Abstract
Traditionally, G protein–coupled receptor antagonists are classified as competitive or noncompetitive and surmountable or insurmountable based on functional antagonism. P2Y1 receptor (P2Y1R) structures showed two antagonists binding to two spatially distinct sites: nucleotide MRS2500 (orthosteric, contacting the helical bundle) and urea BPTU (allosteric, on the external receptor surface). However, the nature of their P2Y1R antagonism has not been characterized. Here we characterized BPTU antagonism at various signaling pathways activated by structurally diverse agonists. BPTU rightward shifted the concentration-response curves of both 2-methylthioadenosine 5′-diphosphate trisodium salt and MRS2365 (5′-diphosphates) in some signaling events, such as extracellular signal-regulated kinase 1/2 and label free, in a parallel manner without affecting the maximum agonist effect (Emax) but antagonized insurmountably (suppressed agonist Emax) in signaling events such as guanosine 5′-3-O-(thio)triphosphate binding and β-arrestin2 recruitment. However, with dinucleotide Ap4A as an agonist, BPTU suppressed the Emax insurmountably in all signaling pathways. By comparison, MRS2500 behaved as surmountable antagonist rightward-shifting concentration-response curves of all three agonists in a parallel manner for all signaling pathways measured. Thus, we demonstrated a previously undocumented phenomenon that P2Y1R antagonism patterns could vary in different signaling pathways, which could be related to conformational selection, signaling amplification, and probe dependence. This phenomenon may apply generally to other receptors considering that antagonism by a specific ligand is often not compared at multiple signaling pathways. Thus, antagonism can be surmountable or insurmountable depending on the signaling pathways measured and the agonists used, which should be of broad relevance to drug discovery and disease treatment.

Introduction
The P2Y1 receptor (P2Y1R), a G protein–coupled receptor (GPCR), is activated by the endogenous agonist ADP to facilitate platelet aggregation (Savi et al., 1998) and serves as an important drug target (Jacobson et al., 2015). Considerable effort has been devoted to research on this receptor, including its recent structural determination by X-ray crystallography (Zhang et al., 2015a). The P2Y1R structures featured the high-affinity nucleotide antagonist (1′R,2′S,4′S,5′S)-4-(2-iodo-6-methylamino-purin-9-yl)-1-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane (MRS2500) (Supplemental Fig. 1) bound in a pocket formed within the seven transmembrane (TM) domains and more external than most small ligands of rhodopsin-like GPCRs. However, an allosteric antagonist 1-(2-(2-(tert-butyl)phenoxy)pyridin-3-yl)-3-(4-(trifluoromethoxy)phenyl)urea (BPTU), a hydrophobic diaryl-urea derivative that arose from a program to develop antithrombotic drugs (Chao et al., 2013), bound on the external receptor surface at the phospholipid membrane interface in contact with TMs 1–3 (Zhang et al., 2015a). This represented the first example in the GPCR field of a ligand located outside the helical bundle or loop regions. BPTU was demonstrated to increase the dissociation rate of an agonist radioligand, [3H]2-methylthioadenosine-5′-diphosphate ([3H]2MeSADP), initiated by the competitive antagonist MRS2500, thus confirming the allosteric property of BPTU pharmacologically. Furthermore, P2Y1R site-directed mutagenesis supported the conclusion that the binding sites of the nucleotide antagonist and negative allosteric modulator were completely mutually exclusive. However, the functional antagonism by this unique allosteric modulator has not been characterized.
As with other GPCRs, the P2Y1R is coupled to multiple G protein–dependent and G protein–independent effectors (Hoffmann et al., 2008; Zhang et al., 2015a). In the present study, we set out to examine the nature of P2Y1R antagonism by BPTU at various signaling pathways, including Gq/11-mediated production of inositol phosphates (IPs), [35S]guanosine 5′-O-(3-thiotriphosphate) (GTPγS) binding to Gq/11, Gq/11-, or β-arrestin2–mediated extracellular signal-regulated kinase 1/2 (ERK1/2) stimulation, β-arrestin2 recruitment, and P2Y1R internalization. The present study demonstrated that BPTU rightward shifted the concentration-response curves of both 2MeSADP and [[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid monoester (MRS2365) in some signaling pathways, such as ERK1/2 activity and label-free dynamic mass redistribution (DMR), in a parallel manner without affecting the maximum agonist effect (Emax), but suppressed the agonist Emax in signaling events, GTPγS binding, β-arrestin2 recruitment, and β-arrestin2–mediated receptor internalization. However, when using a dinucleotide diadenosine tetraphosphate (Ap4A) as an agonist, BPTU suppressed the Emax in all signaling pathways in an insurmountable pattern. By comparison, the orthosteric agonist MRS2500 was shown to behave as a surmountable antagonist shifting concentration-response curves of all three agonists in all signaling pathways measured. Considering that the nature of antagonism at various signaling pathways by a specific antagonist has not been extensively examined previously, these findings could represent a general phenomenon of antagonism with respect to signaling pathways of other GPCRs, and even other membrane receptor classes, and should be of broad relevance to drug discovery and disease treatment.
Materials and Methods
Chemical compounds cited in this article are as follows: MRS2500 [PubChem Compound Identifier (CID): 44448831]; BPTU (PubChem CID: 11510579); Ap4A (PubChem CID: 11957521); 2MeSADP (PubChem CID: 121990); MRS2365 (PubChem CID: 73755043); 3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (GO6983) (PubChem CID: 3499); GTPγS (PubChem CID: 1764); and UBO-QIC (PubChem CID: 14101198).
GO6983, 2MeSADP, MRS2365, and MRS2500 were obtained from Tocris Bioscience (Ellisville, MO). Ap4A was from Sigma-Aldrich (St. Louis, MO). The allosteric antagonist BPTU was synthesized at National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health and was provided by Dr. E. Kiselev (NIDDK, NIH, Bethesda, MD). Reagents for the IP-One HTRF assay were obtained from Cisbio Bioassays (Bedford, MA). Reagents for ALPHAScreen assays and [35S]GTPγS (1250 Ci/mmol) were from PerkinElmer (Waltham, MA). [3H]2MeSADP (7.5 Ci/mmol) was purchased from Moravek Biochemicals, Inc. (Brea, CA). 1321N1 astrocytoma cells expressing the human P2Y1R were from T.K. Harden (University of North Carolina, Chapel Hill, NC). PathHunter U2OS (human osteosarcoma) cells expressing the recombinant human P2Y1R and an engineered β-arrestin2 and reagents for the PathHunter β-arrestin assays were obtained from DiscoverX (Fremont, CA). ON-TARGETplus human β-arrestin2 small interfering RNA (siRNA)-SMARTpool was from Dharmacon (LaFayette, CO). All other materials were from standard commercial sources (Sigma-Aldrich, unless noted) and were of analytical grade.
IP-1 Assay.
IP-1, which is a metabolite of inositol 1,4,5-trisphosphate and downstream of Gq signaling, was detected by the IP-One Tb HTRF Kit (Cisbio Bioassays), as described previously (Violin et al., 2010; Rajagopal et al., 2011). Briefly, after overnight growth, cells were pretreated with an antagonist for 20 minutes before the addition of agonist and incubated for another 60 minutes. Detection reagents were added as instructed by the manual from the manufacturer. The assay plates were read on a Mithras LB940 Reader (Berthold Technologies, Oak Ridge, TN) or a PerkinElmer EnSpire plate reader using a time-resolved fluorescence ratio (665:620 nm).
ERK1/2 Stimulation.
