Abstract
Chronic recurrent multifocal osteomyelitis (CRMO) is an autoinflammatory bone disorder that generally occurs in children and predominantly affects the long bones with marginal sclerosis. We herein report two cases of adult-onset CRMO involving the tibial diaphysis bilaterally, accompanied by polyarthritis. Magnetic resonance imaging (MRI) showed both tibial osteomyelitis and high intensity of the extensive lower leg muscles. Anti-interleukin-6 therapy with tocilizumab (TCZ) effectively controlled symptoms and inflammatory markers in both patients. High intensity of the lower leg muscles detected by MRI also improved. These cases demonstrate that CRMO should be included in the differential diagnosis of adult patients with bone pain, inflammation, and high intensity of the muscles detected by MRI. TCZ may therefore be an effective therapy for muscle inflammation of CRMO.
Keywords: osteomyelitis, polyarthritis, chronic recurrent multifocal osteitis (CRMO), myositis, SAPHO syndrome, tocilizumab
Introduction
Chronic recurrent multifocal osteomyelitis (CRMO) is an autoinflammatory bone disorder characterized by chronic nonbacterial osteomyelitis, multifocal bone lesions, and multiple recurrence (1-3). Generally, CRMO is a pediatric disease, with a mean age at onset of 10 years old and it is seen more frequently in girls (1,3). The metaphyses of the long bones and clavicle are the most commonly affected sites, but the pelvis, vertebrae, and mandible are also frequently involved (1,3,4). Bone lesions with marginal sclerosis are one of the typical feature of CRMO (5). Several diseases have been reported to accompany CRMO, including palmoplantar pustulosis, psoriasis, Crohn's disease, acne, and Sweet syndrome (1). In addition, CRMO has also been categorized as a juvenile form of SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteomyelitis) syndrome, implying that adult-onset CRMO should be included in the definition of SAPHO syndrome. The latter consists of several overlapping diseases, including pustulotic arthro-osteitis, sternocostoclavicular hyperostosis, and CRMO, and a high frequency of cutaneous manifestations (56-84%) (6). In the absence of skin lesions, the diagnosis of SAPHO syndrome, especially in patients with CRMO alone, is often difficult. Furthermore, because CRMO is mainly a pediatric disease, the diagnosis of adult-onset CRMO may be delayed. Indeed, there are only a few reports of adult patients with SAPHO syndrome in which the phenotype was CRMO without any skin manifestations. These cases were characterized by clavicular (7), vertebral, and sacroiliac (8) joint pain or involved the vertebrae and femoral neck (9).
We herein report the cases of two adult patients with CRMO without any typical skin lesions. The diaphyses of the long bones were involved and polyarthritis was present as well. In both patients, high intensity of muscles detected by magnetic resonance imaging (MRI), complicated the diagnosis. These cases demonstrate the importance of including adult-onset CRMO in the differential diagnosis of patients presenting with bone pain, inflammation and high intensity of muscles detected by MRI.
Case Reports
Case 1
A 48-year-old man was admitted to our hospital because of right lower leg pain. From the age of 17, he had been experiencing right lower leg pain of one week's duration that recurrently occurred 2-3 times per year. These episodes were not accompanied by fever. At age 38, he was admitted to a local hospital for swelling and redness of his right lower leg. His C-reactive protein (CRP) level was 3.56 mg/dL and his white blood cell (WBC) count 8,500/μL. A biopsy of the right tibia showed no malignant cells and non-bacterial osteomyelitis was therefore tentatively diagnosed. Although no treatment was started, his right lower leg pain gradually improved, but did not resolve entirely. At age 41, he was admitted to another hospital because of fever, polyarthralgia bilaterally involving his shoulders, and his right elbow and right wrist. His body temperature was 38-39℃ and his right elbow, right wrist, and left ankle were swollen. His CRP was 10 mg/dL; his rheumatoid factor (RF), anti-cyclic citrullinated peptide (CCP), and anti-nuclear antibody were all negative. His matrix metalloproteinase-3 (MMP-3) level was normal (104.0 ng/mL) as well. An MRI scan showed enhancement of the fascia of his anterior right lower leg, suggesting fasciitis. The bone marrow was of slightly heterogeneous intensity, but was not enhanced, consistent with his previous osteomyelitis. Reactive arthritis was considered, and he was started on prednisolone (PSL) at 5 mg/day and salazosulfapyridine (SSZ) therapy. Treatment reduced the polyarthritis, but the abnormal CRP level (1-3 mg/dL) and pain in his right lower leg persisted. After moving to the city where our hospital is located, the patient began treatment under our supervision. Since autoinflammatory disease was suspected, he was started on 0.5 mg colchicine/day, but it was not effective. He was therefore treated with methotrexate (MTX) and minodronate and his PSL dose was increased to 10 mg/day, but his right lower leg pain and elevated CRP continued. Loxoprofen was therefore prescribed for pain relief.
