Abstract
BACKGROUND
Patients with advanced urothelial carcinoma that progresses after platinum-based chemotherapy have a poor prognosis and limited treatment options.
METHODS
In this open-label, international, phase 3 trial, we randomly assigned 542 patients with advanced urothelial cancer that recurred or progressed after platinum-based chemotherapy to receive pembrolizumab (a highly selective, humanized monoclonal IgG4κ isotype antibody against programmed death 1 [PD-1]) at a dose of 200 mg every 3 weeks or the investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine. The coprimary end points were overall survival and progression-free survival, which were assessed among all patients and among patients who had a tumor PD-1 ligand (PD-L1) combined positive score (the percentage of PD-L1–expressing tumor and infiltrating immune cells relative to the total number of tumor cells) of 10% or more.
RESULTS
The median overall survival in the total population was 10.3 months (95% confidence interval [CI], 8.0 to 11.8) in the pembrolizumab group, as compared with 7.4 months (95% CI, 6.1 to 8.3) in the chemotherapy group (hazard ratio for death, 0.73; 95% CI, 0.59 to 0.91; P=0.002). The median overall survival among patients who had a tumor PD-L1 combined positive score of 10% or more was 8.0 months (95% CI, 5.0 to 12.3) in the pembrolizumab group, as compared with 5.2 months (95% CI, 4.0 to 7.4) in the chemotherapy group (hazard ratio, 0.57; 95% CI, 0.37 to 0.88; P=0.005). There was no significant between-group difference in the duration of progression-free survival in the total population (hazard ratio for death or disease progression, 0.98; 95% CI, 0.81 to 1.19; P=0.42) or among patients who had a tumor PD-L1 combined positive score of 10% or more (hazard ratio, 0.89; 95% CI, 0.61 to 1.28; P =0.24). Fewer treatment-related adverse events of any grade were reported in the pembrolizumab group than in the chemotherapy group (60.9% vs. 90.2%); there were also fewer events of grade 3, 4, or 5 severity reported in the pembrolizumab group than in the chemotherapy group (15.0% vs. 49.4%).
CONCLUSIONS
Pembrolizumab was associated with significantly longer overall survival (by approximately 3 months) and with a lower rate of treatment-related adverse events than chemotherapy as second-line therapy for platinum-refractory advanced urothelial carcinoma. (Funded by Merck; KEYNOTE-045 ClinicalTrials.gov number, NCT02256436.)
Urothelial cancer is highly lethal in the metastatic state.1 Platinum-based combination chemotherapy remains the standard first-line treatment for metastatic disease. Carboplatin-based combinations are associated with a median overall survival of 9 months,2 and cisplatin-based combinations with a median overall survival of 12 to 15 months.3 However, after platinum-based chemotherapy, there is no internationally accepted standard of care. Single-agent paclitaxel and docetaxel are commonly used worldwide,4,5 and in Europe, vinflunine has been approved on the basis of an overall survival advantage of 2 months over best supportive care.6,7 Because the median overall survival with second-line therapy is only 6 to 7 months, effective options are needed in patients with previously treated advanced urothelial carcinoma.
Monoclonal antibodies against programmed death 1 (PD-1) and its ligands (PD-L1 and PD-L2) have shown robust antitumor activity and a manageable safety profile in many advanced malignant conditions,8 including urothelial cancer.9–14 Pembrolizumab, a highly selective, humanized monoclonal IgG4κ isotype antibody against PD-1, can disrupt the engagement of PD-1 with its ligands and impede inhibitory signals in T cells. Pembrolizumab showed antitumor activity in patients with advanced urothelial carcinoma in the phase 1b KEYNOTE-012 study9 and the phase 2 KEYNOTE-052 study.12 In the international, randomized, open-label, phase 3 KEYNOTE-045 trial, we compared pembrolizumab with investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine as second-line therapy in patients with advanced urothelial carcinoma that progressed during or after the receipt of platinum-based chemotherapy.
METHODS
PATIENTS
Patients who were 18 years of age or older were eligible for enrollment if they had histologically or cytologically confirmed urothelial carcinoma of the renal pelvis, ureter, bladder, or urethra that showed predominantly transitional-cell features on histologic testing, had progression after platinum-based chemotherapy for advanced disease or recurrence within 12 months after the receipt of platinum-based adjuvant or neoadjuvant therapy for localized muscle-invasive disease, had received two or fewer lines of systemic chemotherapy for advanced disease previously, had at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,15 and had an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0, 1, or 2 (on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating greater disability). Patients who had an ECOG performance-status score of 2 (indicating that the patient is ambulatory and capable of all self-care but is unable to carry out any work activities and is out of bed more than 50% of waking hours) and had one or more of the established poor prognostic factors for second-line therapy (i.e., hemoglobin concentration of <10 g per deciliter, presence of liver metastases, and receipt of the last dose of most recent chemotherapy <3 months before enrollment)16,17 were excluded from enrollment. Patients were ineligible if they had received anti–PD-1, anti–PD-L1, or anti–CTLA-4 therapy previously. Full eligibility criteria are listed in the trial protocol, available with the full text of this article at NEJM.org.
TRIAL DESIGN AND TREATMENT
Patients were randomly assigned in a 1:1 ratio to receive pembrolizumab (at a dose of 200 mg) or investigator’s choice of paclitaxel (at a dose of 175 mg per square meter of body-surface area), docetaxel (at a dose of 75 mg per square meter), or vinflunine (at a dose of 320 mg per square meter), all administered intravenously every 3 weeks. Randomization was stratified according to ECOG performance-status score (0 or 1 vs. 2), presence of liver metastases (yes vs. no), hemoglobin concentration (<10 g per deciliter vs. ≥10 g per deciliter), and time since the last dose of chemotherapy (<3 months vs. ≥3 months). Treatment assignment was not blinded. Treatment was continued until RECIST-defined disease progression,15 development of an unacceptable level of toxic effects, withdrawal of consent, decision by the investigator to discontinue treatment, or the completion of 2 years of pembrolizumab therapy.