The method used was essentially as previously described (Gao et al., 2011, 2014). 1321N1 astrocytoma cells or U2OS cells expressing the human P2Y1R (30,000 cells/100 μl) were seeded in a 96-well plate in complete growth medium. After cell attachment, medium was removed and cells were serum starved overnight in 90 μl of serum-free medium. For Gαq-mediated ERK1/2 stimulation, cells were stimulated with agonist for 5 minutes. For β-arrestin2–mediated ERK1/2 stimulation, cells were incubated with agonist for 30 minutes after a pretreatment with the broad-spectrum protein kinase C (PKC) inhibitor GO6983 (10 µM; Tocris Bioscience) for 20 minutes (Lefkowitz and Shenoy, 2005; Hoffmann et al., 2008; Reiner et al., 2009). In both cases, cells were pretreated with an antagonist 20 minutes before the addition of agonist. After agonist treatment, the medium was removed and cells were lysed with 1× lysis buffer (20 μl) [AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay Kit; PerkinElmer]. Lysate (4 μl/well) was transferred to a 384-well ProxiPlate Plus (PerkinElmer). Acceptor beads were diluted 1:50 in a 1:5 mixture of activation buffer in reaction mix and added to the 384-well plate (5 μl/well). The plate was sealed and incubated for 2 hours at room temperature. Donor beads (2 μl) diluted 1:20 in dilution buffer were added, and the plate was incubated for another 2 hours at room temperature. The plate was measured using an EnVision multilabel reader using standard AlphaScreen settings. Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA) was used for the transfection of β-arrestin2 siRNA (final concentration, 100 nM) according to the manufacturer manual.
[35S]GTPγS Binding Assay.
The preparation of membranes from U2OS cells expressing human P2Y1R was as previously described (Gao et al., 2011). [35S]GTPγS (PerkinElmer, Boston, MA) binding was carried out in duplicate or triplicate by incubation for 30 minutes at 25°C in 200 μl of buffer containing 50 mM Tris HCl (pH 7.4), 1 mM EDTA, 1 mM MgCl2, 1 μM GDP, 1 mM dithiothreitol, 100 mM NaCl, 0.2 nM [35S]GTPγS, 0.5% bovine serum albumin, test agonists, and membrane suspension (10 μg protein/tube). Antagonists were added 20 minutes before the addition of agonists. The reaction was stopped by rapid filtration through Whatman GF/B filters (Brandel, Gaithersburg, MD), presoaked in 50 mM Tris HCl and 5 mM MgCl2 (pH 7.4). The filters were washed twice with 3 ml of the same buffer, and the retained radioactivity was measured using liquid scintillation counting. Nonspecific binding of [35S]GTPγS was measured in the presence of 10 μM unlabeled GTPγS.
Label-Free DMR Measurement.
Label-free DMR measurement was performed on a PerkinElmer EnSpire multimode plate reader based on the EPIC optical biosensor technology using resonance waveguides. For the measurements, 50 μl of P2Y1R-expressing U2OS cells (2 × 104 cells/each well) were seeded into 96-well EnSpire cell assay microplates. The microplates were incubated in a humidified atmosphere containing 5% CO2 at 37°C overnight. Immediately before the experiments, the cells were washed three times with assay buffer (Hank’s balanced salt solution buffer containing 20 mM HEPES; Mediatech, Manassas, VA) and were allowed to equilibrate in the EnSpire multimode reader for 1 hour. After measuring baseline data, 10 μl of the test compounds were added and the cellular response was recorded continuously for 60 minutes.
β-Arrestin2 Recruitment Assays.
The β-arrestin2 recruitment to the P2Y1R was assessed by DiscoverX PathHunter β-arrestin assay as described previously (Gao and Jacobson, 2008; Gao et al., 2014). In this assay, the GPCR is fused in frame with the small enzyme fragment ProLink and coexpressed in U2OS cells stably expressing a fusion protein of β-arrestin2 and the larger N-terminal deletion mutant of β-galactosidase (enzyme acceptor). GPCR activation stimulates binding of β-arrestin2 to the ProLink-tagged GPCR and forces complementation of the two enzyme fragments, resulting in the formation of an active β-galactosidase enzyme. This interaction leads to an increase in enzyme activity that can be measured using chemiluminescent PathHunter Detection Reagents (DiscoverX). For the measurement of P2Y1R-mediated recruitment, PathHunter U2OS cells expressing the human P2Y1R were grown in 96-well plates for 24 hours in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 μmol/ml glutamine. Cells were first treated with antagonists for 20 minutes and then treated with agonists for 60 minutes before adding detection reagents (mixture of 1 part Galacton Star substrate with 5 parts Emerald II Solution and 19 parts of PathHunter Cell Assay Buffer), and incubated at room temperature for 60 minutes before luminescence was measured.
P2Y1R Internalization.
P2Y1R internalization was assessed using a PathHunter eXpress Activated GPCR Internalization Assay as instructed by the manufacturer (DiscoverX). In this assay, PathHunter U2OS cells are engineered to coexpress an untagged GPCR, enzyme acceptor–tagged β-arrestin2, and a ProLink tag localized to the endosomes. Activation of the untagged GPCR induces β-arrestin recruitment, followed by internalization of the receptor/arrestin-enzyme acceptor complex in protein kinase-tagged endosomes, which forces complementation of the two β-galactosidase enzyme fragments, forming a functional enzyme that hydrolyzes substrate to generate a chemiluminescent signal. The method used for this assay was similar to that described in the β-arrestin2 recruitment assay, except that the agonist incubation time was 180 minutes.
Data and Statistical Analysis.
Apparent binding affinities in radioligand binding (Supplemental Methods), according to the formula Ki (inhibition constant) = IC50/(1 + [radioligand]/Kd [dissociation constant]), and functional parameters were calculated using Prism 7.00 software (GraphPad Software, San Diego, CA). Statistical significance of the differences was assessed using a Student’s t test (between two conditions) or a one-way analysis of variance (ANOVA) followed by appropriate post hoc testing. Differences yielding P < 0.05 were considered as statistically different.
Results
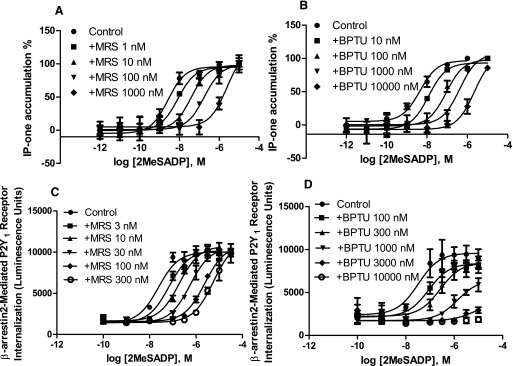
As described previously (Zhang et al., 2015a), BPTU and MRS2500 bind to structurally distinct sites on the P2Y1R. Therefore, we expected different patterns of antagonism of P2Y1R-mediated signaling. Since the P2Y1R is a Gq-coupled receptor, we first measured IP-1 production in 1321N1 astrocytoma cells expressing the recombinant human P2Y1R. Figure 1, a and b shows that both MRS2500 and BPTU produce a rightward shift of the agonist concentration-response curves. The KB (the dissociation equilibrium constant of an antagonist) values for MRS2500 and BPTU calculated from the Schild analysis are 0.86 ± 0.19 and 6.83 ± 1.22 nM, respectively, and their respective slopes are 0.90 ± 0.08 and 0.95 ± 0.06. Thus, the allosteric property of BPTU cannot be manifested using a Schild analysis.
Fig. 1.
