Abstract
Some sea urchins, including the purple sea urchin Strongylocentrotus purpuratus, have been successfully used in aquaculture, but their slow growth and late reproduction are challenging to overcome when developing efficient aquaculture production techniques. S. purpuratus develops via an indirect life history that is characterized by a drastic settlement process at the end of a larval period that lasts for several weeks. During this transition, the bilateral larva is transformed into a pentaradial juvenile, which will start feeding and growing in the benthic habitat. Due to predation and other ecological factors, settlement is typically associated with high mortality rates in juvenile populations. Additionally, juveniles require several days to develop a functional mouth and digestive system. During this perimetamorphic period, juveniles use up larval resources until they are capable to digest adult food. Mechanisms underlying the onset of juvenile feeding and metabolism have implications for the recruitment of natural populations as well as aquaculture and are relatively poorly understood in S. purpuratus. The insulin/insulin-like growth factor signalling (IIS)/Target of Rapamycin (TOR) pathway (IIS/TOR) is well conserved among animal phyla and regulates physiological and developmental functions, such as growth, reproduction, aging and nutritional status. We analyzed the expression of FoxO, TOR, and ILPs in post-settlement juveniles in conjunction with their early growth trajectories. We also tested how pre-settlement starvation affected post-settlement expression of IIS. We found that FoxO provides a useful molecular marker in early juveniles as its expression is strongly correlated with juvenile growth. We also found that pre-settlement starvation affects juvenile growth trajectories as well as IIS. Our findings provide preliminary insights into the mechanisms underlying post-settlement growth and metabolism in S. purpuratus. They also have important implications for sea urchin aquaculture, as they show that pre-settlement nutrient environment significantly affects both early growth trajectories and gene expression. This information can be used to develop new biomarkers for juvenile health in sea urchin population ecology and aquaculture aquaculture.
Keywords: Endocrinology, Developmental biology, Zoology, Ecology, Genetics
1. Introduction
Sea urchin aquaculture has the potential to provide a high value nutrient source that can be harvested in a sustainable manner (Hagen, 1996; Keesing and Hall, 1998; Lesser and Walker, 1998; Lawrence et al., 2001; Andrew et al., 2002; Robinson, 2004). Specifically, edible sea urchins are harvested for their gonads, which are commercialized as a high value food in many areas of the world (Lawrence, 2007). Their gonads are known in markets as ‘roe’ or ‘uni’ (Agatsuma et al., 2005). Since the 1980s, a variety of culturing methods for sea urchins have been developed in Japan and Europe (McBride, 2005). However, there is a need to develop and optimize sea urchin aquaculture as natural populations cannot sustain demand (Hagen, 1996; Andrew et al., 2002; Robinson, 2004). Efforts to grow sea urchins in an aquaculture setting have been implemented in many countries including Canada, the United States, Mexico, Chile, Japan, Ireland Scotland, Belgium, Norway, Israel, New Zealand, and France (McBride, 2005).
Challenges in sea urchin aquaculture emerge as a direct consequence of their bi-phasic life history (Morse, 1991). Specifically, planktotrophic sea urchin larvae are characterized by long larval periods during which they experience high mortality rates. When these larvae settle (i.e. the ecological transition from the plankton into the benthos) in response to environmental cues, competent larvae typically require biofilms and food sources in their benthic environment. The mechanisms driving this transition has been under investigation for many years (e.g. Hadfield and Paul, 2001; Heyland and Moroz, 2005; Hadfield, 2011). In laboratory settings and natural environments, mortality rates are typically high during the first week post-settlement. In fact, for several sea urchin species, over 90% of newly settled juveniles die during these early stages of juvenile development (Highsmith and Emlet 1986; Shimabukuro, 1991; Grosjean et al., 1998). While this is likely a consequence of predation in the benthic habitat, physiological changes occurring on the level of the juvenile sea urchin’s digestive system when it switches from a larval to an adult diet also likely play an important role (Lawrence, 2007). However, little information exists on the physiological and developmental mechanisms underlying early juvenile growth and development. To improve methods for post-settlement survival and to optimize juvenile growth in aquaculture, more information is needed on the maturation of the juvenile digestive system in sea urchins.
The sea urchin S. purpuratus is broadly distributed along the Pacific west coast and its gonadal tissue is edible (Rogers-Bennett, 2007). This species has also been used extensively to study a broad range of research questions from gene regulatory networks in early embryonic development (Martik et al., 2016) to community ecology (Palumbi, 2003), and was the first free-living marine invertebrate to have its complete genome sequenced (Sea Urchin Genome Sequencing Consortium, 2006). In contrast to processes such as fertilization and embryonic development, late larval development and metamorphosis are less understood on a physiological and developmental level (Walker, 1984; Smith et al., 2008; Heyland and Hodin, 2014). Similar, to other sea urchin species, early juvenile stages of S. purpuratus are lacking key feeding structures such as a functional mouth and digestive system (Hinegardner, 1969; Mazur and Miller, 1971; Thet et al., 2004). Therefore, post-settlement sea urchins rely solely on larval reserves in the early days post-settlement (Emlet and Hoegh-Guldberg, 1997). This may impose nutritional stress on juveniles and have consequences on post-settlement performance. Furthermore, it raises the interesting question of how pre-settlement feeding may impact post-settlement performance (Jarrett and Pechenik, 1997; Moran, 1997; 1998).
Little information currently exists on molecular and physiological processes regulating post-settlement growth and development in S. purpuratus. For example, from both, an ecological and aquaculture perspective, it would be beneficial to gain information about transcriptional changes post-settlement that are correlated with juvenile growth and performance as they could then be used to develop molecular markers for the assessment of juvenile health.
The insulin/insulin-like growth factor signalling (IIS)/Target of Rapamycin (TOR) pathway (IIS/TOR) is both conserved and universally important in linking life history traits such as growth, reproduction, and aging to nutritional status (Fig. 1). Specifically, the increase of glucose and amino acid levels lead to the secretion of insulin like peptides (ILPs), which in turn initiates IIS/TOR signaling via protein kinases (Chan and Steiner, 2000). TOR has been shown to regulate cell growth, autophagy and synthesis of proteins. FoxO on the other hand is a transcription factor, which is inhibited by insulin signaling and therefore has opposite effects from TOR. Although, Tu et al., 2006 discussed some of the regulatory functions of FoxO genes in embryonic development of sea urchins and Perillo and Arnone 2014 found that S. purpuratus ILP1 transcript and protein expression level and localization are determined by food, there is little information available on this pathway in the metamorphosis of marine invertebrate species as the majority of information from invertebrates originates from Caenorhabditis elegans (e.g. Murphy and Hu, 2013) and Drosophila melanogaster (e.g. Tatar et al., 2001). It is therefore interesting to explore this pathway in the context of nutrient signaling in the sea urchin.
Fig. 1.
Summary of Ill/TOR signaling. The diagram emphasizes the role of four target genes analyzed in this study (ILPs 1 and 2, TOR and FoxO) in response to nutrient changes in the environment. This scheme was adapted from work done on different organisms: Drosophila melanogaster (Edgar, 2006), Daphnia pulex (Boucher et al., 2010) and Drosophila (Benmimoun et al., 2012).
