Abstract
Background: Artemisinin and partner drug-resistant falciparum malaria is expanding over the Greater Mekong Sub-region (GMS). Eliminating falciparum malaria in the GMS while drugs still retain enough efficacy could prevent global spread of antimalarial resistance. Eliminating malaria rapidly requires targeting the reservoir of asymptomatic parasite carriers.
This pilot trial aimed to evaluate the acceptability, safety, feasibility and effectiveness of mass-drug administration (MDA) in reducing malaria in four villages in Eastern Myanmar.
Methods: Villages with ≥30% malaria prevalence were selected. Long-lasting insecticidal bednets (LLINs) and access to malaria early diagnosis and treatment (EDT) were provided. Two villages received MDA immediately and two were followed for nine months pre-MDA. MDA consisted of a 3-day supervised course of dihydroartemisinin-piperaquine and single low-dose primaquine administered monthly for three months. Adverse events (AE) were monitored by interviews and consultations. Malaria prevalence was assessed by ultrasensitive PCR quarterly for 24 months. Symptomatic malaria incidence,entomological indices, and antimalarial resistance markers were monitored.
Results: MDA was well tolerated. There were no serious AE and mild to moderate AE were reported in 5.6%(212/3931) interviews. In the smaller villages, participation to three MDA courses was 61% and 57%, compared to 28% and 29% in the larger villages. Baseline prevalence was higher in intervention than in control villages (18.7% (95%CI=16.1-21.6) versus 6.8%(5.2-8.7), p<0.0001) whereas three months after starting MDA, prevalence was lower in intervention villages (0.4%(0.04-1.3) versus 2.7%(1.7-4.1), p=0.0014). After nine months the difference was no longer significant (2.0%(1.0-3.5) versus 0.9%(0.04-1.8), p=0.10). M0-M9 symptomatic falciparum incidence was similar between intervention and control. Before/after MDA comparisons showed that asymptomatic P. falciparum carriage and anopheline vector positivity decreased significantly whereas prevalence of the artemisinin-resistance molecular marker remained stable.
Conclusions: This MDA was safe and feasible, and, could accelerate elimination of P. falciparum in addition to EDT and LLINs when community participation was sufficient.
Keywords: Plasmodium falciparum, Plasmodium vivax, Mass Drug Administration, Asymptomatic carriage, submicroscopic infection, artemisinin-resistance, transmission
Introduction
Recent progress in the control of malaria is now under threat because the two pillars of malaria control – antimalarial drugs and insecticides – are falling to resistance 1. Historically, the Greater Mekong sub–region (GMS) has been the source of antimalarial drug resistance, and artemisinin resistant Plasmodium falciparum has also emerged and recently spread in this region 2. Artemisinin resistance has led to the failure of artemisinin based combination treatments (ACT) 3, 4, the cornerstone of uncomplicated P. falciparum malaria therapy worldwide. Without alternative medicines in the near future, the best option to prevent the spread of resistant parasites to Africa is the rapid elimination of P. falciparum parasites from the GMS. Early detection and treatment of clinical cases at the community level is effective in preventing mortality and reduces P. falciparum transmission and the associated morbidity 5– 7. However this approach alone is unlikely to halt the rapid spread of artemisinin resistance because it fails to directly address foci of asymptomatic carriage of P. falciparum. This persistent reservoir represents the main obstacle to the rapid elimination of falciparum malaria in the GMS, where transmission is low, seasonal and unstable 8, 9.
Systematic antimalarial drug treatment of the entire population of these foci may be the only method to achieve a rapid elimination of the parasite reservoir in the absence of a point-of-care test sensitive enough to allow mass screening and treatment of submicroscopic carriers 10– 12. Mass drug administration (MDA) in malaria remains controversial because of its history of success and failure 13. MDA in the ACT era often relies on piperaquine, which provides approximately a one-month protection from reinfection. Adding a single low-dose of primaquine rapidly sterilises P. falciparum infections 14. Promising short term results following MDA were recently obtained in Zambia, albeit in a context of generally decreasing malaria prevalence 15. We present a 24-month pilot study of the safety and effectiveness of MDA in reducing P. falciparum incidence and prevalence in four villages with high prevalence of sub-microscopic infections located on the Thailand–Myanmar border, an area where artemisinin resistance is firmly established 16, 17.
Methods
Study design
This study was the pilot phase of a multicentre cluster-randomized control trial conducted in several sites in the GMS and therefore limited to a sample size of four villages. It is registered at ClinicalTrials.gov: NCT01872702.
The study was conducted in rural Eastern Kayin (Karen) state of Myanmar, a region endemic for P. falciparum and P. vivax malaria 17. In early 2013, 12 villages were screened using a high volume ultra-sensitive qPCR assay method (uPCR) with the original intention of identifying villages with >30% malaria prevalence, of which >30% was P. falciparum ( Supplementary File 1: Table S1). However, the P. falciparum prevalence was lower than expected, so after the survey the threshold proportion of P. falciparum for inclusion was lowered to 10%. Four villages fulfilling this revised entry criterion were selected after community engagement and agreement by the village leaders. A malaria post (MP) was set up at month 0 (M0) in all four villages and long lasting insecticidal nets (LLIN) were provided. Two villages (referred to as A1-KNH and A2-TOT) were randomized using a flipped coin to receive anti-malarial MDA for three consecutive months (M0 to M2) immediately. The other two villages (referred to as B1-TPN and B2-HKT) were monitored for a 9-month control period with MP and LLIN only. The control period was planned originally for 12 months, but MDA had to be expedited because of accessibility concerns during the rainy season, and was given from M9 to M11. All four villages were followed for 24 months in total.
Ethics statement
This study protocol was reviewed and approved by OxTREC (reference no. 1017–13 and 1015–13), by the Tak Community Advisory Board 18, and by village committees. Participation in surveys and MDA activities was voluntary. In addition to group information during community engagement activities, participants received individual information in their language (Karen or Myanmar) and provided written informed consent before inclusion in a survey or MDA. Participants under 18 years provided an assent in addition to the consent of their parent or guardian.
Study participants
Participants were defined as individuals living in the village who provided information during home visits at any time point. Each participant was followed using a unique identification code to collect information on participation, adverse events, malaria carriage during surveys, clinical episodes of malaria, and mobility.
Interventions
Before and during the study, community engagement (CE) activities were conducted (detailed in 19). Briefly, the aim of community engagement was to present the project to the community and involve its members to create understanding and buy-in of the population, and result in a high coverage of the interventions 20. The different activities conducted were: workshops with authorities and local gatekeepers, meetings and activities with different population groups, health-related community incentives (ancillary care and health education; building of water catchment and distribution systems in each of the study villages) 19. Through this mutual understanding, the study activities could be conducted while respecting the life of the community and ensuring maximal benefit for the participants.