Patients with disease progression, according to the investigator’s assessment of radiographic results, and a clinically stable status could continue to receive the therapy at the discretion of the investigator. Patients in the pembrolizumab group who had a complete response could discontinue treatment if they had received pembrolizumab for at least 24 weeks and for at least two doses beyond the time of initial complete response. There was no planned crossover on disease progression. Full information about guidance regarding the treatment decisions is provided in the protocol.
ASSESSMENTS
PD-L1 expression was assessed in formalin-fixed tumor samples at a central laboratory with the use of the commercially available PD-L1 IHC 22C3 pharmDx assay (Dako North America). The kits were purchased at full cost. Archival tumor samples and newly obtained core or excisional biopsy samples from nonirradiated sites were permitted. There were no restrictions on the age of the archival samples or on the number of intervening therapies received after the sample was obtained. All samples, regardless of whether they were archival or newly obtained, were analyzed by the central laboratory during the screening process, and only the patients whose samples could be evaluated for PD-L1 expression were permitted to enroll in the study. PD-L1 expression was categorized as the PD-L1 combined positive score, defined as the percentage of PD-L1–expressing tumor and infiltrating immune cells relative to the total number of tumor cells.12
Tumor imaging was scheduled for week 9, followed by every 6 weeks during the first year and every 12 weeks thereafter. Response to treatment was assessed according to RECIST15 by means of blinded, independent, central radiologic review. During follow-up for survival, patients were contacted every 12 weeks for survival assessment. The full assessment schedule is provided in the trial protocol. All the adverse events and abnormalities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
END POINTS
At the second interim analysis, the coprimary end points were overall survival and progression-free survival, which were assessed in the total population and in the population of patients who had a tumor PD-L1 combined positive score of 10% or more. Overall survival was defined as the time from randomization to death from any cause. Progression-free survival was defined as the time from randomization to disease progression or death from any cause.
Secondary efficacy end points, which were assessed in the total population and in the population of patients who had a tumor PD-L1 combined positive score of 10% or more, included the objective response rate, defined as the percentage of patients who had a confirmed complete or partial response, and the duration of confirmed response, defined as the time from the first documented complete or partial response to disease progression or death. Safety in the total population was also a secondary end point. The full list of end points is provided in the protocol.
Efficacy was assessed in the intention-to-treat population, which included all the patients who were assigned to a treatment group. Safety was assessed in the as-treated population, which included all the patients who received at least one dose of study treatment.
TRIAL OVERSIGHT
The trial was designed by academic advisors and employees of the sponsor (Merck). Data were collected by investigators and their site personnel and analyzed by statisticians who were employees of the sponsor. Results were interpreted by the academic authors, by authors who were employees of the sponsor, and by other employees of the sponsor who did not fulfill all the authorship criteria as outlined by the International Committee of Medical Journal Editors. An external data and safety monitoring committee oversaw the trial and assessed efficacy and safety at the time of prespecified interim analyses that were performed by statisticians from QuintilesIMS, with funding by the sponsor.
The trial protocol and all the amendments were approved by the appropriate ethics body at each center. The trial was conducted in accordance with the protocol and its amendments, with Good Clinical Practice guidelines, and with the provisions of the Declaration of Helsinki. All the patients provided written informed consent before enrollment.
All the authors attest that the trial was conducted in accordance with the protocol and all the amendments, attest that they had access to the data used for the writing of the manuscript, and vouch for the accuracy and completeness of the data and analyses. The first draft of the manuscript was written by the first and last authors, with input from authors who were employees of the sponsor. Assistance with manuscript preparation was provided by a medical writer employed by the sponsor. All the authors participated in reviewing and editing the manuscript and made the decision to submit the manuscript for publication.
STATISTICAL ANALYSIS
Overall survival, progression-free survival, and duration of response were estimated with the use of the Kaplan–Meier method. In the analysis of overall survival, patients who were alive or lost to follow-up had their data censored at the time of last contact. In the analysis of progression-free survival, patients who were alive and without disease progression or who were lost to follow-up had their data censored at the time of last tumor assessment. Between-group differences in overall survival and progression-free survival were calculated with the use of a stratified log-rank test. Hazard ratios and associated 95% confidence intervals were calculated with the use of a stratified Cox proportional-hazards model and Efron’s method of handling ties. Differences in the response rate were calculated with the stratified Miettinen and Nurminen method. The same stratification factors that were used for randomization were applied to all stratified efficacy analyses.
The overall family-wise type I error rate was strictly controlled at a one-sided alpha level of 2.5%. We calculated that enrollment of 470 patients would provide the study with 88% power to show a hazard ratio for death of 0.781 or better in the analysis of overall survival in the pembrolizumab group versus the chemotherapy group in the total population and 86% power to show a hazard ratio of 0.625 or better in the pembrolizumab group versus the chemotherapy group among patients who had a tumor PD-L1 combined positive score of 10% or more.
The protocol specified two interim analyses before the final analysis. After reviewing the first interim analysis, the data and safety monitoring committee recommended continuing the study as planned. The second interim analysis was based on a cutoff date of September 7, 2016, and was performed after 334 deaths had occurred in the total population and 104 deaths had occurred in the population of patients who had a tumor PD-L1 combined positive score of 10% or more. The data and safety monitoring committee reviewed the results of the second interim analysis on October 18, 2016, and recommended early termination of the trial because pembrolizumab met the superiority thresholds for overall survival in the coprimary populations (Table S1 in the Supplementary Appendix, available at NEJM.org). All the data reported herein are those from the second interim analysis. The full statistical analysis plan is available in the protocol.
RESULTS
PATIENTS AND TREATMENT
A total of 748 patients were screened for enrollment at 120 sites in 29 countries. Between November 5, 2014, and November 13, 2015, a total of 542 patients were randomly assigned to pembrolizumab (270 patients) or investigator’s choice of chemotherapy (272). Of these, 266 patients in the pembrolizumab group and 255 in the chemotherapy group received treatment (Fig. S1 in the Supplementary Appendix). In the chemotherapy group, 84 patients received docetaxel, 84 received paclitaxel, and 87 received vinflunine. The demographic and disease characteristics of the patients at baseline were generally balanced between the two treatment groups (Table 1, and Table S2 in the Supplementary Appendix). A total of 164 patients (30.3%), including 74 patients in the pembrolizumab group and 90 in the chemotherapy group, had a tumor PD-L1 combined positive score of 10% or more (Table S3 in the Supplementary Appendix).
