Abstract
Pilocarpine is a prototypical drug used to treat glaucoma and dry mouth and is classified as either a full or partial muscarinic agonist. Here, we report several unexpected results pertaining to its interaction with muscarinic M3 receptor (M3R). We found that pilocarpine was 1000 times less potent in stimulating mouse-eye pupil constriction than muscarinic agonists oxotremorin-M (Oxo-M) or carbachol (CCh), although all three ligands have similar Kd values for M3R. In contrast to CCh or Oxo-M, pilocarpine does not induce Ca2+ mobilization via endogenous M3R in human embryonic kidney cell line 293T (HEK293T) or mouse insulinoma (MIN6) cells. Pilocarpine also fails to stimulate insulin secretion and, instead, antagonizes the insulinotropic effect of Oxo-M and CCh-induced Ca2+ upregulation; however, in HEK293T or Chinese hamster ovary-K1 cells overexpressing M3R, pilocarpine induces Ca2+ transients like those recorded with another cognate G protein–coupled muscarinic receptor, M1R. Stimulation of cells overexpressing M1R or M3R with CCh resulted in a similar reduction in phosphatidylinositol 4,5-bisphosphate (PIP2). In contrast to CCh, pilocarpine stimulated PIP2 hydrolysis only in cells overexpressing M1R but not M3R. Moreover, pilocarpine blocked CCh-stimulated PIP2 hydrolysis in M3R-overexpressing cells, thus, it acted as an antagonist. Pilocarpine activates extracellular regulated kinase 1/2 in MIN6 cells. The stimulatory effect on extracellular regulated kinase (ERK1/2) was blocked by the Src family kinase inhibitor PP2, indicating that the action of pilocarpine on endogenous M3R is biased toward β-arrestin. Taken together, our findings show that pilocarpine can act as either an agonist or antagonist of M3R, depending on the cell type, expression level, and signaling pathway downstream of this receptor.
Introduction
Neurotransmitter acetylcholine plays a fundamental role in the central and peripheral nervous systems. Receptors of acetylcholine and proteins involved in its synthesis, secretion, and degradation are established targets for pharmacologic intervention (reviews: Wess, 2004; Kruse et al., 2014b; Soukup et al., 2017). Acetylcholine receptors that belong to the muscarinic class are G protein–coupled receptors (GPCRs), which are products of five genes (Chrm1–5). Muscarinic M1, M3, and M5 receptors are coupled to cognate G protein (Gq) and are known to mobilize free cytosolic Ca2+, whereas M2 and M4 receptors are coupled to Gi and downregulate cAMP and regulate ion channels (Lechleiter et al., 1989; Burford et al., 1995; Haga, 2013).
M3 receptor (M3R) is interesting in several ways. It is highly expressed in certain areas of the nervous system and many endocrine and exocrine glands, playing a major role in hormone secretion (reviews: Gautam et al., 2008; Kruse et al., 2014a). For example, it is responsible for cholinergic stimulation of insulin release (Kong and Tobin, 2011; Ruiz de Azua et al., 2012). Other notable sites of M3R expression are the vascular endothelial cells and smooth muscle, such as the circular sphincter that closes the eye pupil (Bymaster et al., 2003). At the molecular level, M3R differs from other muscarinic receptors in that it has an unusually large (∼24 kDa) third intracellular loop, which interacts with many unique binding partners (Wu et al., 2000; Simon et al., 2006; Sandiford et al., 2010; Kan et al., 2014). Through stimulation of its cognate G protein, Gq, M3R activates the effector enzyme phospholipase C β (PLCβ), which hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2), leading to generation of second messengers inositol 1,4,5-trisphosphate (IP3), diacylglycerol, and Ca2+. Like many other GPCRs, M3R can also activate protein kinases via β-arrestin and participates in unique interactions with several other proteins (Budd et al., 2000; Wu et al., 2000; Simon et al., 2006; Kan et al., 2014). Recently determined crystal structures of M3R and other muscarinic receptors characterized molecular architecture of these GPCRs, providing valuable insights into organization of their orthosteric binding sites (Kruse et al., 2012; Thal et al., 2016).
The canonical paradigm in pharmacology postulates that an orthosteric ligand of a given receptor can be classified as a full or partial agonist, antagonist, or inverse agonist and either activates or inhibits different signaling pathways mediated by the same receptor and to the same degree. In the past decade, this model has been rapidly evolving to accommodate the effects of many drugs that could not be described solely by these terms. As a single receptor couples to different signal transduction pathways, the degree to which each pathway is activated depends on the nature of the ligand bound to the receptor. Such phenomena are now referred to as functional selectivity, biased signaling, or biased agonism. One of the first observations was an early finding that an antagonist of cholecystokinin receptor D-Tyr-Gly-[(Nle28,31,D-Trp30)cholecystokinin-26-32]-phenethyl ester induces internalization of the receptor (i.e., stimulates the β-arrestin pathway) without activation of a G protein (Roettger et al., 1997). Another notable example is the stimulation of extracellular regulated kinase (ERK) activity via β-adrenergic receptors by such clinically important drugs as β blockers propranolol and carvedilol (Azzi et al., 2003; Wisler et al., 2007). Since those early observations, biased signaling was reported for many receptors and ligands, thus becoming a general concept (Violin and Lefkowitz, 2007; Luttrell and Gesty-Palmer, 2010).
Pilocarpine is an alkaloid that has been used to treat glaucoma since 1875. Historical studies of its agonistic effect on salivary secretion and antagonism toward atropine led to development of the basic concept of a drug receptor (receptive substance) in 1905 (Maehle, 2004). Since that time, numerous physiologic and pharmacologic studies established that pilocarpine selectively stimulates muscarinic receptors and has no nicotinic receptor action. Pilocarpine can activate all five muscarinic receptor subtypes, but most of the therapeutic effects of pilocarpine observed in humans are mediated by M3R. Whereas a substantial number of publications describe the effects of pilocarpine on M2R (e.g., (Gregory et al., 2010), surprisingly few studies have investigated its effects on M3R. Pilocarpine is generally classified as a full or partial agonist (Gurwitz et al., 1994; Sykes et al., 2009; Karpinsky-Semper et al., 2014). In this article, we report previously unappreciated aspects of pilocarpine pharmacology as it relates to M3R. Whereas pilocarpine is a full agonist for M1 muscarinic receptor (M1R), we show that it can act as an antagonist for M3R under certain conditions. We also provide evidence for strong signaling bias of pilocarpine toward arrestin-Src pathway downstream of M3R.
Materials and Methods
Reagents.
Pilocarpine ((3S,4R)-3-ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one), carbachol (2-[(aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride), oxotremorine-M (oxotremorine methiodide, N,N,N,-trimethyl-4-(2-oxo-1-pyrrolidinyl)-2-butyn-1-ammonium iodide), oxotremorine (1-(4-pyrrolidin-1-ylbut-2-yn-1-yl)pyrrolidin-2-one), cevimeline ((2R,2′R)-2'-methylspiro[4-azabicyclo[2.2.2]octane-2,5′-[1,3]oxathiolane]), and acetylcholine (2-acetoxy-N,N,N-trimethylethanaminium) were purchased from Sigma-Aldrich (St. Louis, MO). PP2 (4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-day]pyrimidine) was from Abcam (Cambridge, UK), bisindolylmaleimide I-HCl from ChemCruz (Santa Cruz Biotechnology, Dallas, TX).
Hanks’ balanced salt solution (HBSS) with or without Ca2+ and fura-2, AM were acquired from Life Technologies (Carlsbad, CA). The cDNA encoding human M1R and M3R in pcDNA3.1 were purchased from cDNA.org.
Mouse-Eye Pupil Constriction.
All animal procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and protocols approved by the University of Miami Animal Use and Care Committee. Age-matched (12–18 weeks old) C57Bl6/6J males were used for organ collection. The experiments were conducted at room temperature (20°C). Enucleated eyes were rinsed in HBSS and placed into a well of a custom-designed Styrofoam rack filled with 100 μl of HBSS. Before stimulating constriction, a picture of each eye was taken at the same magnification to record the open pupil diameter at time zero. Then 100 μl of either HBSS or a stimulant in HBSS (at 2× final concentration) was added, and images were taken at the indicated time points. After the images were displayed on a computer monitor, pupil diameter at each time point was compared with the value at time zero (100%). Typically, the pupil of an enucleated eye remained wide open in the absence of a stimulus (pilocarpine or another agonist) for up to 90 minutes.
In Situ RNA Hybridization.
Localization of Chrm3 messenger RNA was done using a custom fluorescence RNAscope probe (Advanced Cell Diagnostics, Newark, CA). Experiments were performed with minor modifications of the manufacturer instructions, as descried earlier (Pronin et al., 2014), using paraffin-embedded slices of the mouse eyes.
Free Intracellular Ca2+ Assays.