A population census was conducted, individuals were linked to houses (with both individual and house identification codes), and geographic references (latitude and longitude) were recorded for each house. Population movement in and out of villages was monitored by census during the quarterly surveys and by home visits. Newcomers intending to stay in the village for more than two weeks were enrolled after consenting to participate in the follow-up surveys. All newcomers arriving after MDA were offered a single curative treatment course of DP-primaquine, irrespective of disease or infection status.
A MP was set up at M0 in all four villages to provide early detection and treatment of clinical cases 7. Fever cases were tested with SD Bioline Pf/Pv rapid diagnostic test (RDT). Uncomplicated P. falciparum cases were given weight-adjusted doses of dihydroartemisinin-piperaquine (DP, Guilin Pharmaceutical Co, Guilin, PRC) for three days and one 0.25mg base/kg dose of primaquine (Governement Pharmaceutical Organization, Thailand). Uncomplicated P. vivax cases were given weight-adjusted doses of chloroquine (Governement Pharmaceutical Organization, Thailand) for three days. Severe illnesses were referred to the nearest clinic. LLINs were distributed to all households at M0.
Blood sampling surveys of the entire village population were conducted at months 0, 3, 6, 9, 12, 15, 18, and 24. Samples of 3 and 0.5 mL of blood were taken by venous puncture from survey participants over and under 5 years respectively. Malaria infection was detected in the laboratory by RDT, microscopy and by a uPCR method with an approximate limit of detection of 20 parasites/mL 8, 21.
Each MDA comprised a standard 3–day treatment course of DP (dihydroartemisinin (7mg/kg) and piperaquine (55 mg/kg) and one 0.25mg base/kg dose of primaquine given orally under supervision. Treatments were given three times at one-month intervals (M0 to M2 or M9 to M11).
Inclusion and exclusion criteria
Infants <6 months, individuals with a known allergy to any of the antimalarial drugs, and pregnant women in their first trimester were excluded from MDA participation. Pregnant women in their second or third trimester and breastfeeding mothers were eligible for DP, but excluded from primaquine treatment. Women of childbearing age were asked if they were or could be pregnant and a pregnancy test was offered if unsure. Infants <6 months were excluded from prevalence surveys.
Adverse event monitoring
Adverse events (AE) were monitored using a structured questionnaire on the second, third, and seventh day after the start of the MDA treatment course. During the three months of MDA, a mobile clinic staffed with a medical assistant was available in the village to provide free consultations to all villagers and to assess MDA participants presenting with AE. Serious adverse events were reported centrally.
Variable collection and definition
Participant follow-up. The individual follow-up time was defined as the number of days spent within the catchment area of the MP, i.e. the village and the surrounding farms.
Participation in MDA. Participation in MDA was measured as the total number of individuals completing one, two or three 3-day DP treatment courses divided by the total number of inhabitants present in the village at least once during the 3-month period, after excluding individuals who visited the village for two weeks or less. Individual participation in MDA was categorized in three groups: no MDA, one or two rounds (i.e. infection cured, but incomplete protection from reinfection by DP), and three rounds MDA (i.e. infection cured and 3-month protection).
Malaria infection prevalence. Prevalence of P. falciparum or P. vivax infection was defined as the proportion of individuals with a positive uPCR for each parasite divided by total number of individuals sampled. When the parasite species could not be determined (because of low DNA content) samples were attributed to species based on the falciparum/vivax ratio measured in clinical cases in the village during the previous period 8.
Incidence of symptomatic malaria. Symptomatic malaria cases were defined as individuals with fever (temperature ≥37.5°C) or history of fever in the past two days and confirmed falciparum or vivax infection by RDT or microscopy. During the period between surveys, cases were detected passively at the MP and confirmed by RDT. During survey periods, individuals with fever or history of fever and a positive RDT or microscopy slide (with uPCR confirmation when available) were considered clinical cases. Incidence of clinical malaria episodes in the village was expressed in cases per 1000 persons per month (number of infections during the month/sum of individual follow-up time during the month). Patients residing in nearby villages and coming to the MP for diagnosis were excluded from the analysis. Mixed infections contributed to both P. falciparum and P. vivax incidence.
Detection of molecular markers of antimalarial resistance
Assessment of mutations in PfKelch13. Polymorphisms in the PfKelch gene were assessed by nested PCR amplification covering the full length of the gene (total 2181 bp) 2, and followed by sequencing of the gene by ABI Sequencer (Macrogen Inc, South Korea). Cross contamination was monitored by adding negative control samples in every run. Sequencing results were aligned against PfKelch13 of reference strain 3D7 (putative 9PF13_0238 NCBI Reference Sequence (3D7): XM_001350122.1), using Bioedit software (Abbott, CA, USA). Polymorphic patterns were assessed by two individuals blinded to the origin of the sample.
PfPlasmepsin2 gene amplification. Pfplasmepsin2 copy number was quantified using Relative-quantitative Real-time PCR based on Taqman probe on a Corbett Rotor-Gene TM Q (Corbett Research, Australia). Primers and probes have been described previously 22. Amplification was performed in triplicate on a total volume of 25 μL as multiplex PCR using Quantitec Multiplex PCR no ROX (QIAgen, Germany). Every amplification run contained 9 replicates of calibrators and triplicates without template as negative controls. β-tubulin served as an internal standard for the amount of sample DNA added to the reactions. Copy numbers were calculated using the formula: copy number= 2 -ΔΔCt with ΔΔ C t denoting the difference between Δ C t of the unknown sample and Δ C t of the reference sample.
Entomology
Entomological surveys to identify malaria vectors species and abundance were conducted monthly in each village for five nights per month, as described elsewhere 23, 24. Briefly, mosquitos were collected using indoor and outdoor human landing catch (HLC) in five sites per village, yielding a total of 50 human.nights per survey. Infection of primary malaria vectors ( Anopheles minimus s.l., An. maculatus s.l., and An. dirus s.l.) by plasmodium parasites was determined using a quantitative real-time PCR (qPCR) assay, confirmed with a second assay amplifying a different DNA target 25. The limit of detection of the whole procedure was 6 P. falciparum sporozoites per mosquito 25. Main entomological indices - human biting rate (HBR), sporozoite index (SI), and entomological inoculation rate (EIR) - were calculated.
Statistical analysis
Statistical analyses were performed using Stata 14.1 (StataCorp, USA). Binomial 95% confidence intervals (CIs) were calculated for prevalence and SI, and Poisson 95% CIs for incidence of clinical episodes, HBR, and EIR. Intervention and control group prevalence were compared using chi-square or Fisher’s exact test, as appropriate.
