Abstract
Importance
Cardiorespiratory fitness (CRF) as assessed by formalized incremental exercise testing is a strong independent predictor of numerous chronic diseases but has received little attention as a predictor of incident cancer or survival following a diagnosis of cancer.
Objective
To assess the association between midlife CRF and incident cancer and survival following a cancer diagnosis.
Design
Prospective, observational cohort study.
Setting
Preventive medicine clinic
Participants and Exposures
The prospective, observational cohort study included 13,949 community-dwelling men who had a baseline fitness examination. All men completed a comprehensive medical examination, a cardiovascular risk factor assessment, and incremental treadmill exercise test to evaluate CRF. We utilized age-sex specific distribution of treadmill duration from the overall CCLS population to define fitness groups as low (lowest 20%), moderate (middle 40%), and high (upper 40%) fit groups. The adjusted multivariable model included: age, exam year, body mass index, smoking, total cholesterol, systolic blood pressure, diabetes, fasting glucose.
Main Outcome Measures
(1) Incident prostate, lung, and colorectal cancer, and (2) all-cause mortality and cause-specific mortality among men who developed cancer are Medicare age (on or after age 65 years).
Results
Compared to low CRF, the adjusted hazard ratio (HR) for incident lung, colorectal, and prostate cancer among men with high CRF was 0.45 (95% CI: 0.29-0.68), 0.56 (95% CI: 0.36-0.87), 1.22 (95% CI: 1.02-1.46), respectively. Among those diagnosed with cancer at Medicare age (65 years), high CRF in mid-life was associated with an adjusted 36% (HR 0.64, 95% CI: 0.45-0.93) risk reduction in all cancer related deaths and a 69% reduction in cardiovascular disease mortality following a cancer diagnosis (HR 0.31, 95% CI: 0.15-0.62) compared to low fit men in mid-life.
Conclusions and Relevance
There is a strong inverse relationship between midlife CRF and incident lung and colorectal cancer but not prostate cancer. High mid-life CRF is also protective against the risk of cause-specific mortality in those diagnosed with cancer at Medicare age.
Introduction
A well-established, strong, graded, inverse relationship exists between cardiorespiratory fitness (CRF) and risk of cardiovascular disease (CVD) as well as all-cause mortality in numerous healthy and clinical adult populations.1-3 Compared to those classified in the lowest CRF category (< 7.9 Metabolic equivalents = METs), individuals in the highest CRF category (≥ 10.9 METs) have between a 1.6 to 1.7-fold lower risk of CVD and all-cause mortality, respectively.4 Accordingly, measurement of CRF via formalized exercise testing provides a wealth of diagnostic and decision-making information in cardiovascular medicine. (REF, Kaminsky)
In stark contrast, the value of CRF for prediction of primary cancer risk has surprisingly received little attention.1-3, 5, 6 The reasons for the paucity of interest are not known, however it is now clear that CVD and cancer account for the majority of deaths in the US,7 with these diseases sharing common risk factors (e.g., tobacco use, poor diet, and insufficient physical activity).8 The powerful value of CRF in the prediction of CVD indicates that such a measure may also be of importance for the prediction of the primary risk of cancer. Evaluation of this question is important for several reasons. First, given that individual risk (of CVD and cancer) is determined by multiple factors, current guidelines advocate for global or multiple-risk factor assessment, using tools such as the Framingham Risk Score. CRF is not currently included as an aspect of general prevention screening guidelines for all average risk adults. However, CRF improves the discrimination and reclassification of CVD mortality risk prediction9, as well as refinement of Framingham Risk Score among adults even among those at low-risk of CVD.10 Second, cancer incidence is projected to increase by approximately 45% over the next two decades,11 largely as a result of the rapidly aging population combined with the fact that the majority of all cancer diagnoses occur in individuals over the age of 65 years.12 Thus, investigation of the predictive value of CRF on primary cancer incidence could have significant public health implications since it will provide medical professionals with a quantitative as well as modifiable risk factor (as opposed to a subjective behavioral risk factor) that simultaneously predicts risk of the most common chronic diseases.13
Third, there is growing evidence that lifestyle behaviors performed years, even decades prior to a cancer diagnosis may strongly influence outcomes after diagnosis. Indeed, midlife body mass index (BMI) and physical activity are strong predictors of cancer-specific as well as all-cause mortality in multiple cancer diagnoses.14-20 To our knowledge, no study to date has investigated whether objective measures of exercise exposures (i.e., CRF) in apparently healthy persons at midlife is predictive of primary risk of cancer as well as cause-specific mortality in those who are subsequently diagnosed with cancer. Prediction of cause-specific mortality after a cancer diagnosis is becoming increasingly important given that individuals diagnosed with certain forms of cancer now have sufficient survival to be at risk for non-cancer competing causes of mortality, primarily CVD due to the chronic and late-effects of treatment.21
Here, we report on a prospective investigation of 13,949 men from the Cooper Center Longitudinal Study (CCLS) to examine the relationship between CRF assessed before age 65 and (1) incidence of lung, colorectal, or prostate cancer and (2) cause-specific mortality in men diagnosed with cancer on or after age 65. We hypothesized that higher midlife CRF would be associated with reduced incidence of lung, colorectal and prostate cancer, and lower risk of cancer and CVD-related mortality in those subsequently diagnosed with cancer.