HEK293T, Chinese hamster ovary (CHO), or MIN6 cells were grown on poly-l-lysine-coated 12-mm glass coverslips, washed with the culture medium, and then incubated at 37°C in the culture medium containing 2 µM fura-2, AM for 60 minutes. After loading fura-2, AM, the cells were kept at ambient temperature for no longer than 1.5 hours before imaging. Coverslips were secured in a flow chamber and mounted on the stage of a Nikon TE2000 (Nikon, Tokyo, Japan) inverted fluorescence microscope. The cells were continuously superfused with HBSS by gravity flow. To stimulate the cells, the flow was switched to agonist-containing HBSS for a time required by a specified experiment and then back to the agonist-free buffer. Images were collected in real time every 5 seconds using a 20× UV objective lens and recorded using MetaFluor software (Molecular Devices, Sunnyvale, CA). The excitation wavelengths were 340 (Ca2+-bound) and 380 nm (Ca2+-free), with the emission set to 510 nm. The 340:380 ratio is representative of intracellular free [Ca2+]. Individual cells or clusters of 10–20 cells were selected as regions of interest for signal quantification. Traces shown in the figures are averages of two to four independent experiments with three replicate coverslips per experiment.
Simultaneous Calcium Imaging Recordings from Cells Transfected with Two Different Receptors.
To simultaneously record Ca2+ responses from cells transfected with different sets of genes, CHO or HEK293T cells were grown in 12-well plates to 70% confluency. In one well, the cells were cotransfected with plasmids containing enhanced yellow fluorescent protein (eYFP) and M1R using FuGENE 6 transfection reagent (Promega Corporation, Madison, WI). In a separate well, the cells were cotransfected with plasmids containing mCherry and M3R. The next day, cells in both wells were trypsinized, mixed together, and plated on poly-l-lysine–coated 12-mm glass coverslips in 24-well plates. The day after, Ca2+ responses from cells were recorded as described already. In addition to fura-2 fluorescence, we recorded fluorescent signals from eYFP (excitation 514 nm, emission 527 nm) and mCherry (excitation 587 nm, emission 610 nm). Individual green and red fluorescent cells were selected as regions of interest for Ca2+ signal quantification. We also selected nonfluorescent cells as a control representing untransfected cells. Ca2+ responses from 30 to 40 cells of the same kind were quantified and averaged.
Live PIP2 Imaging.
We used a protein sensor that increases its fluorescence upon binding of PIP2 (Montana Molecular, Bozeman, MT). It is a fusion between a dimerization-dependent red fluorescent protein and the PH domain from PLCδ. CHO or HEK293T cells were cotransfected with a plasmid containing PIP2 sensor and a plasmid containing either M3R or M1R using FuGENE 6 transfection reagent. The next day, cells were trypsinized and plated on poly-l-lysine–coated 12-mm glass coverslips. The day after, coverslips were secured in a flow chamber and mounted on the stage of a Nikon TE2000 inverted fluorescence microscope. The cells were continuously superfused by gravity flow with HBSS. To stimulate the cells, the flow was switched to agonist-containing HBSS for a specified time and then changed back to the agonist-free buffer. Images were collected in real time every 5 seconds using a 20× objective lens and recorded using MetaFluor software (Molecular Devices). The excitation wavelength was 550 nm, with the emission set to 570 nm. Individual cells were selected as regions of interest for signal quantification. Traces shown here are averages of 10–20 cells from two to four independent experiments with three replicate coverslips per experiment. The peak response below the basal value was used for signal quantification.
MIN6 Culture, Stimulation, and Insulin Enzyme-Linked Immunosorbent Assay.
Cells were cultured in Dulbecco’s minimal essential medium (DMEM) (Invitrogen, Carlsbad, CA) with 25 mM glucose and 4 mM l-glutamine supplemented with 10% fetal bovine serum, 100 μg/ml penicillin per 100 μg/ml streptomycin, 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, and 10 mM Hepes, pH 7.2. For a typical test, cells were suspended in DMEM, seeded at 3 × 105/well in 24-well plates, and grown to 80% confluency. Before application of stimulants, cells were preincubated with serum- and glucose-free DMEM for 1 hour and then washed twice with Krebs-Ringer bicarbonate buffer containing 0.1% bovine serum albumin, after which various agents required by the experiment were added in Krebs-Ringer bicarbonate buffer. The supernatant from the stimulated cells was collected after 30 minutes at 37°C and stored frozen at −80°C until measurement of insulin. Samples were analyzed using mouse insulin “sandwich” enzyme-linked immunosorbent assay kit (Mercodia, Uppsala, Sweden) according to the manufacturer’s protocol and using sample dilutions to ensure that the signal was within the linear range of sensitivity.
ERK1/2 Phosphorylation Assay.
MIN6 cells were grown in 12-well plates to 40% confluency. Before stimulation with muscarinic agonists, the cells were serum-starved for 4 hours. If a protein kinase inhibitor (PP2 and bisindolylmaleimide I) was used in an experiment, it was included in the serum starvation medium. The cells were stimulated for 5 minutes, the culture medium was quickly aspirated, and the cells were harvested by the addition of 160 μl of 1× SDS-PAGE sample buffer. Cell lysates were briefly sonicated to destroy chromosomal DNA and resolved on SDS-polyacrylamide gels, followed by immunoblotting using antibodies against P-ERK1/2 (T202/Y204) (rabbit polyclonal; Cell Signaling Technology, Danvers, MA) and actin (mouse monoclonal; Merck Millipore Corporation, Billerica, MA). The secondary antibodies labeled with infrared IRDye 800CW or 680RD were from LI-COR, Biosciences, Inc., Lincoln, NE). The immune complexes were visualized using Odyssey (LI-COR) infrared fluorescence detection system. For quantitative analysis, the signal in the band of interest (i.e., P-ERK) was normalized to the signal for actin in the same lane on the immunoblot.
Statistics.
Data are reported as means ± S.D. GraphPad Prism software (version 6.07; GraphPad Software, La Jolla, CA) was used for statistical analysis. The EC50 values were determined using nonlinear regression with a four-parameter logistic equation. Groups of data were compared using ANOVA or two-tailed unpaired Student’s t tests, with values of P < 0.05 considered statistically significant.
Results
Effect of Pilocarpine on Pupil Constriction.
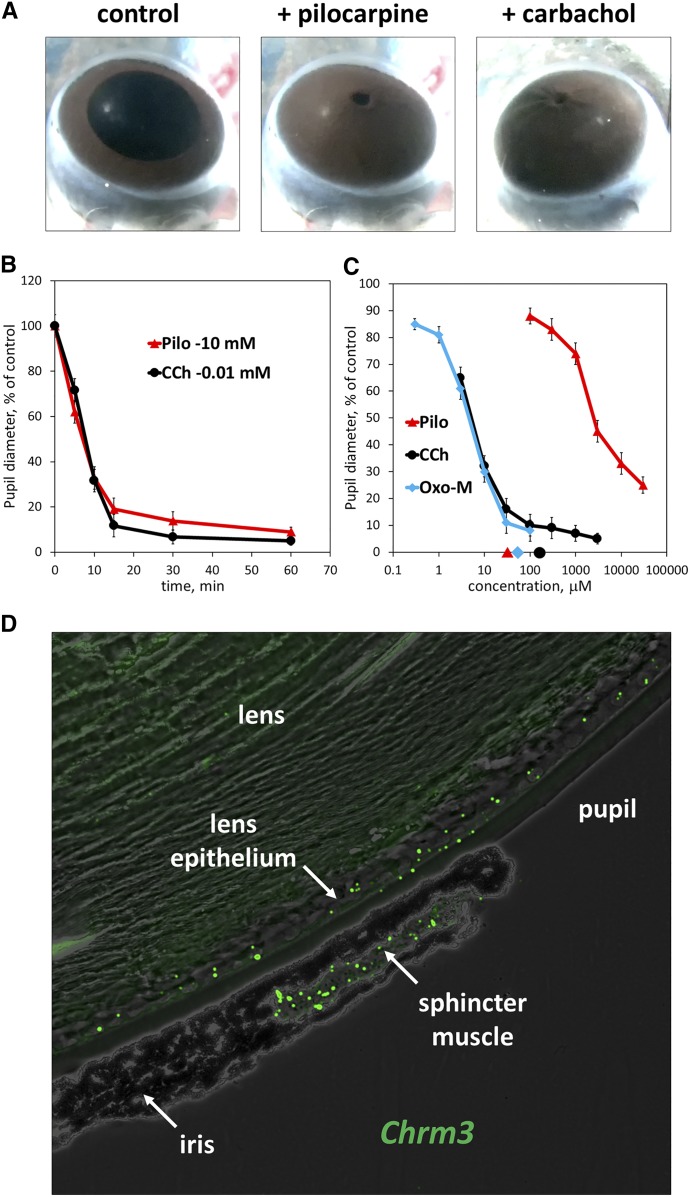
As expected, application of pilocarpine caused constriction of the mouse-eye pupil (miosis), and its full effect was comparable to that of another cholinergic agonist, carbachol CCh (Fig. 1, A and B). However, the estimated EC50 of pilocarpine is about 1000 times greater than the EC50 determined under the same conditions for other cholinergic agonists, CCh or Oxo-M. Furthermore, the EC50 of pilocarpine is three orders of magnitude greater than its reported Kd for M3R (30 μM) (Sykes et al., 2009) (Fig. 1C). M3R was previously shown to be the only acetylcholine receptor mediating constriction of the sphincter muscle by demonstrating a lack of miosis in the Chrm3 knockout mice (Bymaster et al., 2003). Here we confirm by RNA in situ hybridization that the Chrm3 gene is expressed in the sphincter muscle (Fig. 1D). To explain the relatively low potency of pilocarpine compared with other agonists, we hypothesized that it activates different signal transduction pathways and set out to investigate its ability to stimulate canonical intracellular signals in cell models.