Pre-specified endpoints
Because the MDA in control villages was conducted earlier than originally planned (M9 instead of M12), the revised primary endpoints were defined as the prevalence of asymptomatic P. falciparum infection 3 and 9 months after MDA. The secondary endpoints were defined as 1) safety and 2) acceptability of MDA, as evaluated by questionnaires. Additional outcome measures reported here are the incidence of clinical malaria over the first 9 months, number of people participating in MDA, and proportion of artemisinin-resistant P. falciparum infections.
Before/after analysis
A post-hoc before/after analysis of the entire duration of follow-up was conducted to understand the factors influencing the impact of antimalarial MDA and the overall intervention. The effect of the intervention on P. falciparum carriage (recorded in prevalence surveys) was estimated using Generalised Estimating Equations (GEE) with a logit link to estimate Odds Ratios (ORs), an exchangeable correlation structure and robust standard errors to account for repeated measurements at individual level. The change in odds of P. falciparum carriage compared to baseline survey was estimated in the control period and in the after MDA period. To assess the duration of MDA effect, time-dependent changes in the odds of P. falciparum carriage were estimated separately for the control and after MDA periods. Demographic characteristics, village, age group (≤ & >10 years), sex and season, were included as covariates. The analysis was repeated to assess the effect of individual MDA participation. A direct comparison of control and after MDA periods was also conducted in which baseline P. falciparum infection status was added as a covariate in addition to the demographic characteristics.
Results
The study was conducted between May 2013 and June 2015 ( Table 1). Of 3238 individuals recorded in the villages during the 24-month study period, 2941 participated in surveys or MDA at least once ( Table 1). The population structure and mobility were typical of rural villages in this area ( Supplementary File 1: Figure S2). There was substantial turnover of the population in all villages: at any time point, between 19 and 27% of the population corresponded to individuals with <12 months follow-up ( Table 1, Supplementary File 1: Figure S2 and Figure S3). Around 70% of people stayed in the village continuously during the three consecutive months of MDA.
Table 1. Demographics, follow-up of participants, population coverage with mass drug administration (MDA) and duration of intervention, and access to malaria post of the four study villages in Eastern Kayin State, Myanmar.
A map of the villages is presented in Supplementary File 1: Figure S1.
| A1–KNH | A2–TOT | B1–TPN | B2–HKT | |
|---|---|---|---|---|
| Village randomization information | ||||
| Pilot survey P. falciparum prevalence: % (n/N) | 16 (8/51) | 7 (3/41) | 4 (2/54) | 15 (7/48) |
| Pilot survey malaria prevalence: % (n/N) | 42 (22/51) | 42 (17/41) | 32 (17/54) | 42 (20/48) |
| Randomization group | Intervention | Intervention | Control
M0M9 |
Control
M0M9 |
| Study start date (M0) | 12/06/2013 | 27/05/2013 | 20/05/2013 | 02/06/2013 |
| Study end date (M24) | 07/05/2015 | 25/04/2015 | 27/03/2015 | 04/06/2015 |
| Population and follow-up | ||||
| Total population recorded over 24 months | 494 | 940 | 488 | 1316 |
| Participants over 24 months: n (%) | 484 (98) | 799 (85) | 473 (97) | 1185 (90) |
| Median age [IQR] (years) | 23 [9–41] | 19 [7–38] | 20 [6–38] | 19 [8–38] |
| Newcomers before MDA (n) | na | na | 114 | 293 |
| Newcomers during MDA (n) | 83 | 75 | 7 | 112 |
| Newcomers after MDA (n) | 103 | 271 | 46 | 191 |
| Newcomers taking 1 systematic treatment upon arrival,
after MDA: n/N (%) |
63/103 (61) | 34/271 (13) | 25/46 (54) | 30/191 (16) |
| Prevalence of
P. falciparum infection among newcomers
after MDA: n/N (%) |
2/78 (2.6) | 3/150 (2.0) | 0/32 (0) | 1/170 (0.6) |
| Average "real time" population composition: n (%) | ||||
| Number of inhabitants ‡ | 344 | 610 | 317 | 837 |
| Female ‡ | 163 (47) | 301 (49) | 147 (46) | 395 (47) |
| Male ‡ | 181 (53) | 309 (51) | 170 (54) | 442 (53) |
| Participants with >12 months follow-up ‡ | 277 (81) | 463 (76) | 257 (81) | 613 (73) |
| Participants with 3–12 months follow-up ‡ | 58 (17) | 124 (20) | 51 (16) | 192 (23) |
| Participants with <3 months ‡ | 9 (3) | 23 (4) | 9 (3) | 32 (4) |
| MDA implementation | ||||
| MDA start date | 12/06/2013 | 27/05/2013 | 28/01/2014 | 01/04/2014 |
| Study month of MDA start | M0 | M0 | M9 | M9 |
| Duration of round 1; 2; 3 of MDA (days) | 9; 10; 11 | 7; 14; 10 | 6; 9; 4 | 9; 9; 12 |
| Duration to administer 3 doses to >90% of participants in
1 round: mean (days) |
4 | 6 | 4 | 6 |
| Population during MDA period and MDA coverage: n (%) | ||||
| Present at least once during the 3 MDA months | 386 (100) | 662 (100) | 367 (100) | 977 (100) |
| Present continuously during 3 MDA months | 280 (73) | 488 (74) | 283 (77) | 618 (63) |
| Taking 0 round of MDA | 26 (7) | 175 (26) | 28 (8) | 297 (30) |
| Taking 1 round of MDA | 60 (16) | 180 (27) | 64 (17) | 220 (23) |
| Taking 2 rounds of MDA | 64 (17) | 122 (18) | 65 (18) | 180 (18) |
| Taking 3 rounds of MDA | 236 (61) | 185 (28) | 210 (57) | 280 (29) |
| Malaria Post access | ||||
| Consultations from the village | 575 | 770 | 513 | 1496 |
| Consultations from study participants | 515 | 603 | 488 | 889 |
| P. falciparum malaria cases from the village | 6 | 89 | 9 | 8 |
| P. vivax malaria cases from the village | 81 | 139 | 47 | 191 |
| Consultations from outside the village | 465 | 390 | 192 | 1242 |
| P. falciparum malaria cases from outside the village | 11 | 66 | 4 | 20 |
| P. vivax malaria cases from outside the village | 52 | 67 | 11 | 277 |
| Total number of weeks of MP inactivity | 6 | 17 | 5 | 4 |
| Mean consultations per 100/active week | 3.2 | 2.0 | 2.0 | 3.8 |
‡values presented are averages over 10 census occasions during surveys in the village.