Methods
Participants and Procedures
The CCLS is a prospective observational cohort study of participants undergoing a preventive health examination at the Cooper Clinic in Dallas, Texas. Patients enrolled in CCLS signed an informed consent, and The Cooper Institute's Institutional Review Board approved this study. A detailed overview of the methods and procedures of CCLS has been described previously.3, 22, 23 The sampling frame for the present study included 25, 575 individuals in the CCLS completing an incremental treadmill exercise test between 1971 and 2009 and enrolled in Medicare between 1999 and 2009; the available years of claims data at the time of this study. The following participants were excluded: (1) women (n=5,871), (2) those lacking traditional fee-for-service Medicare for whom individual claims data were not available (n=998), (3) individuals without a complete set of baseline variables (n=2,096), (4) participants with myocardial infarction or stroke at their midlife examination visit (n=413), (5) individuals with a cancer diagnosis or death prior to Medicare age (n=1,640), and (6) participants with a first CCLS visit at age 65 years or older (n=552) or a chronic illness requiring Medicare coverage prior to age 67 (n=56). The final cohort included 13,949 men.
Midlife Exposures
The preventive health examination consisted of an extensive medical history, laboratory analysis, blood pressure ascertainment, and an incremental exercise treadmill test. Age, gender, and personal medical history were obtained by self-administered questionnaires; all data was physician verified. Blood pressure was measured with standard auscultatory methods. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Diabetes was defined by self-report or blood glucose ≥ 126 mg/dL. Smoking was categorized into current, former, and never categories. A 12-hour fasting antecubital venous blood sample was obtained and plasma concentrations of glucose and lipids were determined with automated bioassays in the CCLS laboratory.
CRF was assessed by an incremental treadmill test using a modified-Balke protocol as described previously.22 In brief, treadmill speed was set at 3.3 mph (88 m/min) at a grade of 0% in the first minute, followed by 2% in the second minute with an increase of 1% every minute thereafter. After 25 minutes, grade was unchanged while speed increased 0.3 mph (5.4 m/min) every additional minute until volitional exhaustion. Using well-characterized regression equations, treadmill time using the Balke protocol permits estimation of peak Metabolic Equivalents (METs).24 Time to volitional exhaustion is strongly correlated with direct measurement of maximal oxygen uptake (r=0.92).25 CRF was defined as both a continuous and categorical variable. We used our previously published age-sex specific distribution of treadmill duration from the overall CCLS population26 to define CRF categories as follows: low (lowest 20%; mean ± SD: 8.4 ± 1.2 METs), moderate (middle 40%; mean ± SD: 10.4 ± 1.2 METs), and high (upper 40%; mean ± SD: 13.0 ± 1.8 METs). All CRF assessments were performed prior to 2009.