Table 1.
Demographic and Disease Characteristics at Baseline in the Intention-to-Treat Population.*
| Characteristic | Pembrolizumab Group (N = 270) | Chemotherapy Group (N = 272) |
|---|---|---|
| Age — yr | ||
| Median | 67 | 65 |
| Range | 29–88 | 26–84 |
| Male sex — no. (%) | 200 (74.1) | 202 (74.3) |
| ECOG performance-status score — no. (%)† | ||
| 0 | 119 (44.1) | 106 (39.0) |
| 1 | 143 (53.0) | 158 (58.1) |
| 2 | 2 (0.7) | 4 (1.5) |
| Missing data | 6 (2.2) | 4 (1.5) |
| Current or former smoker — no./total no. (%) | 165/269 (61.3) | 186/269 (69.1) |
| Pure transitional-cell features in histologic testing — no./total no. (%) | 186/270 (68.9) | 197/270 (73.0) |
| Tumor PD-L1 combined positive score ≥ 10% — no./total no. (%)‡ | 74/260 (28.5) | 90/266 (33.8) |
| Site of primary tumor in bladder or urethra — no./total no. (%) | 232/270 (85.9) | 234/271 (86.3) |
| Visceral disease — no./total no. (%) | 240/269 (89.2) | 233/271 (86.0) |
| Liver metastases — no./total no. (%) | 91/270 (33.7) | 95/271 (35.1) |
| Hemoglobin concentration <10 g/dl — no./total no. (%) | 43/262 (16.4) | 44/267 (16.5) |
| No. of risk factors — no. (%)§ | ||
| 0 | 54 (20.0) | 44 (16.2) |
| 1 | 96 (35.6) | 97 (35.7) |
| 2 | 66 (24.4) | 80 (29.4) |
| 3 or 4 | 45 (16.7) | 45 (16.5) |
| Missing data | 9 (3.3) | 6 (2.2) |
| Completion or discontinuation of most recent therapy <3 mo previously — no./total no. (%) | 103/269 (38.3) | 104/271 (38.4) |
The intention-to-treat population included all the patients who underwent randomization. There were no significant differences between the two treatment groups. For the characteristics of current or former smoker, pure transitional-cell features on histologic testing, a tumor PD-L1 combined positive score of 10% or more, site of primary tumor in bladder or urethra, visceral disease, liver metastases, hemoglobin concentration, and timing of completion or discontinuation of most recent therapy, percentages are calculated on the basis of the number of patients with available data. The full details regarding the demographic and disease characteristics at baseline are provided in Table S2 in the Supplementary Appendix.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with 0 indicating no symptoms and higher scores indicating greater disability.
The programmed death ligand 1 (PD-L1) combined positive score was defined as the percentage of tumor and infiltrating immune cells with PD-L1 expression out of the total number of tumor cells.
The median duration of follow-up, defined as the time from randomization to September 7, 2016, was 14.1 months (range, 9.9 to 22.1). In the as-treated population, the median duration of study treatment was 3.5 months (range, <0.1 to 20.0) in the pembrolizumab group and 1.5 months (range, <0.1 to 14.2) in the chemotherapy group. A total of 49 patients (18.4%) in the pembrolizumab group and 3 (1.2%) in the chemotherapy group were still receiving study treatment at the time of data cutoff (Fig. S1 in the Supplementary Appendix). In the intention-to-treat population, 68 patients (25.2%) in the pembrolizumab group and 91 (33.5%) in the chemotherapy group received subsequent therapy, including 2 patients (0.7%) and 35 patients (12.9%), respectively, who received subsequent immunotherapy.
OVERALL SURVIVAL
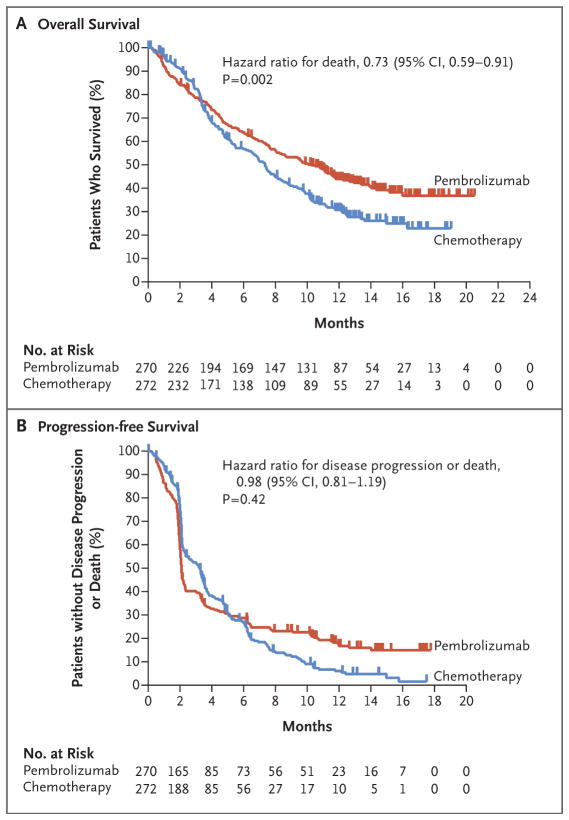
As of September 7, 2016, a total of 334 deaths had occurred in the intention-to-treat population. Overall survival was significantly longer in the pembrolizumab group than in the chemotherapy group (hazard ratio for death, 0.73; 95% confidence interval [CI], 0.59 to 0.91; P = 0.002) (Fig. 1A). The median overall survival was 10.3 months (95% CI, 8.0 to 11.8) in the pembrolizumab group, as compared with 7.4 months (95% CI, 6.1 to 8.3) in the chemotherapy group. The estimated overall survival rate at 12 months was 43.9% (95% CI, 37.8 to 49.9) in the pembrolizumab group, as compared with 30.7% (95% CI, 25.0 to 36.7) in the chemotherapy group.
