Abstract
In chronic mitral regurgitation (MR) left atrium is one of the first cardiac structures that is involved in remodeling and ultrastructural changes for a progressive volume overload. Severe left atrial (LA) dilation on echocardiography and new onset of atrial fibrillation in asymptomatic patients with preserved Left Ventricular (LV) function, appeared as a Class IIb recommendation for consideration for surgical mitral valve repair in the actual guidelines. However, before atrial dilatation and dysfunction, several ultrastructural changes appear in the atrial muscle tissue that are difficult to identify with the standard echocardiography. Speckle tracking echocardiography (STE) can analyze LA function: it has been showed that it can indirectly identify structural tissue modifications from excessive atrial effort in the early stages of MR up to the full depression of atrial function in the late stages where there are advanced ultrastructural alterations.
This review aims to summarize current knowledge on the role of atrial strain identifying early structural alterations of the atrial tissue in the rising stages of MR considering that Left Atrial Peak Longitudinal Strain (PALS) considered useful parameter for a more extensive evaluation of MR patients.
1. Background
Left atrial (LA) enlargement is part of cardiac remodeling observed in various cardiovascular diseases and appears to be associated with poor clinical outcome [1]. LA size is a significant predictor of stroke and death in the general population. LA dilation probably promotes stasis of blood, which in turn predisposes to thrombus formation and the potential for embolization. Another possible mechanism is that left atrial dilation may serve as a marker for other risk factors for stroke and death such as atrial fibrillation, structural heart disease, hypertension, or increased left ventricular mass [2].
In mitral regurgitation (MR), LA enlargement is part of pathophysiological changes: it compensates the volume overload [3] but is also a marker for future heart failure, [4] atrial fibrillation [5] and mortality after surgery [6]. Defining LA remodeling is essential for interpreting LA enlargement.
Echocardiography is widely available for LA size assessment [7]. Echocardiographic parameters of atrial dimension include LA antero-posterior diameter, LA area and volume. These parameters are more globally descriptive and they remain normal in the early phases of disease. The evaluation of LA remodeling is still difficult to obtain.
Speckle tracking echocardiography (STE) is a non–Doppler-based method for the objective quantification of myocardial deformation from standard bidimensional data sets [8] and it allows to obtain the quantification of longitudinal myocardial LA deformation dynamics [9].
2. MR and left atrium
In significant MR during the left ventricular (LV) systole, LV decompresses into the left atrium as the mitral orifice, in parallel with the aortic valve, acts as a pressure-dependent outlet for the ventricle. The physiological consequences to the left atrium are characterized by increases in LA volume and consequently of pressure and size; however, the magnitude of these changes depends chiefly on the rapidity and severity with which the regurgitation develops [10].
In chronic degenerative MR both the left ventricle and left atrium are subject to increased preload [11]. Due to adaptive remodeling of the left ventricular and atrial components and patient adaptation to the disease, patients can remain asymptomatic or minimally symptomatic for prolonged periods, even in the presence of severe MR [12]. LV ejection fraction frequently remains within the normal range in long-term MR even if LV contractile function may begin a slow but progressive deterioration [13]. However during the early phase, the heart compensates for increasing regurgitant volume by LA remodeling and enlargement, reflecting the left atrium as a principal MR target organ damage. This is accompanied by mechanic stress mediated cellular hypertrophy and interstitial fibrosis, that increase vulnerability to atrial fibrillation and LA failure [14]. In addition, LA remodeling has been documented as an important predictor of cardiovascular events [2], which is independently related to stroke and death [16], as well as to systolic and diastolic heart failure [17].
Early identification of atrial damage in MR may be the key to identifying the initial damage of this valvulopathy.
3. Left atrial ultrastructural changes in MR
The progressive increase in MR is associated with further increases in atrial size but diminished atrial stroke volume indicating that this extreme dilation no long provokes a Frank-Starling response and the atrial myocardium is made to operate on the descending limb of function; hence, the atrial chamber is less compliant [10], [11]. The left atrium responds to the excess volume load with a range of adaptive and maladaptive processes. These include myocyte growth, hypertrophy, and, finally, necrosis and apoptosis, as well as alterations in the composition of extracellular matrix, with excessive fibroblast proliferation, leading to myolysis and, consequently, loss of the contractile apparatus [18]. In particular ultrastructural analysis [19] performed on atrial tissues showed that hypertrophied atrial myocytes begin to undergo functional impairment and structural degeneration by alterations of Z-band structure, miofibrillar Iysis with preferential loss of thick filaments. Then marked interstitial fibrosis appear with dissociation of intercellular junctions, progressive loss of myofilaments and replacement by cytoskeletal filaments. The interstitial space is filled with large amounts of collagen fibrils, which often coursed in diverse directions, typical of areas with severe fibrosis. Atrial myocytes in or adjacent to these areas of severe interstitial fibrosis commonly show varying degrees of dissociation from adjacent cells. Both the interstitial fibrosis and the cellular dissociation slow the conduction of the impulse and favor the occurrence of reentry mechanisms, which constitute the electrophysiologic basis of atrial fibrillation and the perpetuation of atrial fibrillation.