Fig. 1.
Pilocarpine is a full agonist in the pupil-constriction assay. Mouse eyes were treated ex vivo with the indicated drugs, and pupil diameter was analyzed as described in Materials and Methods. (A) Photographs of the eyes after 1-hour incubation in 10 mM pilocarpine or 0.01 mM CCh. (B) Time-course of pupil constriction in the presence of 10 mM Pilo or 0.01 mM CCh. (C) Eyes were treated with indicated concentrations of pilocarpine, Oxo-M and CCh for 10 minute. Data show average ± S.D. from three independent experiments. Symbols on the x-axis denote estimated Kd values for pilocarpine (30 μM), Oxo-M (50 μM), and CCh (150 μM) (from Sykes et al., 2009). (D) In situ RNA hybridization of mouse-eye section was performed using RNAscope approach (see Materials and Methods); shown is a representative image. Distinct green fluorescent dots correspond to individual mRNA molecules.
Pilocarpine Antagonizes CCh-Induced Ca2+ Mobilization in HEK293T Cells.
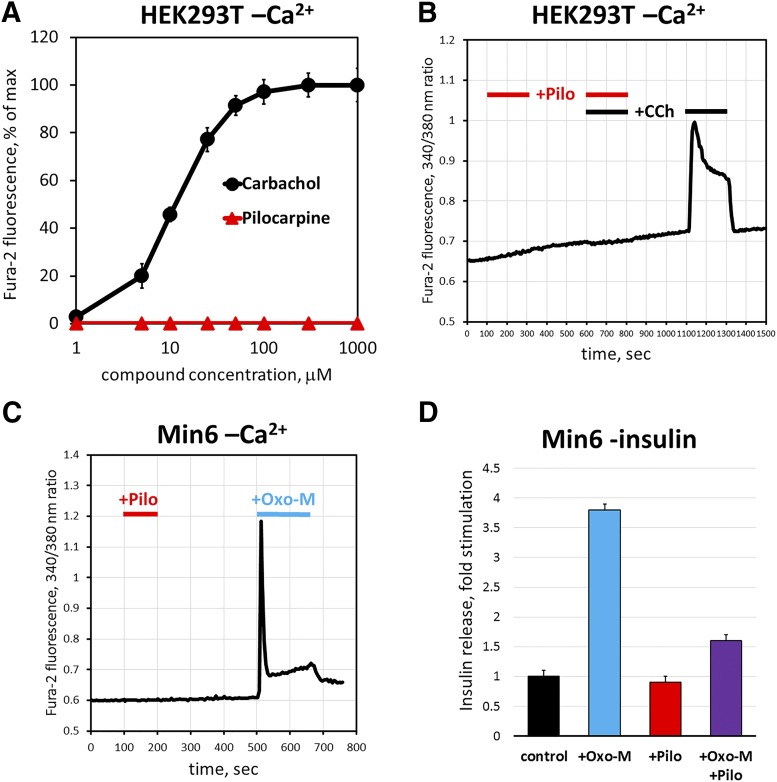
To test signaling mechanisms downstream of M3R, we first turned to HEK293T cells, a system that is more amenable to analysis of second messengers than the sphincter pupillae muscle. Previous studies showed that HEK293T cells express only M3 (not M1) receptor, and cholinergic agonists cause upregulation of Ca2+ via the canonical Gq-mediated pathway (Luo et al., 2008; Atwood et al., 2011). Accordingly, we detected robust increases in free Ca2+ concentration upon application of CCh, with an EC50 of 11 ± 2 μM (Fig. 2, A and B); however, under the same conditions, pilocarpine fails to elicit any Ca2+ response at concentrations up to 1 mM. To determine whether pilocarpine actually interacted with the receptor, we applied it together with CCh and found that pilocarpine completely blocked the CCh-induced signal (Fig. 2B). Thus, we came to an unexpected conclusion that pilocarpine functions as an M3R antagonist by competitively inhibiting CCh-stimulated rise in cytoplasmic free Ca2+.
Fig. 2.
Pilocarpine acts as a cholinergic antagonist for endogenous M3R in HEK293T and MIN6 cells. Cells were grown on glass coverslips, loaded with fura-2, AM, and imaged in a flow cell mounted under a fluorescence microscope. Cells were challenged with CCh and pilocarpine solutions, and fura-2 fluorescence was monitored in real time as described under Materials and Methods. (A) Dose-response curve for intracellular free Ca2+ in HEK293T cells in the presence of indicated concentrations of CCh or pilocarpine. The data points show mean peak response ±S.D., n = 3. (B) Pilocarpine (Pilo, 300 μM) was coapplied with 25 μM CCh, which resulted in blockade of calcium increase. After a 5-minute wash with HBSS, the cells were challenged with 25 μM CCh. The trace shows an average of three experiments recording fura-2 fluorescence from 40 to 60 cells. (C) MIN6 cells were challenged first with 300 μM pilocarpine, then with 100 μM Oxo-M, and free Ca2+ was recorded as in (B). (D) Insulin release from cultured MIN6 cells was determined by enzyme-linked immunosorbent assay as described under Materials and Methods; cells were challenged with 100 μM Oxo-M, 500 μM pilocarpine, or their mixture. Data are shown as the amount of insulin released to the medium compared with unstimulated cells (control; 1 μg/ml). Data show mean ± S.D., n = 3, P < 0.01.
Next, to investigate whether the apparent pilocarpine antagonism can be observed in an alternative system naturally expressing M3R, we examined mouse insulinoma MIN6 cells. The MIN6 cell line is commonly used as a model for studying pancreatic β-cell biology and is known to secrete insulin in response to cholinergic stimulation (Weng et al., 1993; Selway et al., 2012). As expected, application of Oxo-M resulted in a robust increase in free Ca2+ (Fig. 2C) and the amount of insulin released to the medium (Fig. 2D). In contrast, pilocarpine failed to induce Ca2+ response or insulin secretion. Moreover, pilocarpine inhibited the insulinotropic effect of Oxo-M (Fig. 2D). Thus, pilocarpine acts as an M3R antagonist in MIN6 cells, similarly to its effect in HEK293T cells.
The antagonistic effect of pilocarpine toward M3R (Fig. 2) is surprising because this drug has been studied for decades both in vivo and in vitro and has been classified as an agonist. In vivo, the agonistic action of pilocarpine could be, in principle, explained by its effect on a different muscarinic receptor(s) along with M3R; however, numerous experiments with cells that do not have endogenous acetylcholine receptors show that, after overexpression of M3R, pilocarpine acts as an agonist (Sykes et al., 2009; Karpinsky-Semper et al., 2014). We therefore re-examined the behavior of M3R transfected into CHO-K1 cells, which do not express any muscarinic receptors. In these experiments, we compared M3R to M1R, another Gq-coupled muscarinic receptor known to be activated by the same agonists.
Pilocarpine Stimulates Ca2+ Mobilization via Overexpressed M3R.
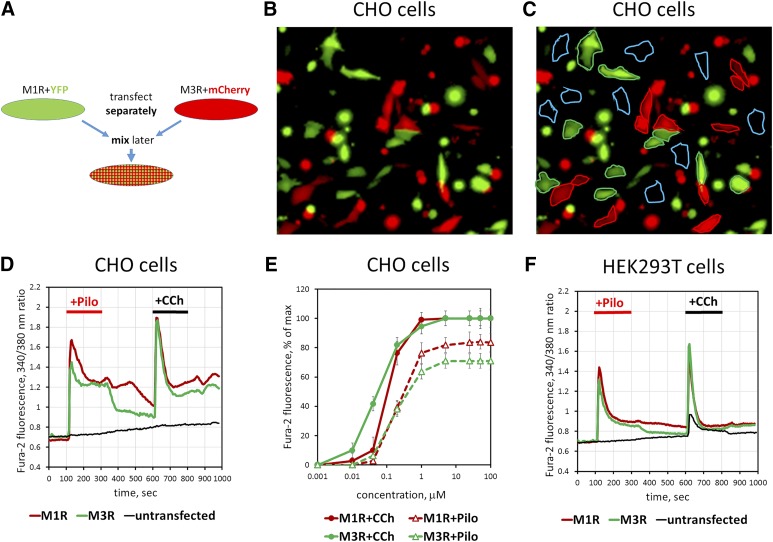
In CHO-K1 cells transiently transfected with M3R, pilocarpine stimulated free Ca2+ increases that appeared to be as robust as Ca2+ transients recorded with transfected M1R (Fig. 3). To quantitatively compare signaling elicited by the two receptors we sought to minimize the assay variability between the M1R- and M3R-transfected cell preparations. For this purpose, we developed a system that allowed us to monitor M1R- and M3R-expressing cells simultaneously on the same glass slide. We cotransfected one batch of CHO-K1 cells with M1R together with a plasmid harboring fluorescent marker eYFP and another batch with M3R together with a different fluorescent marker, mCherry. After transfection, these two cell pools were lifted from the plates, mixed, and replated onto coverslips, so that the “green” and “red” cells could be identified and analyzed within the same visual field (Fig. 3, A–C). Our data show that CCh- or pilocarpine-induced Ca2+ responses of M3R- and M1R-expressing cells were indistinguishable. The EC50 of CCh was only ∼3-fold lower than EC50 of pilocarpine for both M1R and M3R, and no significant difference was noted in the potency of pilocarpine for M1R versus M3R (Fig. 3, D and E). As expected, untransfected CHO-K1 cells, which had neither red nor green fluorescence and were always present in the preparations, showed no response to cholinergic stimulation. We performed similar transfection experiments on HEK293T cells and found that pilocarpine caused Ca2+ signaling via overexpressed M3R (Fig. 3F). The untransfected HEK293T cells present in the same experiment responded only to CCh and not to pilocarpine.
