Abstract
Molecular chaperones direct refolding and triage decisions and support signal transduction responses to cytotoxic stress. The eukaryotic chaperone Hsp110 is represented by the SSE1/2 genes in Saccharomyces cerevisiae, which act as nucleotide exchange factors (NEFs) for cognate cytosolic Hsp70 chaperones. In this report, we present evidence that Sse1 is required for signaling through the cell integrity pathway via partnership with Hsp90 and the terminal MAP kinase Slt2. We found that sse1Δ and sti1Δ mutant cells share the typical cell integrity mutant phenotypes of osmoremediated temperature-sensitive growth and sensitivity to cell wall-damaging agents. Sse1 binds to Slt2 in vivo and similar to Hsp90 mutants, Slt2 stability and phosphorylation is not compromised in sse1Δ cells, whereas activation of the downstream transcription factor Rlm1 is abolished. In addition to Rlm1, Slt2 activates the Swi4/Swi6 heterodimer SBF in response to cell wall damage. SSE1 displayed dramatic synthetic phenotypes when disrupted in combination with mutations in SBF and the related Mbp1/Swi6 heterodimer MBF, characterized by severe growth and morphological defects. These defects were reversed by restoration of Hsp70 NEF activity, providing a mechanistic model wherein Sse1 functionally partners with Hsp90 as an Hsp70 NEF to promote client protein maturation and interaction with downstream effectors.
Keywords: Yeast, Heat shock, Morphogenesis, Cell integrity, Chaperone, Hsp110, Hsp70, Hsp90
Introduction
All cells respond to heat shock and other cytotoxic stresses by production of heat shock proteins (HSPs), many of which function as molecular chaperones. Chaperones typically bind stretches of hydrophobic amino acids not normally exposed to the aqueous cellular milieu, thereby preventing their aggregation and assisting in refolding. Molecular chaperones such as those of the Hsp70 family are required for proper folding of proteins as they emerge from the translating ribosome, for translocation across biological membranes, assembly of multimeric complexes, and are also involved in protein degradation decisions (Mayer and Bukau 2005; Brodsky and Chiosis 2006). In contrast, the Hsp90 chaperone system is distinct from “general” chaperones such as Hsp70 that are involved in bulk protein folding in that its client proteins are typically kinases or transcription factors that rely on Hsp90 for proper signaling activity (Mayer and Bukau 1999; Pearl and Prodromou 2001). Similar to other chaperones, Hsp90 requires the action of several other cochaperones for proper function. The Hsp110 class of eukaryotic chaperones is represented in budding yeast by the essential Sse1 and Sse2 proteins (Mukai et al. 1993). Hsp110/Sse1 chaperones are divergent members of the Hsp70 superfamily, and recent work has established an unique role for this group as nucleotide exchange factors (NEFs) in a stable, heterodimeric complex with yeast and mammalian cytosolic Hsp70 chaperones (Shaner et al. 2004, 2005, 2006; Yam et al. 2005; Dragovic et al. 2006; Raviol et al. 2006; Shaner and Morano 2007). Sse1 was previously shown to be required for maturation and activity of Hsp90 substrates in yeast, consistent with a requirement for regulated transfer of client proteins from Hsp70 to Hsp90 in the early stages of the chaperone cycle (Liu et al. 1999; Goeckeler et al. 2002; Lee et al. 2004). However, the extent to which Sse1 participates in Hsp70-dependent and Hsp90-dependent activities is unknown.
The cell integrity signaling pathway is induced by cell wall perturbations caused by heat and chemical agents including Congo red and caffeine [reviewed in (Levin 2005)]. Cell wall damage is transmitted by protein kinase C to a MAP kinase module terminating in the MAPK Slt2 (Torres et al. 1991; Levin and Bartlett-Heubusch 1992; Paravicini et al. 1992). Slt2 (also known as Mpk1) phosphorylates and activates two transcription factors, Rlm1 and the Swi4/6 cell-cycle box binding factor (SBF), the latter a heterodimer composed of Swi4 and Swi6 proteins (Watanabe et al. 1995; Dodou and Treisman 1997; Madden et al. 1997; Jung et al. 2002). Rlm1 and SBF activate expression of distinct sets of genes with predicted roles in cell wall synthesis and repair (Lagorce et al. 2003; Garcia et al. 2004). In addition to cell wall stress, the pathway is activated during the G1 to S phase transition of the cell cycle due to remodeling of the cell wall at the bud site (Igual et al. 1996; Zarzov et al. 1996). In addition to Swi4, Swi6 partners with the Mbp1 protein to form the transcription factor MBF (MluI cell-cycle box binding factor), also required for G1/S-specific gene expression (Moll et al. 1992; Koch et al. 1993). Mutations of components in the cell integrity pathway share common phenotypes including sensitivity to cell wall-damaging drugs and temperature-sensitive growth at 37°C, both of which can be remediated by osmotic support in the growth medium (Levin 2005). Hsp90 was identified as an Slt2 binding partner in a two-hybrid screen, and multiple lines of evidence demonstrate that the chaperone is a critical component of cell wall integrity signaling: (1) Hsp90 preferentially binds dually phosphorylated (activated) Slt2, (2) mutants defective in Hsp90 function in vivo display osmotically remediated temperature-sensitive growth, and (3) Hsp90 mutants are defective in heat shock-activated expression of Rlm1-dependent target genes (Millson et al. 2005; Piper et al. 2006; Truman et al. 2006, 2007). Unlike many other protein kinase clients of Hsp90, stability and phosphorylation of Slt2 is not impaired in the absence of chaperone function, suggesting a specific chaperone requirement for interaction with the downstream effectors Rlm1 and SBF (Truman et al. 2007). With the exception of Cdc37 and Ydj1, the role of other Hsp90 cochaperones in cell integrity signaling has not been investigated (Hawle et al. 2007; Wright et al. 2007).
In this study, we present evidence that Sse1 is required for signaling through the cell integrity pathway via partnership with Slt2. Cells lacking Sse1 or the Hsp70/Hsp90 organizing protein (HOP) homolog Sti1 share the prototypical cell integrity phenotype of osmoremediated temperature-sensitive growth and sensitivity to cell wall-damaging agents. Sse1 associates with Slt2 in vivo but is not required for its stability or phosphorylation by Mkk1/2. Expression of a synthetic Rlm1 target gene reporter was abolished in cells lacking SSE1, suggesting that the cochaperone is essential for Slt2 kinase maturation. In addition to a role in activating Rlm1, elimination of SSE1 resulted in dramatic synthetic phenotypes in combination with mutations in SBF and MBF, characterized by severe growth and morphological defects. These defects were reversed by restoration of Hsp70 NEF activity, providing a mechanistic model wherein Sse1 functionally partners with Hsp70, and by extension Hsp90, as an NEF to promote Slt2 maturation and interaction with downstream effectors required for cell integrity and morphogenesis.
