Abstract
Cholangiocarcinoma (CCA) is an aggressive malignancy that arises from damaged epithelial cells, cholangiocytes, and possibly de-differentiated hepatocytes. CCA has a poor overall survival rate and limited therapeutic options. Based on this data, it is imperative that new diagnostic and therapeutic interventions be developed. Recent work has attempted to understand the pathological mechanisms driving CCA progression. Specifically, recent publications have delved into the role of cancer stem cells (CSCs), mesenchymal stem cells (MSCs), and microRNAs (miRNAs) during CCA pathology. CSCs are a specific subset of cells within the tumor environment that are derived from a cell with stem-like properties and have been shown to influence recurrence and chemoresistance during CCA. MSCs are known for their anti-inflammatory activity and have been postulated to influence malignancy during CCA, but little is known about their exact functions. miRNAs exert various functions via gene regulation at both the transcriptional and the translational levels, giving miRNAs diverse roles in CCA progression. Additionally, current miRNA-based therapeutic approaches are in clinical trials for various liver diseases, giving hope for similar approaches for CCA. However, the interactions among these three factors in the context of CCA are unknown. In this review, we focus on recently published data (within the last 3 years) that discuss the role of CSCs, MSCs, and miRNAs and their possible interactions during CCA pathogenesis.
Keywords: cholangiocarcinoma, microRNAs, cancer stem cells, mesenchymal stem cells
Introduction
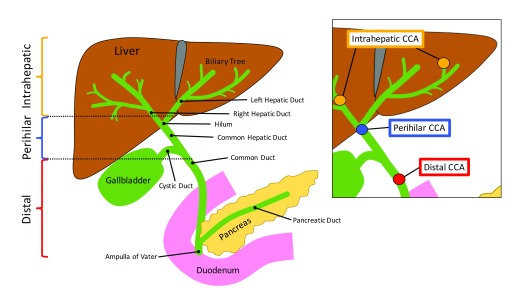
Cholangiocarcinoma (CCA) is a hepatobiliary malignancy with a poor 5-year survival rate and limited treatment options. CCA arises from damaged cholangiocytes, the epithelial cells that line the biliary tree. CCA can be classified into intrahepatic, perihilar, or distal subtypes 1. Intrahepatic CCA, the second most common form of liver cancer, is generally located proximal to the second-order bile ducts, perihilar CCA is located between the second-order bile ducts and the intersection of the cystic duct into the common bile duct, and distal CCA is found in areas between the cystic duct and the ampulla of Vater ( Figure 1) 2. Aside from being categorized by anatomic location, CCA is categorized on the basis of histopathological analysis and by growth-type patterns as well 3, 4. For instance, CCA that has been derived from large cholangiocytes is predominantly categorized as perihilar CCA with well to moderately differentiated mucin-producing cells and periductal infiltrating growth pattern, whereas CCA that is derived from small cholangiocytes is predominantly categorized as intrahepatic CCA and largely contains non–mucin-producing cells and has mass-forming growth patterns 3, 4. CCA is highly heterogeneous not only in initiation and location but also in progression, making it difficult to categorize CCA into distinct subtypes.
Figure 1. Schematic image of the anatomical locations of intrahepatic, perihilar, and distal cholangiocarcinoma (CCA).
CCA is categorized on the basis of its location within the biliary tree. Intrahepatic CCA is found proximal to the second-order bile ducts, perihilar CCA is found between the second-order bile ducts and the convergence of the cystic duct and the common duct, and distal CCA is found between the cystic duct and ampulla of Vater.
CCA incidence has been steadily increasing 5. One of the first lines of treatment for CCA is resection; however, options for resection are limited since the disease is generally too advanced at the time of diagnosis 6. Recently, it has been reported that following resection in patients with intrahepatic CCA the 5-year and 10-year survival rates are 32.3% and 8.4%, respectively 7. If unresectable, liver transplantation is another option for patients with intrahepatic CCA, and post-liver transplantation 5-year survival rates are 51% 8. For patients with perihilar CCA, resection may be an option but outcomes are poor; 5-year survival rates are 10% 9. It has also been suggested that some subsets of perihilar CCA may benefit from neoadjuvant chemoradiation therapy or biliary drainage or both, but outcomes are variable 10– 12. For patients with distal CCA following resection, 5-year survival rates are 23% 9. Furthermore, these patients should not be subjected to biliary drainage, since they tend to have increased complications related to this surgery 13. It is apparent that our current approaches for CCA are lacking, as demonstrated by the poor survival rates and limited treatment options. For these reasons, it is critical that new and effective therapies be developed.
The CCA microenvironment contains a plethora of cell types, extracellular structures, and secreted factors that influence the progression of the tumor. Cancer cells are surrounded by stroma, which is made up of various stromal cells and extracellular matrix 14. Additionally, cytokines, chemokines, growth factors, and proteinases secreted from cancer cells and stromal cells can promote a pro-inflammatory environment 14. This type of tumor microenvironment can lead to myofibroblast activation, cancer stem cell (CSC) initiation, and the recruitment of various inflammatory cell types 14. The CCA microenvironment is a complex network that is influenced by a multitude of soluble factors and cell types.