Figure 1. Overall Survival and Progression-free Survival in the Intention-to-Treat Population.
Shown are Kaplan–Meier estimates of overall survival and progression-free survival according to treatment group. Tick marks represent patients who had data censored at the last time that they were known to be alive (Panel A) or who were known to be alive and without disease progression as assessed per Response Evaluation Criteria in Solid Tumors, version 1.1, by blinded, independent, central radiologic review (Panel B). The intention-to-treat population included all the patients who underwent randomization. The one-sided superiority thresholds for pembrolizumab were a P value of 0.0123 in the analysis of overall survival and a P value of 0.0151 in the analysis of progression-free survival. The median overall survival was 10.3 months (95% CI, 8.0 to 11.8) in the pembrolizumab group, as compared with 7.4 months (95% CI, 6.1 to 8.3) in the chemotherapy group (44.8 weeks [95% CI, 34.8 to 51.3] vs. 32.2 weeks [95% CI, 26.5 to 36.1]). The median progression-free survival was 2.1 months (95% CI, 2.0 to 2.2) in the pembrolizumab group and 3.3 months (95% CI, 2.3 to 3.5) in the chemotherapy group (9.1 weeks [95% CI, 8.7 to 9.6] and 14.3 weeks [95% CI, 10.0 to 15.2], respectively).
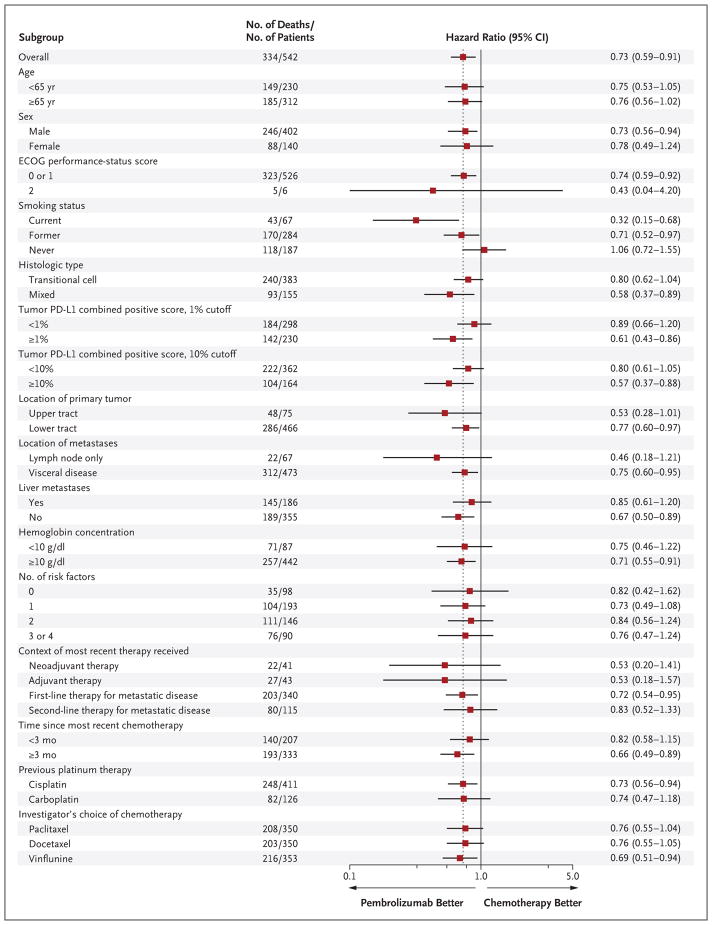
Pembrolizumab was also associated with significantly longer overall survival than chemotherapy in the coprimary population of patients who had a tumor PD-L1 combined positive score of 10% or more (hazard ratio for death, 0.57; 95% CI, 0.37 to 0.88; P = 0.005). The median overall survival was 8.0 months (95% CI, 5.0 to 12.3) in the pembrolizumab group, as compared with 5.2 months (95% CI, 4.0 to 7.4) in the chemotherapy group (Fig. S2A in the Supplementary Appendix). Pembrolizumab was associated with a benefit over chemotherapy in all the subgroups examined, including among patients with liver metastases and those who had a tumor PD-L1 combined positive score of less than 1% (Fig. 2). In an analysis that considered the chemotherapy regimens separately, the benefit of pembrolizumab over chemotherapy was similar for each chemotherapy regimen (Fig. 2).
Figure 2. Analysis of Overall Survival in Key Subgroups.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with 0 indicating no symptoms and higher scores indicating greater disability; a score of 2 indicates that the patient is ambulatory and capable of all self-care but is unable to carry out any work activities and is out of bed more than 50% of waking hours. The programmed death ligand 1 (PD-L1) combined positive score was defined as the percentage of tumor and infiltrating immune cells with PD-L1 expression out of the total number of tumor cells. The upper tract was defined as the renal pelvis or ureter, and the lower tract as the bladder or urethra. Risk factors include the Bellmunt risk factors of an ECOG performance-status score above 0, a hemoglobin concentration of less than 10 g per deciliter, and the presence of liver metastases,16 plus a time since the completion or discontinuation of previous therapy of less than 3 months.17 The number of patients in each of the subgroups of investigator’s choice of chemotherapy represents the number of patients who received at least one dose of that treatment (84 patients received docetaxel, 84 paclitaxel, and 87 vinflunine) plus the number of patients who received at least one dose of pembrolizumab (266). For all other subgroups, the intention-to-treat population was used. Data were missing as follows: ECOG performance-status score (10 patients), smoking status (4), histologic type (2), tumor PD-L1 combined positive score 1% cutoff (14), tumor PD-L1 combined positive score 10% cutoff (16), location of primary tumor (1), location of metastases (2), liver metastases (1), hemoglobin concentration (13), number of risk factors (15), context of most recent therapy received (2), time since most recent chemotherapy (2), and type of previous platinum therapy (2). Data for 2 patients with other histologic type and for 2 patients with other previous platinum therapy are also not shown. The dashed line indicates the rate of overall survival in the entire trial population.