Comparison of antagonism of 2MeSADP-induced IP-1 formation via Gq/11 proteins and receptor internalization mediated by β-arrestin2. IP-1 accumulation was measured in P2Y1R-expressing 1321N1 astrocytoma cells in the presence or absence of MRS2500 (MRS; a) and BPTU (b). Cells were pretreated with MRS or BPTU for 20 minutes before the addition of agonist and incubated for an additional 60 minutes. Receptor internalization was measured using DiscoverX PathHunter U2OS cells, which were pretreated with MRS2500 (c) or BPTU (d) for 20 minutes followed by addition of 2MeSADP for incubated for 180 minutes. Results were expressed as mean ± S.D. from three independent experiments performed in duplicate. The KB values of MRS2500 and BPTU and slopes from Schild analysis from three separate experiments are listed in Table 1.
It has been shown previously that β-arrestin2 mediates P2Y1R internalization (Reiner et al., 2009). Thus, we examined the antagonism by MRS2500 and BPTU in agonist-induced and β-arrestin2–mediated receptor internalization in U2OS cells. Figure 1, c and d shows that MRS2500 shifts the agonist response curve for receptor internalization to the right in a parallel manner with a slope of 1.06 ± 0.05 and a KB value of 1.12 ± 0.23 nM (Table 1). BPTU concentration-dependently suppressed the maximal agonist effect. Thus, apparently, the allosteric antagonist BPTU behaves in a completely different pattern in antagonizing Gq-mediated and β-arrestin2–mediated events, whereas the competitive antagonist MRS2500 behaves similarly for both pathways. The results led us to speculate that BPTU has different antagonistic pattern on Gαq/11- and β-arrestin2–mediated signaling, whereas the antagonism by MRS2500 for these two branches of signaling (G protein and β-arrestin) is in a similar pattern.
TABLE 1 .
Inhibitory effects of the competitive antagonist MRS2500 and the allosteric antagonist BPTU on various P2Y1R-mediated signaling pathways
Data were analyzed using Prism 7.00. Results are expressed as the mean ± S.E.M. from at least three independent experiments performed either in triplicate or in duplicate. Ligand binding affinities and EC50 values of 2MeSADP were expressed in both nanomolar and log scale (CIs). KB values were converted from log scale (Schild analysis) to nanomolar values. The values from individual experiments were calculated separately, and then the mean and S.E.M. of those values were reported. The −logEC50 (CI) or −log Ki (CI) values were calculated with one fit of all the data. NA, not applicable.
| MRS2500 |
BPTU |
2MeSADP |
||||
|---|---|---|---|---|---|---|
| KB or Ki | Slope | KB or Ki (nM) | Slope | EC50 (nM) | −logEC50 (CI) | |
| nM | nM | nM | ||||
| Gq signaling | ||||||
| IP-1a | 0.86 ± 0.19 | 0.94 ± 0.08 | 6.83 ± 1.22 | 0.95 ± 0.06 | 4.83 ± 1.12 | 8.58 (8.86–8.20) |
| ERK1/2a | 0.91 ± 0.13 | 1.03 ± 0.11 | 6.66 ± 1.37 | 0.95 ± 0.07 | 1.74 ± 0.44 | 8.80 (9.04–8.85) |
| GTP bindingb | 5.68 ± 1.22 | 0.96 ± 0.05 | NAc | NAc | 4.62 ± 1.09 | 8.31 (8.58–8.04) |
| β-Arrestin2 signaling | ||||||
| Recruitmentb | 0.85 ± 0.08 | 0.89 ± 0.05 | NAc | NAc | 7.81 ± 2.03f | 8.01 (8.19–7.84) |
| Internalizationb | 1.12 ± 0.23 | 1.06 ± 0.05 | NAc | NAc | 25.8 ± 3.3f | 7.62 (7.83–7.41) |
| ERK1/2a | 7.41 ± 2.23 | 1.10 ± 0.09 | 28.2 ± 6.4 | 1.00 ± 0.06 | 21.3 ± 2.9 | 7.68 (7.80–7.56) |
| Radioligand bindingb,e | ||||||
| 4.90 ± 1.32 | 1.05 ± 0.08 | 116 ± 17 | 1.04 ± 0.03 | 2.13 ± 0.32d | ||
| −log Ki (CI) | 8.28 (8.44–8.12) | 6.73 (7.30–6.16) | ||||
1321N1 cells expressing the human P2Y1R.
U2OS cells expressing the human P2Y1R.
Antagonism by BPTU in these three assays is insurmountable, and thus KB values and slopes are not applicable.
Kd value from saturation binding.
[3H]2MeSADP (2 nM) binding to membranes prepared from U2OS cells expressing the human P2Y1R (Ki or Kd, nM).
The potencies of 2MeSADP in β-arrestin2 recruitment and receptor internalization are significantly different (P < 0.05, student’s test, N = 3).
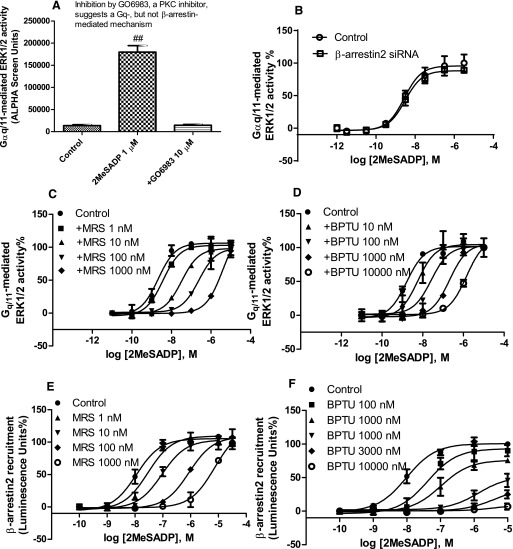
To further prove that BPTU has distinct patterns of antagonism at two signaling pathway branches, we next examined the effect of BPTU on agonist-induced and Gq/11-mediated stimulation of ERK1/2 activity, a known P2Y1R-mediated function (Sellers et al., 2001). Figure 2, a and b shows that the stimulation of ERK1/2 activity for 5 minutes using 2MeSADP (1 µM) is completely PKC sensitive and β-arrestin2 insensitive, suggesting a Gq/11-mediated but not a β-arrestin2–mediated mechanism (Wei et al., 2003; Lefkowitz and Shenoy, 2005; Reiner et al., 2009). 2MeSADP concentration-dependently induced ERK1/2 stimulation corresponding to an EC50 value of 1.74 ± 0.44 nM. Both MRS2500 and BPTU right shifted the agonist response curve in a parallel manner (Fig. 2, c and d), corresponding to KB values of 0.91 ± 0.13 and 6.66 ± 1.37 nM, respectively. The respective slopes are 1.03 ± 0.11 and 0.95 ± 0.07. Again, the allosteric property of BPTU could not be demonstrated with this assay and the patterns of antagonism by MRS2500 and BPTU were indistinguishable (i.e., both produced a parallel rightward shift with slopes close to unity). Thus, the degree of allostery by BPTU for this G protein–dependent pathway is very high, such that by standard criteria of antagonism, its allosteric nature is undetectable. To prove how BPTU inhibits P2Y1 signaling in cells that do not overexpress the recombinant human P2Y1R, the antagonism by BPTU of agonist-induced ERK1/2 activity in HEK293 cells endogenously expressing the human P2Y1R was examined. Supplemental Fig. 2 shows that BPTU rightward shifted the agonist response curve to the right in a parallel manner without affecting the Emax.
Fig. 2.