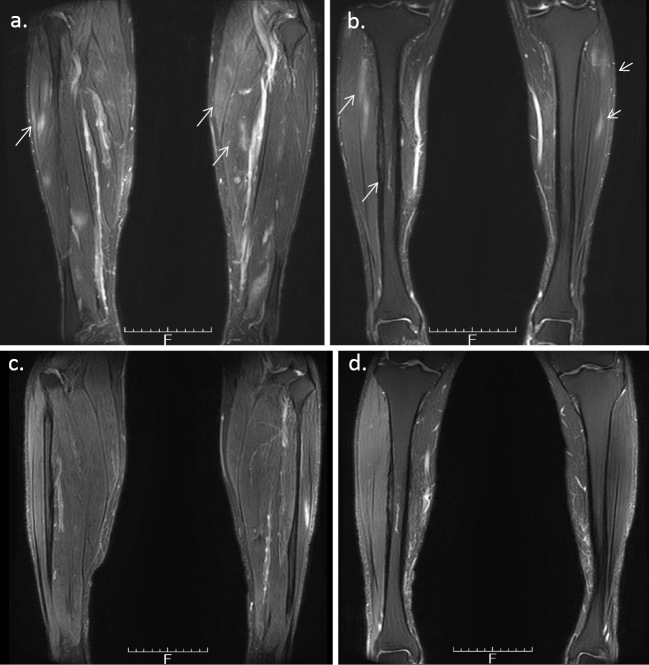
At 48 years of age, he was admitted to our hospital. He did not have fever (36.6℃), but he complained of pain in his right lower leg and right upper arm. His CRP level was 4.14 mg/dL and his WBC count was 10,700/μL. His creatinine kinase (CK) level was low (29 U/L) and he had no elevations in the levels of anti-myeloperoxidase, and proteinase 3 antineutrophil cytoplasmic antibodies. On X-rays, bilateral osteosclerosis of the tibias was observed (right>left) (Fig. 1). On the MRI scan of his lower legs, the tibial bone marrow and muscles enhanced bilaterally (Fig. 2a, b). On 99mTc-hydroxymethylene diphosphonate (HMDP) bone scintigraphy there was an uptake from the proximal metaphysis to the diaphysis of the tibias bilaterally and at the right humeral diaphysis (Fig. 3). His serum interleukin (IL)-6 and tumor necrosis factor (TNF)-α levels were elevated: 57.2 pg/mL (normal range: ≤4.0) and 6.5 pg/mL (normal range: 0.6-2.8), respectively; his serum IL-1β level was ≤10 pg/mL, and his serum IL-10 level was negative (<2 pg/mL). Chronic recurrent non-bacterial osteomyelitis was therefore suspected and he was started on tocilizumab (TCZ, 8 mg/kg) as an intravenous drip over 4 weeks. The CRP level was 5.04 mg/dL just before the second administration of TCZ, but it decreased to 0.31 mg/dL just before the third administration. Loxoprofen could be stopped because his symptoms of pain in his right lower leg and right upper arm improved. After the fourth administration of TCZ, the CRP levels stayed negative and the PSL dose was tapered. The high intensity of lower leg muscles detected in MRI also diminished over eight months. However, the higher linear enhanced intensity in the tibial diaphysis remained and chronic or old osteomyelitis was considered (Fig. 2c, d).
Figure 1.
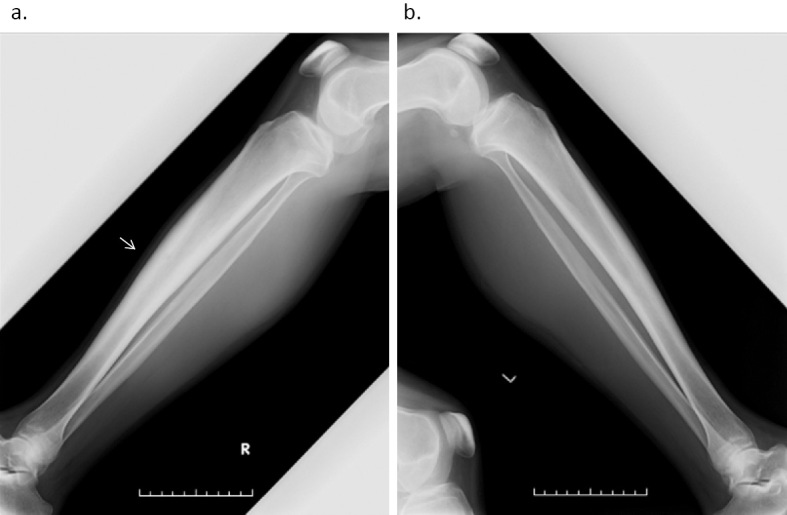
a: lateral right lower leg. b: lateral left lower leg. Bilateral osteosclerosis of the tibias (right>left).