Outcomes
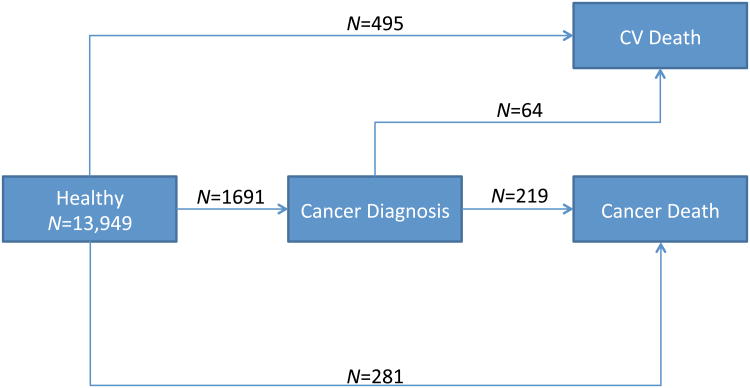
Medicare inpatient claims data were obtained from Centers for Medicare and Medicaid Services (CMS) for participants aged 65 years and older. CMS data contain 100% of claims paid by Medicare for covered inpatient and outpatient health care services. The earliest date of a cancer diagnosis in Medicare age was determined through the Chronic Condition Warehouse (CCW) included in the Beneficiary Annual Summary File. Chronic conditions are defined within the Chronic Condition Warehouse from well-established algorithms.27-30 Three cancer diagnoses (i.e., lung, colorectal, and prostate) were evaluated in the present report for men in this sample of the CCLS. The National Death Index was the primary data source for CVD and all-site cancer mortality outcomes.31 Thus, the outcomes available for analysis were: incident lung, colorectal and prostate cancer as well as death from CVD and all-site cancer. Figure 1 shows the transitions evaluated in this study and includes: 1) healthy men in mid-life who had an interim lung, prostate, or colorectal cancer event at Medicare age and died of cancer (N=219) or CVD (N=64); 2) healthy men in mid-life who subsequently died of cancer but were not diagnosed with prostate, lung, or colorectal cancer at Medicare age (N=281) (for example, a man with a history of prostate cancer without a Medicare claim between 2001-2009 or a man with cancer other than prostate, lung, or colorectal cancer); 3) healthy men in mid-life without prostate, lung, or colorectal cancer at Medicare age who died of CVD (N=495).
Figure 1.
Incident Cancer and Mortality Outcomes in 13,949 Men Followed for a Total of 91,366 Person-Years
*Deaths not attributed to cardiovascular disease or cancer have been treated as censoring events
Statistical Methods
Differences in means and proportions of baseline characteristics across increasing categories of CRF were tested using the Jonckheere-Terpstra nonparametric method. Proportional hazards regression models were used to estimate incident lung, colorectal, and prostate cancer hazard ratios by CRF category, adjusting for age at CCLS examination, BMI, cholesterol, smoking, systolic blood pressure, blood glucose, diabetes, and exam year. Attained age was used as the time scale in the proportional hazards models, which ensures that survival comparisons are among individuals of the same age. Left and right censoring for entry to and exit from Medicare surveillance was implemented using the counting process form of the proportional hazards model, and we assessed the proportional hazards assumption by testing for linear trends in covariate effects across the surveillance period. The analysis of multivariate failures including incident cancer, and CVD or cancer mortality (in those either diagnosed with cancer or not) was constructed from similarly structured marginal proportional hazards models,32 using the robust variance estimate33 to account for the simultaneous presence of the same individual among risk sets of multiple outcomes.
Results
Participant Characteristics
Participant characteristics are presented in Table 1. The mean age and CRF levels were 49 ± 9 years and 11.0 ± 2.3 METs, respectively. For the overall sample, BMI, total cholesterol, smoking, glucose levels, and blood pressure decreased across increasing CRF category (all p <0.001).
Table 1. Baseline Characteristics of Cooper Center Longitudinal Study.
| Cardiorespiratory Fitness Group | ||||
|---|---|---|---|---|
| Low Fit | Moderate Fit | High Fit | ||
| N=2603 | N=5843 | N=5503 | p-value | |
| Age at midlife (years) | 46 (8) | 49 (8) | 51 (8) | <0.001 |
| Median (25, 75 percentile) | 45 (40-51) | 48 (42-55) | 51 (44-57) | |
| Race/ethnicity (n, % Caucasian) | 2556 (98) | 5737 (98) | 5426 (99) | 0.07 |
| Cardiorespiratory Fitness (METs) | 8.4 (1.2) | 10.4 (1.2) | 13.0 (1.8) | <0.001 |
| Body mass index (kg/m2) | 28.6 (4.6) | 26.6 (3.1) | 25.1 (2.6) | <0.001 |
| Total cholesterol (mg/dl) | 221 (41) | 216 (39) | 210 (37) | <0.001 |
| Current smoker, n (%) | 810 (31) | 1117 (19) | 489 (9) | <0.001 |
| Glucose (mg/dl) | 105 (26) | 102 (17) | 100 (13) | <0.001 |
| Systolic blood pressure (mmHg) | 124 (15) | 122 (14) | 122 (14) | <0.001 |
| Diastolic blood pressure (mm Hg) | 83 (10) | 82 (10) | 81 (9) | <0.001 |
| Deaths, n (%) | 527 (20) | 780 (13) | 513 (9) | <0.001 |
| Cancer deaths, n (%) | 125 (5) | 207 (4) | 168 (3) | <0.001 |
| CVD deaths, n (%) | 181 (7) | 229 (4) | 149 (3) | <0.001 |
Values are mean (SD) unless otherwise noted.