Fig. 3.
Pilocarpine stimulates free Ca2+ mobilization in cells overexpressing M3R. CHO-K1 or HEK293T cells were transiently transfected with M3R, M1R, and fluorescent proteins. (A) Schematic of the experiment. Cells transfected to express M1R with eYFP or M3R with mCherry (red and green) are mixed and plated on coverslips. They are subsequently loaded with fura-2, AM and analyzed for sensitivity to cholinergic stimulation. (B and C) A representative image (Original magnification, 200×) of the mixed cell population. Red cells are cotransfected with plasmids encoding M3R and mCherry, and green cells express eYFP together with M1R. (C) Illustration of selection of the regions of interest to collect data on Ca2+. Blue traces denote cells that do not express fluorescent proteins and are visualized by furae staining alone. See Materials and Methods for additional details. (D) Free Ca2+ responses to 10 μM pilocarpine and 10 μM CCh. Traces represent the average of responses recorded from 20 to 30 individual cells per region of interest. Green trace corresponds to the data from M1R-expressing cells, red shows M3R, and black shows untransfected cells. Data shown are representative of at least three such experiments done with independent transfections. (E) Amplitude of Ca2+ responses was measured at the indicated concentrations of CCh or pilocarpine. (F) Experiment on HEK293T cells performed essentially as that done on CHO-K1 cells (A–D). Note that there is a response of untransfected cells to CCh but not to pilocarpine. Representative of two independent transfection experiments.
These results show that—consistent with previous studies (Sykes et al., 2009; Karpinsky-Semper et al., 2014)—pilocarpine acts as a full agonist for overexpressed M3R; however, for endogenous M3R in HEK293T or MIN6 cells, it acts as an antagonist.
Pilocarpine Can Stimulate M3R-Mediated Ca2+ Mobilization but not PIP2 Hydrolysis.
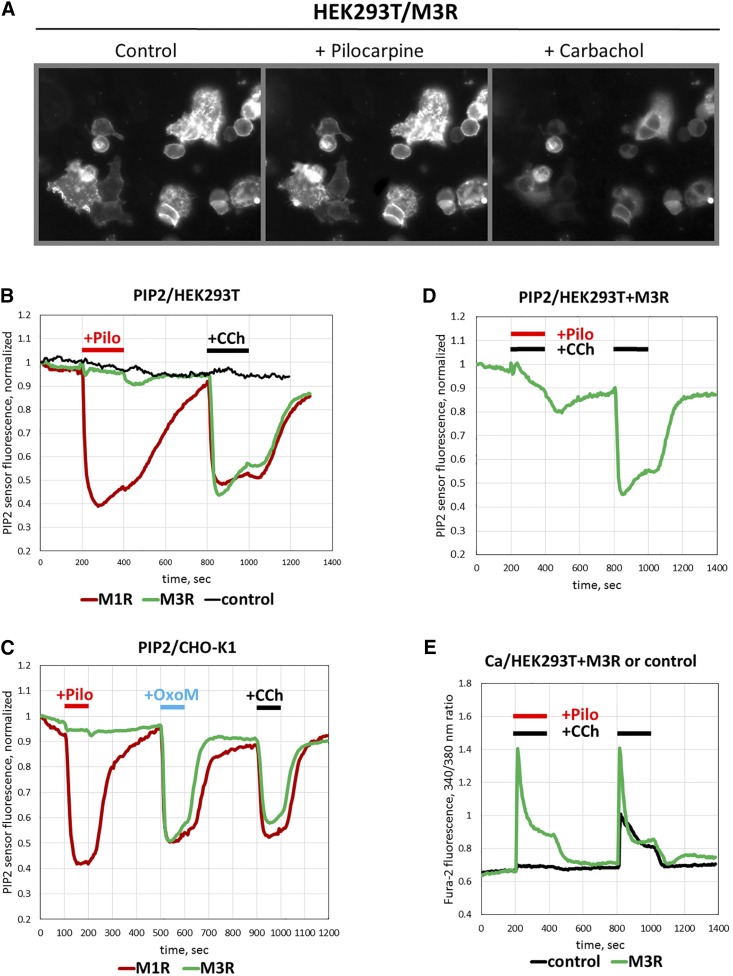
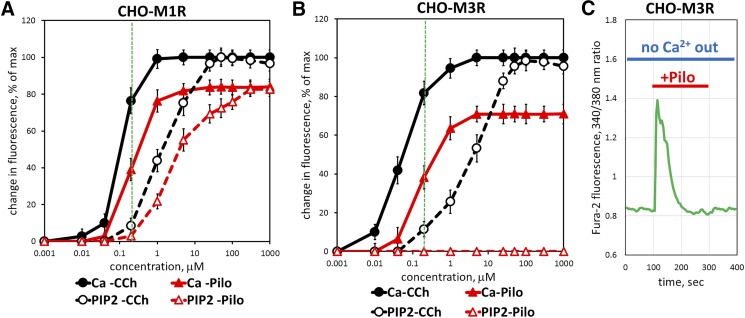
The variability in the effect of pilocarpine on free cytoplasmic Ca2+ in different experimental systems can be explained by activation of distinct pathways. To study signaling downstream of M3R but upstream of Ca2+ release, we measured hydrolysis of the signaling lipid phosphatidylinositol 4,5-bisphosphate (PIP2). For this purpose, we used a novel fluorescent biosensor that consists of mutated dimerization-dependent red fluorescent protein fused to the PH domain of PLCδ. Upon binding to PIP2, this molecule increases fluorescence intensity (Tewson et al., 2012, 2013, 2016). Application of CCh on HEK293T cells cotransfected with the PIP2 sensor and M3R resulted in a notable drop in red fluorescent fluorescence, evidently because of the increase in PIP2 hydrolysis by the M3R-stimulated PLC. In contrast to CCh, pilocarpine did not cause any detectable fluorescence change (Fig. 4, A and B). In cells overexpressing M1R, both CCh and pilocarpine robustly reduced the PIP2 signal (Fig. 4B). Similarly, in transfected CHO-K1 cells, agonists CCh and Oxo-M stimulated PIP2 hydrolysis in cells transfected with either M3R or M1R. Pilocarpine, however, was active only in cells expressing M1R, whereas no effect on PIP2 in M3R-expressing cells was detected (Fig. 4C). Furthermore, pilocarpine blocked CCh-stimulated PIP2 hydrolysis in M3R-overexpressing cells (Fig. 4D). Since either an antagonist or partial agonist can occupy the orthosteric site of a GPCR, they would displace a full agonist and inhibit the functional response. In our experiments, pilocarpine reduced PIP2 hydrolysis below the detection level; in fact, its effect was indistinguishable from that of atropine (data not shown). Therefore, in the M3R-stimulated breakdown of PIP2 assay, pilocarpine acts as an antagonist rather than a partial agonist. On the other hand, the increase in intracellular Ca2+ was still observed when pilocarpine was added together with CCh (Fig. 4E), showing that in this assay pilocarpine acts as an agonist.
Fig. 4.
Effect of pilocarpine on PIP2 hydrolysis. Cells were cotransfected with the PIP2 red biosensor and with the plasmids encoding M1R or M3R. Fluorescence was recorded in real time after cell stimulation with pilocarpine (Pilo) or CCh. (A) Representative images of CHO-K1 cells cotransfected with plasmids to overexpress M3R and the PIP2 sensor. The cells were grown on coverslips and imaged under fluorescence microscope in a flow chamber. (B) Traces show the average of fluorescence response from 20 to 30 HEK293T cells; red trace is response of M1R-expressing cells, green trace is M3R-expressing cells, and black is cells expressing only the PIP2 biosensor. Cells were challenged with the flow of solutions of 100 μM Pilo or CCh at the indicated times. (C) PIP2 responses from CHO-K1 cells transfected with M1R (red) or M3R (green). Cells were stimulated with 100 μM pilocarpine, Oxo-M, or CCh. (D) PIP2 sensor fluorescence recorded from M3R-overexpressing HEK293 cells were first challenged with 25 μM CCh in the presence of 300 μM pilocarpine (red and black horizontal bars) and then washed and stimulated again with 25 μM CCh (black bars). (E) Ca2+ responses from HEK293 cells expressing M3R (green) or control plasmid (black). Cells were stimulated with the mixture of pilocarpine and CCh or CCh alone, as in (D).