4. Speckle tracking echocardiography: left atrial strain
Atria play an important role in ensuring proper performance of the systemic and pulmonary circulation and are also primarily involved in the context of supraventricular tachycardias in particular atrial fibrillation.
From a hemodynamic perspective atria play as booster pomp during late ventricular diastole, as reservoir for the inflow volume received from pulmonary veins during ventricular systole and isovolumic relaxation, and as passive conduits during early ventricular diastole and diastasis [20].
Evaluation of LA size and function can be obtained by two-dimensional echocardiography evaluation of LA antero-posterior diameter, area and volume, Doppler analysis of transmitral and pulmonary vein flow, and Tissue Doppler (TD) assessment of LA myocardial velocities [21, [22], [23]. The assessment of LA size provides prognostic information; however these parameters are morphometric and static. On the other hand information on regional LA function and remodeling may provide more insight in atrial electromechanical remodeling [24].
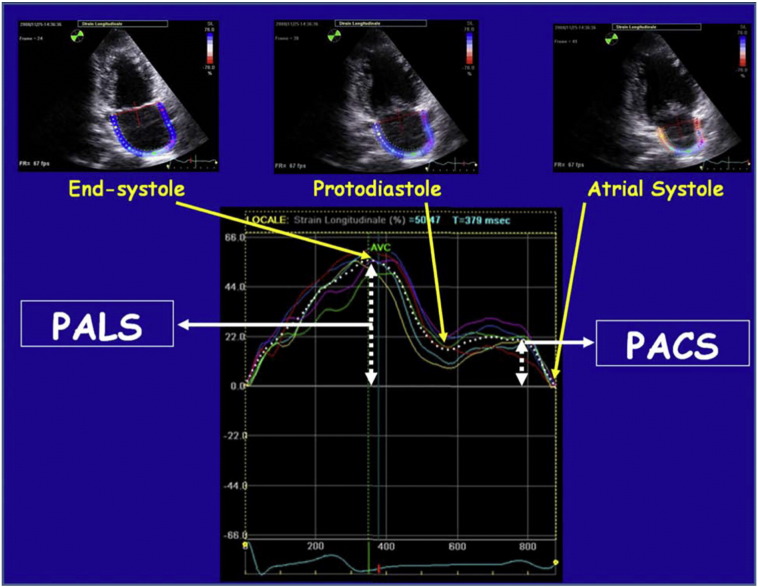
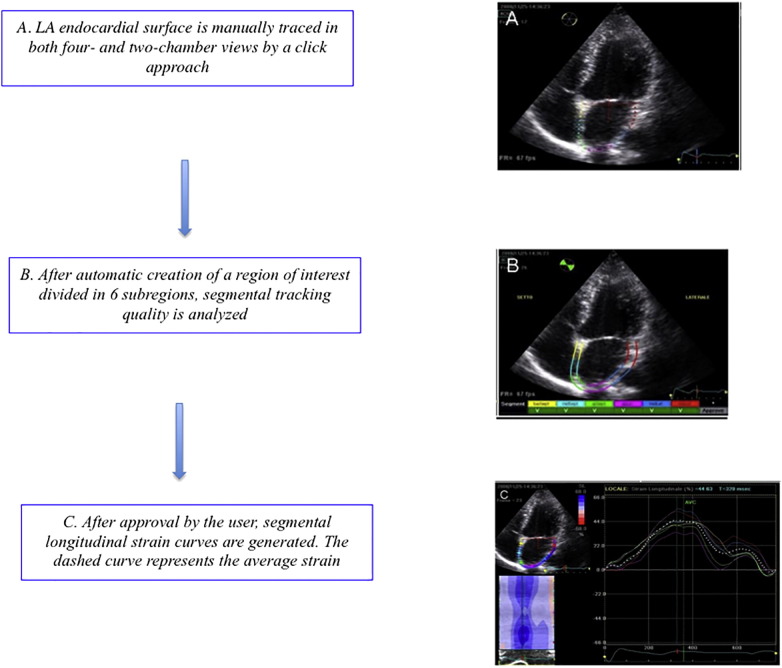
About that, speckle tracking echocardiography (STE) is an echocardiographic technique that uses standard B-mode images for regional and global myocardial function analysis. It is based on an analysis of the spatial dislocation (referred to as tracking) of speckles (defined as spots generated by the interaction between the ultrasound beam and myocardial fibers) on routine 2-dimensional sonograms [25]. The displacement of the speckled pattern is considered to follow myocardial movement, and a change between speckles is assumed to represent myocardial deformation [26]. STE analysis allows an excellent assessment of the atrial deformation profile during an entire cardiac cycle, closely following the LA physiology. In particular LA endocardial surface is manually traced in both four- and two-chamber views by a click approach. The software generates the longitudinal strain curves for each segment and a mean curve of all segments that reflect the pathophysiology of atrial function: peak atrial longitudinal strain (PALS), measured at the end of the reservoir phase, and peak atrial contraction strain (PACS), measured just before the start of the active atrial contractile phase [27] (Fig. 1). They are calculated by averaging values observed in all LA segments (global PALS and PACS), and by separately averaging values observed in four- and two-chamber views (four- and two-chamber average PALS and PACS, respectively). The time to peak longitudinal strain (TPLS) is measured as the average of all 12 segments (global TPLS) and by separately averaging values observed in the two apical views (4- and 2- chamber average TPLS). In contrast to Doppler derived parameters, speckle tracking has the advantage of being angle independent, and to be less affected by reverberations, side lobes and drop out artifacts [8] (Fig. 5).