This review will focus on recent publications (within the last 3 years) that advance our understanding of CCA and will analyze how their findings could lead to new diagnostic or therapeutic targets. Overall, these publications discuss their findings within the context of all three subtypes of CCA (intrahepatic, perihilar, and distal), and for this reason we discuss the findings of the articles in the context of general CCA. Among the recent publications, many of the articles fell into similar categories. Specifically, publications tended to focus on the functional and mechanistic roles of (i) CSCs, (ii) mesenchymal stem cells (MSCs), or (iii) microRNAs (miRNAs) in CCA progression. We will be discussing how these three categories influence the CCA microenvironment, either alone or in relation to one another.
Cancer stem cells
The tumor microenvironment comprises many different cell types with various functions. Recently, CSCs have been identified in the tumor microenvironment and add complexity to our understanding of CCA. Specifically, CSCs have been identified for their role in tumor initiation, progression, and relapse. CSCs function in a manner similar to normal stem cells, wherein they are capable of self-renewal, can differentiate into multiple different cell types, and have unlimited division 15. CSCs promote an inflammatory environment through the activation of stromal cells and the recruitment of inflammatory cells 16. The cell type from which CSCs are derived is still under debate, but it has been hypothesized that CSCs may be derived from a stem-like cell that acquires a cancer-promoting alteration 17. Multiple studies have found that hepatocellular carcinoma (HCC) progression is driven by CSCs 18– 21; however, little is known about the role of CSCs in CCA.
As previously stated, the CCA microenvironment is a complex network of various cell types. Among the various cell types, macrophages are associated with CCA tumor progression and are significantly correlated with a poorer prognosis and metastasis 4. A recent article by Raggi et al. hypothesized that CSCs secrete factors that promote macrophage differentiation toward the tumor-associated macrophage (TAM) subtype 22. First, CCA spheres were cultured and medium was collected and placed on cultured macrophages. Following treatment with CCA sphere medium, macrophages expressed cluster of differentiation 68 (CD68), CD115, human leukocyte antigen-D related, and CD206, indicating an activated phenotype. These activated macrophages had TAM-like features, including increased invasiveness. To identify whether these findings were clinically relevant, macrophages were isolated from human CCA resections, and it was noted that these isolated macrophages expressed a phenotype similar to that of the treated cultured macrophages. The authors then identified the factors secreted from CCA spheres that were driving this TAM-like phenotype and found that interleukin-13 (IL-13), IL-34, and osteoactivin were detected in CCA sphere medium and in serum of patients with CCA. Furthermore, these factors were associated with CSC presence, and the authors concluded that the secreted factors driving the TAM-like phenotype must be derived from CSCs. These findings present a novel mechanism by which CCA-associated CSCs influence the activation of TAMs that promote CCA progression 22. Given that much of the work was done with CCA sphere medium, more work regarding the exact factors secreted from CSCs is necessary to definitively demonstrate that the activation of TAMs is specifically driven by CSCs.
Laminins are a family of extracellular matrix proteins that are mainly found in the basement membrane and are composed of α, β, and γ chains 23, 24. The γ2-chain of laminin-332 (Ln-332) is highly elevated in HCCs expressing the biliary marker cytokeratin-19 (CK-19) and is correlated with a poor prognosis for patients with HCC or intrahepatic CCA 25– 27. The aim of the publication by Govaere et al. was to characterize the CSC niche and determine the possible cell of origin 28. This publication slightly deviates from the others discussed in this review because it looks at CSCs in mixed HCC/CCA samples. It was noted that elevated biliary and hepatic progenitor cell (HPC) markers were seen with increased expression of LAMC2, the gene that encodes the γ2-chain of Ln-332. Immunopositivity for the γ2-chain of Ln-332 was found surrounding small HPC-like tumor cells that had a low proliferation rate, identified as possible CSCs. Lastly, the γ2-chain of Ln-332 was strongly co-expressed in ductular areas with low proliferative capacity but was expressed at low levels in the hepatocyte areas of HCC/CCA. The authors concluded that Ln-332 maintains the CSC niche and supports stem-like properties in these cells 28. While this article is one of a few that looks at the factors that maintain CSCs, future work needs to be performed to look at the role of Ln-332 specifically in CCA samples.
Given these findings, it is evident that the formation of CSCs strongly impacts the surrounding tumor microenvironment, which can impact disease progression. Specifically, these articles identify the pro-inflammatory role of CSCs during CCA. First, CSCs were shown to promote macrophage transition to a TAM-like phenotype. Furthermore, CSC accumulation of laminins, a key component of extracellular matrix, may promote the survival, migration, and invasion of tumor cells as well as stromal activation. It is evident that CSCs are key in maintaining and promoting a pro-inflammatory environment during CCA initiation and progression.
Mesenchymal stem cells
Recently, interest in the role of MSCs in tumor progression and metastasis has increased. MSCs are non-hematopoietic stem cells that primarily reside in the bone marrow but are recruited to injured tissues, inflammatory sites, and primary tumors 29– 31. As stated above, the presence of CSCs promotes an inflammatory environment, which may promote MSC migration and the infiltration of CCA tumors. MSCs have low immunogenicity and, following recruitment to injured tissues, are able to maintain their multi-differentiation capacity 32. Specifically, MSCs may differentiate into cancer-associated fibroblasts, myofibroblasts, or hepatic stellate cells to promote tumor progression 33, 34. These various cell types increase the inflammatory capacity of the stromal environment, further promoting the initiation of CSCs and the recruitment of MSCs. Moreover, MSCs secrete various cytokines that promote an inflammatory microenvironment 35, 36. For these reasons, the role of MSCs in CCA progression and inflammatory response has become a topic of interest.