PROGRESSION-FREE SURVIVAL
A total of 437 events of disease progression or death occurred in the intention-to-treat population, with no significant difference in the duration of progression-free survival between the pembrolizumab group and the chemotherapy group (hazard ratio for death or disease progression, 0.98; 95% CI, 0.81 to 1.19; P = 0.42) (Fig. 1B). The median progression-free survival was 2.1 months (95% CI, 2.0 to 2.2) in the pembrolizumab group and 3.3 months (95% CI, 2.3 to 3.5) in the chemotherapy group. The estimated progression-free survival rate at 12 months was 16.8% (95% CI, 12.3 to 22.0) in the pembrolizumab group and 6.2% (95% CI, 3.3 to 10.2) in the chemotherapy group. There was also no significant between-group difference in the duration of progression-free survival among patients who had a tumor PD-L1 combined positive score of 10% or more (hazard ratio, 0.89; 95% CI, 0.61 to 1.28; P = 0.24) (Fig. S2B in the Supplementary Appendix).
OBJECTIVE RESPONSE
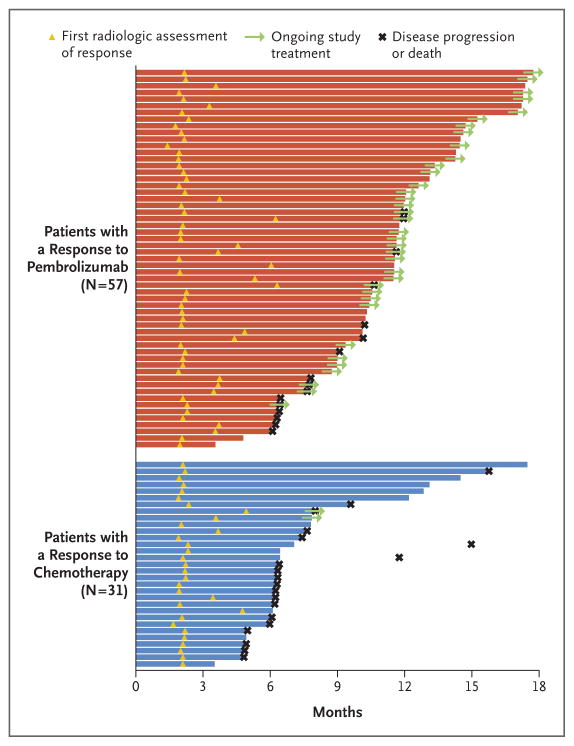
In the total population, the objective response rate was significantly higher in the pembrolizumab group (21.1%; 95% CI, 16.4 to 26.5) than in the chemotherapy group (11.4%; 95% CI, 7.9 to 15.8) (P = 0.001). The median time to response was 2.1 months in each group. The median duration of response was not reached in the pembrolizumab group (range, 1.6+ to 15.6+ months) and was 4.3 months (range, 1.4+ to 15.4+) in the chemotherapy group (Fig. 3). (Plus signs indicate an ongoing response at data cutoff.)
Figure 3. Time to Response and Duration of Response in Patients with a Confirmed Objective Response.
Response and disease progression were assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1, by blinded, independent, central radiologic review. Bars indicate the duration of response at the time of data cutoff.
At the time of data cutoff, 41 of 57 patients (72%) with a response in the pembrolizumab group and 11 of 31 (35%) with a response in the chemotherapy group continued to have a response. Treatment was ongoing in 36 of 57 patients with a response (63%) in the pembrolizumab group and in 2 of 31 (6%) with a response in the chemotherapy group (Fig. 3). The estimated percentage of patients with a duration of response of at least 12 months was 68% in the pembrolizumab group versus 35% in the chemotherapy group. Results were similar in the population of patients who had a tumor PD-L1 combined positive score of 10% or more. Details are provided in Table S4 and Figure S3 in the Supplementary Appendix.
ADVERSE EVENTS
Adverse events that were considered by the investigators to be related to treatment occurred in 60.9% of the patients treated with pembrolizumab, as compared with 90.2% of those who received chemotherapy (Table 2). Treatment-related events of grade 3, 4, or 5 severity were less frequent in the pembrolizumab group than in the chemotherapy group (15.0% vs. 49.4% of patients), as was treatment-related discontinuation of therapy (5.6% vs. 11.0%). One pembrolizumab-treated patient died from treatment-related pneumonitis. Three other deaths in the pembrolizumab group were attributed by the investigators to study treatment, including one death related to urinary tract obstruction, one death related to malignant neoplasm progression, and one death of unspecified cause. In the chemotherapy group, treatment-related deaths were related to sepsis (in two patients), septic shock (in one), and unspecified cause (in one).
Table 2.