Figure 2.
a: Magnetic resonance imaging (MRI) (T2WI) shows higher enhanced intensity of the muscles. b: A higher linear enhanced intensity in the tibial diaphyses bilaterally, indicating osteomyelitis (T2WI). c and d: a higher enhanced intensity of the muscles decreased, but a higher linear enhanced intensity in the tibial diaphysis remained (T2WI).
Figure 3.
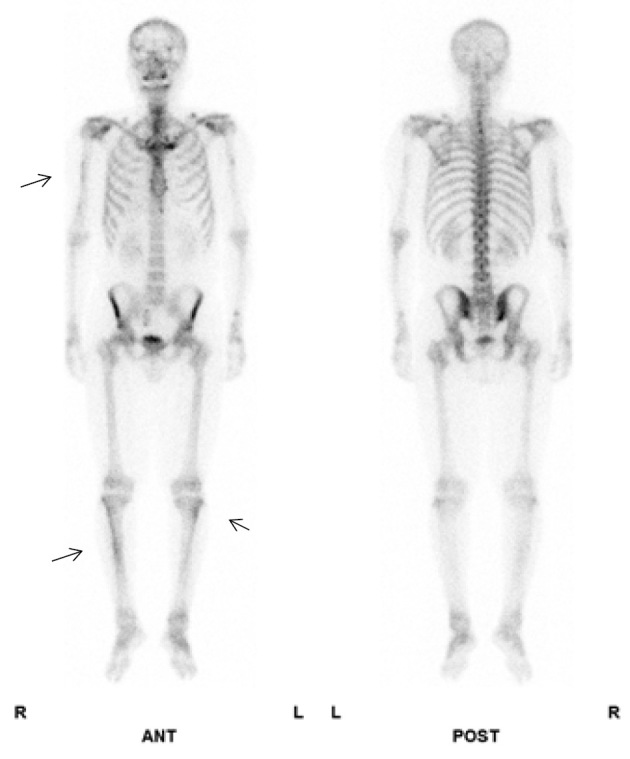
99mTc-HMDP bone scintigraphy shows the uptake from the proximal metaphysis to the diaphysis of the tibias bilaterally and at the right humeral diaphysis. HMDP: hydroxymethylene diphosphonate
Case 2
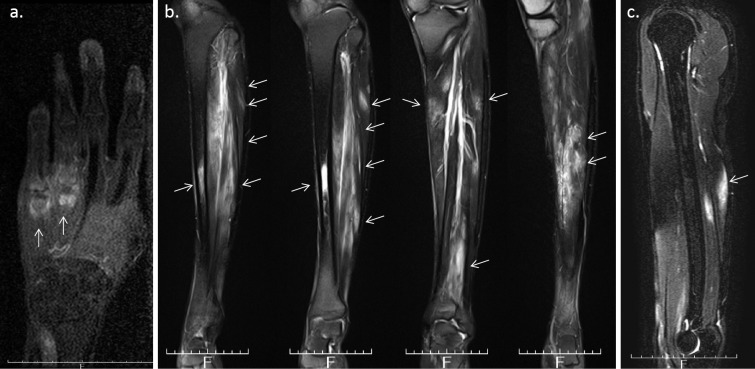
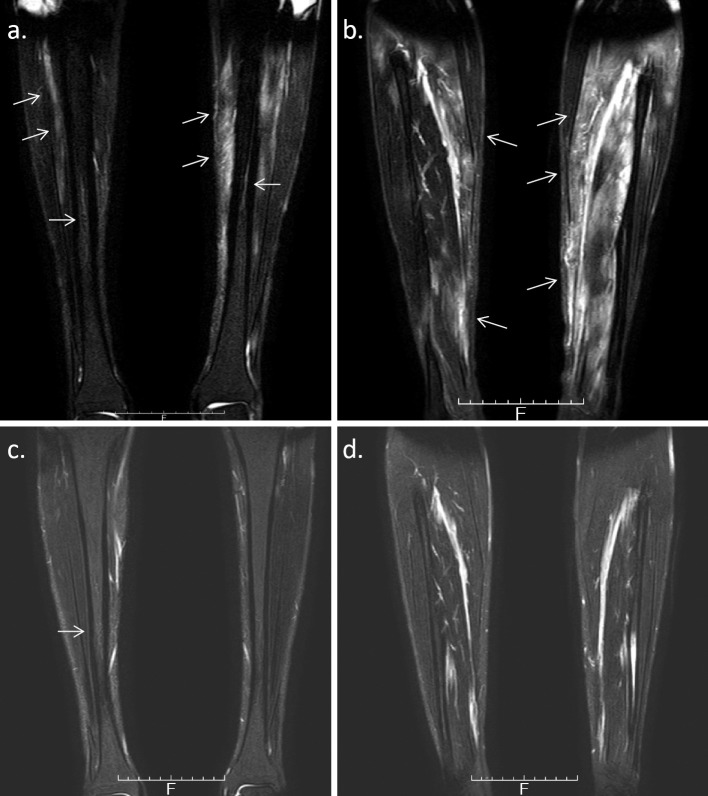
A 26-year-old man was admitted to our hospital because of bilateral lower leg pain and polyarthralgia. He had been taking levetiracetam and valproic acid because of epilepsy, which had been diagnosed when he was 13 years old. At 23 years of age, the patient developed polyarthritis of the third and fourth metacarpophalangeal (MP) joints bilaterally, the right fourth proximal interphalangeal (PIP) joint, and his elbows bilaterally. The pain in his right upper arm was presented. He did not have fever. His CRP level was 4.41 mg/dL, but his RF, anti-CCP and anti-nuclear antibody levels were negative. The MRI scan of his right hand revealed synovitis and bone marrow edema of the fourth and fifth MP joints (Fig. 4a), suggestive of rheumatoid arthritis (RA). He was started on MTX, which improved his arthritis slightly, but his CRP level remained elevated. At 24 years of age, he experienced bilateral lower leg pain. The MRI showed enhanced bone marrow of the right tibial diaphysis associated with cortical bone hypertrophy suggesting chronic osteomyelitis (Fig. 4b). The muscles of his right lower leg (Fig. 4b) and the right triceps brachii muscle (Fig. 4c) were also enhanced, suggesting myositis. The X-ray and CT images showed hypertrophy of the cortical bone of the tibia bilaterally (Fig. 5). 67Ga scintigraphy showed slightly increased uptake in the middle of both lower legs. His CRP level was high (6.35 mg/dL), but his CK (87 IU/L), WBC count (6,730/μL), acetylcholinesterase (11.6 U/L), and soluble IL-2 receptor (279 U/mL) levels were all within the normal range. Anti-Jo-1 antibody was negative (<5.0 index). His electromyogram was normal and a muscle biopsy was not performed. Dermatomyositis, polymyositis, sarcoidosis, and lymphoproliferative syndrome were excluded. A