While MSCs are known to influence the inflammatory environment of tumors, the exact microenvironment necessary for these effects is unknown. Also, it has been postulated that inflammatory conditions are a major activator of MSC-associated immunosuppression 37. Recent work from Zhong et al. found that tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) were able to stimulate the expression of TNF-α, C-C motif chemokine ligand 5 (CCL5), IL-6 and indoleamine 2,3-dioxygenase, and activated nuclear factor kappa B (NF-κB) signaling in MSCs 38. Secreted factors from these stimulated MSCs were able to induce CCA cell migration and metastasis in vitro and in vivo. Furthermore, CCA cells treated with supernatants from the stimulated MSCs had increased expression of C-C motif chemokine receptor 5 (CCR5). Increased CCL5/CCR5 signaling in CCA cells was able to increase the expression of matrix metalloproteinase-2 (MMP-2) and MMP-9. Overall, the authors deduced that TNF-α and IFN-γ stimulate MSCs to secrete CCL5, which in turn activates CCR5 on CCA cells to promote inflammation and metastasis 38. Inhibition of TNF-α or IFN-γ signaling or both could block MSC recruitment, thereby reducing CCA inflammation and metastasis to hopefully contribute to an improved outcome.
Chemoresistance is a major obstacle in cancer treatment. Previously, it was demonstrated that MSC-secreted IL-8 induces doxorubicin resistance in triple-negative breast cancer 39. Considering these findings, Wang et al. examined the role of MSCs in the progression of CCA development 40. Using human umbilical cord–derived MSCs, the authors noted that cultured CCA cells that were co-cultured with these MSCs had increased cell proliferation, metastasis, and resistance to the anti-cancer drug compound K in vivo. In vitro, CCA cells co-cultured with MSCs had increased colony formation and invasion. The tumors from mice injected with CCA cells co-cultured with MSCs demonstrated enhanced Wnt/β-catenin signaling. These findings were corroborated in vitro where MSCs and their secreted factors were shown to stimulate Wnt signaling via nuclear translocation of β-catenin, upregulation of Wnt, and increased expression of the downstream targets MMP-2, cyclin D1, and c-Myc in cultured CCA cells. Overall, this study indicates that MSCs increase CCA metastasis and chemoresistance via increased Wnt/β-catenin signaling 40. Targeting the Wnt/β-catenin signaling pathway may prove therapeutic for patients with CCA.
It is interesting to note that TNF-α and IFN-γ are necessary to drive MSC migration to CCA tumors. Given that CSCs promote an inflammatory environment, this may play a role in the recruitment of MSCs to the CCA tumor. Based on these articles, the presence of MSCs in CCA is indicative of increased CCA migration and invasion, and inhibition of MSC migration or induction of MSC apoptosis may be therapeutic for patients with CCA.
microRNAs
miRNAs are short, non-coding RNAs that regulate the expression of specific mRNAs 41. Specifically, miRNAs will recognize and bind to complementary sequences found within the 3′ untranslated region (3′ UTR) of specific mRNAs to regulate their expression levels 42, 43. The role of miRNAs varies, depending on the cellular process, in both physiological and pathological conditions 44– 46. The function of miRNAs during CCA has increasingly become a topic of interest; multiple human clinical trials evaluating the efficacy of miRNA-based therapeutics during various liver diseases are under way 47, 48. miRNA signaling has been noted in all three subtypes of CCA 49– 51; however, the identification of subtype-specific miRNAs has not been made. It is known that miRNAs can regulate MSC and CSC function 52– 54, but this regulation has not been reported in the context of CCA. Further understanding the impact of miRNAs on CCA development and progression may bring about novel diagnostic tools or therapeutic interventions. In this section, we will be discussing the role of miRNAs during CCA progression but also analyzing their potential impacts on CSC and MSC biology.
Menin is a known tumor-suppressor gene that is expressed in all tissues 55, but its role in CCA is poorly defined. miR-24 has previously been recognized as an oncogene in multiple gastrointestinal cancers and has been shown to target menin, but this interaction in the context of CCA is not understood 56– 59. A recent article by Ehrlich et al. evaluated the role of menin during CCA proliferation and angiogenesis and its regulation by miR-24 51. The authors found that human advanced CCA tumor sections, as well as in vitro human CCA cell lines, had increased miR-24 expression alongside decreased menin expression. In vitro, human CCA cell lines treated with an miR-24 inhibitor showed increased menin levels with a subsequent reduction in cell proliferation, angiogenesis, migration, and invasion. It was then demonstrated that miR-24 negatively regulates menin expression. In vivo, the authors noted that tumor size and the expression of proliferative and angiogenic markers were significantly reduced in the miR-24–inhibited tumor group compared with controls. Interestingly, fibrogenesis was enhanced in the miR-24–inhibited tumor group when compared with controls. From this study, it is evident that miR-24 acts as an oncogene to suppress menin expression, thereby increasing tumor burden, proliferation, angiogenesis, migration, and invasion 51. However, further work is necessary to delineate the exact downstream mechanisms regulating these events as well as understand the primary cellular source of miR-24 during CCA, which may help us understand why fibrogenesis increased even as tumor burden and angiogenesis decreased. While this study focuses on the role of miR-24 in cultured CCA cells, miR-24 has previously been shown to promote stemness in embryonic stem cells 60. In this context, miR-24 may promote the formation of CSCs in CCA, leading to increased CCA migration and invasion.