Primary Cancer Incidence
Medicare surveillance included a total of 91365.5 person-years of follow-up for incident lung, colorectal and prostate cancer in 13,949 men, for an average 6.5 years of surveillance. During this time, 1310 were diagnosed with prostate cancer (14.3 per 1000 person-years), 200 men were diagnosed with lung cancer (incidence 2.2 per 1000 person-years), and 181 were diagnosed with colorectal cancer (2.0 per 1000 person-years).
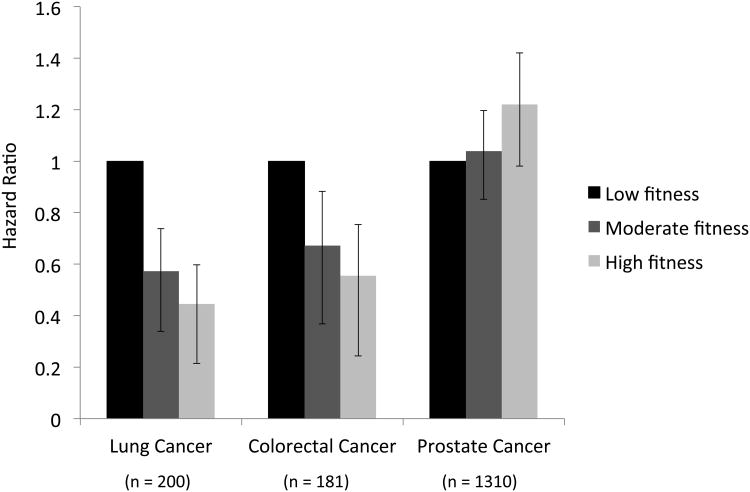
There was a significant inverse and graded relationship across low, moderate and high CRF and incidence of lung (p<0.001) and colorectal cancer (p<0.001) (Figure 2). Compared with men in the low CRF category, the adjusted hazard ratio (HR) for lung cancer incidence was 0.57 (95% CI: 0.41-0.81) for moderate CRF and 0.45 (95% CI: 0.29-0.68) for high CRF. The corresponding HRs for colorectal cancer were 0.67 (95% CI: 0.46-0.98) for moderate CRF and 0.56 (95%CI: 0.36-0.87) for high CRF relative to the lowest CRF category, respectively (Table 2). A 1-MET increase in CRF was associated with a 17% (95% CI: 0.77-0.90) and 9% (95% CI: 0.84-0.99) relative risk reduction in the risk of lung and colorectal cancer, respectively. There was a significant positive and graded relationship across low, moderate and high CRF and incident prostate cancer (p=0.004). Compared with men in the low CRF category, the adjusted HR for prostate cancer incidence was 1.04 (95% CI: 0.88, 1.23) for moderate CRF and 1.22 (95% CI: 1.02, 1.46) for high CRF. Importantly, considering the mixed association of CRF with incident site-specific lung and colorectal versus prostate cancer, the model demonstrated no association between midlife CRF and incident combined lung, colorectal, and prostate cancer [HR = 0.91 (95% CI: 0.80-1.05), p=0.188, moderate versus low CRF; HR = 0.99 (95% CI: 0.86-1.15), p=0.926, high versus low CRF] (Table 3).
Figure 2.
Cardiorespiratory Fitness and Risk of Incident Lung, Colorectal, and Prostate Cancer
*Adjusted for age, exam year, body mass index, smoking, total cholesterol, systolic blood pressure, diabetes, fasting glucose
Table 2. Association Between Mid-life Fitness and Later-life Incident Cancer in CCLS.
| Number of Events | Low Fitness | Moderate Fitness | High Fitness | 1-MET increase | |
|---|---|---|---|---|---|
| Hazard Ratio* (95% Confidence Interval) | Hazard Ratio* (95% Confidence Interval) | Hazard Ratio* (95% Confidence Interval) | |||
| Lung cancer | 200 | referent | 0.57 (0.41, 0.81) | 0.45 (0.29, 0.68) | 0.83 (0.77, 0.90) |
| Colon cancer | 181 | referent | 0.67 (0.46, 0.98) | 0.56 (0.36, 0.87) | 0.91 (0.84, 0.99) |
| Prostate cancer | 1310 | referent | 1.04 (0.88, 1.23) | 1.22 (1.02, 1.46) | 1.03 (1.00, 1.06) |
Adjusted for age, visit date, BMI, smoking, systolic blood pressure, cholesterol, diabetes, fasting glucose
Table 3. Association Between Midlife Cardiorespiratory Fitness and Later-life Incident Cancer and Cause-specific Mortality in CCLS.