Mass antimalarial drug administration (MDA)
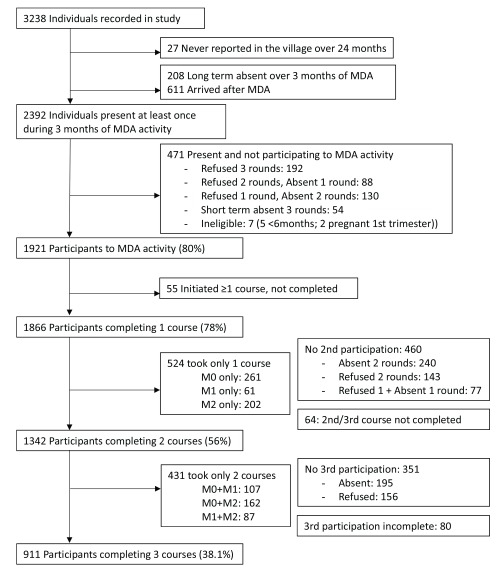
MDA coverage. The target population was 2392 residents of the four villages at the time of MDA. Overall 78% (1866/2392) received at least one 3–day course of DP and 38% (911/2392) received the full course of three treatments. Coverage was higher in the two small villages compared to the two larger villages, for uptake of at least one treatment (A1–KNH: 93%; 360/386 and B1–TPN: 92%; 339/367 compared to A2-TOT: 74%; 487/662 and B2-HKT: 70%; 680/977) and uptake of three treatments (A1–KNH: 61%; 236/386 and B1–TPN: 57%; 210/367 compared to A2-TOT: 28%; 185/662 and B2-HKT:29%; 280/977) ( Table 1; Figure 1).
Figure 1. Flow chart of the participation in antimalarial MDA in the four villages.
Safety and tolerability of MDA. MDA was well accepted and well tolerated. In 3719 of 3931 (94.6%) complete structured interviews, no AE were reported. Reported AE were in majority dizziness (N=192) and pruritus (N=17). There were three reports of black urine. One G6PD-deficient male reported black urine before primaquine and one G6PD-normal male reported black urine 48h after primaquine. Neither were anaemic and both remained well. The third case, a G6PD heterozygote woman with normal G6PD activity, became anaemic but concomitant HIV and TB were considered contributory, as reported previously 26. The percentage of participants reporting AE declined after each MDA round from 8.2% (114/1394) to 3.7% (49/1336). Among the 200 patients who presented at the mobile clinic over the 3-month MDA period, all AE were mild or moderate and most (188/200, 94%) were rated as unrelated or unlikely to be related to treatment ( Table 2). During the 24 months of the study, there were 23 reported serious AE and 15 deaths; none were considered drug related ( Supplementary File 1: Table S2). Haemoglobin decreases in G6PD-deficient individuals were minimal as already reported 26.
Table 2. Safety of mass drug administration (MDA) intervention in the participant population: severity and relation to drugs of adverse events (AE) investigated at the mobile clinic set up during the three months of MDA (M0–M3 or M9–M12).
Severe AEs over the 24-month follow-up are detailed in Supplementary File 1: Table S2.
| Relationship to drug | ||||||
|---|---|---|---|---|---|---|
| Severity | Not related | Unlikely
related |
Possibly
related |
Probably
related |
Highly likely
related |
Total |
| Mild | 134
Infection (71) Weakness (39) Dizziness (4) Allergy (1) Other (19) |
49
Infection (5) Weakness (19) Dizziness (23) Allergy (1) Other (1) |
7
Infection (2) Weakness (3) Dizziness (1) Allergy (1) |
0 | 1
Allergy (1) |
191 |
| Moderate | 5
Infection (4) Other (1) |
0 | 4
Weakness (2) Allergy (2) |
0 | 0 | 9 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 139 | 49 | 11 | 0 | 1 | 200 |
Malaria prevalence
There was a significant difference in baseline P. falciparum prevalence between intervention and control villages (18.7% (150/801) versus 6.8% (58/848); p<0.0001).
The majority of malaria infections detected by uPCR during the cross-sectional surveys were asymptomatic (86%; 1769/2059) and 70% were not detected by microscopy or RDT (1432/2059) ( Supplementary File 1: Table S3). Overall, of 217 individuals positive for P. falciparum, 17 (8%) were still positive in the following survey 3 months later, three were positive after 6 months, and one after 9 months. DP was highly efficacious; no participant receiving at least one complete 3-day DP course was positive for P. falciparum in the survey following MDA (M3 or M12), irrespective of previous infection status ( Supplementary File 1: Table S4 and Table S5). During the follow-up (excluding MDA intervention period), 69% (707/1018) of newcomers to the villages were included in the first survey after their arrival, of whom 1.6% (11/707) were positive for P. falciparum.
Efficacy of MDA
Pilot trial endpoints: MDA+MP versus MP only comparison
Prevalence. At M3 the prevalence of P. falciparum infection had fallen to 0.4% (2/552; 95% CI 0.04 to 1.3), in intervention villages A1-KNH and A2-TOT, and 2.7% (22/812; 1.7 to 4.1) in non-intervention control villages, B1-TPN and B2-HKT (p=0.001). Comparing M0 to M3, prevalence decreased by 98% in MDA villages compared to a 57% decrease in control villages. At M9, the difference in prevalence was no longer significant: 2.0% (11/564; 1.0-3.5) in MDA villages, and 0.9% (9/959; 0.04-1.8) in control villages (p=0.10). Comparing M0 to M9, MDA villages exhibited an 88% decrease in prevalence, compared to an 85% decrease in control villages.
Incidence. Between M0 and M9, falciparum malaria incidence was 1.0 case/1000 person-months (95%CI=0.4-1.9) in MDA villages (8/8352) compared with 1.3 case/1000 person-months (0.6-2.5) in control villages (10/7469, p=0.49). P. vivax malaria incidence was 8.6 cases/1000 person-months (6.8-10.9) in MDA villages (72/8352), compared with 5.6 cases/1000 person-months (4.1-7.6) in control villages (42/7469, p=0.03).
Before versus after MDA comparison
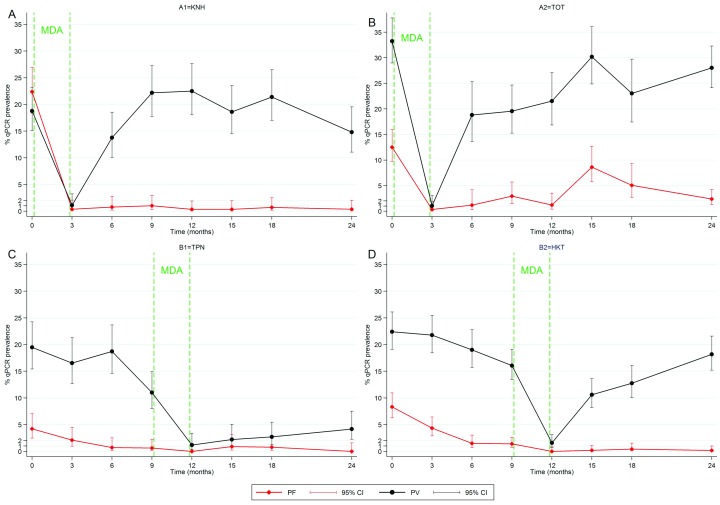
P. falciparum infection. The impact of MDA on P. falciparum carriage was different in the two intervention villages. In A1–KNH, there was a rapid >95% reduction in P. falciparum prevalence, which was sustained from M3 to M24 ( Figure 2). In contrast, in village A2–TOT, prevalence fell between M0 and M3, to reach a peak at M15 during rainy season ( Figure 2). In this village, prevalence was maintained around 5% among individuals who did not take MDA, from M3 to M24 ( Supplementary File 1: Figure S4). Prevalence in the control villages decreased gradually over the control period, dropping at 0% at M12 immediately after MDA, and remained below after MDA ( Figure 2).