Fig. 1.
Composite figure showing the measurement of peak atrial longitudinal strain (PALS) and of peak atrial contraction strain (PACS) using the speckle tracking echocardiography (STE) from an apical two-chamber view, in a representative subject [27].
Fig. 5.
Diagram with a detailed overview on how to perform the procedure of atrial strain [8].
Speckle tracking imaging was found to be a feasible and reproducible method to assess LA longitudinal strain [8]. The reproducibility of measurements was good, with lower variability in comparison with that obtained by Doppler-derived LA strain imaging [30].
5. Correlation between left atrial strain and left Atrial dysfunction in MR
The analysis of atrial strain has been demonstrated useful in many clinical settings [27]. A strong association between decrease of LA myocardial deformation assessed by speckle tracking echocardiography and cardiovascular events was demonstrates [31].
The decrease of LA reservoir and the increase of LA pump functions are the first manifestations of the burden of diastolic dysfunction, appearing before the LA structural changes. Several studies have shown that strain imaging can detect LA dysfunction before the manifestation of LA structural changes [32].
In patients with chronic MR, global PALS is significantly influenced by the degree of MR. A supernormal PALS has been found [14] in mild MR, instead a strong depression has been recorded only in patients with severe MR and it is more depressed in those who experienced an episode of paroxysmal AF (PAF) [33].
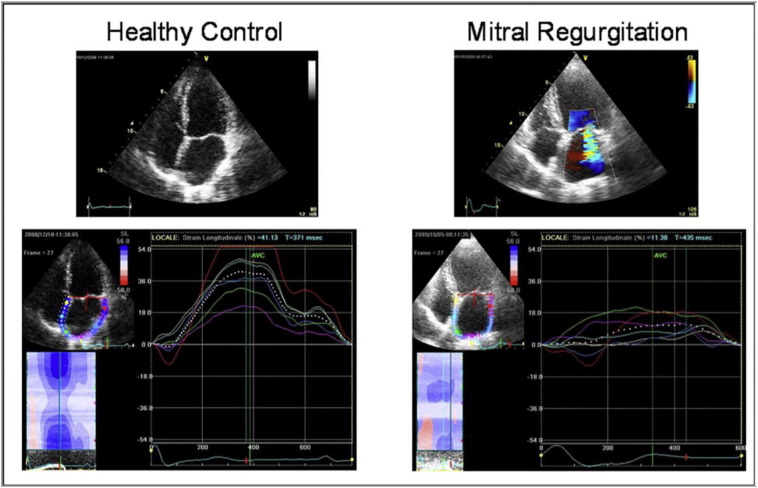
Infact the global PALS is the parameter for the functional evaluation of the reservoir phase that is essential for LV filling by storing energy during ventricular systole [34]. It resulted increased in patients with mild MR, possibly due to enhanced atrial compliance [10]. Instead, it was found a progressive impairment of global PALS in moderate and severe MR groups, potentially explained by the LA ultrastructural abnormalities associated to chronic MR, such as myocyte hypertrophy, interstitial fibrosis, decreased metalloproteinase expression and finally electrical dissociation between muscle bundles that facilitate the initiation and perpetuation of AF [35] (Fig. 2, Fig. 3).