Comparison of antagonism of 2MeSADP-induced ERK1/2 phosphorylation mediated via Gq/11 and β-arrrestin2 recruitment. (a) Agonist-induced ERK1/2 stimulation in 1321N1 astrocytoma cells expressing the P2Y1R is mediated via PKC. Cells were either treated with 2MeSADP for 5 minutes or treated with PKC inhibitor GO6983 (10 µM) for 20 minutes before the addition of 2MeSADP for another 5 minutes. ##Significantly different from the other two groups [P < 0.01, one-way ANOVA with post-hoc test (Turkey), N = 3]. (b) Gq/11-mediated ERK1/2 activity is insensitive to β-arrestin2 siRNA. (c and d) Agonist concentration-response curves in the absence or presence of MRS (MRS2500; a) or BPTU (b). Cells were pretreated with MRS (a) or BPTU (b) for 20 minutes before the addition of agonist and incubated for another 5 minutes. (e and f) β-arrrestin2 recruitment was measured using DiscoverX PathHunter U2OS cells, which were pretreated with MRS2500 (c) or BPTU (d) for 20 minutes followed by the addition of 2MeSADP and incubated for 60 minutes. Results were expressed as the mean ± S.D. from three separate experiments performed in duplicate or triplicate. The KB values of MRS2500 and BPTU and slopes from Schild analysis are listed in Table 1.
It has been shown previously that P2Y1R internalization and desensitization are modulated by different mechanisms (Reiner et al., 2009). Thus, we examined the similarity or differences in antagonism by MRS2500 and BPTU in agonist-induced β-arrestin2 recruitment in addition to β-arrestin2–mediated receptor internalization. In addition to Gq/11-mediated downstream signaling events, P2Y1R activation is known to cause robust recruitment of β-arrestin2, but not β-arrestin1 (Hoffmann et al., 2008; Reiner et al., 2009). However, the pattern of antagonism by various antagonists at this signaling pathway is unknown. Therefore, we compared the antagonism by MRS2500 and BPTU of the agonist-induced β-arrestin2 recruitment using the PathHunter protein complementation assay (DiscoverX), a widely accepted method (Gao and Jacobson, 2008; Violin et al., 2010; Goa et al., 2011, 2014). Figure 2e shows that MRS2500 shifts the agonist concentration-response curve to the right in a parallel manner with a slope of 0.89 ± 0.05 and a KB value of 0.85 ± 0.08 nM. However, unlike its effect on IP-1 or ERK1/2 stimulation, BPTU suppressed the maximal agonist effect in a concentration-dependent manner and at 10 µM completely blocked the agonist effect (Fig. 2f). These results again support that BPTU behaves differently at two branches of signaling pathways.
It is known that β-arrestin2, in addition to its role in receptor desensitization and internalization, may mediate a signaling event such as ERK1/2 phosphorylation that is independent of G proteins (Lefkowitz and Shenoy, 2005). Therefore, we tested this possibility with the P2Y1R expressed in 1321N1 astrocytoma cells by stimulation of cells with 2MeSADP after the pretreatment with the PKC inhibitor GO6983 for 20 minutes (Wei et al., 2003; Shenoy et al., 2006). We first measured the time-course of agonist-induced ERK1/2 stimulation. Figure 3a shows that, in the presence of GO6983, 2MeSADP does not induce stimulation of ERK1/2 activity at 5 minutes, but stimulates the maximal ERK1/2 phosphorylation at 30 minutes. The ERK1/2 activity at 30 minutes was completely abolished by β-arrestin2 siRNA demonstrating a β-arrestin2–mediated effect (Fig. 3a). Thus, in the following experiments, we measured the agonist concentration response and the antagonism by MRS2500 and BPTU under this condition. Figure 3b shows that agonist 2MeSADP concentration-dependently induces a robust stimulation of ERK1/2 activity corresponding to an EC50 value of 21.3 ± 2.9 nM, which was completely abolished by β-arrestin2 siRNA. By contrast, β-arrestin2 siRNA did not affect the concentration-response curve of 2MeSADP-induced ERK1/2 stimulation at 5 minutes in the absence of GO6983 (Fig. 2b), further confirming the difference between Gq- and β-arrestin2–stimulated ERK1/2 activity. Interestingly, unlike the effect of BPTU in blocking agonist-induced β-arrestin2 translocation and receptor internalization, it rightward shifted agonist-induced and β-arrestin2–mediated ERK1/2 activity in a parallel manner in a way similar to that of MRS2500 (Fig. 3, c and d). Thus, the atypical negative allosteric modulator BPTU behaves differently even in different events mediated by β-arrestin2, suggesting that each signaling event is possibly mediated via a specific receptor conformation, a β-arrestin2 conformation, or both (DeWire et al., 2007; Shukla et al., 2008), and BPTU is able to block receptors in a pattern that is possibly dependent on specific conformations. The potencies and slopes for inhibition by MRS2500 and BPTU from Schild analyses are listed in Table 1. However, the results from the antagonism of β-arrestin2–mediated ERK1/2 activity are against our initial notion that BPTU behaves differently at two branches of signaling pathways.
Fig. 3.
(a) β-arrestin2–mediated ERK1/2 stimulation is completely diminished by β-arrestin2 siRNA. β-arrestin2 siRNA-transfected or control 1321N1 astrocytoma cells expressing the P2Y1R were incubated with the PKC inhibitor GO6983 (10 μM) for 20 minutes and then treated with 1 μM 2MeSADP for the indicated time points. The level of β-arrestin2 gene expression was knocked down by 94.6% measured using real-time polymerase chain reaction, as described earlier (Gao et al., 2014). #Significantly different from control value at time 0 [P < 0.01, one-way ANOVA with Multiple Comparisons (Dunnett), N = 3]. (b) Concentration-response curve of 2MeSADP-induced and β-arrestin2–mediated ERK1/2 stimulation (30 minutes) in the presence of the PKC inhibitor GO6983 (10 µM) and in the presence or absence of β-arrestin2 siRNA. (c and d) Comparison of antagonism by MRS (c) and BPTU (d) of β-arrestin2–mediated ERK1/2 stimulation. MRS (c) or BPTU (d) was added together with GO6983 (10 µM) and incubated for 20 minutes before the addition of agonist and incubated for another 30 minutes. Each data point represents the mean ± S.D. from three experiments. The KB values and slopes yielded by Schild analysis from three independent experiments are listed in Table 1.
The above results raised that possibility that differences between MRS2500 and BPTU in some signaling pathways, and different patterns of antagonism by BPTU in different pathways might be due to, for example, the influences of residence time (Guo et al., 2014; Klein Herenbrink et al., 2016), signaling amplification (Hepler, 2014), probe dependence (Kenakin, 2008; Ehlert, 2013), and cell background (Kenakin, 2009), in addition to the possibility of the conformational selective antagonism of BPTU in different pathways (Edelstein and Changeux, 2016).
To probe those possibilities, we examined whether the incubation time with BPTU may or may not affect the different patterns of antagonism. As described in Materials and Methods, in β-arrestin2–mediated receptor internalization assay, the total incubation time for BPTU is 200 minutes (20 minutes of BPTU alone and 180 minutes together with an agonist). In Gαq-mediated ERK1/2 phosphorylation, the total incubation time for BPTU is only 25 minutes (20 minutes alone and 5 minutes together with an agonist). Thus, the antagonist BPTU was preincubated at various time points in the assay of ERK1/2 phosphorylation assay (up to 3 hours, which is comparable to the total incubation time of BPTU in the receptor internalization assay). Supplemental Fig. 3 shows that the prolonged incubation time does not change the pattern of antagonism by BPTU or the EC50 value of agonist 2MeSADP. The EC50 values of 2MeSADP in the presence of BPTU (20, 30, 60, 120, and 180 minutes) are 2.2 ± 0.4, 1.5 ± 0.5, 2.1 ± 0.2, 1.6 ± 0.4, and 2.3 ± 0.5 nM, respectively, which are not significantly different from control (1.8 ± 0.3 nM) [P > 0.05, one-way ANOVA with multiple comparisons (Dunnett’s test), N = 3].