Materials and methods
Strains and plasmids
Standard yeast propagation and transformation procedures were employed. Yeast strains used in this study are described in Table 1. Congo Red was included in solid media at a final concentration of 35 µg/mL and caffeine at a final concentration of 10 mM. Chaperone mutations were engineered into a variety of backgrounds, including W303, S288C (BY4741), and DS10, and are used throughout. While we observed similar responses in all backgrounds, the magnitude of effects, most notably growth phenotypes, varied considerably. Backgrounds are identified in the figure legends for each experiment. The plasmid-based Sse1Δ::LEU2 and Sse1Δ::URA3 disruption cassettes, and pRS413SSE1 have been described previously (Mukai et al. 1993; Shaner et al. 2004). The collection of SSE1 point mutants, corresponding wild type SSE1, and the SSE2 ORFS were all subcloned from previously constructed p414TEF (TRP1) vectors into p415TEF to recreate the series in a LEU2-selectable plasmid (Shaner et al. 2004). A plasmid-based mbp1Δ::HIS3 disruption cassette was created by PCR amplification of a 300-bp fragment upstream and a 400-bp fragment downstream of the MBP1 open reading frame from BY4741 genomic DNA followed by cloning into a plasmid containing the HIS3 gene. The swi4Δ::LEU2 disruption cassettes were constructed in a similar manner, amplifying 300 bp of upstream and 250 bp of downstream genomic sequence. All disruptions were confirmed by PCR and phenotypic analysis. p416GPD-FLAG-SLT2 was constructed by PCR amplification of the SLT2 open reading frame from BY4741 yeast genomic DNA using primers containing the coding sequence for the FLAG epitope followed by cloning into the p416GPD vector. The PST1-lacZ transcriptional fusion was generated by cloning a region −697 to −107 relative to the translational start site of PST1. The SLT2-13xmyc strain (BY2207) and isogenic wild type were generous gifts of B. Andrews (University of Toronto). The ssa1-45 allele and the isogenic wild type strain JN516 were kind gifts of E. Craig (University of Wisconsin) (Becker et al. 1996). The FES1 ORF was subcloned via SpeI and XhoI sites from p413GPDFES1 into plasmid p423GPD to create p423GPDFES1 (Shaner et al. 2006). Sequences of all primers are available upon request.
Table 1.
Yeast strains
| Strain | Genotype | Reference |
|---|---|---|
| W303 | MATa ura3-52 trp1 leu2-3,112 his3-11,15 ade2-1 can1-100 | Rothstein 1991 |
| KMY69 | W303 Sse1Δ::kanMX | Liu et al. 1999 |
| DS10 | MATa ura3-52 lys1 lys2 trp1-1 his3-11,15 leu2-3,112 | Nicolet and Craig 1989 |
| DTY249 | DS10 sse1Δ::kanMX | Liu et al. 1999 |
| LSY1 | DS10 swi4Δ::LEU2 | This study |
| LSY2 | DS10 rlm1Δ::kanMX | This study |
| CN11 | DS10 sti1Δ::kanMX | Nicolet and Craig 1989 |
| BY4741 | MATa his3Δ leu2A metl5A ura3A | Brachmann et al. 1998 |
| LSY4 | BY4741 sse1Δ::kanMX | This study |
| LSY5 | BY4741 swi4Δ::kanMX | This study |
| LSY6 | BY4741 swi6Δ::kanMX | This study |
| LSY7 | BY4741 swi6Δ::kanMX swi4Δ::LEU2 | This study |
| LSY8 | BY4741 swi6Δ::kanMX Sse1Δ::LEU2 | This study |
| LSY9 | BY4741 sse1Δ::kanMX swi4Δ::LEU2 | This study |
| LSY10 | BY4741 mbp1Δ::kanMX | This study |
| LSY11 | BY4741 mbp1Δ::kanMX Sse1Δ::URA3 | This study |
| LSY12 | BY4741 mbp1Δ::kanMX swi4Δ::LEU2 | This study |
| LSY13 | BY4741 swi6Δ::kanMX mbplΔ::HIS3 | This study |
| LSY14 | BY4741 rlm1Δ::kanMX | This study |
| LSY15 | BY4741 rlm1Δ::kanMXswi4A::LEU2 mbp1Δ::HIS3 | This study |
| LSY16 | BY4741 rlm1Δ::kanMX sse1Δ::LEU2 | This study |
| LSY17 | BY4741 mbp1Δ::kanMXswi4Δ::LEU2 sse1Δ::URA3 | This study |
| LSY18 | BY4741 rlm1Δ::kanMX swi4Δ::LEU2 | This study |
| BY2207 | BY263 SLT2-13xMyc::kanMX | B. Andrews |
| LSY19 | BY263 SLT2-13xMyc::kanMX sse1Δ::LEU2 | This study |
| JN516 | MATa leu2-3,-112 his3-1 ura3-5 trp1Δ1 lys2 ssa2::LEU2 ssa3::TRP1 ssa4::LYS2 | E. Craig |
| ssa1-45 | MATa leu2-3,-112 his3-1 ura3-5 trp1Δ1 lys2 ssa1-45 ssa2::LEU2 ssa3::TRP1 ssa4::LYS2 | E. Craig |
Immunoprecipitation and immunoblot analysis
FLAG immunoprecipitations, SDS-PAGE and immunoblot analysis were performed as described (Shaner et al. 2004; Shaner et al. 2005). Anti-Sse1 polyclonal antibody was used at 1:2,000 dilution (kindly provided by J. Brodsky, University of Pittsburgh). M2 monoclonal antibody (anti-FLAG, Sigma, St Louis, MO) was used at 1:1,000 dilution. Anti-myc monoclonal antibody (Invitrogen, Carlsbad, CA) was used at 1:1,000 dilution. Anti-phospho-p44/42 MAPK monoclonal antibody (recognizes dually phosphorylated Slt2) was used at 1:1,000 dilution (Cell Signaling Technology, Danvers, MA).
Microscopy
All microscopy was performed using differential interference contrast (DIC, “Nomarski”) optics with an Olympus BX60 microscope at both 400 × and 1,000 × magnifications using DIC-compatible objectives. Images were captured using a Photometrics CCD camera and QED Imaging software (Media Cybernetics, Bethesda, MD). Cells were grown to logarithmic phase in selective medium where appropriate. Three classes of morphology were defined for the purposes of this study: normal, elongated, and amorphous. Cells of the typical size and round shape of a budding haploid yeast cell were considered normal. Elongated cells were defined as having a long axis at least twice as long as the short axis. Amorphous cells were defined as having multiple elongated buds or aberrant projections per mother cell. In these cases, all unseparated buds or aberrant projections were counted as part of the parent cell. Between 100 and 200 individual cells taken from multiple microscopic fields of view were counted for each strain.