subcutaneous nodule in the left upper arm was palpable and a biopsy was performed. Necrotizing vasculitis of a subfascial small artery was diagnosed pathologically (Fig. 6). Since rheumatoid vasculitis was suspected at that time, he was therefore started on PSL (10 mg/day), with the dose later increased to 20 mg/day. He was also treated with MTX (12 mg/week). At 26 years of age, he was taking 13 mg PSL/day and 12 mg MTX/week, but his bilateral lower leg pain and polyarthralgia became worse. He was admitted to our hospital. His temperature was 36.5°C and his left knee was swollen. He complained of bilateral lower leg pain, which was exacerbated by pressure, but he had no muscle weakness. His CRP was 7.07 mg/dL and his WBC count 8,430/μL. Both his CK and his MMP-3 levels were normal (22 IU/L and 96.7 ng/mL, respectively) and his RF and anti-CCP antibody levels remained negative. On MRI, the muscles of his lower legs were bilaterally enhanced and his bone marrow was of slightly higher than normal intensity (Fig. 7a, b). On X-rays, there was no evidence of the joint destruction or erosion typically associated with RA. His serum IL-6 and TNF-α levels were elevated: 74.4 pg/mL (normal range: ≤4.0) and 22.1 pg/mL (normal range: 0.6-2.8), respectively; his serum IL-1β level was 11 pg/mL (normal range: ≤10) and his serum IL-10 level was negative (<2 pg/mL). Autoinflammatory disease was suspected and he was started on 1 mg colchicine /day, but it was not effective. Chronic recurrent non-bacterial osteomyelitis was suspected, and the patient was then treated with TCZ (162 mg subcutaneously biweekly). After the first administration of TCZ, bilateral lower leg pain improved and the CRP level decreased to normal levels. The PSL dose could be tapered and his left knee swelling decreased after three months. The high intensity of the lower leg muscles detected in MRI also decreased after 10 months (Fig. 7c, d). The high intensity of bone marrow was still slightly higher than normal intensity, which was suggestive of chronic or old osteomyelitis (Fig. 7c).
Figure 4.
a: MRI (T1WI) of the right hand reveals synovitis and bone marrow edema of the fourth and fifth metacarpophalangeal joint. b: MRI (T2WI) shows enhanced bone marrow in the right tibial diaphysis associated with cortical bone hypertrophy. The right lower leg muscle was also enhanced, suggesting myositis. c: MRI (T2WI) reveals an enhancement of the right triceps brachii muscle.