Herein, we present new data on early juvenile growth and the IIS/TOR pathway from S. purpuratus. In addition to documenting early growth trajectories in response to artificially grown biofilm and natural settlement substrates, we analyzed gene expression patterns of marker genes from the IIS pathway, a major regulatory system involved in nutrient signaling. Finally, we tested how pre-settlement nutrition affects post-settlement growth trajectories and expression of marker genes.
2. Materials and methods
2.1. Animal spawning
Adult S. purpuratus (Loma Marine Invertebrate Lab, Lakeside, California) were maintained in the Hagen Aqualab (University of Guelph, Guelph, Ontario) in filtered artificial seawater (FASW) on a 12:12 light cycle in recirculating artificial seawater at 12 °C and 34 ppt salinity. Adult urchins were fed rehydrated kelp (Laminaria sp.) ad libitum throughout the year. For one experiment, sea urchins from Slip Pt Washington were maintained at the Friday Harbor Laboratories (University of Washington, USA) during the summer 2016. Adults were fed fresh kelp and reared in 0.45 μm filtered natural seawater.
2.2. Sea urchin fertilization and culturing
Sea urchins were spawned via gentle shaking and/or intracoelomic injection with 0.5–1 ml of 0.5 M KCl. Females were inverted over a small glass beaker (∼200 ml) filled with 0.45 μm FASW. The gonopores were always in contact with the water while gametes were collected. For males, sperm was collected dry with a small pipette and stored at 4 °C until use. Eggs were allowed to settle and then washed twice before in vitro fertilization. For this, several drops of sperm were diluted in 10 ml of FASW and mixed well. Five drops of this sperm solution were added to the eggs and fertilization success was tested within 2–3 minutes. More sperm was added until fertilization success was higher than 80%. After that, fertilized eggs were distributed to a 600 ml of FASW (hatching beaker).
For culture setup, hatched embryos were collected from the upper layers of the hatching beaker and diluted to an initial density of 1 embryo/ml of FASW at 12 °C with Rhodomonas sp. at 6cells/μl. Water in cultures was changed every two days by reverse filtration using nitex mesh at 50–120 μm depending on developmental stage and size. Larval density was reduced to 1 larva/5 ml of FASW when larvae reached the 4 arm pluteus stage. Larvae were agitated by a stirring system as described previously by Strathmann (1987).
2.3. Competence test and juvenile growth assay
Artificial inducers of metamorphosis and settlement can be used in many marine invertebrate species (Pawlik, 1990; Hadfield, 1998). Specifically, potassium leads to the depolarization of membranes and has been shown to effectively induce settlement in echinoids (sea urchins and sand dollars − Strathmann, 1985). Therefore, larvae were visually assessed for metamorphic competence (i.e. development of juvenile skeletons and tube feet) and then induced with excess 80mMKCl (as an artificial inducer) for one hour. After exposure, larvae were returned to FASW. A total of 8 experiments on juveniles were performed and details on the design of these experiments can be found in Supplement 1.
2.3.1. The effect of artificially grown biofilm on juvenile growth
We tested the effect of A) Biofilm grown from artificial seawater tanks (the same tanks in which the adult urchins were held in Guelph, ON; for details see below). B) Larval food (Rhodomonas lens at 6000 cells/ml) and C) AFSW on the juvenile growth. For more information, see the details in experiments number 1, 2, 3, 4 and 6 in Supplement 1. Fertilization for these experiments was performed during the period from 20th January 2016 until 13th July 2016. Over this period, 2135 larvae were collected from several competent cohorts. The following exposures were accomplished: 1) Pre-induction larvae: larvae collected before exposure to KCL. 2) Non-competent larvae: KCL exposed larvae, which did not settle. 3) Juveniles: KCL exposed larvae, which settled. Juveniles were collected from these experiments following 0, 2, 3, 6 and 11 days post-settlement and RNA was extracted. Biofilm plates for these experiments were prepared on a 12:12 light cycle in recirculating artificial seawater at 12 °C and 34 ppt salinity, where adult urchins were held in. Sterile 6 well plates (Falcon®, 531146, USA; Cell growth area 9.6 cm2) were submerged in tanks for a minimum of 2 weeks. Once ready, plates were visually inspected under the microscope to remove any potential zooplankton predators before competent larvae were exposed.
For each experiment, 20 juveniles were added to a well with 8 ml of FASW. Water in wells was changed every day. Juvenile morphological characters were measured from images taken on a Nikon Ti microscope. Morphological measurements were captured from randomly selected juveniles, which were grown on artificial biofilm, food and no substrate at 0, 2, 4, 6 and 8 days after induction. The effect of different substrates on juvenile growth from experiments 2, 4 and 6 (Supplement 1) were assessed. For measurements, juveniles were photographed in their wells by an inverted microscope and images were analyzed using ImageJ (Fiji − https://fiji.sc/#). Growth was assessed by measuring the juvenile test (calcified body wall) diameter and spine length. Note that we only measured adult spine length (see Miller and Emlet, 1999) (Fig. 2A, B).
Fig. 2.
Juvenile morphology measured in our experiments (A, B). A is a juvenile at 6 days post settlement, while the juvenile in B is a different individual at 13 days post settlement. Different settlement substrates result in different test (C) and spine (D, E) growth in juvenile S. purpuratus. The response of spine growth and test growth is not directly correlated however. Specifically, relative spine length (the ratio of spine length over test area − E) is largest when juveniles are grown on biofilm (BF) and biofilm + food (F). We did not find a statistical interaction between food and biofilm on relative spine length (F). Stars indicate statistically significant difference between substrate and the control (FASW) based on a simple contrast post-hoc comparisons.
2.3.2. Assessing the effect of natural biofilm on the juvenile post settlement
The biofilm from the natural inducer experiment at the Friday Harbor Laboratories (Experiment 5) was grown in sea tables over the course of 4 weeks in natural seawater. For all substrate experiments, juveniles were settled in 6 well multi-well plates (Falcon®, 531146, USA) with the appropriate substrate at 14 °C. Other procedures were identical to those outlined for artificial biofilm above.
2.3.3. Assessing the effect of pre-settlement feeding on post-settlement growth, mortality and gene expression
On October 6th, 2016, 200 larvae from a competent cohort were separated into a starved or fed cohort (Experiment 7: Supplement 1). Larvae from the fed cohort were given Rhodomonas sp. for 5 days while, the other cohort was starved for 5 days. After induction of settlement, juveniles were transferred into replicate plates, and growth as well as mortality were assessed 0, 2, 4, 8 and 14 days post settlement.
For gene expression analysis, a separate experiment was setup with 1358 competent larvae, separated into a starved and fed cohort on November 30th, 2016 (Experiment 8: Supplement 1). After induction of settlement with KCL, juveniles were grown for 3 days and RNA was extracted.