| **Health Status | Number of Events | Low Fitness | Moderate Fitness | High Fitness | 1-MET increase |
|---|---|---|---|---|---|
| Hazard Ratio* (95% Confidence Interval) | Hazard Ratio* (95% Confidence Interval) | Hazard Ratio* (95% Confidence Interval) | |||
| Healthy to Cancer | 1691 | referent | 0.94 (0.83, 1.08) | 1.07 (0.93, 1.24) | 1.01 (0.99, 1.04) |
| Cancer to Cancer Death | 219 | referent | 0.76 (0.53, 1.08) | 0.68 (0.47, 0.98) | 0.90 (0.84, 0.97) |
| Cancer to CVD Death | 64 | referent | 0.59 (0.33, 1.05) | 0.32 (0.16, 0.64) | 0.75 (0.66, 0.87) |
| Healthy to Cancer Death | 281 | referent | 0.73 (0.54, 0.98) | 0.66 (0.48, 0.91) | 0.96 (0.91, 1.02) |
| Healthy to CVD Death | 495 | referent | 0.48 (0.39, 0.59) | 0.38 (0.29, 0.48) | 0.84 (0.80, 0.89) |
Adjusted for age, visit date, BMI, smoking, systolic blood pressure, cholesterol, diabetes, fasting glucose
Healthy defined as having no observed incident cancer or cardiovascular disease at baseline
Cause-Specific Mortality in Men Diagnosed with Lung, Colorectal, or Prostate Cancer
We analyzed the prognostic importance of CRF using a model that allowed for differences in the patterns of mortality following a diagnosis of cancer (Table 3). High midlife CRF was associated with a lower risk of cancer mortality [high versus low CRF HR = 0.68 (95% CI: 0.47-0.98)] and CVD mortality [high versus low CRF HR = 0.32 (95% CI: 0.16-0.64)] following a diagnosis of cancer. Importantly, mid-life fitness remained prognostic of cancer mortality among men diagnosed with cancer who were not captured during the Medicare surveillance period or among those who died of cancers other than prostate, lung, or colorectal cancer [high versus low CRF HR = 0.66 (95% CI: 0.48-0.91)]. Lastly, as expected, there was a strong inverse relationship between mid-life fitness and CVD mortality [high versus low CRF HR = 0.38 (95% CI: 0.29-0.48)], among men without a diagnosis of cancer at Medicare age.
Sensitivity Analysis Among Non-Smokers
We performed a sensitivity analysis to determine associations between CRF and both colorectal and lung cancer as well as survival after a cancer diagnosis among non-smokers. Compared with men in the low CRF category, the adjusted hazard ratio (HR) for lung cancer incidence was 0.74 (95% CI: 0.44-1.24) for moderate CRF and 0.55 (95% CI: 0.31-0.68) for high CRF among non-smokers. The corresponding HRs for colorectal cancer were 0.63 (95% CI: 0.40-0.99) for moderate CRF and 0.42 (95%CI: 0.25-0.70) for high CRF relative to the lowest CRF category. There was a similar trend for lower cancer mortality [high versus low CRF HR = 0.77 (95% CI: 0.49-1.21)] among high fit in mid-life who developed cancer and were non-smokers. Lastly, high midlife CRF was associated with a lower risk of CVD mortality [high versus low CRF HR = 0.34 (95% CI: 0.15-0.77)] following a diagnosis of cancer among non-smoking men.
Discussion
Using a large, prospective cohort study, we found a graded, inverse relationship between midlife CRF and incident lung and colorectal cancers. This association was not demonstrated for midlife CRF and prostate cancer. Importantly, midlife CRF was associated with a lower risk of both cancer and CVD mortality following a diagnosis of lung, colorectal, or prostate cancer in men. Our data suggest that higher levels of mid-life fitness provide a mortality benefit into older age even in the setting of a cancer diagnosis.