Figure 5.
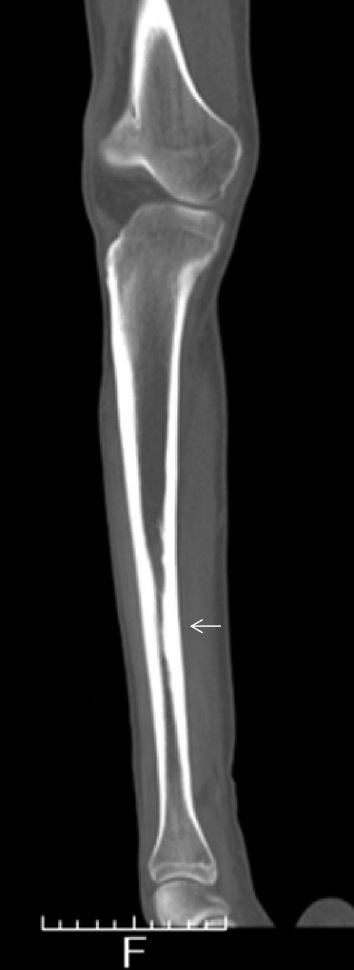
A CT scan of the right tibia show bilateral cortical bone hypertrophy in the tibias.
Figure 6.
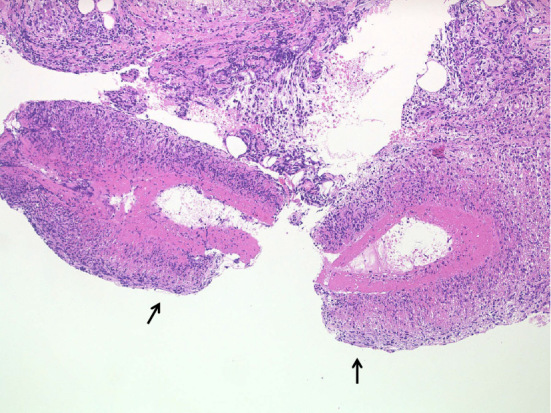
Pathological findings of a biopsy specimen obtained from a subcutaneous nodule in the left upper arm. Necrotizing vasculitis of a subfascial small artery was diagnosed pathologically.
Figure 7.
a/b: MRI (T2WI) shows slightly enhanced bone marrow in the right tibial diaphysis. The muscles were also bilaterally enhanced. c/d: MRI (T2WI) shows a decreased high intensity of the lower leg muscles. The high intensity of bone marrow was still slightly higher than normal intensity.
Discussion
Both of the patients presented herein had adult-onset nonbacterial CRMO of the diaphysis, high intensity of muscles detected by MRI, and polyarthritis. One patient also had cutaneous nodules of necrotizing vasculitis. TCZ was effective for controlling the disease in both cases.
Generally, the bone pain of CRMO is multifocal, typically involving the femoral and tibial metaphyses, the pelvis, clavicles, and vertebrae (1,4,10-12); however, as in our patients, the diaphyses of the long bones may also be involved (11). Arthritis is associated with CRMO in 11-38% of the cases (1,13). In our patients, synovitis was detected on MRI, but no joint destruction, which was seen in patients with RA, was observed.
CRMO is mainly seen in children, with only a few reports of patients with adult-onset CRMO (7,8). SAPHO syndrome covers a broad spectrum of findings (6) and it is diagnosed when a patient has as few as one of the four following nonspecific features: joint lesions accompanying severe acne; joint lesions accompanying palmoplantar pustulosis; osteohypertrophy of the extremities, spine, or sternocostoclavicular joints; and CRMO (9,14). Since CRMO is one of the criteria for SAPHO syndrome, regardless of the presence or absence of skin lesions, our patients with CRMO could also have been diagnosed with SAPHO syndrome. However, skin manifestations are a major feature of SAPHO syndrome and CRMO without skin lesions complicates its diagnosis. Thus, CRMO in adults should be distinguished diagnostically from SAPHO syndrome.
Since bacterial osteomyelitis and malignancy are most important for making a differential diagnosis of CRMO, many patients with CRMO undergo bone biopsies. Jansson et al. proposed a clinical score system for nonbacterial osteitis to avoid unnecessary bone biopsies (5). Wipff et al. used this score system in patients with CRMO, considered to be the most severe form of nonbacterial osteitis (1). The clinical score was based on seven factors, each of which was assigned a score: normal blood cell count (score: 13), symmetric lesions (10), lesions with marginal sclerosis (10), normal body temperature (9), vertebral, clavicular, or sternal lesions (8), radiologically proven lesions ≥2 (7), and CRP ≥1 mg/dL (6). A score ≥39 (out of a maximum of 63) is considered to be diagnostic of nonbacterial osteitis. In our patients, both patients had scores of 55, corresponding to 6 out of 7 risk factors (except vertebral, sternal, or clavicular lesions) (Table). Thus, both of our patients were diagnosed with CRMO.
