitivity pneumonitis (15). Noonan et al. reported transient asymptomatic pulmonary opacities, including ground-glass opacities, peribronchial nodules, and subpleural nodules, developed in 7 of 20 patients during osimertinib treatment (16). Interestingly, these reports suggest that osimertinib-induced ILD has various radiological patterns including unrecognized patterns shown in previous EGFR-TKIs and steroid therapy might not be needed if the severity is below grade 2. In our case, however, the patient had symptomatic ILD requiring oxygen supplementation, and the early use of steroids was thought to be effective considering the mortality rate of the CT patterns described above.
The onset of ILD induced by osimertinib therefore cannot be predicted based on the current data (10,15,16). The chest condition of patients treated with osimertinib should be carefully monitored, considering the possibility of interstitial pneumonitis, even without a prior history of pulmonary toxicity to the same drug class. The continued accumulation of detailed information and further analysis of radiologic and histologic patterns of osimertinib-associated ILD are therefore needed.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Mok TS, Wu YL, Thongprasert S, et al. . Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947-957, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Maemondo M, Inoue A, Kobayashi K, et al. . Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380-2388, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R, et al. . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239-246, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Sequist LV, Yang JC, Yamamoto N, et al. . Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31: 3327-3334, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Yu HA, Arcila ME, Rekhtman N, et al. . Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19: 2240-2247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sequist LV, Waltman BA, Dias-Santagata D, et al. . Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3: 75ra26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janne PA, Yang JC, Kim DW, et al. . AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372: 1689-1699, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Goss G, Tsai CM, Shepherd FA, et al. . Osimertinib for pretreated EGFR Thr790Met- positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single- arm, phase 2 study. Lancet Oncol 17: 1643-1652, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Mok TS, Wu YL, Ahn MJ, et al. . Osimertinib or platinum-pemetrexed in EGFR T790M- positive lung cancer. N Engl J Med 376: 629-640, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khozin S, Weinstock C, Blumenthal GM, et al. . Osimertinib for the treatment of metastatic epidermal growth factor T790M positive non-small cell lung cancer. Clin Cancer Res 2016(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 11. Nishie K, Kawaguchi T, Tamiya A, et al. . Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol 7: 1722-1727, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Cross DA, Ashton SE, Ghiorghiu S, et al. . AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4: 1046-1061, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 13: 39, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endo M, Johkoh T, Kimura K, Yamamoto N. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer 52: 135-140, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Mamesaya N, Kenmotsu H, Katsumata M, Nakajima T, Endo M, Takahashi T. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Invest New Drugs 35: 105-107, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noonan SA, Sachs PB, Camidge DR. Transient asymptomatic pulmonary opacities occurring during osimertinib treatment. J Thorac Oncol 11: 2253-2258, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]