In the current study, high CRF conferred a 55% and 44% reduction in the risk of lung and colorectal cancer, respectively, compared to low-CRF. Every 1-MET increase in CRF was associated with a 17% and 9% relative risk reduction in lung and colorectal cancer risk, respectively. This is similar to the results of the Kuopio Ischemic Heart Disease Risk Factor Study finding a 1-MET increase in CRF was associated with a 20% and 12% reduction in the relative risk of lung and colorectal cancer in 2,268 asymptomatic Finnish men.34 Interestingly, in contrast to lung and colorectal cancer, high CRF was a risk factor for prostate cancer even after adjusting for potential confounding variables. The current results are similar to the two other studies in the literature on CRF and prostate cancer. Laukkanen et al. found a 1-MET increase in CRF was associated with a nonsigificant increase in prostate cancer risk (HR 1.03, 95% CI: 0.94-1.12),34 Byun et al., using data from the Aerobic Center Longitudinal Study, found that compared to men in the lowest CRF category, those of moderate or high CRF had an adjusted hazard ratio of 1.68 (95% CI: 1.13-2.48) and 1.74 (95% CI: 1.15-2.62) for incident prostate cancer, respectively.5
There is conflicting data in the literature regarding the impact of CRF on prostate risk (5, Oliveria et al MSSE). The exact reasons for the observed positive relationship between CRF and incident prostate cancer risk are not known but differences in related health behaviors such as screening may be an important contributing factor. Specifically, men with higher CRF may also be more likely to undergo more frequent preventive health care screening / detection visits and thus, had greater opportunity to be diagnosed with localized prostate cancer relative to men of lower CRF, possibly with less frequent preventive health care visits. Importantly, these findings are also consistent with several studies on physical activity and prostate cancer risk, an important predictor of attained CRF.35 Understanding how screening may affect the relation between CRF and prostate cancer as well as studying the relation between CRF and incident advanced stage prostate cancer are important areas of future research.
A key, novel finding in the current study was that CRF was an independent predictor of the transition from cancer and ultimately death from either cancer or CVD. High CRF was associated with a 36% risk reduction in cancer death among men who developed lung, colorectal, or prostate cancer at Medicare age compared to low CRF. Moreover, CRF was a powerful predictor of CVD death among men. Specifically, high CRF was associated with a 69% reduction in CVD death compared to low CRF among men who developed cancer. Importantly, the number of individuals living with cancer in the United State is projected to increase from 13.7 million in 2012 to 18 million over the next decade.36 Simultaneously, due to significant improvements in screening and adjuvant therapy, the five-year relative survival rate for all cancers has increased from 49% in 1975 to 67% in 2007.37 Consequently, patients with early-stage cancer now have sufficient survival to be at risk for non-cancer competing causes of mortality, particularly CVD. This point is of particular importance given that 70% of cancer-related mortality will occur in individuals ≥65 years.12 As such, the current findings are of timely importance and shed new light on remaining fit throughout the lifespan in an effort to decrease the morbidity and mortality related to cancer.
It is important to note why we chose to focus on CRF as the exposure of interest rather than physical activity. It is well established that level of physical activity significantly influences level of cardiorespiratory fitness (Lakoski, AJC), and structured exercise training is associated with 10% - 25% improvements in measures of CRF (Warburton CMAJ 2006). Moreover, regular physical activity is associated with significant reductions in the risk of certain forms of cancer, with the evidence classified as convincing for breast and colon cancer (Friedenreich and Orenstein Nutr Reviews). Several epidemiological studies suggest that, in general, self-reported regular exercise (e.g., >brisk walking for 30 minutes, 5 d.wk-1) is associated with substantial reductions in the risk of cancer-specific death following a diagnosis cancer (Betof et al. BBI, 2013; Ballard-Barbash et al. JNCI 2013). Importantly, physical activity and CRF are correlated but provide distinct information (ref, Haskell). CRF is also highly reproducible and objectively assessed via incremental exercise tolerance testing compared to physical activity which is largely determined by self-report questionnaires. Prior studies have demonstrated that CRF is be a more potent marker of mortality than physical activity (Blair). As such, given the current study findings and prior evidence, we contend that measurement of CRF should be utilized more frequently in the cancer prevention setting.
Our findings do not address whether improvements in CRF via exercise training interventions is an effective strategy to lower cancer incidence or reduce risk of death following a cancer diagnosis in men. However, there is considerable evidence that aerobic training interventions following standard exercise prescription guidelines are associated with a 15% to 30% CRF improvement in men with chronic conditions but without cancer38, 39 as well as those with cancer.40 In addition, exercise training also has been shown to modulate circulating host pathways postulated to mediate the CRF – cancer incidence / prognosis relationship.41 Nevertheless, the strong predictive value of CRF on cancer incidence and mortality does not necessarily indicate that CRF augmentation will lower cancer / CVD events.42 Adequately powered randomized trials are required to definitively address these questions.