Circadian rhythms are endogenous oscillations that are regulated by clock genes and are present in the central nervous system, peripheral tissues, and single cells 61. While circadian oscillations are a normal physiological process, dysregulation of these oscillations promotes tumor development 62, 63; however, little is known about the role of the circadian rhythm during CCA. A recent publication identified that the expression of Per1, which negatively regulates circadian oscillations, was decreased in human CCA samples and cultured human CCA cell lines 64. In addition, overexpression of Per1 decreased cell proliferation and increased apoptosis in cultured human CCA cells. These findings were mimicked in vivo, wherein immunocompromised mice injected with cultured human CCA cells overexpressing Per1 had decreased tumor growth, proliferation, angiogenesis, and metastasis. Per1 was found to be a target of miR-34a, and following treatment with a miR-34a inhibitor, human cultured CCA cells had decreased proliferation, migration, and invasion. These findings are the first to identify that the disruption of clock genes regulated by miR-34a contributes to CCA malignancy 64. As stated, circadian clock genes are present in all cells and tissues, and previous work has shown that circadian clock genes regulate MSC differentiation, migration, and cell cycle 65. It is possible that the regulation of core clock genes via miR-34a plays a role in MSC migration and activation during CCA. Also, the disruption of core clock genes in CCA cells may contribute to the tumor niche, thereby supporting CSC formation or MSC recruitment or both.
Tumor growth and metastatic potential are regulated by a myriad of factors, including epigenetic changes, such as DNA methylation 66, 67. Specifically, it has been shown that DNA methylation plays a prominent role in the progression of CCA 68. On the basis of this information, Zhou et al. set out to investigate the functional and mechanistic role of miR-191 in CCA 69. It was first noted that miR-191 expression was increased in CCA tumors when compared with adjacent normal bile duct tissue. miR-191 expression was found to be an independent risk factor for a worse prognosis for human CCA patients. In vivo and in vitro analysis revealed that overexpression of miR-191 was associated with enhanced proliferation, invasion, and migration and reduced ten-eleven translocation 1 (TET1) expression, which induces DNA demethylation and was shown to be a direct target of miR-191. The authors then found that TET1 expression allows for the methylation of CpG-rich regions in the gene transcription start site of p53, a tumor suppressor, leading to a reduction of p53 expression and subsequent increase in tumor burden. These findings suggest that the overexpression of miR-191 is associated with CCA progression via miR-191/TET1/p53 signaling 69. Aside from its role in p53 expression 70, miR-191 has been shown to promote CSC-like properties in bronchial epithelial cells, alluding to the fact that miR-191 may influence the CSC niche in CCA as well. This publication identifies a novel therapeutic target for the treatment of CCA and suggests that miR-191 levels may be used as a diagnostic or prognostic factor or both.
Aside from p53, N-myc downstream-regulated gene 2 (NDRG2) is another tumor suppressor that plays a role in the progression of various cancers 71, 72. NDRG2 has been shown to be downregulated during tumor progression, and it has been postulated that NDRG2 inhibits the metastasis of HCC 71– 73. miR-181 is upregulated in several cancers 74– 76; however, miR-181 has also been shown to inhibit tumor formation 77– 79. Leukemia inhibitory factor (LIF) is a member of the IL-6 cytokine family whose dysregulation has been observed in different cancers 79. The potential function of NDRG2, LIF, and miR-181c in the development and progression of CCA is not fully understood, and a recent publication evaluated the potential roles and mechanisms of these factors 80. The authors found that the expression of NDRG2 was decreased and miR-181c and LIF increased in human CCA compared with non-tumor tissues. Furthermore, downregulation of NDRG2 alongside overexpression of miR-181c or LIF indicated a poorer overall survival in patients with CCA. In vivo and in vitro, it was shown that overexpression of NDRG2 was able to inhibit CCA cell proliferation, chemoresistance, and metastasis. LIF was able to activate miR-181c, while NDRG2 inhibited LIF transcription. These findings identify that NDRG2 and LIF/miR-181c counteract each other, and dysregulation of one of these pathways may contribute to carcinogenesis and metastasis 80. Given that LIF is an IL-6 cytokine, miR-181c regulation of LIF may influence the inflammatory properties of the microenvironment so that it favors CSC formation or MSC recruitment. Though complex, this novel pathway may serve as a potential therapeutic target for the treatment of CCA.