Adverse Events in the As-Treated Population.*
| Event | Pembrolizumab Group (N = 266) | Chemotherapy Group (N = 255) | ||
|---|---|---|---|---|
| Any Grade | Grade 3, 4, or 5 | Any Grade | Grade 3, 4, or 5 | |
| number of patients (percent) | ||||
| Treatment-related event† | ||||
|
| ||||
| Any event | 162 (60.9) | 40 (15.0) | 230 (90.2) | 126 (49.4) |
|
| ||||
| Event leading to discontinuation of treatment | 15 (5.6) | 12 (4.5) | 28 (11.0) | 16 (6.3) |
|
| ||||
| Event leading to death | 4 (1.5) | 4 (1.5) | 4 (1.6) | 4 (1.6) |
|
| ||||
| Event occurring in ≥10% of patients in either group‡ | ||||
|
| ||||
| Pruritus | 52 (19.5) | 0 | 7 (2.7) | 1 (0.4) |
|
| ||||
| Fatigue | 37 (13.9) | 3 (1.1) | 71 (27.8) | 11 (4.3) |
|
| ||||
| Nausea | 29 (10.9) | 1 (0.4) | 62 (24.3) | 4 (1.6) |
|
| ||||
| Diarrhea | 24 (9.0) | 3 (1.1) | 33 (12.9) | 2 (0.8) |
|
| ||||
| Decreased appetite | 23 (8.6) | 0 | 41 (16.1) | 3 (1.2) |
|
| ||||
| Asthenia | 15 (5.6) | 1 (0.4) | 36 (14.1) | 7 (2.7) |
|
| ||||
| Anemia | 9 (3.4) | 2 (0.8) | 63 (24.7) | 20 (7.8) |
|
| ||||
| Constipation | 6 (2.3) | 0 | 52 (20.4) | 8 (3.1) |
|
| ||||
| Peripheral sensory neuropathy | 2 (0.8) | 0 | 28 (11.0) | 5 (2.0) |
|
| ||||
| Neutrophil count decreased | 1 (0.4) | 1 (0.4) | 36 (14.1) | 31 (12.2) |
|
| ||||
| Peripheral neuropathy | 1 (0.4) | 0 | 27 (10.6) | 2 (0.8) |
|
| ||||
| Neutropenia | 0 | 0 | 39 (15.3) | 34 (13.3) |
|
| ||||
| Alopecia | 0 | 0 | 96 (37.6) | 2 (0.8) |
|
| ||||
| Event of interest§ | ||||
|
| ||||
| Any event | 45 (16.9) | 12 (4.5) | 19 (7.5) | 4 (1.6) |
|
| ||||
| Hypothyroidism | 17 (6.4) | 0 | 3 (1.2) | 0 |
|
| ||||
| Hyperthyroidism | 10 (3.8) | 0 | 1 (0.4) | 0 |
|
| ||||
| Pneumonitis | 11 (4.1) | 6 (2.3) | 1 (0.4) | 0 |
|
| ||||
| Colitis | 6 (2.3) | 3 (1.1) | 1 (0.4) | 0 |
|
| ||||
| Infusion reaction | 2 (0.8) | 0 | 10 (3.9) | 0 |
|
| ||||
| Nephritis | 2 (0.8) | 2 (0.8) | 0 | 0 |
|
| ||||
| Severe skin reaction | 2 (0.8) | 1 (0.4) | 3 (1.2) | 3 (1.2) |
|
| ||||
| Thyroiditis | 2 (0.8) | 0 | 0 | 0 |
|
| ||||
| Adrenal insufficiency | 1 (0.4) | 1 (0.4) | 0 | 0 |
|
| ||||
| Myositis | 0 | 0 | 1 (0.4) | 1 (0.4) |
The as-treated population included all the patients who received at least one dose of study treatment.
Events were attributed to treatment by the investigator and are listed as indicated by the investigator on the case-report form. Although decreased neutrophil count and neutropenia may reflect the same condition, they were listed by the investigators as two distinct events; this was also the case for peripheral sensory neuropathy and peripheral neuropathy and for fatigue and asthenia.
Events are listed in descending order of frequency in the pembrolizumab group.
The events of interest are those with an immune-related cause and are considered regardless of attribution to study treatment by the investigator. They are listed in descending order of frequency in the pembrolizumab group. In addition to the specific preferred terms listed, related terms were also included.
The most common treatment-related adverse events of any grade were pruritus (19.5% of the patients), fatigue (13.9%), and nausea (10.9%) in the pembrolizumab group and alopecia (37.6%), fatigue (27.8%), and anemia (24.7%) in the chemotherapy group (Table 2). There were no treatment-related events of grade 3, 4, or 5 severity that occurred with an incidence of 5% or more in the pembrolizumab group. In the chemotherapy group, treatment-related events of grade 3, 4, or 5 severity with an incidence of 5% or more were neutropenia (13.3%), decreased neutrophil count (12.2%), anemia (7.8%), febrile neutropenia (7.1%), and decreased white-cell count (5.1%). A summary of all the adverse events with an incidence of 5% or more, regardless of whether they were attributed to treatment by the investigator, is provided in Table S5 in the Supplementary Appendix.
The adverse events of interest with regard to pembrolizumab, regardless of whether they were attributed to study treatment by the investigator, are shown in Table 2. The only events of grade 3, 4, or 5 severity that were observed in two or more patients who were treated with pembrolizumab were pneumonitis (2.3% of the patients), colitis (1.1%), and nephritis (0.8%); there was only one grade 5 event (0.4%), which was pneumonitis.
DISCUSSION
In this randomized, phase 3 study involving patients with advanced urothelial cancer that progressed during or after platinum-based chemotherapy, pembrolizumab resulted in significantly longer overall survival — by approximately 3 months — than the investigator’s choice of paclitaxel, docetaxel, or vinflunine (10.3 months vs. 7.4 months). Pembrolizumab had a better safety profile than did chemotherapy. The benefit of pembrolizumab over chemotherapy was seen in the total population, as well as in the coprimary population of patients who had a tumor PD-L1 combined positive score of 10% or more.
The overall survival benefit with pembrolizumab was observed across almost all the subgroups examined and was similar regardless of investigator’s choice of chemotherapy. In this trial, the benefit of pembrolizumab appeared to be independent of PD-L1 expression on tumor and infiltrating immune cells. The results of ongoing randomized, controlled trials currently being conducted in earlier lines of treatment may help elucidate the role of PD-L1 expression as a biomarker in urothelial carcinoma. A relationship between smoking status and the relative benefit of pembrolizumab was observed. The relationship between smoking and the relative benefit of immunotherapy has also been observed in patients with other advanced cancers8,18 and may reflect a high mutational load in current and former smokers.19 Genomic analysis of this relationship is warranted. The median overall survival of 7.4 months that was observed with chemotherapy is consistent with historical data for second-line, single-agent treatment.4–7
Pembrolizumab resulted in a significantly higher objective response rate than chemotherapy. Most responses in patients in the pembrolizumab group occurred quickly and were reported at the first scheduled imaging assessment. Continued disease regression over time in some patients resulted in radiologically confirmed responses that were reported as long as 6.3 months after the start of therapy. As compared with responses in the chemotherapy group, responses in the pembrolizumab group were durable, with a median duration of response that was not reached over a median follow-up of 14.1 months. On the basis of the Kaplan–Meier estimates, 68% of the responses to pembrolizumab were ongoing at 12 months.