Fig. 2.
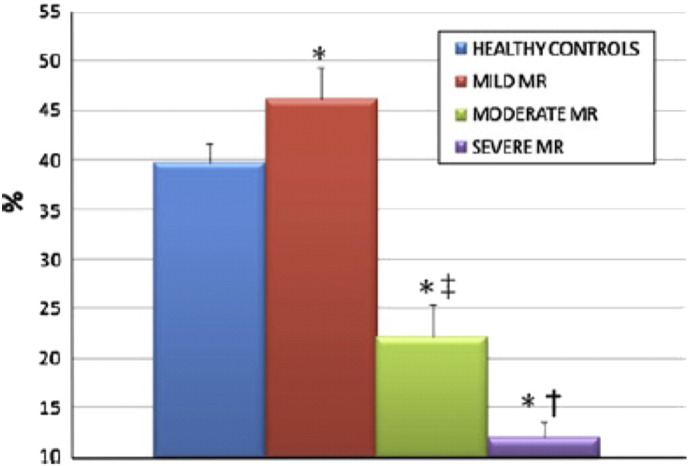
Comparison of global peak atrial longitudinal strain (PALS) among controls and patients with mild, moderate and severe mitral regurgitation (MR) [14].
Fig. 3.
Peak atrial longitudinal strain (PALS): two representative cases of a healthy control (left) and of a patient with severe mitral regurgitation (right) [14].
Strain parameters are influenced by loading conditions and by rhythm irregularity, not only by fibrosis [36]. Left atrial strain is significantly lower in subjects with AF as compared to hypertensive patient. Post-conversion to sinus rhythmus, LA Strain increased significantly, but did not achieve the values found in the controls [37].
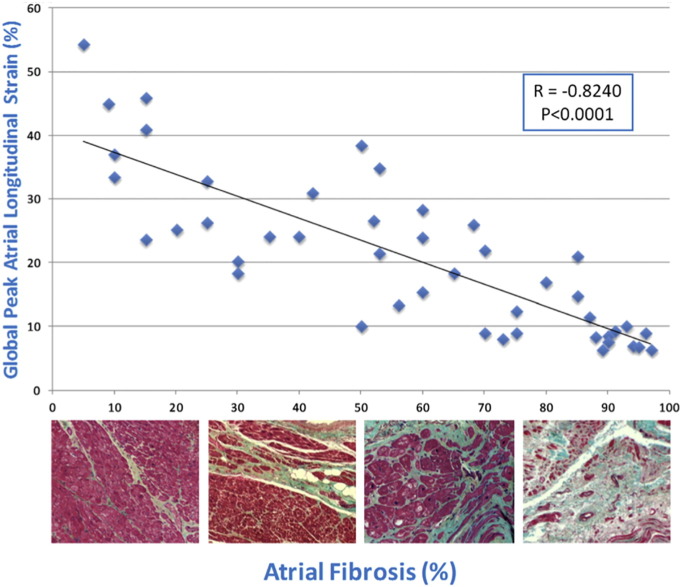
Recently [43] the correlation between the grade of LA fibrosis and the speckle tracking index of LA function (global PALS) was analyzed in patients with severe MR who were referred for mitral surgery. LA tissue samples were obtained from all patients. Masson’s trichrome staining was performed to assess the extent of the fibrosis. The LA endocardial thickness was measured. In this study, speckle tracking echocardiographic analysis showed a stepwise reduction of global PALS with a more depressed value in patients with a major grade of fibrosis at the histologic analysis. Statistical analysis showed a close negative correlation between global PALS measured by STE and LA myocardial fibrosis grade compared to poorer correlations for the LA indexed volume, LA ejection fraction, and E/E [1] ratio. Indeed the increase in interstitial fibrosis in the setting of mitral valvular disease compromises the elastic properties of the atrial myocardium and inevitably leads to impairment of atrial compliance and thus to a reduction of LA reservoir function, as assessed by global PALS [38] (Fig. 4).
Fig. 4.
Correlation between global PALS and LA fibrosis [43].