In the following experiments, to address the impact from signaling amplification, probe dependence, and cell backgrounds, we measured both branches of signaling pathways using the same cell type, U2OS cells, and compared signaling pathways with amplification (ERK1/2 and label-free measurement) and without or with limited amplification (GTP binding and β-arrestin2 recruitment) of signaling using several agonists of distinct chemical structures (2MeSADP, MRS2365, and Ap4A).
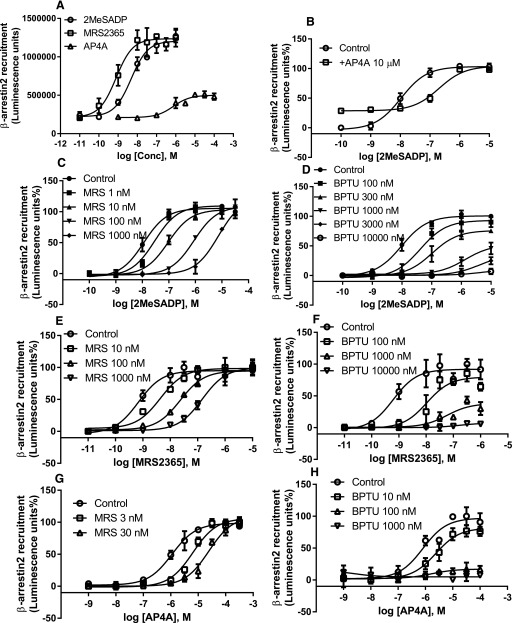
We first compared the initial steps of the two signaling branches, guanine nucleotide binding (Fig. 4) and β-arrestin2 recruitment (Fig. 5), which can be considered as without or only with limited signaling amplification. Figures 4 and 5 show that both 2MeSADP and MRS2365 are potent agonists in the stimulation of [35S]GTPγS binding (Fig. 4a) and β-arrestin 2 recruitment (Fig. 5a). The dinucleotide Ap4A was shown as a partial agonist, with maximum agonist efficacy was about 20% and 30% of that of 2MeSADP in [35S]GTPγS binding and β-arrestin2 recruitment assays, respectively (Fig. 4a; Fig. 5a). In both assays, or both branches of signaling pathways, by using three agonists of distinct structures, it was demonstrated that MRS2500 is a surmountable, whereas BPTU is an insurmountable antagonist. Thus, apparently, there is a possibility that signaling amplification plays a role in the different patterns of antagonism by BPTU, as the results from the initial step of both signaling branches demonstrated that BPTU is insurmountable and MRS2500 is surmountable.
Fig. 4.
(a) Agonist-induced [35S]GTPγS binding to membrane preparations from P2Y1R-expressing U2OS cells. The maximum stimulation by 2MeSADP was expressed as 100%. (b) Effect of MRS2500 and BPTU on Ap4A-stimulated [35S]GTPγS binding. To make the curves discernible, the maximum [35S]GTPγS binding stimulated by Ap4A is expressed as 100% (about 15–20% of the efficacy of 2MeSADP). (c) Antagonism of 2MeSADP-induced [35S]GTPγS binding by MRS2500. (d) Antagonism of 2MeSADP-induced [35S]GTPγS binding by BPTU. (e) Antagonism of MRS2365-induced [35S]GTPγS binding by MRS2500. (f) Antagonism of 2MeSADP-induced [35S]GTPγS binding by BPTU. Results were expressed as the mean ± S.D. from three independent experiments. Conc, concentration.
Fig. 5.
Concentration-response curve for β-arrestin2 recruitment induced by various agonists in the absence or presence of MRS2500 (c, e, and g) or BPTU (d, f, and h). PathHunter U2OS cells expressing the human P2Y1R were first treated with antagonists for 20 minutes and then treated with agonists for 60 minutes before the termination of reaction by adding detection reagents. Data shown are from three independent experiments of similar results performed in duplicate or triplicate. In Fig. 6, g and h, the maximum [35S]GTPγS binding stimulated by Ap4A is expressed as 100% to make the curves discernible. Conc, concentration.
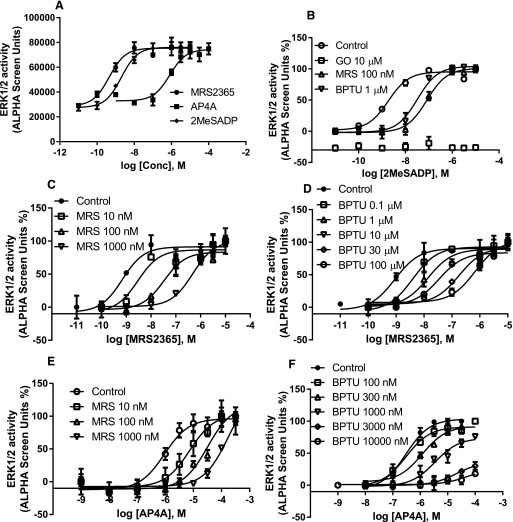
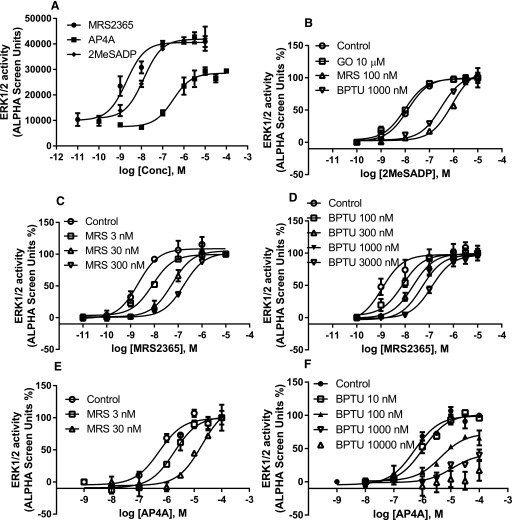
We then compared two later signaling steps after the receptor activation, Gαq-mediated and β-arrestin2–mediated ERK1/2 phosphorylation. Again, both 2MeSADP and MRS2365 are potent and full agonists in both pathways measured (Fig. 6a; Fig. 7a). Ap4A was shown to be a full agonist and high-efficacy partial agonist (65% of the maximum effect of 2MeSADP) in Gαq-mediated and β-arrestin2–mediated ERK1/2 phosphorylation, respectively. Both MRS2500 and BPTU are surmountable antagonists against both 2MeSADP and MRS2365 in both branches of the signaling pathways measured (Figs. 6 and 7). However, interestingly, MRS2500 and BPTU were shown to be a surmountable (Fig. 6e; Fig. 7e) and an insurmountable (Fig. 6f; Fig. 7f) antagonist, respectively, when using the dinucleotide Ap4A as an agonist. Thus, BPTU behaves differently in antagonizing the same signaling event induced by different agonists.
Fig. 6.