Important limitations need to be considered when interpreting the present findings. First, we were unable to determine length and intensity of smoking in the CCLS. To overcome this limitation, we performed a sensitivity analysis among non-smokers, finding similar relationships between fitness and both cancer risk and survival after cancer. Secondly, we were not able to capture outcomes that occurred between study entry and the onset of Medicare eligibility, as the cancer outcome was derived from administrative data from the CMS. However, Medicare data have been shown to be a reliable source of information across multiple clinical cancer outcomes.43, 44 Furthermore, Medicare data represents a cost-effective resource, providing the ability to assess associations between CRF and both cancer incidence and long-term mortality outcomes that would be prohibitively expensive to replicate in a prospective cohort study of comparable size and duration. Third, CRF was assessed years prior to a diagnosis of lung, colorectal, or prostate cancer or death in men diagnosed with cancer. Thus, it is not known how changes in CRF and related behaviors such as physical activity from the initial preventive health care to cancer diagnosis as well as changes in CRF and physical activity after diagnosis may have impacted these current findings. Fourth, it is not known how CRF may differentially impact cancer prognosis among those who are diagnosed at different stages of cancer, as cancer stage was not captured in the current study. Lastly, the specific nature of cancer treatments provided to each patient on an individual level was not characterized, and so the impact of chemotherapy, radiation, and or surgical interventions in the sample could not be quantified.
To our knowledge, this is the first study to demonstrate that CRF is predictive of site-specific cancer incidence as well as risk of death from cancer or CVD following a cancer diagnosis. These findings provide further support for the utility of CRF assessment in preventive health care settings. Future studies are required to determine the absolute level of CRF necessary to prevent site-specific cancer as well as evaluating the long-term effect of cancer diagnosis and mortality in women.
Acknowledgments
SGL is supported in part by the National Institute of General Medical Sciences/ National Institutes of Health (P20GM103644-01A1). LWJ is supported in part by research grants from the National Cancer Institute
Dr. Lakoski had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest: The authors declare no relevant conflicts of interest.
References
- 1.Blair SN, Wei M, Lee CD. Cardiorespiratory fitness determined by exercise heart rate as a predictor of mortality in the Aerobics Center Longitudinal Study. Journal of sports sciences. 1998;16(1):S47–55. doi: 10.1080/026404198366678. [DOI] [PubMed] [Google Scholar]
- 2.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. The American journal of clinical nutrition. 1999;69:373–80. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA : the journal of the American Medical Association. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 4.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA : the journal of the American Medical Association. 2009;301:2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 5.Byun W, Sui X, Hebert JR, Church TS, Lee IM, Matthews CE, Blair SN. Cardiorespiratory fitness and risk of prostate cancer: findings from the Aerobics Center Longitudinal Study. Cancer epidemiology. 2011;35:59–65. doi: 10.1016/j.canep.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA : the journal of the American Medical Association. 1996;276:205–10. [PubMed] [Google Scholar]
- 7.Hoyert DL, Xu J. Deaths: preliminary data for 2011. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;61:1–51. [PubMed] [Google Scholar]
- 8.Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T, Smith RA, Taubert K, Thun MJ, American Cancer S, American Diabetes A, American Heart A. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109:3244–55. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Rohatgi A, Ayers CR, Willis BL, Haskell WL, Khera A, Drazner MH, de Lemos JA, Berry JD. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–83. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow CE, Defina LF, Radford NB, Berry JD, Cooper KH, Haskell WL, Jones LW, Lakoski SG. Cardiorespiratory fitness and long-term survival in “low-risk” adults. Journal of the American Heart Association. 2012;1:e001354. doi: 10.1161/JAHA.112.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 12.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA: a cancer journal for clinicians. 2010;60:120–32. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SL, X J, Kochanek K. Deaths: Final Data for 2010. 2013:61. [PubMed] [Google Scholar]