2.4. Total RNA purification and cDNA synthesis
For all RNA extractions, juvenile, larval and embryonic samples were collected directly in 350 μl TRI Reagent® Solution, (Ambion, The RNA Company, Direct-zol™ RNA Kit (Zymo Research) with the exclusion of as much FASW as possible. The samples were then immediately frozen at −20 °C. A minimum of 15 juveniles per sample was used. The following numbers of larvae were processed depending on developmental age/stage: ∼300 larvae/embryos for early stages (up to 3 weeks); ∼100 larvae for larvae older than 3 weeks; ∼15 larvae per sample for competent larvae. RNA was extracted from frozen samples using the Direct-zol™ RNA MiniPrep kit (Zymo Research #R2050; Lot# ZRC186120) according to the manufacturer’s instructions. Briefly, samples were defrosted and 350 μL 100% EtOH was added to each sample. The sample was then loaded into Zymo-Spin™ IIC column in collection tubes and then centrifuged for 1 minute at maximum speed. Collection tubes were discarded and columns were placed into new collection tubes. Next, 400 μL RNA Wash Buffer was added to each sample. The samples were centrifuged for 30 seconds at maximum speed. Then, 80 μL Master Mix (5 μL DNase 1 plus 75 μL digestion buffer for each samples) was added directly to the column matrix. Columns were incubated at room temperature for 15 minutes. After that, samples were centrifuged for 30 seconds. 400 μL Direct-zol RNA PreWash was added to each column and centrifuged for 1 minute. The flow-was discarded. This step was repeated once. 700 μL of RNA Wash Buffer was added and centrifuged for 1 minute. The samples were then centrifuged for an additional 2 minutes. Columns were transferred to a 1.5 mL tubes. 25 μL DNase/RNase Free Water was added directly to each column matrix. Then, the columns were centrifuged for 1 minute, the quality and concentration of the RNA was assessed using a Nanodrop 8000 Spectrophotometer (Thermo Fisher).
cDNA was synthesized from total RNA immediately after extraction using the Applied Biosystems cDNA synthesis protocol. Specifically, 10 μL RT Master Mix (2 μL 10X Reverse Transcriptase Buffer, 0.8 μL 25X dNTP Mix (100 mM), 1 μL Reverse transcriptase, 2 μL 10X RT random primers, 1 μL MultiScribe Reverse Transcriptase, 4.2 μL Nuclease Free Water) and 10 μL RNA sample were pipetted into the appropriate PCR tube, sealed and placed in the thermocycler. The following cycling conditions were employed: 10 minutes at 25 °C, 37 °C for 120 minutes and 5 minutes at 85 °C. cDNA was stored at −20 °C until it could be used for qRT-PCR.
2.5. qRT-PCR (PCR)
All qRT-PCR experiments were performed using a StepOne Plus™ Real-Time PCR System. Relative expression values were calculated using standard ΔΔCt method and SYBR green cycle with a ramp rate of 100% with ubiquitin as a control gene and different samples as a reference (see summary of experiments below for reference samples). The concentration of all primers was 300 nM and the reaction volume was 20 μL. The NCBI or SPU accession numbers in the herein study for S. purpuratus FoxO, TOR, ILP1 and ILP2 (IGF2) are XM_011669860, XM_011674982, SPU_ SPU_007203 and SPU_030139, respectively. The primer sequences used for qRT-PCR can be found in Supplement 2. Note that in one experiment we directly correlated gene expression with morphological characters in juveniles. These measurements were done on the same individuals.
2.6. Statistical analysis
All statistical tests were done using SPSS v23.0. We used t-test statistic for paired comparisons and ANOVA commands for multiple comparisons. For post-hoc tests, p-values were adjusted using Bonferroni corrections.
3. Results
3.1. Settlement substrate affects juvenile growth
Juvenile morphology measured in our experiments are shown in Fig. 2(A, B). A is the animal at 6 days old, while the animal in B is different individual at 13 days old. Different settlement substrates had no significant effect on test area (F5,241 = 1.56; p = 0.20; Fig. 2C) but did on absolute and relative (ratio of spine length over test area) spine length (F5,241 = 23.62; p < 0.01, Fig. 2; (F5,241 = 17.27; p < 0.01, Fig. 2E). Biofilm (97 ± 13 μm; p < 0.01), biofilm + food (97 ± 13 μm; p < 0.01) and food alone (40 ± 18 μm; p = 0.03) all resulted in longer spines compared to the control. The relative spine length was larger in biofilm (3.5xE−4 5.7xE−5) and biofilm + food (3.7xE−4 5.8xE−5) substrate compared to the control. We also tested whether larval food and biofilm had an interactive effect on relative spine length by using food (present − 1, absent − 0) and biofilm (present − 1, absent − 0) as factors in a two-way ANOVA. 2F). We found a significant effect of biofilm (F1,241 = 36.70; p < 0.01) but not food (F1,241 = 2.76; p = 0.10) on relative spine length. There was no interaction between food and biofilm as substrates on relative spine length (F2,241 = 1.58; p = 0.21).
3.2. FoxO is upregulated in post-settlement juveniles
A survey of temporal gene expression patterns post-settlement was performed using 4 target genes from the IIS/TOR pathway (Fig. 1) over three-time points for four different settlement substrates: FASW, FASW + Food, Biofilm and Biofilm + Food (Fig. 3). As outlined in the Materials and Methods, more than 15 individuals per sample were used to minimize biological variation. We also performed the experiment with two technical replicates. FoxO and ILP2 proved to be consistently up-regulated in 6-day old juveniles compared to 2-day old juveniles for all substrates except food alone, where we observed a drop-in expression over time. Expression levels of TOR and ILP1 were too variable to detect any consistent trends.
Fig. 3.
Survey of target genes expression levels in juvenile S. purpuratus as a function of food (F) and biofilm (BF) 2 and 6 days post-settlement. All expression levels are expressed as fold change relative to ubiquitin and post-induction juveniles (day 0).
In order to statistically analyze target gene expression level changes throughout development (i.e. pre-and post-settlement), we calculated average fold change from three biological replicate samples for juveniles, using non-competent larvae as a reference (expression level of 1–not shown; Fig. 4A). Using a one sampled t-test we found a significant increase of FoxO (t = 2.43; p = 0.05) expression in juveniles. Expression levels of all other genes did not change significantly.
Fig. 4.
Target gene fold change of juvenile S. purpuratus on different substrates. All expression levels are in reference to non-competent larvae A) Average baseline gene expression levels of pre-induction and juveniles (0–6 days). Asterisks indicate significance relative to reference stage (expression level of 1) in one sample t-test. B) Average gene expression levels in response to natural inducer (coralline alga: Calliarthron tuberculosum) decreases for ILP2 and increased for TOR in juveniles compared to non-competent larvae at the same age.
In a separate experiment at the Friday Harbor Laboratories (University of Washington, USA), we tested whether natural inducer, the coralline alga C. tuberculosum, had a significant impact on gene expression patterns post settlement (Fig. 4B). For this, we used non-competent larvae immediately after biofilm exposure as a reference sample and tested expression levels in non-competent larvae and juveniles after 6 days. These results are all based on independent biological replication (3 replicates). We used an independent sample t-test to compare expression levels in juveniles, compared to non-competent larvae of the same age. In comparison to non-competent larvae, juveniles showed reduced levels of FoxO (t = 1.96; p = 0.16) and ILP2 (t = 1.54; p = 0.17); neither was statistically significant. We did, however, detect a significant decrease in ILP1 expression in juveniles compared to non-competent larvae of the same age (t = -2.45; p = 0.05) and an increase in TOR expression levels (t = -2.44; p = 0.05).