(a) Agonist-induced and Gq/11-mediated ERK1/2 stimulation in U2OS cells. (b) Antagonism of 2MeSADP-induced ERK1/2 stimulation by MRS2500, BPTU, and the PKC inhibitor GO6983 (GO). Antagonism of MRS2365-stimulated ERK1/2 activity by MRS2500 (c) and BPTU (d). Antagonism of Ap4A-stimulated ERK1/2 activity by MRS2500 (e) and BPTU (f). Cells were either treated with agonists for 5 minutes or treated with antagonists or PKC inhibitor GO6983 (10 µM) for 20 minutes before the addition of agonists for another 5 minutes. Each data point represents the mean ± S.D. from three experiments. Conc, concentration.
Fig. 7.
(a) Agonist-induced and β-arrestin2–mediated ERK1/2 stimulation in U2OS cells. (b) Antagonism of 2MeSADP-induced ERK1/2 stimulation by MRS2500, BPTU, and the PKC inhibitor GO6983. Antagonism of MRS2365-stimulated ERK1/2 activity by MRS2500 (c) and BPTU (d). Antagonism of Ap4A-stimulated ERK1/2 activity by MRS2500 (e) and BPTU (f). Cells were first treated with the PKC inhibitor GO6983 (GO; 10 µM) and/or antagonists for 20 minutes before the treatment with agonists for another 30 minutes. Maximum stimulation by Ap4A was expressed as 100% to make various curves discernible. Each data point represents the mean ± S.D. from three experiments. Conc, concentration.
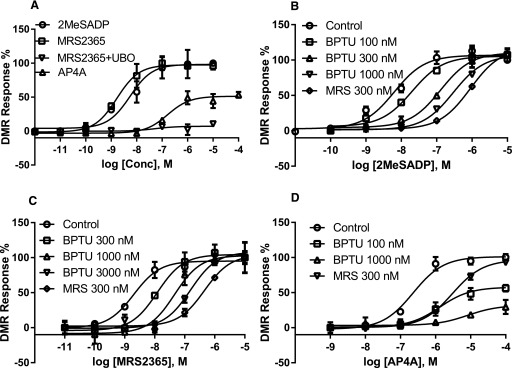
To further test the impact of signal amplification and agonist dependency on the patterns of antagonism, we performed a label-free DMR assay, a kind of end point measurement. Figure 8a shows that Ap4A is a partial agonist compared with 2MeSADP and MRS2365. The agonist effect induced by the selective P2Y1R agonist MRS2365 is completely UBO-QIC sensitive, suggesting a Gαq-mediated event, not a β-arrestin2–mediated event. Again, both MRS2500 and BPTU are surmountable antagonists when tested against 2MeSADP and MRS2365 (Fig. 8, b and c). MRS2500 and BPTU are a surmountable and an insurmountable antagonist, respectively, when tested against the dinucleotide Ap4A.
Fig. 8.
Label-free DMR measurement of agonist potencies and antagonism by MRS2500 and BPTU. (a) Measurement of agonist response and the effect of the Gαq/11 inhibitor UBO-QIC (UBO). (b) Antagonism of 2MeSADP-induced response by MRS or BPTU. (c) Antagonism of MRS2365-induced response by MRS or BPTU. (d) Antagonism of Ap4A-induced response by MRS or BPTU; Ap4A-induced maximum response was expressed as 100% to discern various concentration-response curves. U2OS cells expressing the P2Y1R cells were pretreated with inhibitors for 20 minutes followed by the addition of agonists and measured for 60 minutes. Results are expressed as the mean ± S.D. from three independent experiments performed in duplicate or triplicate. Conc, concentration.
The binding affinity of BPTU at the P2Y1R has been previously reported (Zhang et al., 2015a). In the present study, we used more concentrations to examine the pattern of displacement against the agonist radioligand [3H]2MeSADP, such as the Hill slope. Both MRS2500 and BPTU completely displaced equilibrium [3H]2MeSADP binding with slopes close to one (Table 1). BPTU, but not MRS2500, concentration-dependently accelerated the dissociation of [3H]2MeSADP from the P2Y1R (Supplemental Fig. 4).
Discussion
The present study demonstrated that an antagonist can right shift agonist concentration-response curves in a parallel manner in some signaling pathways with linear Schild plot and slope close to unity, whereas it suppresses the maximal agonist response in an insurmountable pattern in some other pathways, depending on the signaling pathways, the extent of signaling amplification, and the agonists used (Supplemental Table 1). Allosteric modulators have been suggested to have the tendency of being biased (Gao and Jacobson, 2013; Edelstein and Changeux, 2016), but it has not been well documented previously that an allosteric antagonist can be surmountable in one pathway but insurmountable in another pathway.
The dinucleotide Ap4A behaves as a partial agonist in [35S]GTPγS binding, β-arrestin2 recruitment, β-arrestin2–mediated ERK1/2 stimulation, and label-free DMR, but is a full agonist in Gq-mediated ERK1/2 stimulation. Both 5′-diphosphate agonists appear to be full agonists in all activities measured. Because the second nucleoside moiety of the dinucleotides is proposed to reside outside the principle mononucleoside binding site in P2YRs (Jacobson et al., 2015), the bitopic nature of Ap4A suggests functional modulation by this moiety to reduce the intrinsic full agonist response. This partial agonist is particularly susceptible to insurmountable antagonism by BPTU. The probe dependence of the antagonism of BPTU is consistent with the hypothesis that signaling amplification, induced by the full agonists and associated with progression of the signaling cascade, is needed to overcome the allosteric nature of BPTU to shift the antagonism from insurmountable to an apparent surmountable manner. BPTU inhibition of IP accumulation and Gq-mediated ERK1/2 activation may be surmountable since there is a lot of signal amplification in the first assay and may need only a fraction of total signaling to activate PKC in the latter assay.
The degree of P2Y1R reserve has been suggested to play a role in the agonist efficacy of ATP but not ADP (Palmer et al., 1998). It remains to be examined whether the agonist efficacies of 2MeSADP, MRS2365, and AP4A, as well as the antagonistic patterns by BPTU, are susceptible to the degree of receptor reserve. The antagonism of 2MeSADP-induced ERK1/2 phosphorylation by BPTU at three different cell types with variable expression levels shows similar patterns, suggesting that receptor reserve may not play a major role in the surmountable nature of BPTU at this specific signaling pathway. However, it remains to be seen whether the insurmountable nature of BPTU at other pathways and against other agonists is related to the degree of receptor reserve or not.
Biased agonism has been relatively well studied in recent years (Lefkowitz and Shenoy, 2005; Wisler et al., 2014). G protein–biased agonists of glucagon-like peptide 1 receptors were suggested to provide a novel therapeutic approach to type 2 diabetes (Zhang et al., 2015b). Propranolol acting at the β2-adrenergic receptor is an inverse agonist in cAMP accumulation, but it is a partial agonist in ERK1/2 phosphorylation (Azzi et al., 2003; Galandrin and Bouvier, 2006). β-Arrestin pathway-selective angiotensin 1 receptor agonists may promote cell growth via an arrestin-mediated mechanism despite their antagonism of G protein signaling (Kendall et al., 2014). Also, the proteinase-activated receptor 2 ligand GB88 selectively inhibits G(q/11)/Ca2+/PKC signaling but activates cAMP, ERK, and Rho pathways (Suen et al., 2014). Some of those earlier studies suggest that both agonists and antagonists may behave differently in distinct signaling pathways.
Biased antagonism at multiple signaling pathways has not been extensively explored, although it has been suggested that this is a potential area of therapeutic interest (Kenakin, 2014). For example, Muniz-Medina et al. (2009) demonstrated that the allosteric antagonists of CCR5 maraviroc and aplaviroc have a dramatic difference in potency in blocking human immunodeficiency virus entry and CCR5 internalization. Nadeau-Vallée et al. (2015) reported that some interleukin-1 inhibitors inhibit c-jun and RhoGTPase/Rho-associated coiled-coil–containing protein kinase but not nuclear factor-κB signaling, which could prevent inflammation-related conditions without the potential side effects caused by balanced antagonists.