- 14.Zhou Y, Chlebowski R, Lamonte MJ, Bea JW, Qi L, Wallace R, Lavasani S, Walsh BW, Anderson G, Vitolins M, Sarto G, Irwin ML. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: Results from the Women's Health Initiative. Gynecologic oncology. 2014;133:4–10. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keegan TH, Milne RL, Andrulis IL, Chang ET, Sangaramoorthy M, Phillips KA, Giles GG, Goodwin PJ, Apicella C, Hopper JL, Whittemore AS, John EM. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast cancer research and treatment. 2010;123:531–42. doi: 10.1007/s10549-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, Finn S, Graff RE, Penney KL, Rider JR, Nuttall EJ, Martin NE, Sesso HD, Pollak M, Stampfer MJ, Kantoff PW, Giovannucci EL, Loda M, Mucci LA. Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. Journal of the National Cancer Institute. 2013;105:1881–90. doi: 10.1093/jnci/djt332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. American journal of epidemiology. 2000;152:847–54. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:345–53. [PubMed] [Google Scholar]
- 19.Kampert JB, Blair SN, Barlow CE, Kohl HW., 3rd Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Annals of epidemiology. 1996;6:452–7. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 20.Hu G, Tuomilehto J, Silventoinen K, Barengo NC, Peltonen M, Jousilahti P. The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47 212 middle-aged Finnish men and women. Int J Obes (Lond) 2005;29:894–902. doi: 10.1038/sj.ijo.0802870. [DOI] [PubMed] [Google Scholar]
- 21.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. Journal of the American College of Cardiology. 2007;50:1435–41. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA : the journal of the American Medical Association. 1995;273:1093–8. [PubMed] [Google Scholar]
- 23.Lakoski SG, Barlow CE, Farrell SW, Berry JD, Morrow JR, Jr, Haskell WL. Impact of body mass index, physical activity, and other clinical factors on cardiorespiratory fitness (from the Cooper Center longitudinal study) The American journal of cardiology. 2011;108:34–9. doi: 10.1016/j.amjcard.2011.02.338. [DOI] [PubMed] [Google Scholar]
- 24.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. American heart journal. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 25.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. American heart journal. 1982;103:363–73. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 26.Willis BL, Morrow JR, Jr, Jackson AW, Defina LF, Cooper KH. Secular change in cardiorespiratory fitness of men: Cooper Center Longitudinal Study. Medicine and science in sports and exercise. 2011;43:2134–9. doi: 10.1249/MSS.0b013e31821c00a7. [DOI] [PubMed] [Google Scholar]
- 27.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Greenland P, Manheim LM, Dyer AR, Wang R, Lubitz J, Manning WG, Fries JF, Stamler J. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Archives of internal medicine. 2005;165:1028–34. doi: 10.1001/archinte.165.9.1028. [DOI] [PubMed] [Google Scholar]
- 28.Virnig BA, McBean M. Administrative data for public health surveillance and planning. Annual review of public health. 2001;22:213–30. doi: 10.1146/annurev.publhealth.22.1.213. [DOI] [PubMed] [Google Scholar]
- 29.Gorina Y, Kramarow EA. Identifying chronic conditions in Medicare claims data: evaluating the Chronic Condition Data Warehouse algorithm. Health services research. 2011;46:1610–27. doi: 10.1111/j.1475-6773.2011.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of internal medicine. 2002;162:2269–76. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 31.National Death Index. 2013 [Google Scholar]
- 32.Wei L, Lin DY, Weissfield L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. Journal of the American Statistical Association. 1989;84:1065–1073. [Google Scholar]
- 33.Lin Da, W L. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 34.Laukkanen JA, Pukkala E, Rauramaa R, Makikallio TH, Toriola AT, Kurl S. Cardiorespiratory fitness, lifestyle factors and cancer risk and mortality in Finnish men. European journal of cancer. 2010;46:355–63. doi: 10.1016/j.ejca.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Kohl HW, Blair SN, Paffenbarger RS, Jr, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. American journal of epidemiology. 1988;127:1228–39. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 36.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA: a cancer journal for clinicians. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 37.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 38.Sandercock G, Hurtado V, Cardoso F. Changes in cardiorespiratory fitness in cardiac rehabilitation patients: a meta-analysis. International journal of cardiology. 2013;167:894–902. doi: 10.1016/j.ijcard.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 39.Boule NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46:1071–81. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- 40.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of cancer survivorship : research and practice. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 41.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, behavior, and immunity. 2013;30(1):S75–87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauer MS. How will exercise capacity gain enough respect? Circulation. 2011;123:1364–6. doi: 10.1161/CIRCULATIONAHA.111.023218. [DOI] [PubMed] [Google Scholar]
- 43.Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA : the journal of the American Medical Association. 2011;305:1113–8. doi: 10.1001/jama.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willis BL, Gao A, Leonard D, Defina LF, Berry JD. Midlife fitness and the development of chronic conditions in later life. Archives of internal medicine. 2012;172:1333–40. doi: 10.1001/archinternmed.2012.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]