3.3. Juvenile growth correlates positively with IIS expression changes
We tested whether target gene expression levels correlate with morphological characters of juveniles from experiment 4 and 6 (Fig. 5). Both morphological characters were positively correlated with gene expression changes for all tested four target genes (Table 1) on the basis of individual larva.
Fig. 5.
Juvenile growth of sea urchins (A) and spine growth (B) positively correlate with gene expression patterns of all target genes across experiments. For correlation analysis see Table 1.
Table 1.
Analysis of correlation coefficients (CC) between morphological traits and gene expression levels. CC: Pearson Correlation Coefficient; Sig.: 2-tailed significance; N: Number of replicate larvae.
| Spine Length |
Fold Change |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| FoxO | ILP2 | TOR | ILP1 | FoxO | ILP2 | TOR | ILP1 | ||
| Size |
CC | .57 | .57 | .57 | .87 | .71 | .41 | .92 | .50 |
| Sig. | 0.03 | 0.03 | 0.02 | <0.01 | <0.01 | 0.13 | <0.01 | 0.17 | |
| N | 15 | 15 | 15 | 9 | 15 | 15 | 15 | 9 | |
| Spine Length | CC | .29 | .63 | .55 | .32 | ||||
| Sig. | .3 | .01 | .03 | .4 | |||||
| N | 15 | 15 | 15 | 9 | |||||
3.4. Pre-settlement starvation affects post-settlement survival and IIS gene expression
In order to assess the effects of starvation during the competent period on post-settlement performance, we starved competent larvae for 5 days and then compared their growth and spine growth to larvae that were fed normal food ration (6 cells/μl) during the competent period (Fig. 6A-C). Using an independent sample t-test, we found that starvation during the pre-competent period had no effect on test area (Fig. 6A − t = 0.12; p = 0.91) but had a significant effect on absolute spine length (Fig. 6B − t = 5.71; p < 0.01). We also found that juvenile survival curves post-settlement diverged notably 2–8 days post-settlement in that larvae starved during the competent period showed high mortality during that post-settlement period. Specifically, both pre-settlement treatment (i.e. fed versus starved) and time post-settlement had a significant effect on mortality (Food treatment − F1 = 23.00; p < 0.01; Time post-settlement − F10 = 18.62; p < 0.01). We did not find a significant interaction between food treatment and time post-settlement (F1,10 = 1.23; p = 0.27). In gene expression analysis, inconsistent results for ILP1 in late larvae and early juveniles were noticed. This inconsistency is probably due to very low expression levels and we therefore decided to focus on ILP2, which was expressed at higher levels. When we analyzed the effect of pre-settlement starvation on gene expression (Fig. 7) and found that larval gene expression of FoxO and TOR was reduced post-starvation, while ILP2 expression was increased post-starvation. We detected a decrease in FoxO expression and an increase in TOR and ILP2 expression post-settlement as a consequence of starving larvae pre-settlement (Table 2).
Fig. 6.
Competent larvae that are starved before settlement grow the same test size (A) but longer spines (B, C) post-settlement. Starved larvae show significantly higher mortality 2–8 days post-settlement (D).
Fig. 7.
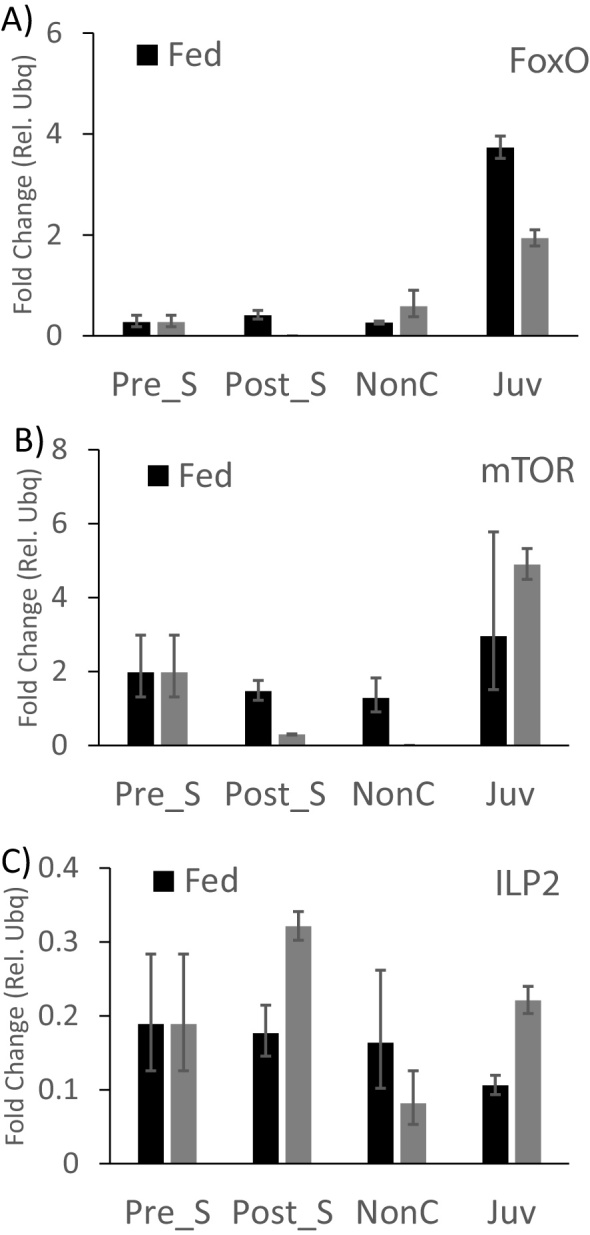
Gene expression levels for FoxO, TOR ad ILP2 as a consequence of pre-settlement starvation. We tested gene expression levels using qRT-PCR for three target genes as a consequence of a five-day starvation of competent larvae pre-settlement. Larval gene expression of FoxO and TOR was reduced post-starvation while ILP2 expression was increased post-starvation. We detected a decrease in FoxO expression and an increase in TOR and ILP2 expression post-settlement as a consequence of starving larvae pre-settlement.
Table 2.
The results of the expression profile of TOR, FoxO and ILP2.