Enzymatic assays
Liquid β-galactosidase assays were performed on yeast strains grown to mid-log phase (OD600 ~ 0.6) at 30°C, followed by determination of β-galactosidase activity as described (Trott et al. 2005). The alkaline phosphatase cell leakage assay was performed as described with minor modifications (Paravicini et al. 1992). Cells were suspended to a density of approximately 106 cells/mL in 180 µL of YPD in a sterile 96-well plate, and a multiprong replicator (Sigma) was used to spot each strain onto YPD plates. The plates were incubated at 30°C for 1 day, and then shifted to 39°C for two additional days. Next, 10 mL of the alkaline phosphatase assay solution [1% agar, 0.05 M glycine (pH = 9.5), 10 mM 5-bromo-4-chloro-3-indolyl phosphate disodium salt (BCIP)] was overlaid onto each plate, allowed to solidify, and incubated at room temperature. Plates were photographed at multiple time intervals. In repeated experiments, initial coloration was observed within 20 min, while intact spots remained white for over 80 min.
Results
The Hsp90/Hsp70 cochaperones Sse1 and Sti1 are required for yeast cell integrity
Hypomorphic alleles of Hsp90 (HSC82 or HSP82 genes) or the heat shock transcription factor HSF1 exhibit temperature-sensitive growth at 37°C that can be remediated by the addition of the osmotic stabilizers sorbitol or sodium chloride (Piper et al. 2006; Truman et al. 2007). Although deletion of both SSE1 and SSE2 is lethal, sse1Δ strains are likewise temperature-sensitive at 37°C(Mukai et al. 1993; Trott et al. 2005). Given that Sse1 is required for Hsp90 chaperone activity in S. cerevisiae, we sought to determine if the temperature-sensitive growth phenotype reflected a role in cell integrity signaling. Wild type and sse1Δ cells were serially diluted and plated on YPD medium with or without 1 M sorbitol or 0.4 M NaCl and incubated at 30, 37, or 39°C (Fig. 1a). Strikingly, sse1Δ temperature sensitivity was completely suppressed in the presence of 0.4 M NaCl or 1 M sorbitol, consistent with phenotypes exhibited by strains compromised in cell integrity signaling. Likewise, an isogenic strain lacking the Hsp90 cochaperone STI1 displayed temperature-sensitive growth remediated by osmotic stabilization (Nicolet and Craig 1989). This observation suggests that loss of these Hsp70 and Hsp90 protein chaperone cofactors renders cells temperature-sensitive not because of a global protein folding defect, but rather due to specific attenuation of cell integrity pathway function.
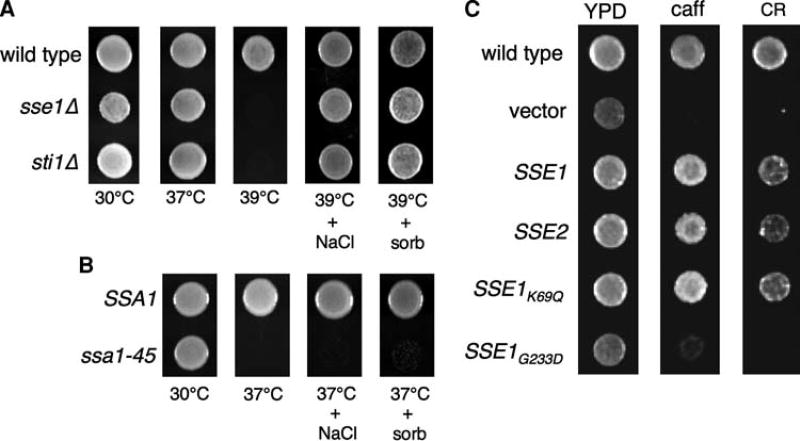
Fig. 1.
SSE1 and STI1 mutants exhibit defects in cell integrity. a Serial dilutions of wild type (DS10) and the indicated isogenic knockouts, sse1Δ (DTY249) and sti1Δ (CN11), were spotted onto YPD, YPD containing 1 M sorbitol, or YPD containing 0.4 M NaCl plates and incubated at 30, 37, or 39°C for 2 days. A single equivalent dilution representing approximately 103 cells is shown for each condition. b A strain containing the ssa1-45 allele as the sole copy of Ssa Hsp70 and its isogenic SSA1 wild type parent were serially diluted and grown at 30 or 37°C on YPD plates containing 1 M sorbitol or 0.4 M NaCl as described in (a). c Serial dilutions of wild type (BY4741) and the isogenic sse1Δ disruption (LSY4) containing the indicated alleles were spotted onto YPD, YPD plus caffeine, or YPD plus Congo Red and incubated at 30°C for 2 days. A single dilution is shown as in (a)
Because the temperature-sensitive growth phenotypes of HSF1, SSE1, STI1, and some HSP82/HSC82 mutants are suppressed by osmotic stabilization, we next asked whether loss of function of the cytosolic Ssa family of Hsp70 chaperones could be suppressed by osmotic stabilization. The ssa1-45 allele of SSA1 is inactivated at high temperature, and in the absence of the partially redundant SSA2, SSA3 and SSA4 genes results in inviability at 37°C (Becker et al. 1996). Growth of this mutant at the restrictive temperature, or a strain lacking both SSE1 and SSE2, was not restored in the presence of salt or sorbitol, demonstrating that osmotic stabilization does not indiscriminately rescue loss of global chaperone function, but rather compensates for a specific defect in cell integrity signaling (Fig. 1b and data not shown).
Cell integrity pathway mutants are commonly sensitive to compounds that damage the cell wall. Therefore, we sought to determine if this was the case for sse1Δ cells growing on media containing Congo red or caffeine, two compounds known to interfere with cell wall assembly and stability. As shown in Fig. 1c, growth of sse1Δ cells was strongly inhibited in the presence of both compounds when compared to wild type. Sse1 and its paralog Sse2 function as nucleotide exchange factors for the cytosolic families of Hsp70, Ssa, and Ssb (Dragovic et al. 2006; Raviol et al. 2006; Shaner et al. 2006). Likewise, metazoan Hsp110 performs a similar role for its cognate Hsp70 (Dragovic et al. 2006). Mutations in SSE1 that perturb association with Hsp70 abrogate NEF function and phenocopy the sse1Δ null mutation (Shaner et al. 2004, 2005). We therefore asked whether the observed cell integrity defects were consistent with loss of Hsp70 NEF activity using a previously generated series of SSE1 alleles. The sse1-K69Q, allele, defective in ATP hydrolysis, and the SSE2 gene have been shown to functionally complement all known Sse1Δ phenotypes at 30°C (Shaner et al. 2004). In contrast, the G233D mutation abolishes Sse1–Hsp70 interaction and fails to complement (Shaner et al. 2004). These results were recapitulated with respect to tolerance of cell wall-inhibiting compounds, as shown in Fig. 1c. These results demonstrate that Sse1 is likely acting in the cell integrity pathway as an Hsp70 NEF, possibly in concert with the Hsp90 chaperone complex.