Table.
Characteristics of the Patients.
| Patient 1 | Patient 2 | |
|---|---|---|
| Age at onset (years) | 17 | 23 |
| Age at diagnosis of CRMO | 48 | 26 |
| Periodic fever | - | - |
| Family history | - | - |
| Polyarthritis | +, Bilateral shoulders, right elbow and right wrist | +, MP and PIP joints, bilateral elbows and left knee |
| Joint destruction in X-rays | Poor | Poor |
| Higher intensity of muscles on MRI | + | + |
| Osteomyelitis | + | + |
| Sternoclavicular joint abnormality | - | - |
| Skin lesion | - | Subcutaneous nodule, (biopsy specimen showed necrotizing vasculitis of subfascial artery) |
| RF/ACPA/ANA/ANCA | -/-/-/- | -/-/-/- |
| MMP-3 | WNR | WNR |
| WBC count | 8,500/μL 10,700/μL (PSL 13 mg/day) | 6,730/μL 8,430/μL (PSL 13 mg/day) |
| CRP ≥1.0 mg/dL | + | + |
| PSL | NE (max dose; 10 mg) | NE (max dose; 20 mg) |
| Colchicine | NE (max dose; 0.5 mg) | NE (max dose; 1 mg) |
| MTX | NE (max dose; 8 mg/week) | NE (max dose; 12 mg/week) |
| NSAIDs | NE | NE |
| Bisphosphonate | NE (Minodronate) | - |
| TCZ | Effective | Effective |
| Clinical score system for CRMO1) | 55 (≥39 out of a maximum of 63 is considered CRMO) | 55(≥39 out of a maximum of 63 is considered CRMO) |
CRMO: chronic recurrent multiple osteomyelitis, MP joints: metacarpophalangeal joints, PIP joint, proximal interphalangeal joint, MRI: magnetic resonance imaging, CK: creatinine kinase, RF: rheumatoid factor, ACPA: anti-cyclic citrullinated peptide antibody, ANA: anti-nuclear antibody, ANCA: myeloperoxidase and proteinase 3 antineutrophil cytoplasmic antibody, MMP-3: matrix metalloproteinase-3, WBC: white blood cell, CRP: C-reactive protein, WNR: within the normal range, PSL: prednisolone, NE: not effective, MTX: methotrexate, NSAIDs: non-steroidal anti-inflammatory drugs, TCZ: tocilizmab
The high intensity of muscles detected on MRI, suggested the presence of myositis in both patients, but the CK levels were normal and muscle weakness was absent. There have been several reports of patients with CRMO and myositis. Whole-body MRI was performed in nine pediatric patients with CRMO and in one patient with myositis of the tibias and femurs (15). A soft-tissue reaction along the femoral diaphysis was detected by MRI in a pediatric patient with CRMO (11). These reports suggest an association of either myositis or soft-tissue inflammation with osteomyelitis because of the close anatomic relationships of the foci of myositis and osteomyelitis in these cases. A patient with CRMO associated with interstitial myositis has also been reported (16). In an 11-year-old boy, CRMO associated with myositis of the quadriceps femoris and gastrocnemius muscles, detected on MRI and fluorodeoxyglucose (FDG)-positron emission tomography/CT, was diagnosed, but the osteomyelitis did not involve his femurs and tibias. His CK level was normal and muscle weakness was not appreciable. A muscle biopsy revealed interstitial myositis. Similarly, in our patients, myositis was present not along, but extensively around the areas of osteomyelitis, and the CK levels were normal. An electromyogram of patient 2 was normal, as were his muscle fibers. Interstitial myositis may be associated with systemic and local inflammation, but muscle biopsies of both patients were not performed because muscle weakness was absent and the CK levels were normal. Further studies are needed to clarify the mechanism of myositis in patients with CRMO.
In patient 2, necrotizing vasculitis was detected following a biopsy of the subcutaneous nodule. There have been few reports of patients with necrotizing vasculitis and CRMO/SAPHO syndrome. In a patient with cutaneous necrotizing vasculitis and familial Mediterranean fever (FMF), one of the most frequent autoinflammatory diseases in adults, the subcutaneous nodules arising from the necrotizing vasculitis were successfully controlled with colchicine (17). In another patient with FMF and polyarteritis nodosa, interferon-alpha therapy was successful (18). Since, like FMF, CRMO is an autoinflammatory disease, necrotizing vasculitis can be considered to be a feature of CRMO, as was the case in our patient.