| Stage | Gene | Mean (Gene) | Sd (Gene) | Mean (Control) | Sd (Control) | dCt (Gene) | sd-dCt (Gene) | ddCt (Sample) | sd-ddCt (Sample) | fold-mean |
|---|---|---|---|---|---|---|---|---|---|---|
| Juv0 | FoxO | 33.11 | 0.27 | 27.8 | 0.26 | 5.31 | 0.38 | 0 | 0.38 | 1 |
| Pre-starvation1 | FoxO | 27.88 | 0.04 | 20.69 | 0.58 | 7.19 | 0.58 | 1.88 | 0.58 | 0.27 |
| Pre-induction N1 | FoxO | - | 32.71 | 0.09 | 0.09 | 0.09 | ||||
| Pre-induction F2 | FoxO | 27.06 | 0.15 | 20.46 | 0.26 | 6.6 | 0.3 | 1.29 | 0.3 | 0.41 |
| Non-competent N1 | FoxO | 36.40 | 0.04 | 30.32 | 0.62 | 6.08 | 0.62 | 0.77 | 0.62 | 0.59 |
| Non-competent F1 | FoxO | 33.31 | 0.14 | 26.07 | 0.09 | 7.24 | 0.16 | 1.93 | 0.16 | 0.26 |
| 3Days-settelment N2 | FoxO | 26.33 | 0.10 | 21.97 | 0.07 | 4.36 | 0.12 | -0.95 | 0.12 | 1.94 |
| 3Days-settelment F1 | FoxO | 27.78 | 0.02 | 24.37 | 0.08 | 3.41 | 0.09 | -1.9 | 0.09 | 3.73 |
| Juv0 | TOR | 30.46 | 0.92 | 27.8 | 0.26 | 2.66 | 0.95 | 0 | 0.95 | 1 |
| Pre-starvation1 | TOR | 22.37 | 0.12 | 20.69 | 0.58 | 1.68 | 0.59 | -0.98 | 0.59 | 1.98 |
| Pre-induction N1 | TOR | 37.15 | 32.71 | 0.09 | 4.43 | 0.09 | 1.77 | 0.09 | 0.29 | |
| Pre-induction F2 | TOR | 22.57 | 0.06 | 20.46 | 0.26 | 2.11 | 0.26 | -0.55 | 0.26 | 1.46 |
| Non-competent N1 | TOR | - | 30.32 | 0.62 | 0.62 | 0.62 | ||||
| Non-competent F1 | TOR | 28.37 | 0.50 | 26.07 | 0.09 | 2.3 | 0.51 | -0.36 | 0.51 | 1.28 |
| 3Days-settelment N2 | TOR | 22.34 | 0.10 | 21.97 | 0.07 | 0.37 | 0.12 | -2.29 | 0.12 | 4.89 |
| 3Days-settelment F1 | TOR | 25.46 | 0.96 | 24.37 | 0.08 | 1.1 | 0.97 | -1.56 | 0.97 | 2.95 |
| Juv0 | ILP2 | 30.55 | 0.14 | 27.8 | 0.26 | 2.75 | 0.3 | 0 | 0.3 | 1 |
| Pre-starvation1 | ILP2 | 25.85 | 0.08 | 20.69 | 0.58 | 5.16 | 0.59 | 2.41 | 0.59 | 0.19 |
| Pre-induction N1 | ILP2 | 37.10 | 32.71 | 0.09 | 4.39 | 0.09 | 1.64 | 0.09 | 0.32 | |
| Pre-induction F2 | ILP2 | 25.72 | 0.12 | 20.46 | 0.26 | 5.25 | 0.28 | 2.5 | 0.28 | 0.18 |
| Non-competent N1 | ILP2 | 36.68 | 30.32 | 0.62 | 6.37 | 0.62 | 3.62 | 0.62 | 0.08 | |
| Non-competent F1 | ILP2 | 31.44 | 0.67 | 26.07 | 0.09 | 5.37 | 0.68 | 2.61 | 0.68 | 0.16 |
| 3Days-settelment N2 | ILP2 | 26.90 | 0.10 | 21.97 | 0.07 | 4.93 | 0.12 | 2.18 | 0.12 | 0.22 |
| 3Days-settelment F1 | ILP2 | 30.36 | 0.16 | 24.37 | 0.08 | 5.99 | 0.18 | 3.24 | 0.18 | 0.11 |
4. Discussion
The transition of planktonic sea urchin larvae into the benthic habitat is poorly understood from a mechanistic perspective and analyses so far have not focused on markers of early juvenile growth. Here, we analyzed growth trajectories of settled juveniles before the onset of feeding as well as their morphology using simple morphometrics. This study also evaluated the usability of molecular markers from the insulin/insulin-like growth factor signalling (IIS)/Target of Rapamycin (TOR) axis and found that Forkhead Box class O (FoxO), a negative regulator of IIS/TOR is predominantly upregulated post-settlement. These data provide new insights into mechanisms underlying the onset of feeding in juvenile sea urchins and provides evidence for carry-over effects of larval feeding on early juvenile growth trajectories and performance. Our data have implications for sea urchin aquaculture, as metamorphosis is a determining factor for benthic recruitment and with that juvenile population size (Grosjean et al., 1998; Shimabukuro, 1991).
4.1. IIS/TOR signaling genes as markers for juvenile development
IIS/TOR signaling is a critical regulator of body size in animals (Grewal, 2009) as it provides a link between nutrient intake and the hormonal regulation of growth (Hartfelder and Engels, 1998; Koyama et al., 2013). While increase of glucose and amino acid levels lead to the secretion of insulin like peptides (ILPs) (Chan and Steiner, 2000), food restriction reduces the synthesis and secretion of ILPs (Brogiolo et al., 2001; Ikeya et al., 2002; Geminard et al., 2009). The TOR pathway reacts to intracellular amino acid levels to control Akt in a cell autonomous mode (Hietakangas and Cohen, 2007). Because of this interaction between the two pathways they are frequently referred to as the IIS/TOR pathway. FoxO is generally considered a negative regulator of growth and its activity is inhibited by the IIS pathway (Junger et al., 2003; Zanella et al., 2010; Webb and Brunet, 2014). In sea urchin pluteus larvae, this pathway has recently been implicated in nutrient signaling Perillo and Arnone (2014), and Carrier et al. (2015) hypothesized that it may be linked to the phenotypically plastic response of pluteus larvae and exogenous food levels. During the peri-metamorphic period, juvenile sea urchins develop a functional digestive system and gain the capacity to feed in the benthic environment. Hence, they deplete larval reserves during these early days post-settlement (Gosselin and Jangoux, 1998; Miller and Emlet, 1999). We hypothesized that changes in the IIS/TOR pathway would reflect these changes in feeding mode and potentially correlate with depletion of endogenous resources during the first days of juvenile development. Specifically, we predicted that FoxO would be up-regulated as juveniles deplete their resources while TOR and ILP expression should be suppressed. These predictions are based on numerous studies, showing that FoxO is negatively regulated by insulin signaling (see above).
We found that FoxO is consistently up-regulated post-settlement in sea urchin juveniles. In contrast to that, TOR and ILP expression levels were generally low and more variable in post-settlement juveniles. These results suggest that FoxO may provide a useful biomarker of post-settlement nutrient stress. FoxO genes have many different regulatory functions in sea urchins, some of which have been discussed for embryonic development (Tu et al., 2006). Little to no information, however, exists on the function of these genes in juvenile development. Future studies may assess exactly what the function of FoxO is post-settlement. As a regulator of growth rate, it may play an important role in coordinating post-settlement growth and metabolism and is therefore suitable as a marker of juvenile growth in aquaculture.
4.2. Biofilm exposure post-settlement induces a long spine morphology and increases FoxO expression
Marine habitats provide a diversity of settlement substrates for competent larvae, many of which likely contain specific chemical or mechanical cues, indicative of the suitability of the habitat for juvenile growth and survival. These substrates can be coralline algae, kelps as well as marine and microbial biofilms (Grosjean et al., 1998; Rahim et al., 2004; Dworjanyn and Pirozzi, 2008; Juinio-Meñez and Bangi, 2010). Still, the composition of these substrates and their contribution to juvenile growth and development has rarely been analyzed (see Miller and Emlet, 1999; Takahashi et al., 2002). Bacterial biofilms can provide cues for the settlement of larvae (Lau et al., 2002). Huggett et al. (2006) found that the rate of settlement was reduced by autoclaving algae and rocks, and by applying antibiotic treatments reduced the settlement rate of the sea urchin Heliocidaris erythrogramma. This research emphasizes the role of bacteria in the settlement process of marine invertebrates.