Several studies showed that quantitative assessment of atrial function by echocardiography as an additional tool to guide the optimum timing of surgery for MR. [39]
Analysis of cardiac mechanics through speckle-tracking imaging has been proposed for risk stratification in asymptomatic patients with chronic primary MR. Compared to asymptomatic patients, those with events at baseline showed more severe MR, larger and spherical ventricles, diastolic dysfunction, and greater systolic pulmonary arterial pressure. Moreover, patients with events had decreased LA reservoir so LA reservoir evaluation significantly improved risk stratification and is considered an independent predictors of outcome [40]. Furthermore in patients with asymptomatic severe primary MR, reduced Global peak positive strain of the left atrium and strain rate in the LA filling phase predicted a worse prognosis. These findings may offer additional information to guide early surgery [41].
The clinical utility of LA functional indexes in patients with mitral valve prolapse (MVP) and MR was also investigated using 2D speckle imaging, maximal left atrial volume (LAVmax) and minimal left atrial volume (LAVmin) and total left atrial emptying fraction (TLAEF). By multivariate analysis, TLAEF and atrial strain were independent predictors of severe MR requiring surgery.39 In this regard, volume overload generally diminishes after mitral valve surgery and LA size and shape are expected to recover. On the other hand the volume overload for extended periods may result in irreversible ultrastructural changes and reverse remodeling is said to be related with prognosis and mortality. It was demonstrated that Left atrial PALS, in conjunction with preoperative LA volume index (LAVI) and age is a predictor of LA reverse remodeling in patients undergoing surgery for severe MR. LA reservoir strain is an independent predictor and has a high accuracy to identify patients with indications for mitral surgery [42]. Moreover impaired LA reservoir strain in patients with severe organic MR relates to long-term survival after mitral valve surgery, independently of and incremental to current guidelines-based indications for mitral surgery (symptoms, left ventricular ejection fraction ≤ 60%, left ventricular end-systolic diameter ≥ 40 mm, atrial fibrillation, or systolic pulmonary arterial pressure > 50 mmHg). Hence PALS may be used as a valid preoperative prognostic marker [43], [44].
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels increased among all moderate or severe chronic MR. It has already shown that in MR, changes in volume load may be paralleled by changes in the N-terminal brain natriuretic peptide (NT-proBNP) level and that the fall in NT-proBNP was related to corrections in volume and removal of the diastolic run-off into the left atrium [45], [46]. However there are no data on the relationship between levels of ANP, BNP, NT-proBNP and atrial strain in patients with mitral regurgitation. LA wall fibrosis, implied by delayed enhancement on MRI, is inversely related to LA strain and strain rate and these are related to the AF burden. Lower midlateral strain and strain rate and midseptal strain rate were independent predictors of a larger extent of fibrosis. Echocardiographic assessment of LA structural and functional remodeling is quick and feasible and may be helpful in predicting outcomes in AF [47].
The two-dimensional speckle tracking (2DT) method is based on the measurements of strain on two-dimensional (2D) images, ignoring actual three-dimensional (3D) myocardial movements. 3D–STE has not been a routine tool for cardiac function analysis, despite of various advantages and potentials compared to 2-dimensional (2D) imaging. 3D–STE can simultaneously provide the three orthogonal strain values, radial strain (RS), longitudinal strain (LS), and circumferential strain (CS). In addition, 3D–STE provides a novel deformation parameter, area change ratio, which have the potential for more accurate assessment of overall and regional myocardial function. 3D–STE has great promise as a clinical feasible tool for evaluating myocardial function, and could provide novel pathophysiological insights based on 3D cardiac mechanics. However, the clinical uses are not enough. Therefore, information about the current status of 3D–STE should be spread [48], [49].