Taken together, our findings indicate that for M3R, pilocarpine can behave as either agonist or antagonist, depending on the expression level of the receptor and the downstream signaling. For M1R, pilocarpine is a full agonist regardless of the functional readout.
To further investigate the relationship between PIP2 hydrolysis and Ca2+ mobilization, we examined the effects of pilocarpine and CCh on M3R and M1R overexpressed in CHO-K1 cells under the same conditions (Fig. 5, A and B; Table 1). Our results with overexpressed M1R show that the EC50 in the Ca2+ assay was about 10-fold lower than the EC50 determined in the PIP2 assay for both CCh and pilocarpine. Thus, Ca2+ assay is significantly more sensitive to agonists compared with PIP2 assay. For example, at 0.2 μM of either CCh or pilocarpine, PIP2 response is barely detectable (<10% of maximal), whereas Ca2+ increase is already at 40%–80% of the maximum (Fig. 5A, vertical green line). These results are consistent with earlier work (e.g., (Evans et al., 1985) reporting higher potency of muscarinic agonists in stimulating Ca2+ versus PIP2 responses. The Ca2+ assay can be expected to be more sensitive than PIP2 hydrolysis because of signal amplification in the cascade, where a relatively small number of IP3 molecules can trigger the release of numerous Ca2+ ions from the stores.
Fig. 5.
Ca2+ assay is more sensitive to agonist stimulation than is the PIP2 assay. CHO-K1 cells were transiently transfected with M1R- or M3R-encoding plasmids and analyzed for pilocarpine and CCh-stimulated Ca2+ increase and PIP2 hydrolysis. (A) M1R transfected cells were stimulated with indicated concentrations of CCh (black lines) or pilocarpine (red). Live-cell imaging of free Ca2+ (solid lines) or PIP2 responses (dashed lines) was performed as described in Materials and Methods. Data points denote the maximal amplitude of the response and expressed at the percentage of the maximal response (mean ± S.D.); n = 3 or more. (B) CHO-K1 cells were transfected to overexpress M3R and analyzed as in (A). (C) CHO-K1 cells were transfected with M3R, stimulated with 1 μM pilocarpine in the absence of extracellular Ca2+, and analyzed for their Ca2+ response. Data shown are representative of at least three such experiments done with independent transfections.
TABLE 1 .
Analysis of Ca2+ and PIP2 responses to CCh and pilocarpine in M1R- and M3R-expressing cells
CHO-K1 or HEK293T cells were transfected with either M1R or M3R-expressing plasmids. Nontransfected HEK293 cells were used to study the endogenous M3R present in these cells. The EC50 and Emax were determined for pilocarpine (Pilo) and CCh in two functional assays: free Ca2+ and PIP2 measurements. The experiments were performed as discussed in the text and in the Materials and Methods. Shown are means ± S.D. for the values determined in three to four independent experiments.
| CHO-K1 |
HEK293T |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ |
PIP2 |
Ca2+ |
PIP2 |
||||||
| EC50 | Emax | EC50 | Emax | EC50 | Emax | EC50 | Emax | ||
| μM | % | μM | % | μM | % | μM | % | ||
| Endogenous M3R | Pilo | n.a. | n.a. | n.a. | n.a. | — | 0 | — | 0 |
| CCh | n.a. | n.a. | n.a. | n.a. | 11 ± 2 | 44 ± 5 | — | 0 | |
| Transfected M3R | Pilo | 0.19 ± 0.06 | 71 ± 6 | — | 0 | n.d. | n.d. | — | 0 |
| CCh | 0.06 ± 0.02 | 100 | 4 ± 1 | 100 | 0.04 ± 0.01 | 100 | 4 ± 1 | 100 | |
| Transfected M1R | Pilo | 0.21 ± 0.07 | 84 ± 7 | 2 ± 0.6 | 83 ± 8 | n.d. | n.d. | n.d. | n.d. |
| CCh | 0.11 ± 0.04 | 100 | 1.2 ± 0.4 | 100 | n.d. | n.d. | n.d. | n.d. | |
n.a., not applicable; n.d, not determined; —, no functional response was detected under these conditions.
For M3R, the difference between the Ca2+ and PIP2 assays is more pronounced than for M1R. The EC50 determined for CCh in the Ca2+ assay with overexpressed M3R was about 70-fold lower than the EC50 value obtained in the PIP2 assay. The most striking difference is the complete inability of pilocarpine to induce PIP2 hydrolysis. Even if the concentration of pilocarpine was 1000 times greater than that required for saturation of the Ca2+ response, no change in PIP2 level was detected. These results led us to conclude that stimulation of M3R with pilocarpine does not cause PIP2 hydrolysis and, presumably, IP3 production. To test whether the increase in cytosolic free Ca2+ occurs through the influx of extracellular Ca2+, we stimulated CHO-K1 cells overexpressing M3R in the absence of Ca2+ in the culture medium. There was still a robust Ca2+ response to pilocarpine (Fig. 5C) under these conditions, demonstrating that Ca2+ is released from an intracellular source(s).
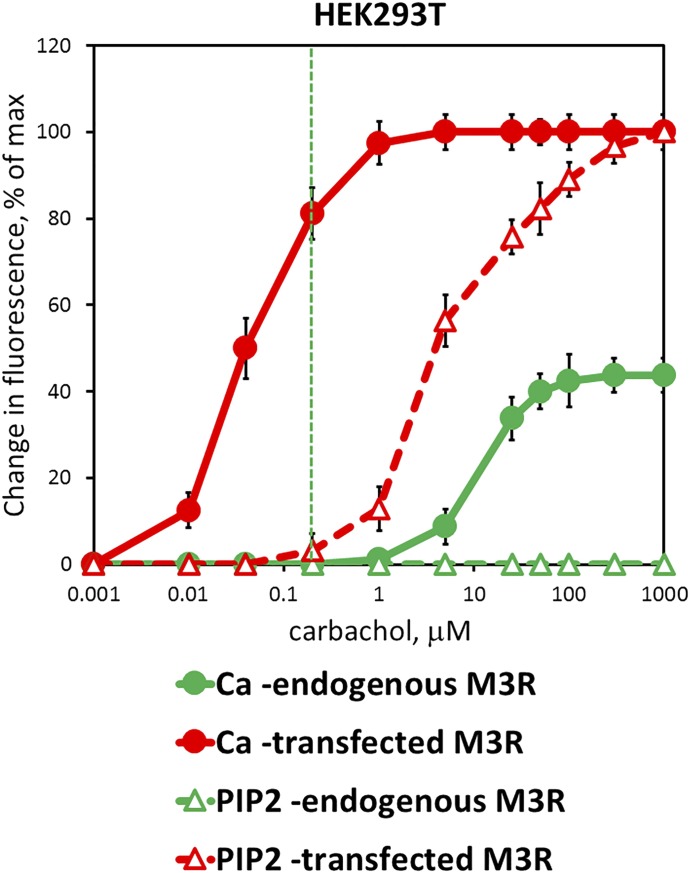
Because of the differences in behavior of endogenous M3R versus overexpressed in CHO-K1 cells (Fig. 2; Fig. 3; Fig. 4; Fig. 5), we also used the free Ca2+ and PIP2 assays to compare the effect of CCh on endogenous versus overexpressed M3R in in the same cell line, HEK293T (Fig. 6; Table 1). As one could expect, Ca2+ responses were much stronger with the transfected M3R: the determined EC50 for CCh was more than 250 times lower and the Emax was more than two times greater than with the endogenous receptor. Similar to CHO-K1 cells, the EC50 measured in the Ca2+ assay with overexpressed M3R was about 100 times lower than with PIP2 hydrolysis. As a result, at 0.2 μM of CCh, the PIP2 response is barely detectable (<5% of maximal), whereas the Ca2+ increase is already at 80% of the maximum (Fig. 6, the vertical green line). The most striking result is that even though CCh induces Ca2+ responses via the endogenous receptor (EC50 = 11 ± 2 μM), we were unable to detect any PIP2 hydrolysis, even at millimolar concentrations of CCh. This finding is consistent with an early finding that pilocarpine upregulated Ca2+, but it did not measurably increase inositol-phosphate accumulation in 1321N1 astrocytoma cells (Evans et al., 1985). A likely explanation is that at the endogenous level of M3R expression, even high doses of agonist cause hydrolysis of only a small fraction of PIP2; this fraction is sufficient to stimulate the Ca2+ release, but it is too low to be detected using either radioactively labeled PIP2 precursor or the fluorescent PIP2 biosensor.
Fig. 6.