Figure 2. Prevalence of P. falciparum and P. vivax carriage over 24 months measured by uPCR in quarterly cross sectional surveys conducted in the four study villages.
A steep decrease in prevalence immediately followed MDA in all 4 villages. The reduction in P. falciparum prevalence was sustained in 3 of 4 villages stabilizing to a <1% prevalence after MDA. In contrast P. vivax prevalence rebounded in 3 of 4 villages. In village A2-TOT, P. falciparum prevalence increased at M15 and M18, mostly from clinical cases occurring while vector densities and thus transmission was increased ( Table 5). A decrease in P. falciparum prevalence was observed in the control period (M0–M9) in villages B1 and B2, possibly attributable to the effect of early diagnosis and treatment provided by MP.
Adjusting for age group, sex, village and season, MDA was associated with an overall 12-fold decrease in odds of P. falciparum carriage over the full observation period (21 month for intervention and 15 months for controls) compared to baseline (OR [95%CI]=0.08 [0.05–0.11]) ( Table 3). The control period before MDA was also associated with a significant decrease in odds of P. falciparum carriage (0.4 [0.3–0.7]) ( Table 3). This translated into a significant 5-fold odds reduction after MDA compared to control period (0.2 [0.1–0.3]).
Table 3. Multivariable analysis of factors associated with Plasmodium falciparum carriage during uPCR surveys over the 24-month study period and quantification of the impact of mass drug administration (MDA).
Two specifications of the MDA impact are presented showing overall impact by period (model 1) or including temporal trends (model 2). The impact of individual participation is presented in Supplementary File 1: Table S7 (see also Supplementary File 1: Table S6 for univariable analysis). The direct comparison between control and intervention adjusting for baseline P. falciparum infection in presented in Supplementary File 1: Table S8 and Supplementary File 1: Table S9. Baseline refers to M0 survey at the beginning of the study. Control period refers to the period between M0 and M9 in the two control villages after implementation of Malaria Post and LLIN distribution at M0. After MDA refers to the period where MDA had been conducted in addition to implementation of Malaria Post and LLIN distribution (M3 to M24 in intervention villages, M12 to M24 in control villages).
| Variable | Categories | Adjusted OR | 95%CI | p–value | |
|---|---|---|---|---|---|
| Age | ≤10 years | 1 | Reference | <0.0001 | |
| >10 years | 2.8 | 1.9–4.1 | |||
| Sex | Female | 1 | Reference | <0.0001 | |
| Male | 2.3 | 1.7–3.2 | |||
| Village | B2–HKT | 1 | Reference | <0.0001 | |
| B1–TPN | 0.8 | 0.4–1.4 | |||
| A2–TOT | 4.0 | 2.4–6.5 | |||
| A1–KNH | 2.8 | 1.9–4.0 | |||
| Season | Cold | 1 | Reference | <0.0001 | |
| Hot | 1.0 | 0.6–1.6 | |||
| Wet | 2.5 | 1.6–4.1 | |||
| Model 1. Study period | Baseline (M0 survey) | 1 | Reference | <0.0001 | |
| Control period | 0.4 | 0.3–0.7 | |||
| After MDA period | 0.08 | 0.05–0.11 | |||
|
Model 2. Interaction between
study period and time |
Study period | Baseline (M0 survey) | 1 | Reference | <0.0001 |
| Control period | 0.4 | 0.2–0.8 | |||
| After MDA period | 0.03 | 0.01–0.05 | |||
| Time (for each additional month) | during control period | 1.03 | 0.91–1.16 | ||
| during period after MDA | 1.08 | 1.03–1.12 | |||
Compared to baseline, the decrease in odds of P. falciparum carriage was 30-fold immediately after MDA (0.03 [0.02–0.06]). Each additional month after MDA was associated with a minimal increase in odds of P. falciparum carriage (1.08 [1.03–1.12] for one additional month). For example, 12 months after MDA, a 15-fold reduction persisted (0.06 [0.04–0.09]). Similar results were obtained when estimating odds for each quarter instead of assuming a monthly linear trend ( Supplementary File 1: Figure S5).
During the pre-MDA control period, individuals with baseline P. falciparum infections had a significantly increased odds of subsequent P. falciparum carriage compared to baseline uninfected individuals (17.2 [6.5–45.6]) ( Supplementary File 1: Table S8). Irrespective of their baseline infection status, individuals taking one or two rounds and individuals completing the three rounds of MDA had a significantly decreased odds of being infected with P. falciparum compared to individuals in the control period (0.2 [0.1–0.6] and 0.1 [0.03–0.4] respectively). In contrast, individuals who did not receive MDA in villages allocated to it had no decrease in the odds of P. falciparum carriage (0.4 [0.1–1.9]) compared to the control period ( Supplementary File 1: Table S8).
P. falciparum clinical malaria. A total of 113 P. falciparum symptomatic cases were detected among study participants, of which 91 were detected passively by the MP and 22 were detected during surveys. An additional 19 cases were detected among non-participants (individuals who reported living in the village but were not recorded in the study, e.g. visitors).
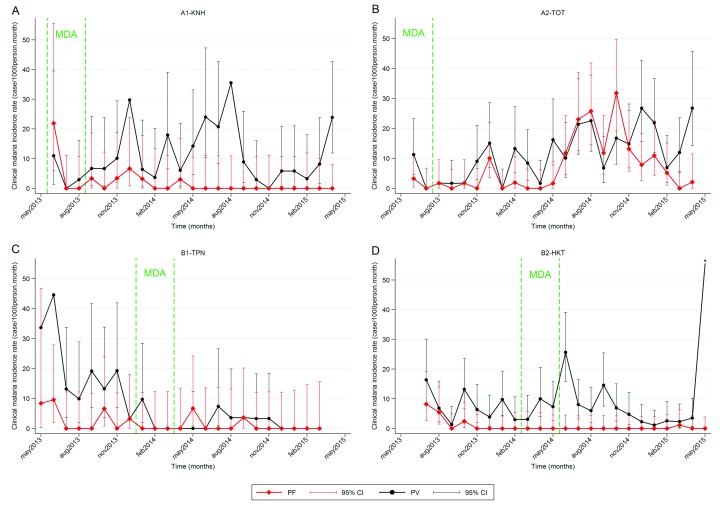
In 3 out of 4 villages, the combined intervention of early diagnosis and treatment through the MP and MDA resulted in a dramatic and sustained decrease in the incidence of P. falciparum clinical cases, which was close to zero after 12 months ( Figure 3). Most of the clinical episodes (75/113, 66%) occurred during a 7–month period in village A2–TOT between July 2014 and January 2015 (M14 to M20), corresponding to the rainy and following cool seasons.