The pathogenesis of CRMO is still not well understood. Familial cases of CRMO have been reported and the susceptibility gene has been pinpointed to chromosome (18q21.3-18q22) (19), but this does not pathologically explain the non-familial cases. Recently, an imbalance between pro-inflammatory (TNF-α, IL-6) and anti-inflammatory (IL-10) cytokines was proposed to play a key role in CRMO (20). Lipopopolysaccharide stimulation increases IL-10 expression by monocytes from patients with CRMO, but the level of elevation is significantly lower than that seen in normal controls (21), which may reflect an impaired recruitment of the transcription factor specificity protein (Sp)-1 to the IL-10 promoter and reduced H3 serin 10 (H3S10) phosphorylation (21). Thus, a reduced activation of mitogen-activated protein (MAP) kinase, which is upstream of Sp1 and H3S10 phosphorylation signaling, was suggested to be involved in CRMO (22). In both of our patients, the serum IL-6 and TNF-α levels were elevated while the serum IL-10 levels were negative, and these findings are also in line with those of a previous study (21).
In children with CRMO, non-steroidal anti-inflammatory drugs (NSAIDs) are the first-line treatment and they are effective in 73% of cases (1). Naproxen results in a symptom-free status in 6 months in 43% of treated patients (13). Pamidronate also dramatically improves symptoms in patients with CRMO (23). The authors of the latter study suggested that pamidronate changes the relative proportions of cytokines (23). In addition to NSAIDs and pamidronate, patients with refractory CRMO have been treated with glucocorticoids, MTX, sulfasalazine, and anti-TNF therapy (1,3,13,24). Wipff et al. performed a cluster analysis of a CRMO cohort; all of the patients with the severe phenotype were male and 97% of them had multifocal disease (1). These severe phenotype patients also had the worst prognosis, with a remission rate of 22%, despite a higher rate of treatment with bisphosphonate and/or anti-TNF antibodies (33%) (1). Both of our patients were male and they had multifocal lesions; their symptoms and inflammation had continued for many years, even after treatment with NSAIDs, PSL, and MTX. Thus, male patients with adult-onset CRMO characterized by multifocal lesions might develop more severe forms of predominantly pediatric CRMO. Despite many reports in which autoinflammatory diseases were successfully treated using TCZ (25-27), ours is the first report of the successful use of TCZ for the treatment of CRMO. Our decision to use TCZ was based on the high serum IL-6 levels in our patients and previous reports of lower remission rates with anti-TNF antibodies in severe phenotype patients. The reductions in both the symptoms and inflammatory parameters indicate that TCZ may be effective for treating severe forms of CRMO.
The high intensity of the lower leg muscles detected by MRI significantly improved by TCZ therapy, but otherwise the high intensity of the bone marrow did not change in our patients.
In Case 2, the bone marrow enhancement in MRI prior to TCZ therapy (Fig. 7a) had already improved by PSL and MTX therapy, as shown in Fig. 4b. In Case 1, the bone marrow showed a slightly heterogeneous intensity without any treatment at 41 years of age, although a biopsy of the right tibia showed non-bacterial osteomyelitis at 38 years of age. MRI at 48 years of age showed a slightly higher intensity of bone marrow with PSL, and MTX therapy and TCZ did not improve the intensity. In our cases, adding TCZ to PSL and MTX did not significantly improve osteomyelitis according to the MRI findings. However, PSL could be tapered without an exacerbation of osteomyelitis and TCZ was therefore thought to have successfully controlled osteomyelitis in our patients.