6. Conclusions
A routine measurement of LA strain might guarantee the detection of LA remodeling, providing useful adjunctive information in patients undergoing echocardiography for cardiac surgery. Thus, this novel imaging method can be considered a promising index for the better quantification of LA function in patients with chronic MR, allowing the potential identification of LA impairment, useful for deciding the timing of surgery [18]. Infact this method has proven to be able to investigate noninvasively the early and asymptomatic ultrastructural changes appearing in the atrial tissue in a similar way to a tissue biopsy. This allows us to early identification of patients whose valve disease has started to create irreversible damage to the atrial myocardium and it can be considered a useful marker to predict good or poor survival after surgery.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Messika-Zeitoun D., Bellamy M., Avierinos J.F., Breen J., Eusemann C., Rossi A., Behrenbeck T., Scott C., Tajik J., Enriquez-Sarano M. Left atrial remodelling in mitral regurgitation- methodologic approach, physiological determinants, and outcome implications: A prospective quantitative Doppler-echocardiographic and electron beamcomputed tomographic study. Eur. Heart J. 2007:1773–1781. doi: 10.1093/eurheartj/ehm199. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., D'Agostino R.B., Belanger A.J., Wolf P.A., Levy D. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E., Awe W. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation. 1963;27:29–35. doi: 10.1161/01.cir.27.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Ling H., Enriquez-Sarano M., Seward J., Tajik A., Schaff H., Bailey K., Frye R. Clinical outcome of mitral regurgitation due to flail leaflets. N. Engl. J. Med. 1996;335:1417–1423. doi: 10.1056/NEJM199611073351902. [DOI] [PubMed] [Google Scholar]
- 5.Grigioni F., Avierinos J.F., Ling L.H., Scott C.G., Bailey K.R., Tajik A.J., Frye R.L., Enriquez-Sarano M. Atrial fibrillation complicating the course of degenerative mitral regurgitation: Determinants and long-term outcome. J. Am. Coll. Cardiol. 2002;40:84–92. doi: 10.1016/s0735-1097(02)01922-8. [DOI] [PubMed] [Google Scholar]
- 6.Reed D., Abbott R., Smucker M., Kaul S. Prediction of outcome after mitral valve replacement in patients with symptomatic chronic mitral regurgitation. The importance of left atrial size. Circulation. 1991;84:23–34. doi: 10.1161/01.cir.84.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Lester S.J., Ryan E.W., Schiller N.B., Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am. J. Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 8.Cameli M., Caputo M., Mondillo S. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound. 2009;7:6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muranaka A., Yuda S., Tsuchihashi K. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography. 2009;26(3):262–271. doi: 10.1111/j.1540-8175.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- 10.Kihara Y., Sasayama S., Miyazaki S., Onodera T., Susawa T., Nakamura Y., Fujiwara H., Kawai C. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ. Res. 1988;62:543–553. doi: 10.1161/01.res.62.3.543. [DOI] [PubMed] [Google Scholar]
- 11.Ren B., de Groot-de Laat L.E., Geleijnse M.L. Left atrial function in patients with mitral valve regurgitation. Am. J. Physiol. Heart Circ. Physiol. 2014 Nov 15;307(10):H1430–7. doi: 10.1152/ajpheart.00389.2014. [DOI] [PubMed] [Google Scholar]
- 12.Raymond L., Marwick T.H. Assessment of subclinical left ventricular dysfunction in asymptomatic mitral regurgitation. Eur. J. Echocardiogr. 2007;8:175–184. doi: 10.1016/j.euje.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Starling M.R., Kirsh M.M., Montgomery D.G. Impaired left ventricular contractile function in patients with longterm mitral regurgitation and normal ejection fraction. J. Am. Coll. Cardiol. 1993;22:239–250. doi: 10.1016/0735-1097(93)90840-w. [DOI] [PubMed] [Google Scholar]
- 14.Cameli M., Lisi M., Giacomin E., Caputo M., Navarri R., Malandrino A., Ballo P., Agricola E., Mondillo S. Chronic mitral regurgitation: Left atrial deformation analysis by two-dimensional speckle tracking echocardiography. Echocardiography. 2011 Mar;28(3):327–334. doi: 10.1111/j.1540-8175.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 16.Kizer J., Bella J., Palmieri V. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The strong heart study (SHS) Am. Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Gottdiener J., Kitzman D., Aurigemma G. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons.65 Years of age (the cardiovascular health study) Am. J. Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 18.Cameli M., Lisi M., Righini F.M., Massoni A., Natali B.M., Focardi M., Tacchini D., Geyer A., Curci V., Di Tommaso C., Lisi G., Maccherini M., Chiavarelli M., Massetti M., Tanganelli P., Mondillo S. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am. J. Cardiol. 2013 Feb 15;111(4):595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Thiedemann K.U. Ferrans VJ left atrial ultrastructure in mitral valvular disease. Am. J. Pathol. 1977 Dec;89(3):575–604. [PMC free article] [PubMed] [Google Scholar]