Pilocarpine-stimulated Ca2+ and PIP2 signaling via endogenous and overexpressed M3R in HEK293 cells. HEK293 cells were transfected to overexpress M3R (red) or a control plasmid (lacZ, green), plated on glass coverslips, and stimulated with indicated concentrations of CCh. Ca2+ (solid line, filled circles) or PIP2 (dashed line, empty triangles) was measured in real time using a fluorescence microscope. Data points represent peak amplitude (mean ± S.D., n = 3) measured as average from 20 to 40 cells in a visual field.
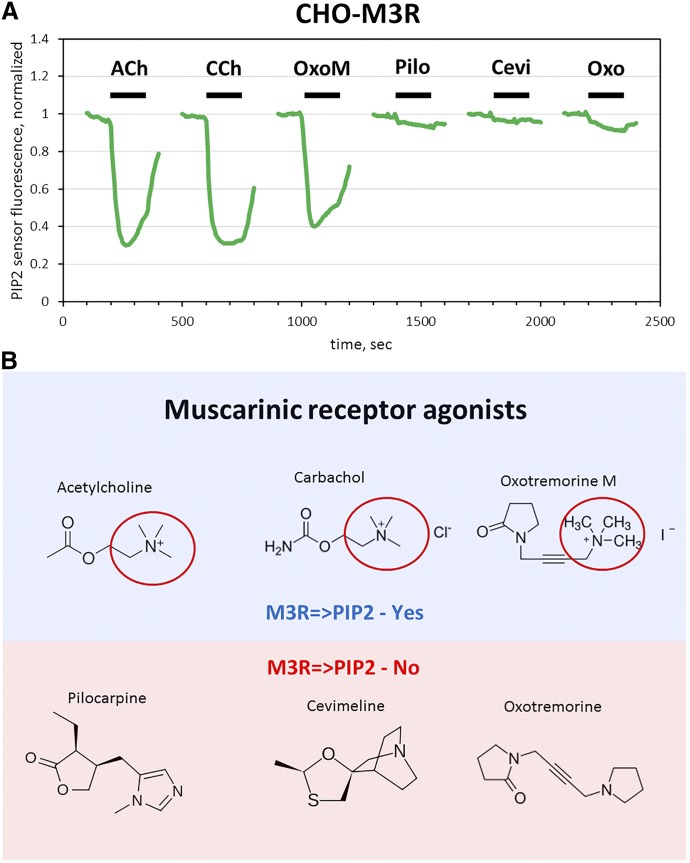
To determine whether the inability to stimulate PIP2 hydrolysis via overexpressed M3R is unique to pilocarpine, we performed pilot experiments with two other muscarinic agonists, oxotremorine (Oxo) and cevimeline. Like pilocarpine, they stimulated Ca2+ responses (data not shown); and, similar to pilocarpine, neither Oxo nor cevimeline induced notable reduction in PIP2 (Fig. 7A). Under identical conditions, Oxo-M, CCh and acetylcholine stimulated robust PIP2 hydrolysis. One obvious common feature in the last three agonists is the quarternary amine of the choline moiety (Fig. 7B). We speculate that this amine is the pharmacophore determining the ability of the drug to stabilize the conformation of overexpressed M3R in which it can activate both Ca2+ mobilization and PIP2 hydrolysis.
Fig. 7.
Oxotremorine and cevimeline do not induce PIP2 hydrolysis via overexpressed M3R. (A) CHO-K1 cells were transfected to overexpress M3R and the PIP2 reporter, plated on glass coverslips, and stimulated with 100 μM of the indicated drugs. PIP2 responses (green lines) were measured in real time using a fluorescence microscope. The traces show an average of three experiments recording fluorescence from 20 to 30 cells for each compound. (B) Structures of the tested muscarinic agonists. ACh, acetylcholine; Pilo, pilocarpine; Oxo, oxotremorine.
Pilocarpine Stimulates ERK Phosphorylation in MIN6 Cells with a Bias toward the Src-Mediated Pathway.
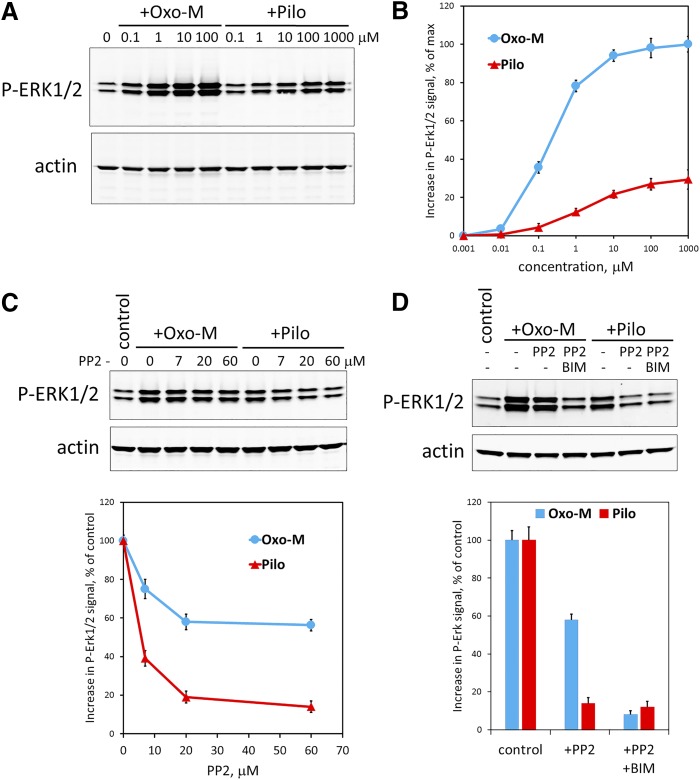
Like other GPCRs, M3R is known to activate extracellular signal-regulated kinases, ERK1/2 (Luo et al., 2008; Selway et al., 2012; Guerra et al., 2014). Activation of ERK can occur via distinct mechanisms that can involve G protein- and β-arrestin–mediated pathways and result in ERK phosphorylation. We found that, like other muscarinic agonists, pilocarpine causes ERK1/2 phosphorylation in MIN6 cells (Fig. 8, A and B); however, the EC50 for pilocarpine was about 10 times greater than that induced by Oxo-M, and the maximal level of pilocarpine-induced phosphorylation was only about 32% of that induced by Oxo-M. Since there is more than one signaling pathway that can couple M3R to ERK1/2 activation, we hypothesized that pilocarpine-bound M3R could activate only one of these mechanisms, for example, β-arrestin–mediated activation of Src kinase. We tested this idea by applying an inhibitor of Src family kinases, 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine, known as PP2 (Fig. 8C), and found that it almost completely eliminated pilocarpine-induced ERK1/2 phosphorylation. In contrast, when MIN6 cells were stimulated with Oxo-M, more than 55% of ERK1/2 phosphorylation occurred even in the presence of the saturating (60 μM) concentration of PP2. This remaining ERK1/2 phosphorylation was almost completely blocked when an inhibitor of PKC (bisindolylmaleimide I, BIM) was included in the mix (Fig. 8D). These results are consistent with the model (Fig. 9) that in MIN6 cells, pilocarpine acts on M3R as a partial agonist that is biased toward a pathway sensitive to PP2, likely the β-arrestin-Src pathway.
Fig. 8.
Pilocarpine stimulates ERK1/2 phosphorylation in MIN6 cells via a PP2-sensitive pathway. (A) MIN6 cells were serum-starved for 4 hours and then stimulated for 5 minutes with the indicated concentrations of Oxo-M or pilocarpine (Pilo). The amount of phosphorylated ERK1/2 was determined by Western blot using anti-P-ERK1/2(T202/Y204) antibody. The same membrane was also stained with an anti-actin antibody used for signal normalization. Shown is a representative immunoblot. (B) Quantification of ERK1/2 phosphorylation in response to stimulation with Oxo-M (blue) or pilocarpine (Pilo, red) was done as described in Materials and Methods. Data show mean ± S.D. from three independent experiments. (C) PP2, a Src family kinase blocker, inhibits pilocarpine- and Oxo-M-stimulated ERK1/2 phosphorylation. MIN6 cells were serum-starved and preincubated with the indicated concentrations of PP2 for 4 hours. Then they were stimulated for 5 minutes with either 1 μM Oxo-M (blue) or 100 μM pilocarpine (Pilo, red). ERK1/2 phosphorylation was determined as in (A and B). Shown is a representative immunoblot and data quantification (mean ± S.D. from three independent experiments). (D) A PKC inhibitor bisindolylmaleimide I (BIM, 10 μM) almost completely blocked Oxo-M-stimulated ERK1/2 phosphorylation when combined with PP2 (60 μM). The experiment was performed and quantified as in (C). Data show mean ± S.D. from three independent experiments.