The role of miR-16 in tumorigenesis has been studied in various cancers 81– 83, but its role in CCA is unknown. Yes-associated protein 1 (YAP1) is inhibited by the hippo signaling pathway, but in the absence of this inhibition, YAP1 acts as an oncogene in numerous cancers 84– 88; however, mechanisms regulating YAP1 expression in human CCA are largely unknown. Recent work found that in human CCA tissues miR-16 expression was significantly downregulated and correlated with tumor size, metastasis, and staging 89. Furthermore, downregulation of miR-16 was strongly associated with increased tumor progression and worsened overall survival in human CCA patients. In vivo and in vitro experimentation demonstrated that overexpression of miR-16 inhibited CCA cell proliferation, invasion, and metastasis. The authors then found that YAP1 was a direct target of miR-16. In support of these findings, it was noted that YAP1 levels are greatly enhanced in human CCA tissues, which was inversely correlated with the above findings that miR-16 is largely downregulated in human CCA. This publication concludes that miR-16 acts as a tumor suppressor in CCA through downregulation of YAP1 89. The miR-16/YAP1 interaction may not only be a therapeutic target but also act as a reliable prognostic marker for CCA. Additionally, YAP1 has been shown to control the self-renewal and differentiation of MSCs; therefore, miR-16 regulation of YAP1 may influence MSC function during CCA 90.
It has long been known that miRNAs play a role in the initiation and progression of CCA, but miRNA regulation of MSCs and CSCs during CCA is unknown. While these publications discuss the role of miRNAs in CCA cells and tissues, we can draw comparisons to the impact that they may have on MSC and CSC function. Furthermore, we can note that miRNA signaling may manipulate the microenvironment to become pro-inflammatory, which will favor the initiation of CSC formation as well as the recruitment of MSCs to the injured tissue. However, the discussion of miRNA regulation of MSCs and CSCs during CCA is largely speculative, and further research is necessary to fully understand this signaling process.
Conclusions
Currently, therapeutic options for patients with CCA are extremely limited, a worrying predicament given the increase in incidence. In addition, options for patients with CCA are further limited since the disease tends to be at an advanced stage at the time of diagnosis. These facts indicate the imperative need to develop better diagnostic tools to help identify CCA at an earlier time point as well as more sophisticated therapeutic tools to improve survival rates. The roles of MSCs and CSCs during CCA are a new area of study with little information known about these cells. For this reason, more studies are needed to fully elucidate the impact that MSCs and CSCs have on CCA development and progression. It is possible that therapies targeting these cells will reduce chemoresistance and recurrence, but only future studies will be able to fully define this. There are a large number of publications regarding miRNA regulation of CCA progression; however, miRNA regulation of MSCs and CSCs during CCA is unknown. Given that miRNA therapies for various liver diseases are currently in clinical trials, there is hope that new miRNA-based therapies are developed for CCA, specifically those that may impact MSC and CSC function. While we have made strides in terms of understanding the CCA tumor environment and the pathways regulating progression, few translational findings have been developed. In the future, translational studies are necessary to help find diagnostic, prognostic, and therapeutic tools for the treatment of CCA.
Abbreviations
CCA, cholangiocarcinoma; CCL5, C-C motif chemokine ligand 5; CCR5, C-C motif chemokine receptor 5; CD, cluster of differentiation; CSC, cancer stem cell; HCC, hepatocellular carcinoma; HPC, hepatic progenitor cell; IFN-γ, interferon-gamma; IL, interleukin; LIF, leukemia inhibitory factor; Ln-332, laminin-332; miRNA, microRNA; MMP, matrix metalloproteinase; MSC, mesenchymal stem cell; NDRG2, N-myc downstream-regulated gene 2; TAM, tumor-associated macrophage; TET1, ten-eleven translocation 1; TNF-α, tumor necrosis factor-alpha; YAP1, yes-associated protein 1.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jesper B Anderson, University of Copenhagen, Copenhagen, Denmark
Li Wang, University of Connecticut, Storrs, Connecticut, USA
Funding Statement
Portions of this work were supported by (i) a VA Merit Award (1I01BX003031) from the US Department of Veterans Affairs, Biomedical Laboratory Research and Development Service and (ii) the National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK108959). This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System (Temple, TX, USA). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the US Government.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Oliveira IS, Kilcoyne A, Everett JM, et al. : Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdom Radiol (NY). 2017;42(6):1637–49. 10.1007/s00261-017-1094-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Razumilava N, Gores GJ: Combination of gemcitabine and cisplatin for biliary tract cancer: a platform to build on. J Hepatol. 2011;54(3):577–8. 10.1016/j.jhep.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 3. Cardinale V, Bragazzi MC, Carpino G, et al. : Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2(5):272–80. 10.3978/j.issn.2304-3881.2013.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banales JM, Cardinale V, Carpino G, et al. : Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–80. 10.1038/nrgastro.2016.51 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Bridgewater J, Galle PR, Khan SA, et al. : Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–89. 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 6. Guglielmi A, Ruzzenente A, Campagnaro T, et al. : Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33(6):1247–54. 10.1007/s00268-009-9970-0 [DOI] [PubMed] [Google Scholar]