- 20.Di Salvo G., Galderisi M., Rea A., Ansalone G., Dini F.L., Gallina S., Mele D., Montisci R., Sciomer S., Mondillo S., Di Bello V., Marino P.N. Gruppo di Lavoro di Ecocardiografia; Società Italiana di Cardiologia: Evaluation of atrial function by echocardiography. G. Ital. Cardiol. 2007;8:225–235. [PubMed] [Google Scholar]
- 21.Nakao F., Wasaki Y., Kimura M., Iwami T., Iida H., Wakeyama T., Miura T., Ogawa H., Matsuzaki M. Evaluation of left atrial function by the functional volume change curve derived from Doppler flow spectra. Jpn. Circ. J. 2001;65:953–957. doi: 10.1253/jcj.65.953. [DOI] [PubMed] [Google Scholar]
- 22.Marino P., Faggian G., Bertolini P., Mazzucco A., Little W. Early mitral deceleration and left atrial stiffness. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1172–1178. doi: 10.1152/ajpheart.00051.2004. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Paredes M., Gonzálvez M., Ruiz Ros J.A., Giménez D.M., Carnero A., Carrillo A., Cubero T., Martínez-Corbalán F.R., García Almagro F. Assessment of left Atrial Wall velocities by pulsed wave tissue Doppler imaging. A new approach to the study of atrial function. Rev. Esp. Cardiol. 2004;57:1059–1066. [PubMed] [Google Scholar]
- 24.Tops L.F., Van der Wall E.E., Schalij M.J., Bax J.J. Multimodality imaging to assess left atrial size, anatomy and function. Heart. 2007;93:1461–1470. doi: 10.1136/hrt.2007.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondillo S., Galderisi M., Mele D., Cameli M., Lomoriello V.S., Zacà V., Ballo P., D'Andrea A., Muraru D., Losi M., Agricola E., D'Errico A., Buralli S., Sciomer S., Nistri S., Badano L., Echocardiography Study Group Of The Italian Society Of Cardiology (Rome, Italy) Speckletracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011;30(1):71–83. doi: 10.7863/jum.2011.30.1.71. [DOI] [PubMed] [Google Scholar]
- 26.Teske A.J., De Boeck B.W., Melman P.G., Sieswerda G.T., Doevendans P.A., Cramer M.J. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc. Ultrasound. 2007;5:27. doi: 10.1186/1476-7120-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameli M., Lisi M., Righini F.M., Mondillo S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc. Ultrasound. 2012;1:10e14. doi: 10.1186/1476-7120-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirbu C., Herbots L., D'hooge J., Claus P., Marciniak A., Langeland T., Bijnens B., Rademakers F.E., Sutherland G.R. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: A study in normal subjects. Eur. J. Echocardiogr. 2006;7:199–208. doi: 10.1016/j.euje.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Cameli M., Lisi M., Focardi M., Reccia R., Natali B.M., Sparla S., Mondillo S. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am. J. Cardiol. 2012 Jul 15;110(2):264–269. doi: 10.1016/j.amjcard.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Kokubu N., Yuda S., Tsuchihashi K., Hashimoto A., Nakata T., Miura T., Ura N., Nagao K., Tsuzuki M., Wakabayashi C., Shimamoto K. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible benificial effect of Renin-Angiotensin System Inhibition on left atrial function. Hypertens. Res. 2007;30:13–21. doi: 10.1291/hypres.30.13. [DOI] [PubMed] [Google Scholar]
- 33.Cameli M., Lisi M., Righini F.M., Focardi M., Alfieri O., Mondillo S. Left atrial speckle tracking analysis in patients with mitral insufficiency and history of paroxysmal atrial fibrillation. Int. J. Card. Imaging. 2012 Oct;28(7):1663–1670. doi: 10.1007/s10554-011-9987-y. [DOI] [PubMed] [Google Scholar]
- 34.Grant C., Bunnell I.L., Greene D.G. The reservoir function of the left atrium during ventricular systole. Anangiocardiographic study of atrial stroke volume and work. Am. J. Med. 1964;37:36–43. doi: 10.1016/0002-9343(64)90210-4. [DOI] [PubMed] [Google Scholar]