We tested the effects of biofilm and larval food on juvenile development post-settlement, before the onset of feeding, (i.e., the peri-metamorphic period sensu Gosselin and Jangoux, 1998). While biofilm grown from artificial seawater appears to change the ratio of arm length to test diameter, it does not affect juvenile test size. These results are indicative of an early juvenile plasticity related to the substrate composition. Previous studies have identified extensive spine growth plasticity in juvenile sea urchins in areas of high wave action (shorter spines − Rogers-Bennett et al., 1995) or in response to interspecific competition (McClanahan, 1988). However, neither of these factors apply to our experiment and future studies will have to determine which signal from the substrate is responsible for this plastic response. This is particularly important, as we observed this plastic response before the opening of the juvenile mouth. Therefore, the cue contributing to this plasticity is unlikely to be dependent on the nutrient content of the biofilm or algae.
In order to separate the effect of larval food from juvenile substrate, we also tested whether the exposure of juveniles to algae can affect spine length. We did not find any evidence that the addition of algae affects spine length morphology. In fact, the factorial analysis of food x biofilm, clearly showed that the increased spine length phenotype is a consequence of biofilm and not larval food. This suggests that increasing the spine length is a response to either the settlement substrate itself or to declining endogenous resources. Intriguingly, we did find that pre-settlement starvation leads to a long juvenile spine phenotype, suggesting that the response may be related to the nutritional status of juveniles. It is for example conceivable that the artificial biofilm signals that the substrate is unsuitable for growth and the increased spine length would enhance juvenile protection and/or mobility. Future studies will have to address the question of early juvenile spine length plasticity.
In addition to the phenotypic changes of the juvenile we also found that FoxO expression increased in response to artificially grown biofilm. While FoxO expression appears to generally increase in juveniles’ post-settlement, independently of settlement substrate, we did find a reduction of expression if only food is provided. These findings suggest that although post-settlement juveniles are morphologically different from larvae, their ability to sense planktonic food may remain.
4.3. The Effect of larval starvation on juvenile survival and growth
Nutrient levels are directly linked with IIS/TOR signaling (Fig. 1) as this pathway regulates metabolic as well as behavioral processes. These processes can change plastically in order for the organism to adjust to changing nutrient environments. We found that a short period of starvation during the competent period does not affect juvenile size but significantly increased juvenile spine length. While Hart and Strathmann (1994), Boidron-Metairon (1988), Adams et al. (2011) and others found extensive phenotypic plasticity in sea urchin larval arms in response to nutrient levels, nobody has so far described this plasticity in juvenile spines. Furthermore, the starvation treatment also resulted in a slightly different mortality trajectory in that starved larvae produced juveniles experiencing increased mortality during the first week post-settlement. Juvenile growth rates can be negatively affected by short periods of larval starvation (Qian and Chia, 1993; Pechenik et al., 1996a, b). This reduction is typically due to either starvation for short times or food restriction during larval development. Juveniles require several days to develop functional mouth and digestive system. During this period, they use up their larval reserves. Starvation before settlement may lead to the reduction of these reserves. Indicators of reduced larval reserves can be found in lipid stores in the larval stomach or can be reflected by reduced juvenile size (Byrne et al., 2008). Note that these parameters were however not measured in our experiments.
Miller and Emlet (1999) found that short periods of starvation pre-settlement resulted in longer spines relative to test size, these findings largely correspond to our findings. This time period roughly overlaps with the juvenile pre-feeding period and it may indicate that larvae, which have been starved pre-settlement are exhausting their internal (larval) reserves earlier and therefore experience higher mortality, as we observed in out experiments.
Our data also show that FoxO, a potential marker of nutritional stress (Carrier et al., 2015) is expressed at lower levels in juveniles, which experienced a starvation treatment during the competent period in comparison to those that did not. This is exactly the opposite of what we would have expected based on FoxO function in starvation in other organisms (Rena et al., 1999). However, the developmental rate of juveniles metamorphosed from starved larvae was reduced compared to juveniles metamorphosed from fed larvae. Therefore, juveniles at the same time may not be at the same developmental stage. While this hypothesis could be further assessed by a detailed morphological analysis of the development of Aristotle's lantern, we found preliminary support from our findings that FoxO expression generally increases post-settlement, likely because juveniles are depleting larval reserves (see above). This hypothesis is further supported by the fact that ILP2 expression is higher in juveniles, which were starved as larvae compared to juveniles that were fed.
4.3. Implication for sea urchin aquaculture
High mortality of newly settled sea urchin juveniles is a major challenge in sea urchin aquaculture (Highsmith and Emlet 1986; Shimabukuro, 1991; Grosjean et al., 1998) and our results provide evidence that some of that mortality may be caused by both pre-settlement nutrient environment and indirectly, settlement substrate. Specifically, we found that upregulation of FoxO as larval reserves are depleted, before the juvenile is capable of feeding. FoxO could also be used as an indicator of stress. For example, larvae grown on biofilm from natural seawater showed no upregulation of FoxO in comparison to non-competent larvae (which are likely nutrient deprived). Together these findings provide preliminary evidence that FoxO may be a useful marker for post-settlement growth and health and this information is useful when screening cohorts of juveniles in an aquaculture setting.
Declarations
Author contribution statement
Alyaa Elsaid Abdelaziz Fadl: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data and wrote the manuscript.
Magdy Elsayed Mahfouz, Mona Mabrouk Taha El- Gamal: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Andreas Heyland: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Ministry of Higher Education, Egypt and NSERC Discovery Grant (400230 to AH).
Competing interest statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at http://dx.doi.org/10.1016/j.heliyon.2017.e00412.
No additional information is available for this paper.