Sse1 is required for Slt2 MAP kinase activity
Hsp90 is required for activity of Slt2, the terminal MAP kinase in the cell integrity kinase cascade (Piper et al. 2006; Truman et al. 2007). Multiple findings support the idea that the relationship between Hsp90 and Slt2 is unique. Hsp90 and the associated cochaperone Cdc37 are required for the stability of a majority of protein kinases, and loss of chaperone activity results in lowered client protein abundance (Farrell and Morgan 2000; Lee et al. 2004; Hawle et al. 2007; Mandal et al. 2007). However, Slt2 stability is unaffected in numerous mutants with reduced Hsp90 activity (Piper et al. 2006; Truman et al. 2007). Moreover, dual phosphorylation of Slt2 (Thr190/Tyr192) by the MAPK kinases Mkk1 and Mkk2 is likewise unaffected (Piper et al. 2006; Hawle et al. 2007; Truman et al. 2007). Lastly, Hsp82 is preferentially recruited to dually phosphorylated Slt2, consistent with a role postactivation (Piper et al. 2006). Because Sse1 has been shown to participate in Hsp90 signal transduction activities, we sought to determine if Sse1 physically interacts with Slt2. To this end, we performed coimmunoprecipitation analysis using a functional FLAG-epitope tagged Slt2 (Fig. 2a). Indeed, when FLAG-Slt2 was immunoprecipitated from yeast protein extracts, Sse1 was found to copurify with the kinase and was not enriched from control extracts.
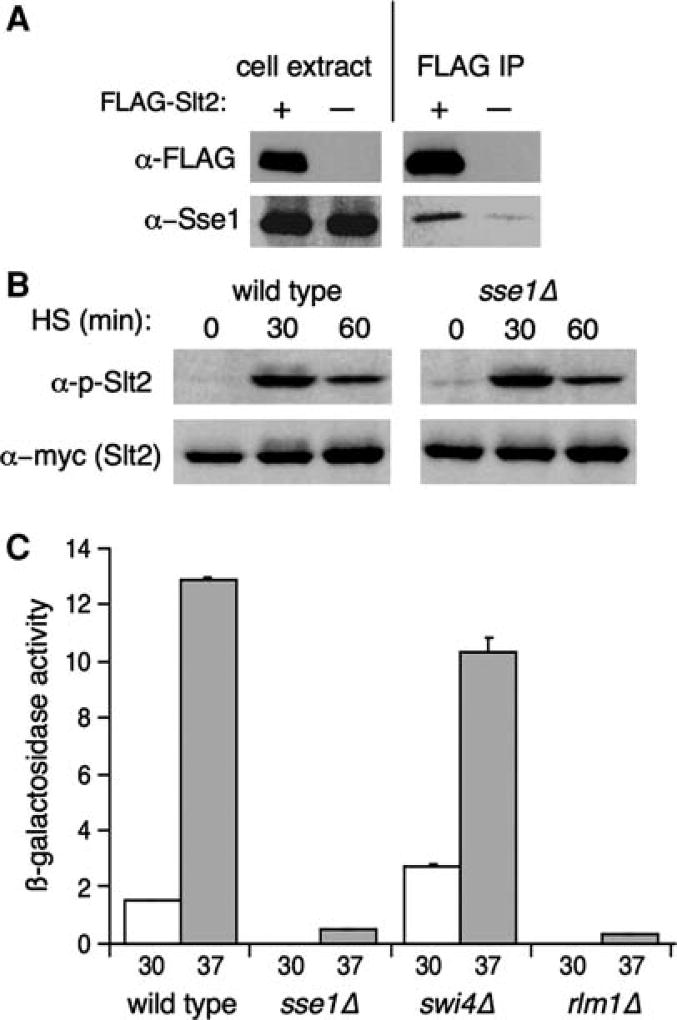
Fig. 2.
Sse1 physically interacts with Slt2 and is required for its activation. a FLAG immunoprecipitations were performed on protein extracts derived from W303 cells harboring p416GPD-FLAG-SLT2 or p416GPD as described in “Materials and methods.” Immunoblot analysis using anti-Sse1 and anti-FLAG antibodies was carried out on the extracts and immunoprecipitates. The extract lanes represent 5% of the total amount of extract used for the immunoprecipitation. b A strain carrying an integrated 13×-myc-tagged SLT2 allele (wild type, BY2207) and the isogenic SSE1 deletion (sse1Δ, LSY19) were grown to mid-log phase at 25°C followed by shifting of the cultures to 37°C and removal of aliquots at 0, 30, and 60 min postshift. Protein extracts were prepared followed by anti-myc and anti-phospho-Slt2 immunoblot analysis. c Wild type (DS10), sse1Δ (KMY69), swi4Δ (LSY1), and rlm1Δ (LSY2) cells were transformed with the PST1-lacZ reporter plasmid to measure transcriptional activation by Rlm1 and β-galactosidase activity assayed before and after a 1.5 h heat shock at 37°C
To further probe for signaling defects through the cell integrity MAP kinase cascade in sse1Δ cells during heat shock, we examined stability and phosphorylation of Slt2. A functional myc-tagged genomic allele of SLT2 was utilized in this experiment, along with a commercially available antibody that specifically recognizes Slt2 dually phosphorylated at Thr190/Tyr192. Wild type and sse1Δ cells bearing the SLT2-13xMyc allele were subject to a 1-h heat shock, and protein extracts were made from aliquots taken at the indicated time points and subjected to SDS-PAGE and immunoblot. Slt2 protein levels remained unchanged in response to the heat shock and loss of SSE1, as shown in Fig. 2b. In addition, Slt2 was phosphorylated in response to heat shock in sse1Δ cells to levels indistinguishable from wild type. This experiment indicates that signaling through the cell integrity pathway is intact through phosphorylation of the terminal MAP kinase, Slt2, and that any phenotypic consequences of SSE1 mutation must result from the inability of Slt2 to phosphorylate its downstream targets.
Slt2 is known to phosphorylate and activate at least two transcription factors, Rlm1 and the Swi4/Swi6 heterodimer SBF (Watanabe et al. 1995; Dodou and Treisman 1997; Madden et al. 1997; Jung et al. 2002)). To determine if Rlm1 function is impaired in sse1Δ cells, we constructed a transcriptional reporter using promoter sequences amplified from the known Rlm1 target gene PST1 and β-galactosidase (lacZ). PST1 induction in response to heat shock requires Slt2, and thus directly reports on cell integrity pathway signaling (Garcia et al. 2004). Although Slt2 phosphorylation attenuates rapidly, Rlm1-dependent transcription as assayed by reporter fusion increases over time up to 15 h (Jung et al. 2002). In wild type cells, expression of PST1-lacZ was low at 30°C but induced nearly eightfold by a 1.5-h heat shock at 37°C. Consistent with previous reports, both basal and heat shock-induced expression of PST1-lacZ was abolished in rlm1Δ cells (Garcia et al. 2004). Interestingly, an identical result was observed in sse1Δ cells, demonstrating that Sse1 is also required for stress signaling between Slt2 and Rlm1. Cells lacking SBF (swi4Δ) displayed nearly wild type levels of induction, consistent with a previous report that PST1 expression is independent of this transcription factor (Garcia et al. 2004). In contrast to the dramatic reduction in Rlm1-dependent gene expression observed in sse1Δ cells, no defects in heat shock expression of the Slt2/SBF target gene PCL1 (Baetz et al. 2001) were detected (data not shown), suggesting differential involvement of Sse1 in cell integrity-regulated transcription.