Figure 3. Changes in the incidence of P. falciparum and P. vivax clinical cases.
Clinical falciparum malaria cases persisted up to 9 months in villages achieving stable <1% P. falciparum prevalence after MDA (A1, B1 and B2), before zero incidence was achieved. In village A2, where the intervention with MDA was not considered successful, a significant increase in incidence was observed during the rainy and cold seasons from July 2014 to January 2015.* P. vivax incidence rate in B2-HKT for June 2015 was 100 cases/1000 person-month (95%CI=81-122).
Table 4. Detection of molecular markers of antimalarial resistance in Plasmodium falciparu m positive samples according to period before and after mass drug administration (MDA).
| Before MDA | After MDA | Overall | |
|---|---|---|---|
| PF positive samples by uPCR | 191 | 56 | 247 |
| Sample with PfK13 result | 77 (40.3%) | 30 (53.6%) | 107 (43.3%) |
| Samples with PfK13 mutation | 66 | 17 | 83 |
| PfK13 Mutation prevalence [95% CI] | 85.6% [75.9–92.6] | 56.7% [37.4–74.5] | 77.6% [68.5–85.1] |
| Sample with Piperaquine resistance result | 53 (27.7%) | 16 (28.6%) | 69 (27.9%) |
| Samples with Piperaquine resistance marker | 0 | 0 | 0 |
| Piperaquine resistance prevalence [95% CI] | 0% [0–6.7] | 0% [0–20.6] | 0% [0–5.2] |
P. vivax infection and clinical malaria. In 3 out of 4 villages, P. vivax prevalence was reduced only transiently by MDA ( Figure 2). Even when MDA participation was high, P. vivax prevalence rebounded quickly to reach initial levels within 3 to 6 months. In village B1–TPN, P. vivax prevalence did not rebound as rapidly. P. vivax symptomatic malaria incidence was unaffected beyond the 3-month MDA period in three villages, with seasonal peaks of clinical cases during transmission seasons both before and after MDA ( Figure 3). In village B1–TPN, the incidence did not return to pre-MDA levels.
Prevalence of molecular markers of antimalarial drug resistance
Data on the artemisinin resistance marker Pfkelch13 (PfK13) was available for 107 of 247 detected P. falciparum infections. The prevalence of PfK13 mutations was 77.6% (83/107) ( Table 4). The most frequent mutations were C580Y (37.4%; 31/83) and G538V (24.1%; 20/83). The prevalence of PfK13 mutants was 85.6% (95%CI=75.9–92.6) before, and 56.7% (37.4–74.5) after MDA.
No plasmepsin 2 amplification (the molecular marker of piperaquine resistance) was found in the 69 samples that could be analysed (53 before MDA and 16 after) ( Table 4).
Entomological findings
The main anopheles vectors in the villages belonged to the Minimus Complex (41% of total anopheles), Maculatus Group (11%) and Dirus Complex (1%). Overall, the SI of primary malaria vectors was 2.2/1000 for P. vivax and 0.4/1000 for P. falciparum ( Table 5). During the period before MDA, the HBR was between 200 and 300 bites per human per month in all four villages. After MDA, the HBR was unchanged in A1-KNH and B2-HKT villages, decreased in B1-TPN, and increased in A2-TOT. In spite of these variations in abundance of vectors, SI and EIR decreased after MDA in A2-TOT. Before MDA, P. falciparum SI was 0.9/1000 (95%CI=0.5-1.6) compared to 0.2 (95%CI=0.05-0.4) after (p=0.0001). Likewise, before MDA, P. falciparum EIR was 0.25 infective bites/human/month (95%CI=0.13-0.43) compared to 0.06 (95%CI=0.02-0.15) after (p=0.005).
Table 5. Human biting rate (HBR, bites/person/month), sporozoite index (SI, positive/1000) and entomological inoculation rate (EIR, infective bites/person/month) for primary malaria vectors ( Anopheles minimus, An. maculatu s and An. dirus) by study village according to period before and after mass drug administration (MDA) intervention.