Acknowledgements
We would like to thank Jason Hodin and Cory Bishop for insightful feedback on earlier versions of this manuscript. We would also like to thank Jason Hodin for sharing is laboratory at the Friday Harbor Laboratories (University of Washington) when conducting some experiments there during the summer 2016.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Adams D.K., Sewell M.A., Angerer R.C., Angerer L.M. Rapid adaptation to food availability by a dopamine-mediated morphogenetic response. Nat. Commun. 2011;2:592. doi: 10.1038/ncomms1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatsuma Y., Nakabayashi N., Miura N., Taniguch K. Growth and gonad production of the sea urchin Hemicentrotus pulcherrimus in the fucoid bed and algal turf in northern Japan. Mar. Ecol. 2005;26(2):100–109. [Google Scholar]
- Andrew N.L., Agatsuma Y., Ballesteros E., Bazhin A.G., Creaser E.P., Barnes D.K.A., Botsford L.W., Bradbury A., Campbell A., Dixon J.D., Einarsson S., Gerring P., Hebert K., Hunter M., Hur S.B., Johnson C.R., Junio-Menez M.A., Kalvass P., Miller R.J., Moreno C.A., Palleiro J.S., Rivas D., Robinson S.M.L., Schroeter S.C., Steneck R.S., Vadas R.I., Woodby D.A., Xiaoqi Z. Status and management of world sea urchin fisheries. Oceanogr Mar. Biol. Annu. Rev. 2002;40:343–425. [Google Scholar]
- Benmimoun B., Polesello C., Waltzer L., Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- Boidron-Metairon I.F. Morphological plasticity in laboratory-reared echinoplutei of Dendraster excentricus (Eschscholtz) and Lytechinus variegatus (Lamarck) in response to food conditions. J. Erp. Mar. Biol. Ecol. 1988;119:31–41. [Google Scholar]
- Boucher P., Ditlecadet D., Dubé C., Dufresne F. Unusual duplication of the insulin-like receptor in the crustacean Daphnia pulex. BMC Evol. Biol. 2010;10:305. doi: 10.1186/1471-2148-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Byrne M., Sewell M.A., Prowse T.A.A. Nutritional ecology of sea urchin larvae: influence of endogenous and exogenous nutrition on echinopluteal growth and phenotypic plasticity in Tripneustes gratilla. Funct. Ecol. 2008;22:643–648. [Google Scholar]
- Carrier T.J., King B.L., Coffman J.A. Gene expression changes associated with the developmental Plasticity of Sea Urchin Larvae in Response to Food Availability. Biol. Bull. 2015;228:171–180. doi: 10.1086/BBLv228n3p171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.J., Steiner D.F. Insulin through the ages: Phylogeny of a growth promoting and metabolic regulatory hormone. Am. Zool. 2000;40:213–222. [Google Scholar]
- Dworjanyn S.A., Pirozzi I. Induction of settlement in the sea urchin Tripneustes gratilla by macroalgae, biofilms and conspecifics: A role for bacteria? Aquaculture. 2008;274:268–274. [Google Scholar]
- Edgar A.B. How flies get their size: genetics meets physiology. Nature Rev. Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Emlet R.B., Hoegh-Guldberg O. Egg size and post-larval performance: experimental evidence from a sea urchin. Evolution. 1997;51:141–152. doi: 10.1111/j.1558-5646.1997.tb02395.x. [DOI] [PubMed] [Google Scholar]
- Geminard C., Rulifson E.J., Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Gosselin P., Jangoux M. From competent larva to exotrophic juvenile: a morphofunctional study of the perimetamorphic period of Paracentrotus lividus (Echinodermata: Echinoida) Zoomorph. 1998;118:31–43. [Google Scholar]
- Grewal S.S. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int. J. Biochem. Cell Biol. 2009;41:1006–1010. doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Grosjean P., Spirlet C., Gosselin P., Vaïtilingon D., Jangoux M. Land-based, closed-cycle echiniculture of Paracentrotus lividus (Lamarck) (Echinoidea: Echinodermata): a long-term experiment at a pilot scale. J. Shellfish. Res. 1998;17:1523–1531. [Google Scholar]
- Hadfield M.G. The D P Wilson lecture: Research on settlement and metamorphosis of marine invertebrate larvae: past, present and future. Biofouling. 1998;12:9–29. [Google Scholar]
- Hadfield M.G. Biofilms and Marine Invertebrate Larvae: What Bacteria Produce That Larvae Use to Choose Settlement Sites. Ann. Rev. Mar. Sci. 2011;3:453–470. doi: 10.1146/annurev-marine-120709-142753. [DOI] [PubMed] [Google Scholar]
- Hadfield M.G., Paul V.J. Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. In: McClintock J.B., Baker W., editors. Marine Chemical Ecology. CRC Press; Boca Raton: 2001. pp. 431–461. [Google Scholar]
- Hagen N.T. Echinoculture: from fishery enhancement to closed cycle cultivation. World Aquacult. 1996;27(4):6–19. [Google Scholar]
- Hart M.W., Strathmann R.R. Functional consequences of phenotypic plasticity in echinoid larvae. Biol. Bull. 1994;186:291–299. doi: 10.2307/1542275. [DOI] [PubMed] [Google Scholar]
- Hartfelder K., Engels W. Social insect polymorphism: hormonal regulation of plasticity in development and reproduction in the honeybee. Curr. Top Dev. Biol. 1998;40:45–77. doi: 10.1016/s0070-2153(08)60364-6. [DOI] [PubMed] [Google Scholar]
- Heyland A., Hodin J. A detailed staging scheme for late larval development in Strongylocentrotus purpuratus focused on readily-visible juvenile structures within the rudiment. BMC. Dev. Biol. 2014;14:22. doi: 10.1186/1471-213X-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyland A., Moroz L.L. Cross-kingdom hormonal signaling: an insight from thyroid hormone functions in marine larvae. J. Exp. Biol. 2005;208:4355–4361. doi: 10.1242/jeb.01877. [DOI] [PubMed] [Google Scholar]
- Hietakangas V., Cohen S.M. Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 2007;21(6):632–637. doi: 10.1101/gad.416307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith R.C., Emlet R.B. Delayed metamorphosis: effect on growth and survival of juvenile sand dollars (Echinoidea: Clypeasteroida) Bull. Mar. Sci. 1986;39:347–361. [Google Scholar]
- Hinegardner R.T. Growth and development of the laboratory cultured sea urchin. Biol. Bull. 1969;137(3):465–475. doi: 10.2307/1540168. [DOI] [PubMed] [Google Scholar]
- Huggett M.J., Williamson J.E., de Nys R., Kjelleberg S., Steinberg P.D. Larval settlement of the common Australian sea urchin Heliocidaris erythrogramma in response to bacteria from the surface of coralline algae. Oecologia. 2006;149:604. doi: 10.1007/s00442-006-0470-8. [DOI] [PubMed] [Google Scholar]
- Ikeya T., Galic M., Belawat P., Nairz K., Hafen E. Nutrient dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Jarrett J.N., Pechenik J.A. Temporal variation in cyprid quality and juvenile growth capacity for an intertidal barnacle. Ecology. 1997;78:1262–1265. [Google Scholar]
- Juinio-Meñez M.A., Bangi H.G.P. Extrinsic and intrinsic factors affecting the metamorphic rate of Tripneustes gratilla (Echinodermata: Echinoidea) Mar. Ecol. Prog. Ser. 2010;402:137–145. [Google Scholar]
- Junger M.A., Rintelen F., Stocker H., Wasserman J.D., Vegh M., Radimerski T., Greenberg M.E., Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing J.K., Hall K.C. Review of harvests and status of world sea urchin fisheries point to opportunities for aquaculture. J. Shellfish. Res. 1998;17:1505–1506. [Google Scholar]