It was reported that β-arrestin2 but not β-arrestin1 is critical in P2Y1R desensitization and internalization (Hoffmann et al., 2008; Reiner et al., 2009). Also, P2Y1R desensitization and internalization are mediated by different phosphorylation sites and kinases (Reiner et al., 2009). The present study also demonstrated the greater P2Y1R agonist potency in β-arrestin2 translocation compared with β-arrestin2–mediated receptor internalization and β-arrestin2–mediated ERK1/2 stimulation. Independent ERK1/2 signaling mediated via β-arrestin2 and Gq also may suggest that a distinct “active” P2Y1R conformation is coupled to each effector (Wisler et al., 2014; Edelstein and Changeux, 2016). The different patterns of antagonism at different signaling pathways, and against events induced by different agonists, raise the possibility of BPTU as a conformationally selective antagonist, which, in theory, could be more therapeutically advantageous and result in fewer side effects.
It is known that GPCRs can adopt multiple conformations that have signaling implications (Kahsai et al., 2011). Shukla et al. (2008) showed that β-arrestins can also adopt multiple conformations to inhibit classic G protein signaling and to initiate distinct β-arrestin–mediated signaling (Shukla et al., 2008; Nobles et al., 2011). Thus, distinct receptor conformations induced or stabilized by different ligands can promote distinct and functionally specific conformations in arrestins, which could partly explain the differential antagonism by BPTU at three β-arrestin2–mediated events demonstrated in the present study. To our knowledge, the antagonism of various β-arrestin signaling pathways is still a largely unexplored area. It seems that the different patterns of antagonism may also be related to different extents of signaling amplification in different pathways induced by different agonists.
Traditionally, GPCR antagonist affinity has been considered constant, regardless of the different agonists used in different assays. Recent evidence suggested that an antagonist can show different affinities if measured under distinct experimental conditions (Baker and Hill, 2007; Kenakin, 2014). One reason that has been ascribed to this phenomenon is the possible involvement of more than one binding site on the receptor. However, this assumption so far is based only on pharmacological data but not on structurally elucidated information showing distinct binding sites in a receptor. The present study is the first clear pharmacological example to show differential antagonism resulting from the binding to two structurally distinguished sites on the receptor. Thus, antagonists may not only have different affinities in distinct measurement conditions, they may also show completely different patterns of antagonism in different signaling pathways, which has not been demonstrated previously. Even in different events mediated via β-arrestin2, BPTU showed different potencies and patterns of antagonism, suggesting that both receptors and β-arrestin2 can adopt multiple conformations.
In classic pharmacology, Schild analysis of antagonist-induced shifts of agonist concentration-response curves has long been used to define the competitive property of antagonists (Schild, 1949; Arunlakshana and Schild, 1959; Kenakin, 1984; Wyllie and Chen, 2007). It is often assumed that if the pattern of a rightward shift is parallel with a slope close to one, competitive antagonism is defined. On the other hand, the definition of noncompetitive or allosteric antagonism is often based on nonlinear Schild plots, insurmountable inhibitory effects, and/or the incomplete displacement of radioligand binding. However, the present study demonstrates that none of them are strict criteria for characterizing the mode of antagonism. Thus, the conventional definition of antagonist action has to be combined with knowledge of the binding sites involved and signaling pathways measured, as demonstrated in the present study. Also, allosteric modulators may resemble competitive antagonists in both competitive binding and functional assays. It might additionally require one of the two criteria, structurally elucidated or pharmacologically demonstrated by an effect on the dissociation rate of an orthosteric agonist, to define a novel antagonist in terms of competitive or allosteric action.
A strict comparison of the pharmacological consequences of antagonists binding to different sites on a specific receptor is rare in the literature, partly due to the fact that only limited information is currently available for diverse ligand binding sites on a receptor. Most of the previous structural information derived from crystallized GPCR complexes indicated that both competitive antagonists and allosteric antagonists bind at or near the pocket formed by seven TM domains, not exclusively on the external surface of the receptor. However, it should be noted that only very few allosteric antagonists have been used in crystallization compared with the total population of GPCR antagonists available. Even for the urea class of P2Y1R antagonists related to BPTU, the binding location was initially predicated by molecular modeling to be within the helical bundle (Chao et al., 2013; Qiao et al., 2013). Thus, potentially biased functional antagonism remains to be explored for other P2Y1R antagonists and for diverse antagonists, especially allosteric modulators, of other GPCRs. Also, it remains to be seen whether the pattern of antagonism at platelet aggregation induced by various P2Y1R agonists, which is an end point functional measurement, by this unique allosteric antagonist is surmountable or insurmountable.
Regarding the molecular mechanisms of insurmountable antagonism, the slow dissociation of an antagonist-receptor complex or long residence time has been traditionally considered as a major mechanism (Guo et al., 2014; Klein Herenbrink et al., 2016). Allosteric sites, distinct conformations, and receptor internalization have also been used as alternative explanations. Insurmountable antagonism is also related to signaling amplification (Hepler, 2014), probe dependence (Ehlert, 2013), cell types. and cell background (Kenakin, 2009). Our results suggest that the insurmountable nature of BPTU can only be demonstrated in some signaling pathways and by using some agonists to initiate the receptor activation, which seems to be related to signaling amplification, agonist probes, and possibly the degree of receptor reserve. Thus, to define the nature of a ligand in terms of “surmountable” or “insurmountable,” one has to measure multiple signaling pathways by using agonists of diverse or distinct structures. Although both MRS2500 and BPTU inhibit [3H]2MeSADP binding completely in a similar pattern based on the displacement binding curves, inhibition by MRS2500 probably occurs via the direction competition of the same binding pocket, whereas the inhibition by BPTU occurs indirectly via the acceleration of the [3H]2MeSADP dissociation rate. The exact, detailed mechanism of inhibition remains to be defined.
In summary, the present study illustrates a previously undocumented example to challenge the dogma in traditional pharmacology in defining competitive versus allosteric antagonism. First, a parallel rightward shift of agonist concentration-response curves and complete inhibition of radioligand binding may or may not be evidence in support of competitive antagonism. Second, the nature of functional antagonism for various signaling pathways may be different in terms of being competitive and noncompetitive (or surmountable or insurmountable), which could be especially true for allosteric antagonists and for the largely uncharacterized antagonism at various β-arrestin pathways. Furthermore, the present study demonstrated a clear example of the implications of signaling amplification and probe dependence in the discovery and characterization of P2Y1R allosteric modulators, which could be a general phenomenon for other receptors, considering that the antagonism at various signaling pathways was largely unexplored. The findings suggest that an antagonist can be surmountable or insurmountable depending on the signaling pathways measured and the agonists used, which should be of broad relevance to drug discovery and disease treatment.