Synthetic genetic interaction between SSE1 and SBF
To further investigate the relationship between Sse1 and SBF, we constructed a series of mutant strains with deletions in SSE1, SWI4, and SWI6 (see Table 1). In the BY4741 strain background, the swi4Δ and swi6Δ single deletion strains exhibited wild-type growth at 30°C and 37°C, while the sse1Δ mutant displayed a slow growth defect without additional temperature sensitivity (Fig. 3a). A strain containing mutant alleles of both SWI4 and SSE1 grew at 30°C at a rate similar to the sse1Δ single mutant strain, but was temperature-sensitive at 37°C. A more dramatic phenotype was observed in the swi6Δ sse1Δ strain, which was nearly inviable at both temperatures. Intriguingly, we were successful in obtaining a swi4Δ swi6Δ strain, previously thought to be inviable, in the BY4741 background that also displayed a severe growth defect (Koch et al. 1993). To ask whether these growth phenotypes correlated with defects in cell integrity, we assessed leakage of endogenous alkaline phosphatase. This assay provides a colorimetric readout of cell lysis using the chromogenic substrate BCIP (Paravicini et al. 1992). All the single mutants, as well as the swi4Δ sse1Δ double mutant strain, exhibited little to no alkaline phosphatase activity after 2 days of growth at 37°C. In contrast, but in keeping with the shared severe growth phenotypes, both the swi6Δ sse1Δ and the swi4Δ swi6Δ strains displayed substantial leakage, demonstrating that cell integrity was severely compromised (Fig. 3a, “BCIP”).
Fig. 3.
Synthetic growth and morphology defects between SSE1 and SBF components. a Wild type (BY4741), and the indicated isogenic derivative strains (see Table 1) were serially diluted, plated on YPD and incubated at 30 and 37°C for 2 days. A parallel dilution series was subject to the alkaline phosphatase cell leakage assay after 2 days of growth at 37°C as described in “Materials and methods,” and a representative dilution spot is shown for all strains (BCIP). b The same strains were analyzed for morphology using Normarski microscopy. Representative micrographs are shown. c Quantitation of the morphological types observed in (b). Populations of at least 100 cells were categorized as normal (white bars), elongated (gray bars), or amorphous (black bars) as described in “Materials and methods”
When these strains were examined by differential interference contrast microscopy, both the swi6Δ sse1Δ (LSY8) and the swi4Δ swi6Δ strains (LSY7) exhibited striking morphological defects typified by extremely large, amorphous cells suggestive of defects in cell cycle regulation and septum formation (Fig. 3b). The swi6Δ and swi4Δ sse1Δ strains displayed only moderately perturbed morphologies that included elongated daughter cells. The distribution of morphological classes (normal, elongated, and amorphous) was quantified for a minimum of 100 cells per strain and is shown in Fig. 3c. By this analysis, nearly 95% of swi6Δ sse1Δ cells examined were amorphous (black bars) or exhibited an elongated phenotype (gray bars). Similarly, approximately 77% of swi4Δ swi6Δ cells exhibited abnormal morphology. Loss of SSE1 therefore appears to phenocopy loss of SWI4 with respect to synthetic genetic interaction with the swi6Δ mutation.
Loss of Hsp70 NEF activity is responsible for morphological defects
Because sensitivity to cell wall-inhibiting compounds in sse1Δ cells could be suppressed by SSE2, but not by the sse1-G233D allele incapable of acting as an Hsp70 NEF, we asked whether the NEF activity of Sse1 was likewise required for proper morphogenesis in cells lacking SWI6. Plasmids carrying wild type SSE1, SSE2, or mutant alleles were introduced into sse1Δ swi6Δ cells and the morphological distribution of each resulting transformant is calculated, as shown in Fig. 4. sse1Δ swi6Δ cells harboring either an empty vector or the sse1-G233D allele exhibited significant morphological defects, with slight variance in the percentages of normal versus abnormal daughter cells. In contrast, reintroduction of wild type SSE1 or SSE2, or the functional SSE1-K69Q allele, restored normal cell growth and morphology.
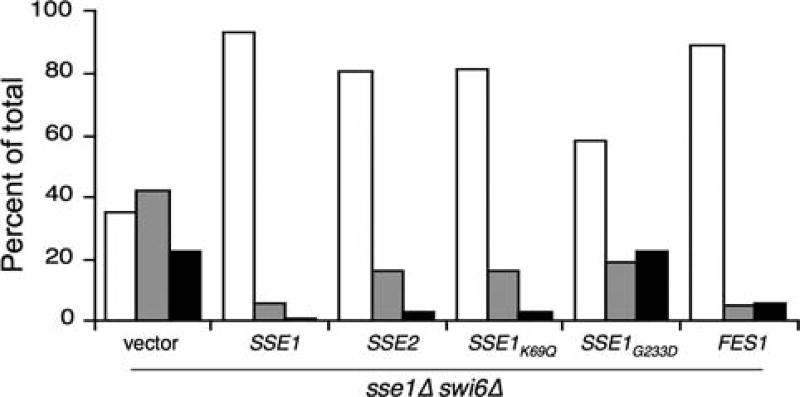
Fig. 4.
Hsp70 nucleotide exchange factor activity is required for normal growth and morphology in swi6Δ mutants. sse1Δ swi6Δ cells were transformed with the indicated plasmids bearing SSE1 alleles, SSE2 or FES1 (see text for explanation), and morphological defects assessed as in Fig. 3. Populations of at least 200 cells for each strain were categorized as normal (white bars), elongated (gray bars), or amorphous (black bars) as described in “Materials and methods”
To further validate that loss of Hsp70 NEF activity is a significant cause of the growth and morphology defects in sse1Δ swi6Δ cells, we asked whether overexpression of another cytosolic NEF, Fes1, would suppress the observed phenotypes (Kabani et al. 2002). As shown in Fig. 4, over-expressed Fes1 reversed the morphology defects to a level nearly indistinguishable from overexpressed Sse1. These data indicate that either NEF may partner with Hsp70, and by extension, Hsp90, to facilitate cell integrity signaling and proper proliferation and morphogenesis.