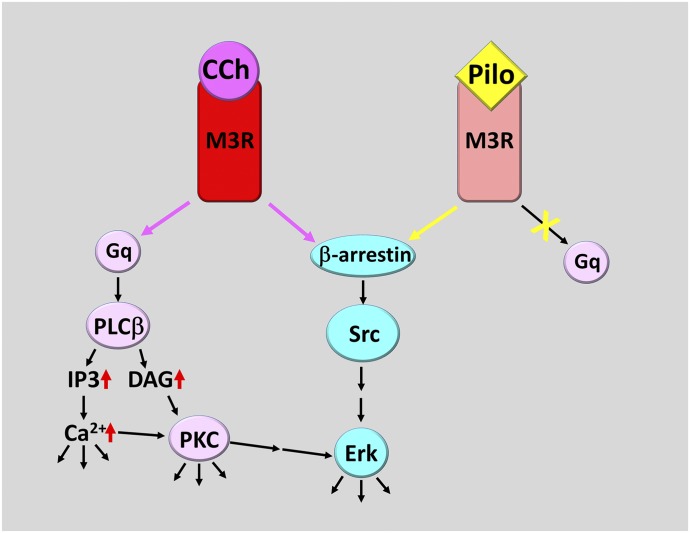
Fig. 9.
A proposed model for CCh and pilocarpine (Pilo) action via M3R. When either CCh (or other agonists such as acetylcholine and Oxo-M) or Pilo bind to M3R, the receptor assumes two similar but distinct conformations. The CCh-bound M3R is able to activate Gq, leading to stimulation of PLCβ and subsequent signaling cascade steps. It also activates β-arrestin–mediated signaling cascade, leading to stimulation of ERK1/2. In contrast, the Pilo-bound M3R conformation is unfavorable for Gq activation. In fact, by competing with CCh, Pilo can antagonize the Gq-dependent signaling cascade; however, Piloc is still able to activate β-arrestin-dependent signaling that leads to ERK1/2 phosphorylation.
Discussion
Pilocarpine is a prototypical cholinergic drug present on the World Health Organization List of Essential Medicines. Its ability to activate secretion by exocrine glands has been used for many decades to treat dry mouth and dry-eye syndromes. Because it constricts smooth muscles in the eye releasing intraocular pressure, topical pilocarpine has been the treatment of choice for glaucoma. Early physiologic experiments on pilocarpine-atropine competition led to the development of one of the most important biochemical and pharmacologic concepts, the concept of a drug receptor. Our article reveals previously unappreciated aspects of pilocarpine pharmacology by showing that its effect on M3R cannot be described solely by full or partial agonism. According to our data, pilocarpine can also act as an antagonist or biased agonist for this muscarinic receptor, depending on cellular environment and the readout used to study molecular events downstream of M3R stimulation.
We compared our M3R data with a similar Gq-coupled muscarinic receptor, M1R. Our results on M1R are consistent with the common knowledge that pilocarpine is a full muscarinic agonist. It is worth noting the multiplex technique that we developed to compare the two receptors. The M1R- and M3R-expressing cells were marked by cotransfection with red and green fluorescent proteins, which allowed us to examine the two cell populations simultaneously under identical conditions in real time (Fig. 3). We recommend this simple method for comparing other receptors as well, particularly when the expected differences in the downstream signaling are small. For example, one could evaluate the effects of drugs on closely related receptors, examine receptor mutants or the effects of coexpressed regulatory proteins.
In the Ca2+ mobilization assay, we did not detect a significant difference between the responses of overexpressed M1R and M3R to either CCh or pilocarpine (Fig. 4; Table 1). When we analyzed PIP2 hydrolysis in the same system, however, the difference between M1R and M3R was remarkable. For M1R, both CCh and pilocarpine acted as full agonists, eliciting a robust reduction in PIP2 level. M3R was also fully activated by CCh, but with pilocarpine, we did not detect any change in PIP2 signal (Figs. 4 and 5). Thus, pilocarpine is strikingly selective for pathways downstream of M3R. For the free Ca2+ increase, it works as a full agonist. For PIP2 hydrolysis, it does not elicit any response by itself and completely blocks the stimulatory effect of CCh. This apparent antagonism is unique for M3R-stimulated PIP2 hydrolysis. For other receptors and readouts, pilocarpine works as a partial agonist with considerable efficacy; for example, it can stimulate M2R to about 70% the maximal effect of CCh (Gregory et al., 2010). Our initial survey of cholinergic agonists shows that cevimeline and oxotremorine also fail to stimulate PIP2 hydrolysis via overexpressed M3R. We speculate that the quaternary amine absent in these compounds, but present in acetylcholine, Oxo-M, and CCh, is responsible for stimulation of Gq and PIP2 hydrolysis.
Another important finding of our study is the very different effect of pilocarpine on overexpressed versus endogenous M3R. We analyzed M3R function in three biologic systems where it is known to be the sole muscarinic receptor: HEK293T cells, MIN6 cells, and the constrictor muscle of the pupil. Surprisingly, pilocarpine did not stimulate Ca2+ mobilization in HEK293T or MIN6 cells at all (Fig. 2) unless M3R was overexpressed. Thus, pilocarpine-induced Ca2+ response in M3R-transfected cells can be interpreted as an artifact of the abnormally high receptor level. This notion is likely to have implications for other GPCRs, as transfected cells are widely used for receptor deorphanization, drug screening, and delineation of signaling and regulatory mechanisms. Our results show that even for such a well known pharmaceutical as pilocarpine, the answer to the basic question of whether it is an agonist or antagonist could be different for the native versus the overexpressed form of the same receptor. Clearly, drugs and receptors that have been investigated less than pilocarpine and the muscarinic family must be analyzed in the native context.
Pilocarpine also failed to stimulate insulin secretion in MIN6 cells and blocked insulin responses elicited by Oxo-M, and so it works as an M3R antagonist in the pancreatic β-cell model. On the other hand, pilocarpine stimulates pupil constriction, and even though it is much less potent than other agonists, it is a full agonist in this system. It has an unusually high EC50 that is three orders of magnitude greater than the reported Kd of pilocarpine for M3R. Accordingly, the concentration of pilocarpine in eye drops is extremely high: 2%–4% (80–160 mM), and there is no explanation for why the therapeutic dose is that high. An abnormally low ability of pilocarpine to cause activation of Gq was noticed earlier, when it was shown that pilocarpine-stimulated GTPγS binding in M3R-transfected cells was several-fold lower than with other agonists (Sykes et al., 2009). Data on pilocarpine-stimulated generation of IP3 are controversial: some investigators reported robust overexpressed M3R-mediated production (Ehlert et al., 1999), whereas others found pilocarpine to be a virtually ineffective stimulant of overexpressed or endogenous M3R (Evans et al., 1985; Gurwitz et al., 1994).
Together with the earlier observations, our results show that pilocarpine does not activate all signaling pathways triggered by M3R, which led us to propose that, unlike CCh and Oxo-M, pilocarpine can act as a biased agonist (Fig. 9). The following data in HEK293T and MIN6 cells support this model: pilocarpine does not activate Ca2+ via the endogenous M3R present in these cells, but it does stimulate ERK, evidently via the β-arrestin-Src kinase mechanism. For ERK activation, pilocarpine fits under the definition of a partial agonist since the maximal level of ERK phosphorylation in the presence of pilocarpine is about three times less than that reached with Oxo-M. For the G protein pathway, application of pilocarpine causes inhibition of signaling induced by other agonists down to the basal level (Fig. 2); thus, it acts as an antagonist.
The model presented in Fig. 9 can explain much of our current data and possibly earlier observations (Gurwitz et al., 1994; Sykes et al., 2009); however, our study also exposed some phenomena where the underlying mechanisms are unclear. For example, it is puzzling why, in the basic assay of pupil constriction, pilocarpine works as a full agonist but requires an extremely high concentration. Classic pharmacology cannot explain the observed difference in the effects of 1 (30-fold above the Kd, ∼99% receptor occupancy) and 10 mM (300-fold above the Kd, ∼99.9% receptor occupancy) pilocarpine. One hypothesis explaining why pilocarpine can act both as an agonist and antagonist toward M3R is the existence of two binding sites. The high-affinity site would be the orthosteric site where it competes with CCh but does not activate Gq, (i.e., works as an antagonist). At the second, low-affinity site, pilocarpine could additionally change the receptor conformation, switching it to the active form. The second pilocarpine molecule could occupy the outside vestibule area revealed by the M3R crystal structure (Kruse et al., 2012); however, the two binding sites model does not explain why even the millimolar concentrations of pilocarpine elicit no detectable Ca2+ responses via endogenous M3R in HEK293T cells (Fig. 2).
Another observation that we cannot yet explain is how pilocarpine can increase Ca2+ via overexpressed M3R without inducing detectable PIP2 hydrolysis. In theory, Ca2+ can come from a source that does not require IP3 but instead is activated, for example, by phosphorylation initiated by β-arrestin or another mechanism downstream of M3R. Thus far, we found that, in the absence of extracellular Ca2+, pilocarpine can still induce Ca2+ transients in CHO-K1 cells overexpressing M3R (Fig. 5C), which points to an intracellular Ca2+ source such as mitochondria. Currently, however, we favor a simpler explanation that is based on the assumption that little IP3 is sufficient to trigger a full Ca2+ release from the endoplasmic reticulum. Indeed, there is a significant (one to two orders of magnitude) shift to the right in the PIP2 compared with Ca2+ dose-response curves measured with overexpressed M3 even with CCh (Fig. 5B); it is possible that for pilocarpine this difference is even greater. This hypothesis suggests that some IP3 is generated locally, whereas the biosensor assay we use in this study can detect only changes in global PIP2. Imaging techniques such as total internal reflection fluorescence (Wuttke et al., 2016) and a knockdown of potentially relevant signaling components can test these ideas in the future.