Abbreviations
- ANOVA
analysis of variance
- Ap4A
diadenosine tetraphosphate
- BPTU
1-(2-(2-(tert-butyl)phenoxy)pyridin-3-yl)-3-(4-(trifluoromethoxy)phenyl)urea
- CID
Compound Identifier
- DMR
dynamic mass redistribution
- Emax
maximum agonist effect
- ERK1/2
extracellular signal-regulated kinase 1/2
- GO6983
3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione
- GPCR
G protein–coupled receptor
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- IP
inositol phosphate
- Kd
dissociation constant
- Ki
inhibition constant
- 2MeSADP
2-methylthioadenosine 5′-diphosphate trisodium salt
- MRS2365
[[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid monoester
- MRS2500
(1′R,2′S,4′S,5′S)-4-(2-iodo-6-methylamino-purin-9-yl)-1-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane
- P2Y1R
P2Y1 receptor
- PKC
protein kinase C
- siRNA
small interfering RNA
- TM
transmembrane
- UBO-QIC
L-threonine, (3R)-N-acetyl-3-hydroxy-L-leucyl-(αR)-α-hydroxybenzenepropanoyl-2,3-didehydro-N-methylalanyl-L-alanyl-N-methyl-L-alanyl-(3R)-3-[[(2S,3R)-3-hydroxy-4-methyl-1-oxo-2-[(1-oxopropyl)amino]pentyl]oxy]-L-leucyl-N,O-dimethyl-, (7→1)-lactone
Authorship Contributions
Participated in research design: Gao, Jacobson.
Conducted experiments: Gao.
Performed data analysis: Gao.
Wrote or contributed to the writing of the manuscript: Gao, Jacobson.
Footnotes
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health [ZIA DK031116].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br Pharmacol Chemother 14:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. (2003) Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hill SJ. (2007) Multiple GPCR conformations and signalling pathways: implications for antagonist affinity estimates. Trends Pharmacol Sci 28:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H, Turdi H, Herpin TF, Roberge JY, Liu Y, Schnur DM, Poss MA, Rehfuss R, Hua J, Wu Q, et al. (2013) Discovery of 2-(phenoxypyridine)-3-phenylureas as small molecule P2Y1 antagonists. J Med Chem 56:1704–1714. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510. [DOI] [PubMed] [Google Scholar]
- Edelstein SJ, Changeux JP. (2016) Biased allostery. Biophys J 111:902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ. (2013) What ligand-gated ion channels can tell us about the allosteric regulation of G protein-coupled receptors. Prog Mol Biol Transl Sci 115:291–347. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Bouvier M. (2006) Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol 70:1575–1584. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Balasubramanian R, Kiselev E, Wei Q, Jacobson KA. (2014) Probing biased/partial agonism at the G protein-coupled A(2B) adenosine receptor. Biochem Pharmacol 90:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA. (2008) Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacol Res 57:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Jacobson KA. (2013) Allosteric modulation and functional selectivity of G protein-coupled receptors. Drug Discov Today Technol 10:e237–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, Ijzerman AP, Jacobson KA. (2011) Functionally biased modulation of A(3) adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol 82:658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Hillger JM, IJzerman AP, Heitman LH. (2014) Drug-target residence time--a case for G protein-coupled receptors. Med Res Rev 34:856–892. [DOI] [PubMed] [Google Scholar]
- Hepler JR. (2014) G protein coupled receptor signaling complexes in live cells. Cell Logist 4:e29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. (2008) Agonist-selective, receptor-specific interaction of human P2Y receptors with β-arrestin-1 and -2. J Biol Chem 283:30933–30941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Paoletta S, Katritch V, Wu B, Gao ZG, Zhao Q, Stevens RC, Kiselev E. (2015) Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol 88:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai AW, Xiao K, Rajagopal S, Ahn S, Shukla AK, Sun J, Oas TG, Lefkowitz RJ. (2011) Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat Chem Biol 7:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (2008) Functional selectivity in GPCR modulator screening. Comb Chem High Throughput Screen 11:337–343. [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2014) What is pharmacological “affinity”? Relevance to biased agonism and antagonism. Trends Pharmacol Sci 35:434–441. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (1984) The classification of drugs and drug receptors in isolated tissues. Pharmacol Rev 36:165–222. [PubMed] [Google Scholar]
- Kenakin TP. (2009) Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat Rev Drug Discov 8:617–626. [DOI] [PubMed] [Google Scholar]
- Kendall RT, Lee MH, Pleasant DL, Robinson K, Kuppuswamy D, McDermott PJ, Luttrell LM. (2014) Arrestin-dependent angiotensin AT1 receptor signaling regulates Akt and mTor-mediated protein synthesis. J Biol Chem 289:26155–26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Herenbrink C, Sykes DA, Donthamsetti P, Canals M, Coudrat T, Shonberg J, Scammells PJ, Capuano B, Sexton PM, Charlton SJ, et al. (2016) The role of kinetic context in apparent biased agonism at GPCRs. Nat Commun 7:10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. (2005) Transduction of receptor signals by beta-arrestins. Science 308:512–517. [DOI] [PubMed] [Google Scholar]
- Muniz-Medina VM, Jones S, Maglich JM, Galardi C, Hollingsworth RE, Kazmierski WM, Ferris RG, Edelstein MP, Chiswell KE, Kenakin TP. (2009) The relative activity of “function sparing” HIV-1 entry inhibitors on viral entry and CCR5 internalization: is allosteric functional selectivity a valuable therapeutic property? Mol Pharmacol 75:490–501. [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallée M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, Sanchez M, Leimert K, Boudreault A, Duhamel F, et al. (2015) Novel noncompetitive IL-1 receptor-biased ligand prevents infection- and inflammation-induced preterm birth. J Immunol 195:3402–3415. [DOI] [PubMed] [Google Scholar]
- Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, et al. (2011) Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal 4:ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. (1998) Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol 54:1118–1123. [PubMed] [Google Scholar]
- Qiao JX, Wang TC, Ruel R, Thibeault C, L’Heureux A, Schumacher WA, Spronk SA, Hiebert S, Bouthillier G, Lloyd J, et al. (2013) Conformationally constrained ortho-anilino diaryl ureas: discovery of 1-(2-(1′-neopentylspiro[indoline-3,4′-piperidine]-1-yl)phenyl)-3-(4-(trifluoromethoxy)phenyl)urea, a potent, selective, and bioavailable P2Y1 antagonist. J Med Chem 56:9275–9295. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. (2011) Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol 80:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S, Ziegler N, Leon C, Lorenz K, von Hayn K, Gachet C, Lohse MJ, Hoffmann C. (2009) beta-Arrestin-2 interaction and internalization of the human P2Y1 receptor are dependent on C-terminal phosphorylation sites. Mol Pharmacol 76:1162–1171. [DOI] [PubMed] [Google Scholar]
- Savi P, Beauverger P, Labouret C, Delfaud M, Salel V, Kaghad M, Herbert JM. (1998) Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett 422:291–295. [DOI] [PubMed] [Google Scholar]
- Schild HO. (1949) pAx and competitive drug antagonism. Br Pharmacol Chemother 4:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA. (2001) Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem 276:16379–16390. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. (2006) beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281:1261–1273. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. (2008) Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA 105:9988–9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen JY, Cotterell A, Lohman RJ, Lim J, Han A, Yau MK, Liu L, Cooper MA, Vesey DA, Fairlie DP. (2014) Pathway-selective antagonism of proteinase activated receptor 2. Br J Pharmacol 171:4112–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. (2010) Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther 335:572–579. [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100:10782–10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. (2014) Recent developments in biased agonism. Curr Opin Cell Biol 27:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Chen PE. (2007) Taking the time to study competitive antagonism. Br J Pharmacol 150:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Gao ZG, Zhang K, Kiselev E, Crane S, Wang J, Paoletta S, Yi C, Ma L, Zhang W, et al. (2015a) Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sturchler E, Zhu J, Nieto A, Cistrone PA, Xie J, He L, Yea K, Jones T, Turn R, et al. (2015b) Autocrine selection of a GLP-1R G-protein biased agonist with potent antidiabetic effects. Nat Commun 6:8918. [DOI] [PMC free article] [PubMed] [Google Scholar]