SSE1 and RLM1 are required for growth in the absence of SBF and MBF
Results from the preceding experiments suggest that Sse1, as an Hsp70 NEF, is required for proper morphogenesis under normal growth conditions as well as gene expression governed by the cell integrity pathway, likely through Slt2. We next asked whether these genetic interactions extended to the other major Slt2-controlled factor, Rlm1. In contrast to the growth and morphological defects observed with deletions of SSE1 and SBF components, deletion of RLM1 caused no detectable growth impairment in combination with SSE1 at 30 or 37°C (Fig. 5 and data not shown). Surprisingly, rlm1Δ swi4Δ cells also exhibited no growth defects, indicating that Slt2-activated cell integrity signaling requiring these transcription factors (but perhaps not Slt2-dependent, Rlm1/SBF-independent gene expression, see “Discussion”) is largely dispensable in the BY4741 genetic background.
Fig. 5.
SSE1 genetically interacts with RLM1 and MBF in addition to SBF. Wild type (BY4741) and the indicated isogenic derivative strains (see Table 1) were serially diluted, spotted onto YPD plates and incubated at 30°C for 2 days
Because deletion of SSE1 had dramatic consequences in the context of cells lacking SWI6, we also generated multiple deletion strains to test for genetic interactions with the heterodimeric transcription factor MBF, composed of the SWI6 and MBP1 gene products. When gene disruption combinations affecting all three transcription factors (Rlm1, SBF, and MBF) were constructed, several interesting results were obtained. First, we were able to successfully obtain a swi4Δ mbp1Δ strain, previously reported to be inviable (Fig. 5) (Koch et al. 1993). This result suggests that cells from the BY4741 strain background are able to regulate expression of genes important for the G1 to S phase transition in an unknown manner. Importantly, when all three transcription factors were disrupted (rlm1Δ swi4Δ mbp1Δ), severe growth impairment was observed at 30°C. A nearly identical phenotype was obtained when SSE1, MBP1, and SWI4 were deleted in combination (Fig. 5), demonstrating that loss of the Sse1 protein chaperone is functionally equivalent to loss of the cell integrity-specific transcription factor Rlm1 in combination with inactivation of the Swi6-containing cell cycle-regulated transcription factors (SBF, MBF). This result is consistent with the hypothesis that Hsp90/Hsp70 and the essential cochaperone Sse1 are required for proper activation of Rlm1 by Slt2.
Discussion
In this report, we have demonstrated that the Hsp70 and Hsp90 cochaperone Sse1 is required for yeast cell integrity signaling through the Slt2 MAP kinase. Moreover, Sse1 genetically interacts with the SBF and MBF cell cycle-regulated transcription factors to support growth and proliferation. A number of recent reports have linked conclusively protein chaperone function to the cell integrity pathway, with Slt2 as the likely nexus of interaction (Millson et al. 2005; Piper et al. 2006; Truman et al. 2006). Hsp90 physically associates with the kinase, and Hsp90 mutants display phenotypes consistent with impaired cell integrity signaling. Moreover, identical results are observed with the HSF(1-583) truncation mutant of Hsf1, previously shown to be deficient in Hsp90 production (Morano et al. 1999; Truman et al. 2007). Pharmacological inhibition of Hsp90 by radicicol likewise mirrors these mutant phenotypes (Truman et al. 2006, 2007). Our results documenting cell integrity pathway phenotypes with SSE1 and STI1 mutants are completely consistent with these findings. In addition, with the recent assignment of Sse1 as an Hsp70 nucleotide exchange factor, we have been able to extend our investigation to show that Hsp70 NEF activity is required for both cell integrity signaling and morphogenesis (Dragovic et al. 2006; Raviol et al. 2006; Shaner et al. 2006; Shaner and Morano 2007).
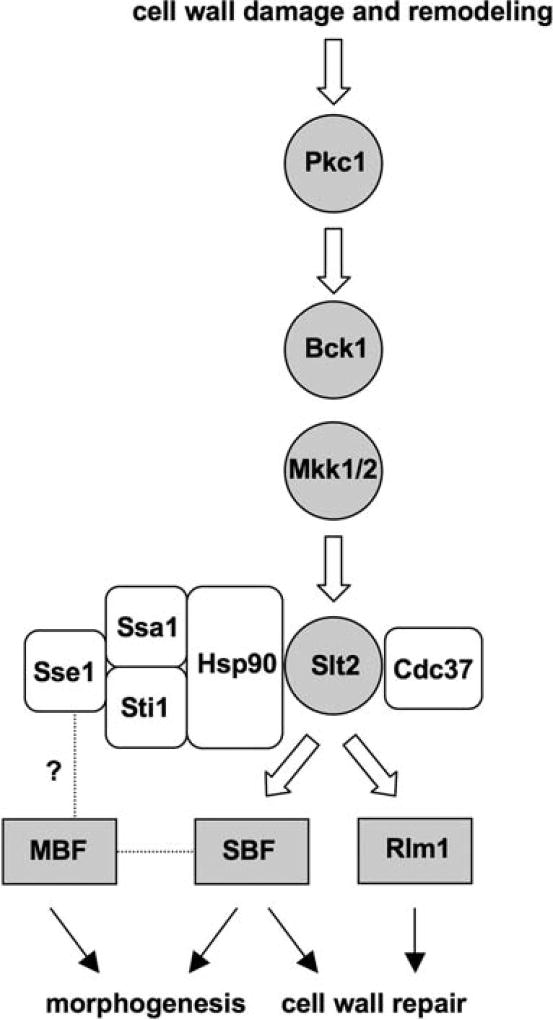
Unlike most other Hsp90 clients that require the chaperone for stability in vivo, the role of Hsp90 and its associated cochaperones in Slt2 function appears to be more subtle. Slt2 stability and activation via phosphorylation of Thr190/Tyr192 in response to heat shock was unaffected in sse1Δ cells and in cells defective in Hsp90 function. In contrast, Slt2 kinase activity as measured using the model substrate myelin basic protein (MBP) was abolished in the presence of radicicol (Truman et al. 2007). These interactions are likely to be conserved in higher eukaryotes, as the human MAP kinase ERK5 can functionally substitute for Slt2 and is also Hsp90-dependent (Truman et al. 2006). We have not observed major defects in Slt2 kinase activity in sse1Δ cells using a similar assay, nor is Slt2-dependent; heat shock-induced phosphorylation of Swi6 diminished in vivo (data not shown). However, Rlm1-dependent transcriptional activation in response to heat shock is strongly reduced both in Sse1Δ cells and after treatment with radicicol (Truman et al. 2007). Together, these data suggest that while Hsp90 and the dedicated kinase chaperone Cdc37 are directly responsible for supporting cell integrity signaling through Slt2, Sse1, Sti1, and other cochaperones may play a more ancillary role that is amplified in vivo during heat shock conditions. A model summarizing these interactions is shown in Fig. 6. The cell integrity pathway is initially activated by a chemical or physical assault to the cell wall leading to signaling through protein kinase C and the MAP kinase pathway, ultimately resulting in the dual Thr/Tyr phosphorylation and activation of Slt2. Hsp90 and its cochaperones are required for Slt2 to phosphorylate and activate SBF and Rlm1 to induce a battery of cell wall integrity genes, with a greater bias toward Rlm1 function. In addition, Hsp110 appears to influence cellular morphogenesis in a pathway tightly linked to SBF and MBF, although the data presented in this report do not conclusively link Slt2 to this phenomenon. It is possible that the Hsp90 chaperone complex may differentially promote productive interaction between kinase and substrates, potentially as a scaffold. Because the strongest transcriptional defects in sse1Δ cells were observed with the Rlm1-dependent reporter, more precise investigation of these later stages in the pathway await development of an in vivo assay for Rlm1 phosphorylation by Slt2 (Jung et al. 2002).