- 35.Kirchhof P, Bax J, Blomstrom-Lundquist C, Calkins H, Camm AJ, Cappato R, Cosio F, Crijns H, Diener HC, Goette A, Israel CW, Kuck KH, Lip GY, Nattel S, Page RL, Ravens U, Schotten U, Steinbeck G, Vardas P, Waldo A, Wegscheider K, Willems S, Breithardt G: Early and comprehensive management of atrial fibrillation: proceedings from the 2nd AFNET/EHRA consensus conference on atrial fibrillation entitled “research perspectives in atrial fibrillation”. Europace 11:860–885. [DOI] [PubMed]
- 36.Di Salvo G., Caso P., Lo Piccolo R., Fusco A., Mar-tiniello A.R. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external CV of recent-onset lone atrial brillation: A color Doppler myocardial imaging and transtho-racic and transesophageal echocardiographic study. Circulation. 2005;112(3):387–395. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 37.Gyalai-Korpos I., Andor M., Tomescu M.C., Bordejevic A., Marincu I. Speckle echocardiographic left atrial strain as predictor of atrial fibrillation recurrence in hypertensive patients. HealthMED. 2014;8(6) [Google Scholar]
- 38.Cameli M., Lisi M., Mondillo S., Padeletti M., Ballo P., Tsioulpas C., Bernazzali S., Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc. Ultrasound. 2010;8:14. doi: 10.1186/1476-7120-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ring L., Rana B.S., Wells F.C., Kydd A.C., Dutka D.P. Atrial function as a guide to timing of intervention in mitral valve prolapse with mitral regurgitation. JACC Cardiovasc. Imaging. 2014 Mar;7(3):225–232. doi: 10.1016/j.jcmg.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Zito C., Manganaro R., Khandheria B., Oreto G., Cusmà-Piccione M., Todaro M.C., Caprino A., Pugliatti P., Di Bella G., Carerj S. Usefulness of left atrial reservoir size and left ventricular untwisting rate for predicting outcome in primary mitral regurgitation. Am. J. Cardiol. 2015 Oct 15;116(8):1237–1244. doi: 10.1016/j.amjcard.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 41.Yang L.T., Liu Y.W., Shih J.Y., Li Y.H., Tsai L.M., Luo C.Y., Tsai W.C. Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J. Am. Soc. Echocardiogr. 2015 Nov;28(11):1309–1317. doi: 10.1016/j.echo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Candan O., Ozdemir N., Aung S.M., Hatipoglu S., Karabay C.Y., Guler A., Gecmen C., Dogan C., Omaygenc O., Bakal R.B. Atrial longitudinal strain parameters predict left atrial reverse remodeling after mitral valve surgery: A speckle tracking echocardiography study. Int. J. Card. Imaging. 2014 Aug;30(6):1049–1056. doi: 10.1007/s10554-014-0433-9. [DOI] [PubMed] [Google Scholar]
- 43.Debonnaire P., Leong D.P., Witkowski T.G., Al Amri I., Joyce E., Katsanos S., Schalij M.J., Bax J.J., Delgado V., Marsan N.A. Left atrial function by two-dimensional speckle-tracking echocardiography in patients with severe organic mitral regurgitation: association with guidelines-based surgical indication and postoperative (long-term) survival. J. Am. Soc. Echocardiogr. 2013 Sep;26(9):1053–1062. doi: 10.1016/j.echo.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Dayal N.B., Müller H. ESC (European Society of Cardiology): Guidelines on the management of valvular heart disease (version 2012) Rev. Med. Suisse. 2014 May 28;10(432) (1166, 1168–72) [PubMed] [Google Scholar]
- 45.Shimamoto K., Kusumoto M., Sakai R., Watanabe H., Ihara S., Koike N., Kawana M. Usefulness of the brain natriuretic peptide to atrial natriuretic peptide ratio in determining the severity of mitral regurgitation. Can. J. Cardiol. 2007 Mar 15;23(4):295–300. doi: 10.1016/s0828-282x(07)70758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prakaschandra D.R., Naidoo D.P., Esterhuizen T. The time-course changes of NT-proBNP and tissue Doppler indices in patients undergoing mitral valve replacement. Cardiovasc. J. Afr. 2012 May;23(4):200–205. doi: 10.5830/CVJA-2011-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuppahally S., Akoum N., Burgon N.S., Badger T.J., Kholmovski E.G., Vijayakumar S., Rao S.N., Blauer J., Fish E.N., DiBella E.V.R., MacLeod R.S., McGann C., Litwin S.E., Marrouche N.F. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation relationship to left atrial structural remodeling detected by delayed-enhancement MR. Circ. Cardiovasc. Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 48.Saito K., Okura H., Watanabe N., Hayashida A., Obase K., Imai K., Maehama T., Kawamoto T., Neishi Y., Yoshida K. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J. Am. Soc. Echocardiogr. 2009 Sep;22(9):1025–1030. doi: 10.1016/j.echo.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Seo Y., Ishizu T., Aonuma K. Current Status of 3-Dimensional Speckle Tracking Echocardiography: A review from our experiences. J. Cardiovasc. Ultrasound. 2014 Jun;22(2):49–57. doi: 10.4250/jcu.2014.22.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]