| Before MDA ‡ | After MDA | All follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N | index | 95%CI | n/N | index | 95%CI | n/N | index | 95%CI | ||
| A1-KNH | Human.nights | 200 | 850 | 1050 | ||||||
| HBR | 1615/6.67 * | 242 | [231-254] | 7803/28.33 * | 275 | [269-282] | 9418/35.00 * | 269 | [264-275] | |
| Pf-SI | 3/1574 † | 1.9 | [0.4-5.6] | 1/7307 † | 0.1 | [0.004-0.8] | 4/8881 † | 0.5 | [0.1-1.2] | |
| Pf-EIR | 0.46 | [0.01-1.36] | 0.04 | [0.001-0.21] | 0.12 | [0.03-0.31] | ||||
| A2-TOT | Human.nights | 150 | 900 | 1050 | ||||||
| HBR | 1776/5.00 * | 355 | [339-372] | 22438/30.00 * | 748 | [738-758] | 24214/35.00 * | 692 | [683-701] | |
| Pf-SI | 3/1729 † | 1.7 | [0.4-5.1] | 3/20742 † | 0.1 | [0.03-0.4] | 6/22471 † | 0.3 | [0.1-0.6] | |
| Pf-EIR | 0.61 | [0.13-1.88] | 0.10 | [0.02-0.30] | 0.17 | [0.07-0.39] | ||||
| B1-TPN | Human.nights | 600 | 400 | 1000 | ||||||
| HBR | 4500/20.00 * | 225 | [218-232] | 1419/13.33 * | 106 | [101-112] | 5919/33.33 * | 178 | [173-182] | |
| Pf-SI | 2/4265 † | 0.5 | [0.1-1.7] | 1/1271 † | 0.8 | [0.02-4.4] | 3/5536 † | 0.5 | [0.1-0.6] | |
| Pf-EIR | 0.12 | [0.01-0.38] | 0.08 | [0.002-0.46] | 0.11 | [0.02-0.28] | ||||
| B2-HKT | Human.nights | 670 | 350 | 1020 | ||||||
| HBR | 6771/22.33 * | 302 | [296-310] | 3870/11.67 * | 332 | [321-342] | 10641/34.00 * | 312 | [307-319] | |
| Pf-SI | 5/6632 † | 0.8 | [0.2-1.8] | 0/3809 † | 0 | [0-1] | 5/10441 † | 0.5 | [0.2-1.1] | |
| Pf-EIR | 0.21 | [0.07-0.54] | 0 | [0-0.36] | 0.14 | [0.05-0.36] | ||||
| 4 villages | Human.nights | 1620 | 2500 | 4120 | ||||||
| HBR | 14662/54.00 * | 272 | [267-276] | 35530/83.33 * | 426 | [422-431] | 50192/137.33 * | 365 | [362-369] | |
| Pf-SI | 13/14200 † | 0.9 | [0.5-1.6] | 5/33129 † | 0.2 | [0.05-0.4] | 18/47329 † | 0.4 | [0.2-0.6] | |
| Pf-EIR | 0.25 | [0.13-0.43] | 0.06 | [0.02-0.15] | 0.14 | [0.08-0.22] | ||||
‡ 1 to 2 surveys conducted during MDA intervention are included for each village
* n captured/N person.time exposed (human.months)
† n positive/N tested
Discussion
The rapid emergence of artemisinin resistance followed by ACT partner drug resistance in P. falciparum parasites in the GMS leaves few options to prevent worsening malaria and spread of resistance westwards, as has occurred before. This preliminary evaluation of the feasibility, safety and effectiveness of MDA in accelerating the elimination of artemisinin-resistant P. falciparum in communities with a high prevalence of asymptomatic malaria shows that MDA with DP and a single low dose of primaquine was safe and well accepted 26. Most participants did not report any AE, and those who did reported only mild and transient AE which declined in frequency after each successive round. Access to diagnosis and treatment, an essential component of malaria control, was deployed at baseline and had a substantial impact on malaria, which was augmented by MDA. When coverage was high, MDA provided a marked and long-lasting impact on P. falciparum malaria prevalence and incidence, but these benefits were transient in one village (A2-TOT). Overall, the odds of P. falciparum carriage decreased by >95% immediately after MDA in comparison with baseline and an 80% decrease was maintained for 24 months.
The effect of these interventions on P. vivax malaria was substantially less important, as expected. The MDA did not target hypnozoites, so relapses of vivax were not prevented and P. vivax incidence and prevalence rose rapidly after the period of post treatment prophylaxis. Because of the relatively high prevalence of G6PD deficiency in this population and the unavailability of rapid testing, radical cure with primaquine was not used in P. vivax malaria treatment. Interestingly, in village B1-TPN only, the prevalence of P. vivax infection fell and did not rebound.
Limitations of the study
The main limitation of this study is that, being a pilot investigation, it is underpowered to draw definitive conclusions on the overall benefit of this approach. The comparison between intervention and control is indeed strongly influenced by individual village characteristics: although the four villages had similar malaria prevalence in the pre-survey ( Table 1, Supplementary File 1: Table S1), they were significantly different in the baseline exhaustive survey with a higher prevalence of asymptomatic malaria in the villages receiving MDA first.
A second limitation was that the beneficial effects of MDA could not be evaluated independently from the continuing effects of the malaria post and the LLINs. Their benefits are illustrated in the control villages where there was three-fold reduction in P. falciparum prevalence over a nine month observation period until MDA was conducted. As the two control villages had a relatively low baseline P. falciparum prevalence, the impact in a higher prevalence setting of instituting a malaria post and distributing LLIN without providing MDA cannot be predicted from these observations. Nevertheless providing MDA in these control villages after nine months reduced prevalence by 100% immediately after MDA, showing an additional effect of MDA to enhanced control measures, as shown recently in Zambia 15.
Third, those who refused MDA were less likely to participate in surveys (as in village A2-TOT). This led to underestimation of the true prevalence of malaria, which was close to zero in the general survey population but 5% in the group of survey participants who did not take MDA. Individual participation in MDA was therefore included in the statistical models of impact to take this into account.
Interpretation
The sustained reduction in P. falciparum prevalence in village A1-KNH, where MDA coverage was high, suggests a reduction of malaria transmission. This is supported by finding reductions of similar magnitude in P. falciparum SI and EIR, while HBR remained stable. By contrast, in A2–TOT, P. falciparum prevalence and incidence increased as vector abundance rose during the rainy and cold seasons of 2014, 14 to 20 months after MDA ( Table 5). In this community broken by years of conflict, participation with MDA was low and the reservoir of sub-microscopic malaria parasite carriage was reduced only partially. The non-MDA participants may have contributed as a reservoir for P. falciparum, as indicated by a continuously high prevalence of asymptomatic P. falciparum carriage (5%) in this group. In addition, the MP was not functioning properly during the first year, with frequent interruptions of activity and the lowest rate of consultations among the four villages ( Table 1 and Supplementary File 1: Figure S6). These observations illustrate the ‘real-life’ challenges that would confront a programme aiming for rapid elimination, and confirm the well described necessity of ensuring high coverage in any MDA campaign. They also underline the essential importance of providing diagnosis and effective treatment, both immediately after MDA and in an outbreak context as experienced in A2-TOT between M14 and 20. The low prevalence observed at M24 (2%) is likely the result of the adequate treatment of clinical episodes by the MP during the period M12 to M24, contrasting with the dysfunctions observed between M0 and M12.
Importantly this intervention does not appear to have modified the resistance profile of P. falciparum in the area. The prevalence of mutations in the artemisinin resistance marker PfK13 did not increase and there was no indication that piperaquine resistance was selected, although numbers were small. No markers of piperaquine resistance were found either in >200 samples from clinics covering the same area and period, where DP was used for routine treatment of clinical cases (Miotto O, unpublished data).
Conclusion
This pilot study shows that MDA with three rounds of a treatment course of DP and a single low dose of primaquine at one month interval is well tolerated, safe and feasible once the community has been successfully engaged in participation to activities and ownership of the program. It provides evidence that in a remote malaria endemic area, following implementation and support of a functioning MP, and distribution of LLIN, MDA eliminates the sub-microscopic parasites in those who take it. If a sufficiently high proportion of the community participates and if early diagnosis and treatment of clinical episodes remains available, this has a long lasting effect on falciparum malaria reservoir. By contrast, P. vivax returns rapidly, presumably because of relapse, which occurs frequently in the GMS. These preliminary findings need confirmation and exploration in further studies. Scaling up to provide MDA to larger populations will introduce new challenges of feasibility, but may improve overall effects as human migration from untreated areas becomes less likely. From a community perspective, elimination of malaria means elimination of all malaria, and to achieve that rapidly will require targeting the vivax hypnozoite reservoir.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Landier J et al.