Our current work showed for the first time that pilocarpine acts on M3R, not only as a full or partial agonist, as it is known to act on other muscarinic receptors, but also as an antagonist and a functionally selective ligand. Since these behaviors are particularly apparent with the endogenous M3R, we speculate that these properties might explain why pilocarpine has fewer side effects than CCh when used to treat dry mouth or glaucoma. Understanding the structure-activity relationship in cholinergic drugs and receptors may expand their use for other diseases, such as diabetes, where biased signaling via M3R can improve the function and viability of β cells.
Acknowledgments
We thank Dr. Daniel Isom for careful reading of the manuscript and excellent suggestions.
Abbreviations
- CCh
carbachol
- CHO
Chinese hamster ovary
- ERK
extracellular regulated kinase
- eYFP
enhanced yellow fluorescent protein
- GPCR
G protein-coupled receptor
- Gq
cognate G protein
- HBSS
Hanks’ balanced salt solution
- HEK293T
human embryonic kidney cell line 293T
- IP3
inositol 1,4,5-trisphosphate
- M1R
M1 muscarinic receptor
- M3R
M3 muscarinic receptor
- Oxo
oxotremorine
- Oxo-M
oxotremorine-M
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- PLC
phospholipase C
Authorship Contributions
Participated in research design: Pronin, Slepak.
Conducted experiments: Pronin, Wang.
Performed data analysis: Pronin, Wang, Slepak.
Wrote or contributed to the writing of the manuscript: Pronin, Slepak.
Footnotes
This work was supported by the National Institutes of Health [Grants RO1DK105427 and O1DK111538].
References
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. (2003) Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd DC, McDonald JE, Tobin AB. (2000) Phosphorylation and regulation of a Gq/11-coupled receptor by casein kinase 1alpha. J Biol Chem 275:19667–19675. [DOI] [PubMed] [Google Scholar]
- Burford NT, Tobin AB, Nahorski SR. (1995) Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3′,5′-cyclic monophosphate accumulation in Chinese hamster ovary cells. J Pharmacol Exp Ther 274:134–142. [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. (2003) Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci 17:1403–1410. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ, Griffin MT, Sawyer GW, Bailon R. (1999) A simple method for estimation of agonist activity at receptor subtypes: comparison of native and cloned M3 muscarinic receptors in guinea pig ileum and transfected cells. J Pharmacol Exp Ther 289:981–992. [PubMed] [Google Scholar]
- Evans T, Hepler JR, Masters SB, Brown JH, Harden TK. (1985) Guanine nucleotide regulation of agonist binding to muscarinic cholinergic receptors. Relation to efficacy of agonists for stimulation of phosphoinositide breakdown and Ca2+ mobilization. Biochem J 232:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam D, Jeon J, Li JH, Han SJ, Hamdan FF, Cui Y, Lu H, Deng C, Gavrilova O, Wess J. (2008) Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review. J Recept Signal Transduct Res 28:93–108. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Hall NE, Tobin AB, Sexton PM, Christopoulos A. (2010) Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J Biol Chem 285:7459–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra ML, Wauson EM, McGlynn K, Cobb MH. (2014) Muscarinic control of MIN6 pancreatic β cells is enhanced by impaired amino acid signaling. J Biol Chem 289:14370–14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D, Haring R, Heldman E, Fraser CM, Manor D, Fisher A. (1994) Discrete activation of transduction pathways associated with acetylcholine m1 receptor by several muscarinic ligands. Eur J Pharmacol 267:21–31. [DOI] [PubMed] [Google Scholar]
- Haga T. (2013) Molecular properties of muscarinic acetylcholine receptors. Proc Jpn Acad, Ser B, Phys Biol Sci 89:226–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan W, Adjobo-Hermans M, Burroughs M, Faibis G, Malik S, Tall GG, Smrcka AV. (2014) M3 muscarinic receptor interaction with phospholipase C β3 determines its signaling efficiency. J Biol Chem 289:11206–11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinsky-Semper D, Volmar CH, Brothers SP, Slepak VZ. (2014) Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol Pharmacol 85:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KC, Tobin AB. (2011) The role of M(3)-muscarinic receptor signaling in insulin secretion. Commun Integr Biol 4:489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Hu J, Kobilka BK, Wess J. (2014a) Muscarinic acetylcholine receptor X-ray structures: potential implications for drug development. Curr Opin Pharmacol 16:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, et al. (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Kobilka BK, Gautam D, Sexton PM, Christopoulos A, Wess J. (2014b) Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov 13:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J, Peralta E, Clapham D. (1989) Diverse functions of muscarinic acetylcholine receptor subtypes. Trends Pharmacol Sci (Suppl Subtypes of Muscarinic Receptors IV):34–38. [PubMed] [Google Scholar]
- Luo J, Busillo JM, Benovic JL. (2008) M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol 74:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Gesty-Palmer D. (2010) Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev 62:305–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehle AH. (2004) “Receptive substances”: John Newport Langley (1852-1925) and his path to a receptor theory of drug action. Med Hist 48:153–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin A, Levay K, Velmeshev D, Faghihi M, Shestopalov VI, Slepak VZ. (2014) Expression of olfactory signaling genes in the eye. PLoS One 9:e96435 DOI: 10.1371/journal.pone.0096435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, Miller LJ. (1997) Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol Pharmacol 51:357–362. [PubMed] [Google Scholar]
- Ruiz de Azua I, Gautam D, Jain S, Guettier JM, Wess J. (2012) Critical metabolic roles of β-cell M3 muscarinic acetylcholine receptors. Life Sci 91:986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiford SL, Wang Q, Levay K, Buchwald P, Slepak VZ. (2010) Molecular organization of the complex between the muscarinic M3 receptor and the regulator of G protein signaling, Gbeta(5)-RGS7. Biochemistry 49:4998–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selway JL, Moore CE, Mistry R, John Challiss RA, Herbert TP. (2012) Molecular mechanisms of muscarinic acetylcholine receptor-stimulated increase in cytosolic free Ca(2+) concentration and ERK1/2 activation in the MIN6 pancreatic β-cell line. Acta Diabetol 49:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Guidry J, Gettys TW, Tobin AB, Lanier SM. (2006) The proto-oncogene SET interacts with muscarinic receptors and attenuates receptor signaling. J Biol Chem 281:40310–40320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup O, Winder M, Killi UK, Wsol V, Jun D, Kuca K, Tobin G. (2017) Acetylcholinesterase inhibitors and drugs acting on muscarinic receptors- potential crosstalk of cholinergic mechanisms during pharmacological treatment. Curr Neuropharmacol 15:637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes DA, Dowling MR, Charlton SJ. (2009) Exploring the mechanism of agonist efficacy: a relationship between efficacy and agonist dissociation rate at the muscarinic M3 receptor. Mol Pharmacol 76:543–551. [DOI] [PubMed] [Google Scholar]
- Tewson P, Westenberg M, Zhao Y, Campbell RE, Quinn AM, Hughes TE. (2012) Simultaneous detection of Ca2+ and diacylglycerol signaling in living cells. PLoS One 7:e42791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewson PH, Martinka S, Shaner NC, Hughes TE, Quinn AM. (2016) New DAG and cAMP sensors optimized for live-cell assays in automated laboratories. J Biomol Screen 21:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewson PH, Quinn AM, Hughes TE. (2013) A multiplexed fluorescent assay for independent second-messenger systems: decoding GPCR activation in living cells. J Biomol Screen 18:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Sun B, Feng D, Nawaratne V, Leach K, Felder CC, Bures MG, Evans DA, Weis WI, Bachhawat P, et al. (2016) Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 531:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422. [DOI] [PubMed] [Google Scholar]
- Weng L, Davies M, Ashcroft SJ. (1993) Effects of cholinergic agonists on diacylglycerol and intracellular calcium levels in pancreatic beta-cells. Cell Signal 5:777–786. [DOI] [PubMed] [Google Scholar]
- Wess J. (2004) Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol 44:423–450. [DOI] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. (2007) A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA 104:16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bogatkevich GS, Mukhin YV, Benovic JL, Hildebrandt JD, Lanier SM. (2000) Identification of Gbetagamma binding sites in the third intracellular loop of the M(3)-muscarinic receptor and their role in receptor regulation. J Biol Chem 275:9026–9034. [DOI] [PubMed] [Google Scholar]
- Wuttke A, Yu Q, Tengholm A. (2016) Autocrine signaling underlies fast repetitive plasma membrane translocation of conventional and novel protein kinase C isoforms in β cells. J Biol Chem 291:14986–14995. [DOI] [PMC free article] [PubMed] [Google Scholar]