- 7. Si A, Li J, Xiang H, et al. : Actual over 10-year survival after liver resection for patients with intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(27):44521–32. 10.18632/oncotarget.17815 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Elshamy M, Presser N, Hammad AY, et al. : Liver transplantation in patients with incidental hepatocellular carcinoma/cholangiocarcinoma and intrahepatic cholangiocarcinoma: a single-center experience. Hepatobiliary Pancreat Dis Int. 2017;16(3):264–70. 10.1016/S1499-3872(17)60016-X [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. DeOliveira ML, Cunningham SC, Cameron JL, et al. : Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62. 10.1097/01.sla.0000251366.62632.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darwish Murad S, Kim WR, Harnois DM, et al. : Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98.e3; quiz e14. 10.1053/j.gastro.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deviere J, Baize M, de Toeuf J, et al. : Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34(2):95–101. 10.1016/S0016-5107(88)71271-7 [DOI] [PubMed] [Google Scholar]
- 12. Bhat M, Hathcock M, Kremers WK, et al. : Portal vein encasement predicts neoadjuvant therapy response in liver transplantation for perihilar cholangiocarcinoma protocol. Transpl Int. 2015;28(12):1383–91. 10.1111/tri.12640 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. van der Gaag NA, Rauws EA, van Eijck CH, et al. : Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362(2):129–37. 10.1056/NEJMoa0903230 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Brivio S, Cadamuro M, Strazzabosco M, et al. : Tumor reactive stroma in cholangiocarcinoma: The fuel behind cancer aggressiveness. World J Hepatol. 2017;9(9):455–68. 10.4254/wjh.v9.i9.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pattabiraman DR, Weinberg RA: Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13(7):497–512. 10.1038/nrd4253 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Romano M, De Francesco F, Gringeri E, et al. : Tumor Microenvironment Versus Cancer Stem Cells in Cholangiocarcinoma: Synergistic Effects? J Cell Physiol. 2016;231(4):768–76. 10.1002/jcp.25190 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Raggi C, Invernizzi P, Andersen JB: Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol. 2015;62(1):198–207. 10.1016/j.jhep.2014.09.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Marquardt JU, Raggi C, Andersen JB, et al. : Human hepatic cancer stem cells are characterized by common stemness traits and diverse oncogenic pathways. Hepatology. 2011;54(3):1031–42. 10.1002/hep.24454 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Ma S, Chan KW, Hu L, et al. : Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–56. 10.1053/j.gastro.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 20. Raggi C, Factor VM, Seo D, et al. : Epigenetic reprogramming modulates malignant properties of human liver cancer. Hepatology. 2014;59(6):2251–62. 10.1002/hep.27026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamashita T, Forgues M, Wang W, et al. : EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68(5):1451–61. 10.1158/0008-5472.CAN-07-6013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Raggi C, Correnti M, Sica A, et al. : Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66(1):102–15. 10.1016/j.jhep.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Kallis YN, Robson AJ, Fallowfield JA, et al. : Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60(4):525–33. 10.1136/gut.2010.224436 [DOI] [PubMed] [Google Scholar]
- 24. Lorenzini S, Bird TG, Boulter L, et al. : Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59(5):645–54. 10.1136/gut.2009.182345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Govaere O, Komuta M, Berkers J, et al. : Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63(4):674–85. 10.1136/gutjnl-2012-304351 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Sulpice L, Rayar M, Desille M, et al. : Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013;58(6):1992–2000. 10.1002/hep.26577 [DOI] [PubMed] [Google Scholar]
- 27. Giannelli G, Fransvea E, Bergamini C, et al. : Laminin-5 chains are expressed differentially in metastatic and nonmetastatic hepatocellular carcinoma. Clin Cancer Res. 2003;9(10 Pt 1):3684–91. [PubMed] [Google Scholar]
- 28. Govaere O, Wouters J, Petz M, et al. : Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J Hepatol. 2016;64(3):609–17. 10.1016/j.jhep.2015.11.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Karp JM, Leng Teo GS: Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16. 10.1016/j.stem.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 30. Chamberlain G, Fox J, Ashton B, et al. : Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–49. 10.1634/stemcells.2007-0197 [DOI] [PubMed] [Google Scholar]
- 31. Spaeth E, Klopp A, Dembinski J, et al. : Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15(10):730–8. 10.1038/gt.2008.39 [DOI] [PubMed] [Google Scholar]
- 32. Yagi H, Soto-Gutierrez A, Parekkadan B, et al. : Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667–79. 10.3727/096368910X508762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spaeth EL, Dembinski JL, Sasser AK, et al. : Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4(4):e4992. 10.1371/journal.pone.0004992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sawitza I, Kordes C, Götze S, et al. : Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci Rep. 2015;5:13320. 10.1038/srep13320 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Keating A: Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–16. 10.1016/j.stem.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 36. Nauta AJ, Fibbe WE: Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. 10.1182/blood-2007-02-069716 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Krampera M, Cosmi L, Angeli R, et al. : Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–98. 10.1634/stemcells.2005-0008 [DOI] [PubMed] [Google Scholar]