Durable responses in patients with advanced urothelial cancer have also been observed with the anti–PD-L1 antibodies atezolizumab,10 durvalumab,13 and avelumab14 and the anti–PD-1 antibody nivolumab.11 Atezolizumab and nivolumab are approved only in the United States for the treatment of advanced urothelial cancer that progressed during or after receipt of previous platinum-based chemotherapy. Accelerated approval of these two antibodies was based on objective-response data from single-group studies.10,11 Pembrolizumab has now been shown to result in a significant survival benefit over standard-of-care therapy in a large, randomized trial in this population.
Overall, there was no significant difference between pembrolizumab and chemotherapy with regard to progression-free survival. Beyond 6 months, the progression-free survival curves diverged in favor of pembrolizumab. Similar findings have been observed with checkpoint inhibitors in other tumor types20–24 and suggest that in contrast to its use as a surrogate end point in historical chemotherapy studies, progression-free survival may not be a reliable surrogate end point for the clinical benefit of immunotherapy. The prolonged duration of response was seen only in patients who had a response to pembrolizumab, and because less than half the patients had a response, the therapy did not exert an effect on median progression-free survival. However, the duration of response in patients who had a response was much longer than the duration of response that was seen with chemotherapy.
The safety profiles that were observed with pembrolizumab and chemotherapy were as expected, with no new or unexpected toxic effects. Immune-mediated adverse events with pembrolizumab were relatively infrequent and were mostly of grade 1 or 2 severity. Overall, the incidence of treatment-related adverse events was substantially lower with pembrolizumab than with standard chemotherapy, including fewer events of grade 3, 4, or 5 severity and fewer events that resulted in the discontinuation of treatment. The number of treatment-related deaths was the same in the pembrolizumab group and the chemotherapy group, which probably reflects the poor prognosis of patients in this population. The better safety profile of pembrolizumab than of standard chemotherapy is important, considering that patients with recurrent or refractory urothelial carcinoma are generally older and have poor performance status and multiple coexisting conditions.
The survival benefit that was observed with pembrolizumab in this previously treated population of patients with a poor prognosis supports its study in earlier stages of disease. Currently, pembrolizumab and other PD-1 and PD-L1 inhibitors are being evaluated as adjuvant therapy and as first-line therapy for advanced disease in ongoing clinical trials (e.g., ClinicalTrials.gov numbers, NCT02450331, NCT02516241, NCT02632409, NCT02807636, and NCT02853305).
In conclusion, pembrolizumab resulted in significantly longer overall survival — by approximately 3 months — than the investigator’s choice of paclitaxel, docetaxel, or vinflunine and was associated with a higher rate of objective response and a lower rate of treatment-related adverse events than chemotherapy as second-line therapy in patients with advanced urothelial carcinoma that progressed during or after platinum-based chemotherapy, regardless of tumor PD-L1 expression status.
Supplementary Material
Acknowledgments
Supported by Merck (Kenilworth, NJ).
Dr. Bellmunt reports receiving consulting fees from Merck and Genentech; Dr. de Wit, receiving fees for serving on an advisory board from Eli Lilly; Dr. Vaughn, receiving fees for serving on a data and safety monitoring board from Astellas Pharma; Dr. Fradet, receiving consulting fees from Astellas Pharma, Bayer, Amgen, Merck, Roche, Sanofi, and AstraZeneca and grant support from Astellas Pharma, Amgen, and AstraZeneca; Dr. Lee, receiving fees for serving on advisory boards from Astra-Zeneca, Astellas Pharma, Eisai, and Pfizer; Dr. Fong, receiving grant support from Dendreon, Bristol-Myers Squibb, Roche/Genentech, AbbVie, and Amgen; Dr. Climent, receiving fees for serving on advisory boards from Roche, Pierre Fabre Laboratories, and Bristol-Myers Squibb; Dr. Petrylak, receiving consulting fees from Millennium Pharmaceuticals, Dendreon, Sanofi Aventis, Roche, Bayer, Johnson & Johnson, Exelixis, Ferring Pharmaceuticals, Medivation, Pfizer, Bellicum Pharmaceuticals, and Tyme Pharmaceuticals, grant support from Merck, Celgene, Agensys, Eli Lilly, Millennium Pharmaceuticals, Dendreon, Sanofi Aventis, and Roche Laboratories, and having ownership interest or involvement in Bellicum Pharmaceuticals and Tyme Pharmaceuticals; Dr. Choueiri, receiving fees for serving on an advisory board from Merck; Dr. Necchi, receiving consulting fees from Merck Sharp & Dohme, Roche, AstraZeneca, and Bayer and grant support from Millennium Pharmaceuticals, Amgen, Novartis Pharmaceuticals, Merck Sharp & Dohme, Roche, and AstraZeneca; Dr. Gerritsen, receiving fees for serving on advisory boards from Amgen, Astellas Pharma, Janssen-Cilag, CureVac, Merck Sharp & Dohme, and Bayer, lecture fees from Bristol-Myers Squibb, Janssen-Cilag, and Bayer, and grant support from Astellas Pharma and Janssen-Cilag; Dr. Gurney, receiving fees for serving on advisory boards from Bristol-Myers Squibb, Astellas Pharma, and Roche and grant support from Pfizer; Dr. Quinn, receiving fees for serving on advisory boards from Merck, Bristol-Myers Squibb, Genentech, AstraZeneca, Pfizer, Novartis Pharmaceuticals, Astellas Pharma, Peloton Therapeutics, Dendreon, Exelixis, Bayer, Piramal Life Sciences, and Sanofi; Dr. Sternberg, receiving honoraria from OncoGeneX, Eli Lilly, Merck, and Bristol-Myers Squibb and grant support from Eli Lilly, Janssen Pharmaceuticals, AstraZeneca, and Roche/ Genentech; Drs. Mai, Poehlein, and Perini, being employees of and holding stock or stock options in Merck; Dr. Poehlein, having been employed in the past by and holding stock options in Bayer Health Care Oncology and holding a patent related to the augmentation of immune response to a cancer vaccine (U.S. patent number, 20090317407A1); Dr. Perini, holding pending patents related to the combination of a programmed death 1 (PD-1) antagonist and a listeria-based vaccine for treating prostate cancer (U.S. patient application number, 20160022814 A1) and treating cancer with a combination of a PD-1 antagonist and a vascular endothelial growth factor receptor (VEGFR) inhibitor (U.S. patent application number, 15101409); and Dr. Bajorin, receiving lecture fees and travel support from Merck. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families and caregivers for participating in this trial; all the investigators and site personnel; the staff of Dako North America for developing the PD-L1 IHC 22C3 pharmDx assay; the staff of QuintilesIMS for performing the PD-L1 testing; the staff of BioClinica for performing the radiologic assessments; Kijoeng Nam, Arkendu Chatterjee, and Xueying Ji (Merck) for assistance with statistical analyses; David Kaufman, Jill Lindia, Eric Rubin, Melissa Shakour, Swati Sharma, and Diana Waterbury (Merck) for contributions to trial design and conduct; Sharon Blimm (Merck) for logistic coordination; Mary Savage (Merck) for input on biomarker analyses; Oswaldo Bracco (Merck) for input on safety analyses; Roger Dansey, Tara Frenkl, and Markus Puhlmann (Merck) for critical review of an earlier version of the manuscript; and Melanie A. Leiby (Merck) for medical writing and editorial assistance with an earlier version of the manuscript.