- Koyama T., Mendes C., Mirth C. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 2013;4:263. doi: 10.3389/fphys.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.C.K., Mak K.K.W., Chen F., Qian P.Y. Bioactivity of bacterial strains isolated from marine biofilms in Hong Kong waters for the induction of larval settlement in the marine polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. 2002;226:301–310. [Google Scholar]
- Lawrence J.M. Edible sea urchins: biology and ecology. Elsevier Science; 2007. Sea Urchin Roe Cuisine. [Google Scholar]
- Lawrence J.M., Lawrence A.L., McBride S.C., George S.B., Watts S.A., Plank L.R. Development in the use of prepared feeds in sea-urchin aquaculture. World Aquacult. 2001;32(3):34–39. [Google Scholar]
- Lesser M.P., Walker C.W. Introduction to the special section on sea urchin aquaculture. J. Shellfish. Res. 1998;17:1505–1506. [Google Scholar]
- Martik M.L., Lyons D.C., McClay D.R. Developmental gene regulatory networks in sea urchins and what we can learn from them [version 1; referees: 3 approved] F1000Research 5(F1000FacultyRev) 2016;203 doi: 10.12688/f1000research.7381.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur J.E., Miller J.W. A description of the complete metamorphosis of the sea urchin Lytechinus variegatus cultured in synthetic sea water. Ohio J. Sci. 1971;71(1):30–36. [Google Scholar]
- McBride S.C. Sea urchin aquaculture. In: Kelly A.M., Silvertein J., editors. Vol. 46. American Fisheries Society Symposium; Bethesda, Maryland: 2005. pp. 179–208. (Aquaculture in the 21st Century). [Google Scholar]
- McClanahan T.R. Predation and the distribution and abundance of tropical sea urchin populations. J. Exp. Mar. Biol. Ecol. 1988;221:231–255. [Google Scholar]
- Miller B.A., Emlet R.B. Development of newly metamorphosed juvenile sea urchins (Strongylocentrotus franciscanus and S. purpuratus): morphology, the effects of temperature and larval food ration, and a method for determining age. J. Exp. Mar. Biol. Ecol. 1999;235(1):67–90. [Google Scholar]
- Moran A.L. University of Oregon; Eugene, Oregon, USA: 1997. Size, form, and function in the early life histories of the gastropod genera Nucella and Littorina Dissertation. [Google Scholar]
- Moran A.L. Juvenile invertebrates in the marine benthos: mortality, size and performance. Am. Zool. 1998 in press. [Google Scholar]
- Morse A.N.C. How do planktonic larvae know where to settle? Am Sci. 1991;79:154–167. [Google Scholar]
- Murphy C.T., Hu P.J. WormBook Dec 26; The C elegans Research Community. WormBook; 2013. Insulin/insulin-like growth factor signaling in C elegans; pp. 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi S.R. Population genetics, demographic connectivity, and the design of marine reserves. Ecol. Appl. 2003;13:S146–S158. [Google Scholar]
- Pawlik J.R. Natural and artificial induction of metamorphosis of Phragmatopoma lapidosa californica (Polychaeta Sabellariidae), with a critical look at the effects of bioactive compounds on marine invertebrate larvae. Bull. mar. Sci. 1990;46:512–536. [Google Scholar]
- Pechenik J.A., Estrella S., Hammer K. Food limitation stimulates metamorphosis of competent larvae and alters postmetamorphic growth rate in the marine prosobranch gastropod Crepidula fornicata. Mar. Biol. 1996;127:267–275. [Google Scholar]
- Pechenik J.A., Hammer K., Weise C. The effect of starvation on acquisition of competence and postmetamorphic performance in the marine prosobranch gastropod Crepidula fornicata (L) J. Exp. Mar. Biol. Ecol. 1996;199:137–152. [Google Scholar]
- Perillo M., Arnone M.I. Characterization of insulin-like peptides (ILPs) in the sea urchin Strongylocentrotus purpuratus: Insights on the evolution of the insulin family. Gen. Comp. Endocrinol. 2014;205:68–79. doi: 10.1016/j.ygcen.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Qian P.Y., Chia F.S. Larval growth and development as influenced by food limitation in two polychaetes: Capitella sp: and Polydora ligni. J. Exp. Mar. Biol. Ecol. 1993;166:93–105. [Google Scholar]
- Rahim S., L. i J.Y., Kitamura H. Larval metamorphosis of the sea urchins: Pseudocentrotus depressus and Anthocidaris crassispina in response to microbial films. Mar. Biol. 2004;144:71–78. [Google Scholar]
- Rena G., Guo S., Cichy S.C., Unterman T.G., Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 1999;274(24):17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Robinson S.M. The evolving role of aquaculture in the global production of sea urchins. In: Lawrence J.M., Guzmán O., editors. Sea Urchins: Fisheries and Ecology. DEStech Publications, Inc.; Lancaster: 2004. pp. 343–357. [Google Scholar]
- Rogers-Bennett L. Chapter 19, The Ecology of Strongylocentrotus franciscanus and Strongylocentrotus purpuratus. In: Lawrence J.M., editor. Edible Sea Urchins: Biology and Ecology. Elsevier Science; 2007. [Google Scholar]
- Rogers-Bennett L., Bennett W.A., Fastenau H.C., Dewees C.M. Spatial variation in red sea urchin reproduction and morphology: implications for harvest refugia. Ecol. Appl. 1995;5:1171–1180. [Google Scholar]
- Shimabukuro S. Tripneustes gratilla. In: Shokita S., Yamaguchi M., Masashi M., editors. Aquaculture in Tropical Areas. Midori Shobo; Tokyo: 1991. pp. 313–328. [Google Scholar]
- Smith M.M., Cruz Smith L., Cameron R.A., Urry L.A. The larval stages of the sea urchin, Strongylocentrotus purpuratus. J. Morphol. 2008;269:713–733. doi: 10.1002/jmor.10618. [DOI] [PubMed] [Google Scholar]
- Sodergren E., Weinstock G.M., Davidson E.H., Cameron R.A., Gibbs R.A., Angerer R.C., Angerer L.M., Arnone M.I., Burgess D.R. The Genome of the Sea Urchin Strongylocentrotus purpuratus: Sea Urchin Genome Sequencing Consortium. Science. 2006;2006(314):941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann M.F. University of Washington Press; Seattle: 1987. Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast: Data and Methods for the Study of Eggs, Embryos, and Larvae. [Google Scholar]
- Strathmann R.R. Feeding and nonfeeding larval development and life history evolution in marine invertebrates. Annu. Rev. Ecol. Syst. 1985;16:339–361. [Google Scholar]
- Takahashi Y., Itoh K., Ishii M., Suzuki M., Itabashi Y. Induction of larval settlement and metamorphosis of the sea urchin Strongylocentrotus intermedius by glycoglycerolipids from the green algae Ulvella lens. Marine Bio. 2002;140:763–777. [Google Scholar]
- Tatar M., Kopelman A., Epstein D., Tu M.P., Yin C.M., Garofalo R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Thet M.M., Noguchi M., Yazaki I. Larval and juvenile development of the echinometrid sea urchin Colobocentrotus mertensii: emergence of the peculiar form of spines. Zool. Sci. 2004;21(3):265–274. doi: 10.2108/zsj.21.265. [DOI] [PubMed] [Google Scholar]
- Tu Q., Brown T.C., Eric H., Davidson H., Oliveri P. Sea urchin Forkhead gene family: Phylogeny and embryonic expression. Dev. Biol. 2006;300(1):49–62. doi: 10.1016/j.ydbio.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Walker M.M. Larval life span, larval settlement, and early growth of Evechinus chloroticus (Valenciennes) New Zeal. J. Mar. Fresh. 1984;18:4–397. [Google Scholar]
- Webb A.E., Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem. Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella F., Link W., Carnero A. Understanding FOXO, new views on old transcription factors. Curr. Cancer Drug Targets. 2010;10:135–146. doi: 10.2174/156800910791054158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