- 38. Zhong W, Tong Y, Li Y, et al. : Mesenchymal stem cells in inflammatory microenvironment potently promote metastatic growth of cholangiocarcinoma via activating Akt/NF-κ B signaling by paracrine CCL5. Oncotarget. 2017;8:73693–73704. 10.18632/oncotarget.17793 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Chen DR, Lu DY, Lin HY, et al. : Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed Res Int. 2014;2014: 532161. 10.1155/2014/532161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang W, Zhong W, Yuan J, et al. : Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015;6(39):42276–89. 10.18632/oncotarget.5514 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Friedman RC, Farh KK, Burge CB, et al. : Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pillai RS, Artus CG, Filipowicz W: Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10(10):1518–25. 10.1261/rna.7131604 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Bartel DP: MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katsumi T, Ninomiya M, Nishina T, et al. : MiR-139-5p is associated with inflammatory regulation through c-FOS suppression, and contributes to the progression of primary biliary cholangitis. Lab Invest. 2016;96(11):1165–77. 10.1038/labinvest.2016.95 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Francis H, McDaniel K, Han Y, et al. : Regulation of the extrinsic apoptotic pathway by microRNA-21 in alcoholic liver injury. J Biol Chem. 2014;289(40):27526–39. 10.1074/jbc.M114.602383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kennedy LL, Meng F, Venter JK, et al. : Knockout of microRNA-21 reduces biliary hyperplasia and liver fibrosis in cholestatic bile duct ligated mice. Lab Invest. 2016;96(12):1256–67. 10.1038/labinvest.2016.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shibata C, Otsuka M, Kishikawa T, et al. : Current status of miRNA-targeting therapeutics and preclinical studies against gastroenterological carcinoma. Mol Cell Ther. 2013;1:5. 10.1186/2052-8426-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baek J, Kang S, Min H: MicroRNA-targeting therapeutics for hepatitis C. Arch Pharm Res. 2014;37(3):299–305. 10.1007/s12272-013-0318-9 [DOI] [PubMed] [Google Scholar]
- 49. Palumbo T, Poultsides GA, Kouraklis G, et al. : A functional microRNA library screen reveals miR-410 as a novel anti-apoptotic regulator of cholangiocarcinoma. BMC Cancer. 2016;16(1):353. 10.1186/s12885-016-2384-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Haga H, Yan I, Takahashi K, et al. : Emerging insights into the role of microRNAs in the pathogenesis of cholangiocarcinoma. Gene Expr. 2014;16(2):93–9. 10.3727/105221614X13919976902174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ehrlich L, Hall C, Venter J, et al. : miR-24 Inhibition Increases Menin Expression and Decreases Cholangiocarcinoma Proliferation. Am J Pathol. 2017;187(3):570–80. 10.1016/j.ajpath.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Du K, Li Z, Fang X, et al. : Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting microRNA-340 to induce β-catenin expression through hypoxia. Eur J Cell Biol. 2017;96(6):496–503. 10.1016/j.ejcb.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 53. Pakravan K, Babashah S, Sadeghizadeh M, et al. : MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr). 2017;40(5):457–470. 10.1007/s13402-017-0335-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Yu M, Xue Y, Zheng J, et al. : Linc00152 promotes malignant progression of glioma stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol Cancer. 2017;16(1):110. 10.1186/s12943-017-0677-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; F1000 Recommendation
- 55. Chandrasekharappa SC, Guru SC, Manickam P, et al. : Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404–7. 10.1126/science.276.5311.404 [DOI] [PubMed] [Google Scholar]
- 56. Vijayaraghavan J, Maggi EC, Crabtree JS: miR-24 regulates menin in the endocrine pancreas. Am J Physiol Endocrinol Metab. 2014;307(1):E84–92. 10.1152/ajpendo.00542.2013 [DOI] [PubMed] [Google Scholar]
- 57. Meng FL, Wang W, Jia WD: Diagnostic and prognostic significance of serum miR-24-3p in HBV-related hepatocellular carcinoma. Med Oncol. 2014;31(9):177. 10.1007/s12032-014-0177-3 [DOI] [PubMed] [Google Scholar]
- 58. Liu YX, Long XD, Xi ZF, et al. : MicroRNA-24 modulates aflatoxin B1-related hepatocellular carcinoma prognosis and tumorigenesis. Biomed Res Int. 2014;2014:482926. 10.1155/2014/482926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dong W, Li B, Wang Z, et al. : Clinical significance of microRNA-24 expression in esophageal squamous cell carcinoma. Neoplasma. 2015;62(2):250–8. 10.4149/neo_2015_030 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Lee SH, Chen TY, Dhar SS, et al. : A feedback loop comprising PRMT7 and miR-24-2 interplays with Oct4, Nanog, Klf4 and c-Myc to regulate stemness. Nucleic Acids Res. 2016;44(22):10603–18. 10.1093/nar/gkw788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Balsalobre A, Damiola F, Schibler U: A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93(6):929–37. 10.1016/S0092-8674(00)81199-X [DOI] [PubMed] [Google Scholar]
- 62. Filipski E, King VM, Li X, et al. : Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94(9):690–7. 10.1093/jnci/94.9.690 [DOI] [PubMed] [Google Scholar]