Fig. 6.
A multichaperone complex supports cell integrity signaling. A multichaperone complex that includes at least Hsp90, Hsp70 (Ssa), Hsp110 (Sse), and Stil interacts with the terminal Slt2 MAP kinase and is required for proper signaling to the effector transcription factors SBF and Rlm1. SBF and Rlm1 differentially regulate genes involved in cell wall repair and morphogenesis. Chaperone components may also be required for Slt2-independent functions of MBF
We also observed strong genetic interactions between SSE1 and genes encoding the two MBF subunits, SWI6 and MBP1. Cells lacking SSE1 and SWI6 displayed severe growth inhibition and morphological defects at 30°C, whereas sse1Δ swi4Δ mutants exhibited only minor temperature sensitivity. Cells lacking MBP1 and SWI4 displayed no apparent growth defects, whereas the additional deletion of SSE1 rendered these cells nearly inviable, as did disruption of RLM1. A possible explanation for these observations is that deletion of SSE1 (and by extension inhibition of Hsp90 function) results in diminished activity of Slt2, which would then manifest as “hypomorphism” in Rlm1 and SBF, both Slt2-dependent factors. Further deletion of MBP1 may reduce cell cycle-regulated transcription below a critical threshold. Interestingly, Slt2 itself possesses transcriptional activation potential that requires physical interaction with SBF at target promoters but not kinase activity (Baetz et al. 2001; Kim et al. 2007, 2008). In this scenario, Slt2 acts as a DNA-binding factor to recruit Swi4, and ultimately Swi6, to activate gene expression. Our inability to detect defects in Slt2 kinase activity in sse1Δ mutants but clear cell wall integrity signaling phenotypes leads us to speculate that Sse1, in partnership with Hsp70, may be involved in this novel transcriptional activation complex.
Because Swi6 is a component of both SBF and MBF, swi6Δ mutants are simultaneously defective in transcription dependent on both factors, whereas loss of SWI4 or MBP1 is expected to eliminate SBF or MBF, respectively. Therefore, sse1Δ swi6Δ mutants are predicted to be genetically equivalent to sse1Δ swi4Δ mbp1Δ mutants, consistent with the growth phenotypes obtained in these studies. In addition, the identical growth defects observed in rlm1Δ swi4Δ mbp1Δ and sse1Δ swi4Δ mbp1Δ cells demonstrates that in the absence of SBF and MBF function, SSE1 and RLM1 are genetically equivalent, consistent with the similar reduction of PST1-lacZ induction in these strains (Fig. 2). Additional consideration must be given to the finding that HSF(1-583) mutants are defective in at least one synthetic transcriptional target of MBF, and this defect is remediated by overexpression of Hsp90 (Truman et al. 2007). While this result may be explained by the fact that Slt2 phosphorylates Swi6, the observed remediation also occurs in an slt2Δ background, suggesting that Hsp90 may interact with MBP in an Slt2-independent manner. It is also noteworthy to point out that many of our observations would not have been possible in other strain backgrounds. For example, we were able to engineer several mutant strains in the BY4741 strain that were previously reported to be inviable (swi4Δ swi6Δ, mbp1Δ swi4Δ), allowing us to uncover novel phenotypes (Koch et al. 1993). At this time, it is not clear if these differences reflect general strain “robustness” or differential reliance on SBF, MBF, or the novel Slt2-SBF transcription factors.
An important conclusion that can be drawn from our findings is that many temperature-sensitive growth phenotypes of Hsp90 and cochaperone mutants [HSF(1-583), sse1Δ, sti1Δ, ydj1Δ] are not simply due to indiscriminate destabilization or aggregation of cellular proteins (Truman et al. 2007; Wright et al. 2007). Instead, these phenotypes are due to improper signaling through Slt2 resulting in weakened cell walls leading to cell lysis. This view is supported by the fact that osmotic stabilization of the growth medium could not replace Hsp70 (ssa1-45) or Hsp110 (sse1Δ sse2Δ) functions to allow viability. This is almost certain because these chaperones have other essential functions in the cell, such as protein biogenesis, that cannot be rescued by this treatment. In summary, our results demonstrate that in addition to well-documented duties in protein refolding and repair, protein chaperones, especially Hsp110, Hsp90, Hsp70, and their associated cochaperones, play important roles in supporting stress-responsive signal transduction critical for high-temperature growth.
Acknowledgments
We thank Drs. Brenda Andrews, Jeffrey Brodsky, Betty Craig, Peter Piper, and Jill Johnson for advice, reagents, and helpful discussion. We also thank Dr. William Margolin for microscopy support. This work was supported by National Institutes of Health grant GM074696 to K.A.M.