In summary, we herein described the cases of two patients with adult-onset CRMO involving the diaphyses of the long bones and accompanied by high intensity of muscles on MRI and polyarthritis. Necrotizing vasculitis in one patient was also evident, based on a biopsy of a cutaneous nodule. In adult-onset CRMO, anti-IL-6 therapy should therefore be considered in patients with muscle inflammation not responding to NSAIDs, MTX and PSL.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Wipff J, Costantino F, Lemelle I, et al. A large national cohort of French patients with chronic recurrent multifocal osteitis. Arthritis Rheumatol 67: 1128-1137, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Stern SM, Ferguson PJ. Autoinflammatory bone diseases. Rheum Dis Clin North Am 39: 735-749, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borzutzky A, Stern S, Reiff A, et al. Pediatric chronic nonbacterial osteomyelitis. Pediatrics 130: e1190-e1197, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Khanna G, Sato TS, Ferguson P. Imaging of chronic recurrent multifocal osteomyelitis. Radiographics 29: 1159-1177, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Jansson AF, Muller TH, Gliera L, et al. Clinical score for nonbacterial osteitis in children and adults. Arthritis Rheum 60: 1152-1159, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum 42: 254-265, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Chow LT, Griffith JF, Kumta SM, Leung PC. Chronic recurrent multifocal osteomyelitis: a great clinical and radiologic mimic in need of recognition by the pathologist. APMIS 107: 369-379, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Hong CW, Hsiao EC, Horvai AE, Link TM. Chronic recurrent multifocal osteomyelitis with an atypical presentation in an adult man. Skeletal Radiol 44: 1359-1364, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi K, Matsusita M, Takagishi K. A case of SAPHO (synovitis-acne-pustulosis-hyperostosis-osteomyelitis) syndrome in which [18F]fluorodeoxyglucose positron emission tomography was useful for differentiating from multiple metastatic bone tumors. Mod Rheumatol 17: 67-71, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Surendra G, Shetty U. Chronic recurrent multifocal osteomyelitis: a rare entity. J Med Imaging Radiat Oncol 59: 436-444, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Wipff J, Adamsbaum C, Kahan A, Job-Deslandre C. Chronic recurrent multifocal osteomyelitis. Joint Bone Spine 78: 555-560, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Girschick HJ, Zimmer C, Klaus G, Darge K, Dick A, Morbach H. Chronic recurrent multifocal osteomyelitis: what is it and how should it be treated? Nat Clin Pract Rheumatol 3: 733-738, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Beck C, Morbach H, Beer M, et al. Chronic nonbacterial osteomyelitis in childhood: prospective follow-up during the first year of anti-inflammatory treatment. Arthritis Res Ther 12: R74, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 6: 109-112, 1988. [PubMed] [Google Scholar]
- 15. Guerin-Pfyffer S, Guillaume-Czitrom S, Tammam S, Kone-Paut I. Evaluation of chronic recurrent multifocal osteitis in children by whole-body magnetic resonance imaging. Joint Bone Spine 79: 616-620, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Nagashima S, Nozawa T, Kizawa T, et al. [Chronic recurrent multifocal osteomyelitis with interstitial myositis]. Nihon Rinsho Meneki Gakkai Kaishi (Jpn J Clin Immunol) 36: 52-57, 2013(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 17. Komatsu S, Honma M, Igawa S, et al. Cutaneous necrotizing vasculitis as a manifestation of familial Mediterranean fever. J Dermatol 41: 827-829, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Calguneri M, Apras S, Ozbalkan Z, Ozturk MA. The efficacy of interferon-alpha in a patient with resistant familial Mediterranean fever complicated by polyarteritis nodosa. Intern Med 43: 612-614, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Golla A, Jansson A, Ramser J, et al. Chronic recurrent multifocal osteomyelitis (CRMO): evidence for a susceptibility gene located on chromosome 18q21.3-18q22. Eur J Hum Genet 10: 217-221, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Hedrich CM, Hofmann SR, Pablik J, Morbach H, Girschick HJ. Autoinflammatory bone disorders with special focus on chronic recurrent multifocal osteomyelitis (CRMO). Pediatr Rheumatol Online J 11: 47, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofmann SR, Schwarz T, Moller JC, et al. Chronic non-bacterial osteomyelitis is associated with impaired Sp1 signaling, reduced IL10 promoter phosphorylation, and reduced myeloid IL-10 expression. Clin Immunol 141: 317-327, 2011. [DOI] [PubMed] [Google Scholar]
- 22. Hofmann SR, Roesen-Wolff A, Hahn G, Hedrich CM. Update: cytokine dysregulation in chronic nonbacterial osteomyelitis (CNO). Int J Rheumatol 2012: 310206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simm PJ, Allen RC, Zacharin MR. Bisphosphonate treatment in chronic recurrent multifocal osteomyelitis. J Pediatr 152: 571-575, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Walsh P, Manners PJ, Vercoe J, Burgner D, Murray KJ. Chronic recurrent multifocal osteomyelitis in children: nine years' experience at a statewide tertiary paediatric rheumatology referral centre. Rheumatology (Oxford) 54: 1688-1691, 2015. [DOI] [PubMed] [Google Scholar]
- 25. Koga T, Migita K, Kawakami A. Biologic therapy in familial Mediterranean fever. Mod Rheumatol 26: 637-641, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Hamanoue S, Suwabe T, Hoshino J, et al. Successful treatment with humanized anti-interleukin-6 receptor antibody (tocilizumab) in a case of AA amyloidosis complicated by familial Mediterranean fever. Mod Rheumatol 26: 610-613, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Hosoya T, Mizoguchi F, Hasegawa H, et al. A case presenting with the clinical characteristics of tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS) without TNFRSF1A mutations successfully treated with tocilizumab. Intern Med 54: 2069-2072, 2015. [DOI] [PubMed] [Google Scholar]