The data is available upon request to the Mahidol Oxford Tropical Medicine Research Unit Data Access Committee ( Supplementary File 2; http://www.tropmedres.ac/data-sharing) and following the Mahidol Oxford Tropical Medicine Research Unit data access policy ( http://www.tropmedres.ac/_asset/file/data-sharing-policy-v1-0.pdf).
Acknowledgements
The authors would like to thank the population from the communities of the four villages, the Karen Department of Health and Welfare and all Karen community-based organisations providing health services to these communities. The authors also thank the investigators of the other study sites for fruitful discussions and collaborations. The Shoklo Malaria Research Unit is part of the Mahidol Oxford University Research Unit supported by the Wellcome Trust of Great Britain.
Funding Statement
This work was supported by the Wellcome Trust [101148]; and the Bill and Melinda Gates Foundation [GH OPP 1081420].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
Supplementary material
Supplementary File 1: Supplementary results.
Supplementary File 2: Request form for Mahidol Oxford Tropical Medicine Research Unit Data Access Committee.
References
- 1. White NJ, Pukrittayakamee S, Hien TT, et al. : Malaria. Lancet. 2014;383(9918):723–35. 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 2. Ashley EA, Dhorda M, Fairhurst RM, et al. : Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411–23. 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phyo AP, Ashley EA, Anderson TJC, et al. : Declining Efficacy of Artemisinin Combination Therapy Against P. Falciparum Malaria on the Thai-Myanmar Border (2003–2013): The Role of Parasite Genetic Factors. Clin Infect Dis. 2016;63(6):784–791. 10.1093/cid/ciw388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amaratunga C, Lim P, Suon S, et al. : Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: A multisite prospective cohort study. Lancet Infect Dis. 2016;16(3):357–65. 10.1016/S1473-3099(15)00487-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes KI, Chanda P, Ab Barnabas G: Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia. Malar J. 2009;8(suppl 1):S8. 10.1186/1475-2875-8-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrara VI, Sirilak S, Thonglairuam J, et al. : Deployment of early diagnosis and mefloquine-artesunate treatment of falciparum malaria in Thailand: the Tak Malaria Initiative. PLoS Med. 2006;3(6):e183. 10.1371/journal.pmed.0030183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landier J, Parker DM, Thu AM, et al. : The role of early detection and treatment in malaria elimination. Malar J.BioMed Central;2016;15:363. 10.1186/s12936-016-1399-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imwong M, Stepniewska K, Tripura R, et al. : Numerical Distributions of Parasite Densities During Asymptomatic Malaria. J Infect Dis. 2016;213(8):1322–9. 10.1093/infdis/jiv596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imwong M, Nguyen TN, Tripura R, et al. : The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar J.BioMed Central;2015;14:381. 10.1186/s12936-015-0906-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Seidlein L, Dondorp A: Fighting fire with fire: mass antimalarial drug administrations in an era of antimalarial resistance. Expert Rev Anti Infect Ther. 2015;13(6):715–30. 10.1586/14787210.2015.1031744 [DOI] [PubMed] [Google Scholar]
- 11. Lwin KM, Imwong M, Suangkanarat P, et al. : Elimination of Plasmodium falciparum in an area of multi-drug resistance. Malar J.BioMed Central;2015;14:319. 10.1186/s12936-015-0838-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook J, Xu W, Msellem M, et al. : Mass screening and treatment on the basis of results of a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis. 2015;211(9):1476–83. 10.1093/infdis/jiu655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newby G, Hwang J, Koita K, et al. : Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg. 2015;93(1):125–134. 10.4269/ajtmh.14-0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dicko A, Brown JM, Diawara H, et al. : Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis. 2016;16(6):674–84. 10.1016/S1473-3099(15)00479-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisele TP, Bennett A, Silumbe K, et al. : Short-term Impact of Mass Drug Administration With Dihydroartemisinin Plus Piperaquine on Malaria in Southern Province Zambia: A Cluster-Randomized Controlled Trial. J Infect Dis. 2016;214(12):1831–1839. 10.1093/infdis/jiw416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phyo AP, Nkhoma S, Stepniewska K, et al. : Emergence of artemisinin-resistant malaria on the western border of Thailand: A longitudinal study. Lancet.Elsevier Ltd;2012;379(9830):1960–1966. 10.1016/S0140-6736(12)60484-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carrara VI, Lwin KM, Phyo AP, et al. : Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai-Myanmar border, 1999–2011: an observational study. PLoS Med. 2013;10(3):e1001398. 10.1371/journal.pmed.1001398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheah PY, Lwin KM, Phaiphun L, et al. : Community engagement on the Thai-Burmese border: rationale, experience and lessons learnt. Int Health. 2010;2(2):123–9. 10.1016/j.inhe.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kajeechiwa L, Thwin MM, Nosten S, et al. : Community engagement for the rapid elimination of malaria: the case of Kayin State, Myanmar [version 1; referees: 1 approved]. Wellcome Open Res. 2017;2:59 10.12688/wellcomeopenres.12051.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kajeechiwa L, Thwin MM, Shee PW, et al. : The acceptability of mass administrations of anti-malarial drugs as part of targeted malaria elimination in villages along the Thai-Myanmar border. Malar J. 2016;15(1):494. 10.1186/s12936-016-1528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imwong M, Hanchana S, Malleret B, et al. : High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol. 2014;52(9):3303–9. 10.1128/JCM.01057-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amato R, Lim P, Miotto O, et al. : Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17(2):164–173. 10.1016/S1473-3099(16)30409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwansomboon N, Chaumeau V, Kittiphanakun P, et al. : Vector bionomics and malaria transmission along the Thailand-Myanmar border: a baseline entomological survey. J Vector Ecol. 2017;42(1):84–93. 10.1111/jvec.12242 [DOI] [PubMed] [Google Scholar]
- 24. Ya-umphan P, Cerqueria D, Parker DM, et al. : Use of an Anopheles Salivary Biomarker to Assess Malaria Transmission Risk Along the Thailand-Myanmar Border. J Infect Dis. 2017;215(3):396–404. 10.1093/infdis/jiw543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaumeau V, Andolina C, Fustec B, et al. : Comparison of the Performances of Five Primer Sets for the Detection and Quantification of Plasmodium in Anopheline Vectors by Real-Time PCR. PLoS One. 2016;11(7):e0159160. 10.1371/journal.pone.0159160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bancone G, Chowwiwat N, Somsakchaicharoen R, et al. : Single Low Dose Primaquine (0.25 mg/kg) Does Not Cause Clinically Significant Haemolysis in G6PD Deficient Subjects. PLoS One. 2016;11(3):e0151898. 10.1371/journal.pone.0151898 [DOI] [PMC free article] [PubMed] [Google Scholar]