APPENDIX
The authors’ full names and academic degrees are as follows: Joaquim Bellmunt, M.D., Ph.D., Ronald de Wit, M.D., Ph.D., David J. Vaughn, M.D., Yves Fradet, M.D., Jae-Lyun Lee, M.D., Ph.D., Lawrence Fong, M.D., Nicholas J. Vogelzang, M.D., Miguel A. Climent, M.D., Daniel P. Petrylak, M.D., Toni K. Choueiri, M.D., Andrea Necchi, M.D., Winald Gerritsen, M.D., Ph.D., Howard Gurney, M.D., David I. Quinn, M.D., Ph.D., Stéphane Culine, M.D., Ph.D., Cora N. Sternberg, M.D., Yabing Mai, Ph.D., Christian H. Poehlein, M.D., Rodolfo F. Perini, M.D., and Dean F. Bajorin, M.D.
The authors’ affiliations are as follows: Dana–Farber Cancer Institute, Boston (J.B., T.K.C.); Parc de Salut Mar, Hospital del Mar Medical Research Institute, Barcelona (J.B.), and Fundación Instituto Valenciano de Oncología, Valencia (M.A.C.) — both in Spain; Erasmus MC Cancer Institute, Rotterdam (R.W.), and Radboud University Medical Center, Nijmegen (W.G.) — both in the Netherlands; Abramson Cancer Center, University of Pennsylvania, Philadelphia (D.J.V.); Centre Hospitalier Universitaire de Québec–Université Laval, Quebec, QC, Canada (Y.F.); Asan Medical Center and University of Ulsan College of Medicine, Seoul, South Korea (J.-L.L.); the University of California, San Francisco, San Francisco (L.F.); Comprehensive Cancer Centers of Nevada, Las Vegas (N.J.V.); Smilow Cancer Hospital, Yale University, New Haven, CT (D.P.P.); Fondazione IRCCS Istituto Nazionale dei Tumori, Milan (A.N.); Westmead Hospital and Macquarie University, Sydney (H.G.); the University of Southern California Norris Comprehensive Cancer Center and Hospital, Los Angeles (D.I.Q.); Hôpital Saint-Louis, Paris (S.C.); San Camillo and Forlanini Hospitals, Rome (C.N.S.); Merck, Kenilworth, NJ (Y.M., C.H.P., R.F.P.); and Memorial Sloan Kettering Cancer Center, New York (D.F.B.).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seront E, Machiels JP. Molecular biology and targeted therapies for urothelial carcinoma. Cancer Treat Rev. 2015;41:341–53. doi: 10.1016/j.ctrv.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Oing C, Rink M, Oechsle K, Seidel C, von Amsberg G, Bokemeyer C. Second line chemotherapy for advanced and metastatic urothelial carcinoma: vinflunine and beyond — a comprehensive review of the current literature. J Urol. 2016;195:254–63. doi: 10.1016/j.juro.2015.06.115. [DOI] [PubMed] [Google Scholar]
- 5.Raggi D, Miceli R, Sonpavde G, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:49–61. doi: 10.1093/annonc/mdv509. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–61. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 7.Bellmunt J, Fougeray R, Rosenberg JE, et al. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol. 2013;24:1466–72. doi: 10.1093/annonc/mdt007. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget. 2016;7:73068–79. doi: 10.18632/oncotarget.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017 Jan 9; doi: 10.1016/S1470-2045(17)30007-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17:1590–8. doi: 10.1016/S1470-2045(16)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balar AV, Bellmunt J, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible unresectable or metastatic urothelial cancer: interim results for KEYNOTE-052. Ann Oncol. 2016;27(Suppl 6) abstract. [Google Scholar]
- 13.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–25. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apolo AB, Infante JR, Hamid O, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34(Suppl) abstract. [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–5. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 17.Sonpavde G, Pond GR, Fougeray R, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63:717–23. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Pang Z, Ding N, et al. The efficacy and potential predictive factors of PD-1/PD-L1 blockades in epithelial carcinoma patients: a systematic review and meta analysis. Oncotarget. 2016;7:74350–61. doi: 10.18632/oncotarget.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson NB, Maker AV. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther. 2016 Nov 11; doi: 10.1038/cgt.2016.63. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 23.Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol. 2016;27(Suppl 6) abstract. [Google Scholar]
- 24.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.