- 63. Viswanathan AN, Schernhammer ES: Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281(1):1–7. 10.1016/j.canlet.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Han Y, Meng F, Venter J, et al. : miR-34a-dependent overexpression of Per1 decreases cholangiocarcinoma growth. J Hepatol. 2016;64(6):1295–304. 10.1016/j.jhep.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boucher H, Vanneaux V, Domet T, et al. : Circadian Clock Genes Modulate Human Bone Marrow Mesenchymal Stem Cell Differentiation, Migration and Cell Cycle. PLoS One. 2016;11(1):e0146674. 10.1371/journal.pone.0146674 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Dawson MA, Kouzarides T: Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. 10.1016/j.cell.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 67. You JS, Jones PA: Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22(1):9–20. 10.1016/j.ccr.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sandhu DS, Shire AM, Roberts LR: Epigenetic DNA hypermethylation in cholangiocarcinoma: potential roles in pathogenesis, diagnosis and identification of treatment targets. Liver Int. 2008;28(1):12–27. 10.1111/j.1478-3231.2007.01624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li H, Zhou ZQ, Yang ZR, et al. : MicroRNA-191 acts as a tumor promoter by modulating the TET1-p53 pathway in intrahepatic cholangiocarcinoma. Hepatology. 2017;66(1):136–51. 10.1002/hep.29116 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Xu W, Ji J, Xu Y, et al. : MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015;54 Suppl 1:E148–61. 10.1002/mc.22221 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Lee DC, Kang YK, Kim WH, et al. : Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68(11):4210–20. 10.1158/0008-5472.CAN-07-5040 [DOI] [PubMed] [Google Scholar]
- 72. Tepel M, Roerig P, Wolter M, et al. : Frequent promoter hypermethylation and transcriptional downregulation of the NDRG2 gene at 14q11.2 in primary glioblastoma. Int J Cancer. 2008;123(9):2080–6. 10.1002/ijc.23705 [DOI] [PubMed] [Google Scholar]
- 73. Wang J, Yin D, Xie C, et al. : The iron chelator Dp44mT inhibits hepatocellular carcinoma metastasis via N-Myc downstream-regulated gene 2 (NDRG2)/gp130/STAT3 pathway. Oncotarget. 2014;5(18):8478–91. 10.18632/oncotarget.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tong SJ, Liu J, Wang X, et al. : microRNA-181 promotes prostate cancer cell proliferation by regulating DAX-1 expression. Exp Ther Med. 2014;8(4):1296–300. 10.3892/etm.2014.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Swanson JM, Wigal S, Greenhill LL, et al. : Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37(5):519–26. 10.1097/00004583-199805000-00014 [DOI] [PubMed] [Google Scholar]
- 76. Mansueto G, Forzati F, Ferraro A, et al. : Identification of a New Pathway for Tumor Progression: MicroRNA-181b Up-Regulation and CBX7 Down-Regulation by HMGA1 Protein. Genes Cancer. 2010;1(3):210–24. 10.1177/1947601910366860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang P, Ye B, Yang Y, et al. : MicroRNA-181 functions as a tumor suppressor in non-small cell lung cancer (NSCLC) by targeting Bcl-2. Tumour Biol. 2015;36(5):3381–7. 10.1007/s13277-014-2972-z [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Conti A, Aguennouz M, La Torre D, et al. : miR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumors. J Neurooncol. 2009;93(3):325–32. 10.1007/s11060-009-9797-4 [DOI] [PubMed] [Google Scholar]
- 79. Pichler M, Winter E, Ress AL, et al. : miR-181a is associated with poor clinical outcome in patients with colorectal cancer treated with EGFR inhibitor. J Clin Pathol. 2014;67(3):198–203. 10.1136/jclinpath-2013-201904 [DOI] [PubMed] [Google Scholar]
- 80. Wang J, Xie C, Pan S, et al. : N-myc downstream-regulated gene 2 inhibits human cholangiocarcinoma progression and is regulated by leukemia inhibitory factor/MicroRNA-181c negative feedback pathway. Hepatology. 2016;64(5):1606–22. 10.1002/hep.28781 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Chatterjee A, Chattopadhyay D, Chakrabarti G: MiR-16 targets Bcl-2 in paclitaxel-resistant lung cancer cells and overexpression of miR-16 along with miR-17 causes unprecedented sensitivity by simultaneously modulating autophagy and apoptosis. Cell Signal. 2015;27(2):189–203. 10.1016/j.cellsig.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 82. Tang X, Jin L, Cao P, et al. : MicroRNA-16 sensitizes breast cancer cells to paclitaxel through suppression of IKBKB expression. Oncotarget. 2016;7(17):23668–83. 10.18632/oncotarget.8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen L, Wang Q, Wang GD, et al. : miR-16 inhibits cell proliferation by targeting IGF1R and the Raf1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS Lett. 2013;587(9):1366–72. 10.1016/j.febslet.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 84. Perra A, Kowalik MA, Ghiso E, et al. : YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61(5):1088–96. 10.1016/j.jhep.2014.06.033 [DOI] [PubMed] [Google Scholar]
- 85. Lee K, Lee SS, Kim SB, et al. : Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients. Clin Cancer Res. 2015;21(2):357–64. 10.1158/1078-0432.CCR-14-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xia Y, Chang T, Wang Y, et al. : YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9(3):e91770. 10.1371/journal.pone.0091770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun D, Li X, He Y, et al. : YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget. 2016;7(49):81062–76. 10.18632/oncotarget.13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wu C, Xu B, Yuan P, et al. : Genome-wide interrogation identifies YAP1 variants associated with survival of small-cell lung cancer patients. Cancer Res. 2010;70(23):9721–9. 10.1158/0008-5472.CAN-10-1493 [DOI] [PubMed] [Google Scholar]
- 89. Han S, Wang D, Tang G, et al. : Suppression of miR-16 promotes tumor growth and metastasis through reversely regulating YAP1 in human cholangiocarcinoma. Oncotarget. 2017;8(34):56635–56650. 10.18632/oncotarget.17832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tang Y, Weiss SJ: Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle. 2017;16(5):399–405. 10.1080/15384101.2017.1280643 [DOI] [PMC free article] [PubMed] [Google Scholar]