References
- Baetz K, Moffat J, Haynes J, Chang M, Andrews B. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol Cell Biol. 2001;21:6515–6528. doi: 10.1128/MCB.21.19.6515-6528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Walter W, Yan W, Craig EA. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol Cell Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann CB, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, Chiosis G. Hsp70 molecular chaperones: emerging roles in human disease and identification of small molecule modulators. Curr Top Med Chem. 2006;6:1215–1225. doi: 10.2174/156802606777811997. [DOI] [PubMed] [Google Scholar]
- Dodou E, Treisman R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1848–1859. doi: 10.1128/mcb.17.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J. 2006;25:2519–2528. doi: 10.1038/sj.emboj.7601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A, Morgan DO. Cdc37 promotes the stability of protein kinases Cdc28 and Cakl. Mol Cell Biol. 2000;20:749–754. doi: 10.1128/mcb.20.3.749-754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, et al. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J Biol Chem. 2004;279:15183–15195. doi: 10.1074/jbc.M312954200. [DOI] [PubMed] [Google Scholar]
- Goeckeler JL, Stephens A, Lee P, Caplan AJ, Brodsky JL. Over-expression of yeast Hsp110 homolog Sse1p suppresses ydj1–151 thermosensitivity and restores Hsp90-dependent activity. Mol Biol Cell. 2002;13:2760–2770. doi: 10.1091/mbc.02-04-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawle P, Horst D, Bebelman JP, Yang XX, Siderius M, van der Vies SM. Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p) Eukaryot Cell. 2007;6:521–532. doi: 10.1128/EC.00343-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igual JC, Johnson AL, Johnston LH. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 1996;15:5001–5013. [PMC free article] [PubMed] [Google Scholar]
- Jung US, Sobering AK, Romeo MJ, Levin DE. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol Microbiol. 2002;46:781–789. doi: 10.1046/j.1365-2958.2002.03198.x. [DOI] [PubMed] [Google Scholar]
- Kabani M, Beckerich JM, Brodsky JL. Nucleotide exchange factor for the yeast Hsp70 molecular chaperone Ssa1p. Mol Cell Biol. 2002;22:4677–4689. doi: 10.1128/MCB.22.13.4677-4689.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Cosano IC, Levin DE, Molina M, Martin H. Dissecting the transcriptional activation function of the cell wall integrity MAP kinase. Yeast. 2007;24:335–342. doi: 10.1002/yea.1475. [DOI] [PubMed] [Google Scholar]
- Kim KY, Truman AW, Levin DE. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol Cell Biol. 2008;28:2579–2589. doi: 10.1128/MCB.01795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, MoU T, Neuberg M, Ahorn H, Nasmyth K. A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase. Science. 1993;261:1551–1557. doi: 10.1126/science.8372350. [DOI] [PubMed] [Google Scholar]
- Lagorce A, et al. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J Biol Chem. 2003;278:20345–20357. doi: 10.1074/jbc.M211604200. [DOI] [PubMed] [Google Scholar]
- Lee P, Shabbir A, Cardozo C, Caplan AJ. Stil and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol Biol Cell. 2004;15:1785–1792. doi: 10.1091/mbc.E03-07-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992;116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Morano KA, Thiele DJ. The yeast Hsp110 family member, Sse1, is an Hsp90 cochaperone. J Biol Chem. 1999;274:26654–26660. doi: 10.1074/jbc.274.38.26654. [DOI] [PubMed] [Google Scholar]
- Madden K, Sheu YJ, Baetz K, Andrews B, Snyder M. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science. 1997;275:1781–1784. doi: 10.1126/science.275.5307.1781. [DOI] [PubMed] [Google Scholar]
- Mandal AK, et al. Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J Cell Biol. 2007;176:319–328. doi: 10.1083/jcb.200604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Molecular chaperones: the busy life of Hsp90. Curr Biol. 1999;9:R322–R325. doi: 10.1016/s0960-9822(99)80203-6. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millson SH, Truman AW, King V, Prodromou C, Pearl LH, Piper PW. A two-hybrid screen of the yeast proteome for Hsp90 interactors uncovers a novel Hsp90 chaperone requirement in the activity of a stress-activated mitogen-activated protein kinase, Slt2p (Mpk1p) Eukaryot Cell. 2005;4:849–860. doi: 10.1128/EC.4.5.849-860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll T, Dirick L, Auer H, Bonkovsky J, Nasmyth K. SWI6 is a regulatory subunit of two different cell cycle START-dependent transcription factors in Saccharomyces cerevisiae. J Cell Sci Suppl. 1992;16:87–96. doi: 10.1242/jcs.1992.supplement_16.11. [DOI] [PubMed] [Google Scholar]
- Morano KA, Santoro N, Kock KA, Thiele DJ. A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol Cell Biol. 1999;19:402–411. doi: 10.1128/mcb.19.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Kuno T, Tanaka H, Hirata D, Miyakawa T, Tanaka C. Isolation and characterization of SSE1 and SSE2, new members of the yeast HSP70 multigene family. Gene. 1993;132:57–66. doi: 10.1016/0378-1119(93)90514-4. [DOI] [PubMed] [Google Scholar]
- Nicolet CM, Craig EA. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini G, et al. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol Cell Biol. 1992;12:4896–4905. doi: 10.1128/mcb.12.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure, function, and mechanism of the Hsp90 molecular chaperone. Adv Protein Chem. 2001;59:157–186. doi: 10.1016/s0065-3233(01)59005-1. [DOI] [PubMed] [Google Scholar]
- Piper PW, Truman AW, Millson SH, Nuttall J. Hsp90 chaperone control over transcriptional regulation by the yeast Slt2(Mpk1) p and human ERK5 mitogen-activated protein kinases (MAPKs) Biochem Soc Trans. 2006;34:783–785. doi: 10.1042/BST0340783. [DOI] [PubMed] [Google Scholar]
- Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B. Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J. 2006;25:2510–2518. doi: 10.1038/sj.emboj.7601139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. In: Guthrie C, Fink G, editors. Methods in enzymology. Academic Press; San Diego: 1991. pp. 281–301. [DOI] [PubMed] [Google Scholar]
- Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner L, Sousa R, Morano KA. Characterization of hsp70 binding and nucleotide exchange by the yeast hsp110 chaperone Sse1. Biochemistry. 2006;45:15075–15084. doi: 10.1021/bi061279k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner L, Trott A, Goeckeler JL, Brodsky JL, Morano KA. The function of the yeast molecular chaperone Sse1 is mechanistically distinct from the closely related hsp70 family. J Biol Chem. 2004;279:21992–22001. doi: 10.1074/jbc.M313739200. [DOI] [PubMed] [Google Scholar]
- Shaner L, Wegele H, Buchner J, Morano KA. The yeast Hsp110 Sse1 functionally interacts with the Hsp70 chaperones Ssa and Ssb. J Biol Chem. 2005;280:41262–41269. doi: 10.1074/jbc.M503614200. [DOI] [PubMed] [Google Scholar]
- Torres L, et al. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol Microbiol. 1991;5:2845–2854. doi: 10.1111/j.1365-2958.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Trott A, Shaner L, Morano KA. The molecular cchaperone Sse1 and the growth control protein kinase Sch9 collaborate to regulate protein kinase A activity in Saccharomyces cerevisiae. Genetics. 2005;170:1009–1021. doi: 10.1534/genetics.105.043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman AW, et al. Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Eukaryot Cell. 2006;5:1914–1924. doi: 10.1128/EC.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman AW, Millson SH, Nuttall JM, Mollapour M, Prodromou C, Piper PW. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryot Cell. 2007;6:744–752. doi: 10.1128/EC.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Irie K, Matsumoto K. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol Cell Biol. 1995;15:5740–5749. doi: 10.1128/mcb.15.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CM, Fewell SW, Sullivan ML, Pipas JM, Watkins SC, Brodsky JL. The Hsp40 molecular chaperone Ydj1p, along with the protein kinase C pathway, affects cell-wall integrity in the yeast Saccharomyces cerevisiae. Genetics. 2007;175:1649–1664. doi: 10.1534/genetics.106.066274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam AY, Albanese V, Lin HT, Frydman J. Hsp110 cooperates with different cytosolic HSP70 systems in a pathway for de novo folding. J Biol Chem. 2005;280:41252–41261. doi: 10.1074/jbc.M503615200. [DOI] [PubMed] [Google Scholar]
- Zarzov P, Mazzoni C, Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]