Abstract
Background
We have previously reported a high incidence of colorectal cancer (CRC) in carriers of pathogenic MLH1 variants (path_MLH1) despite follow-up with colonoscopy including polypectomy.
Methods
The cohort included Finnish carriers enrolled in 3-yearly colonoscopy (n = 505; 4625 observation years) and carriers from other countries enrolled in colonoscopy 2-yearly or more frequently (n = 439; 3299 observation years). We examined whether the longer interval between colonoscopies in Finland could explain the high incidence of CRC and whether disease expression correlated with differences in population CRC incidence.
Results
Cumulative CRC incidences in carriers of path_MLH1 at 70-years of age were 41% for males and 36% for females in the Finnish series and 58% and 55% in the non-Finnish series, respectively (p > 0.05). Mean time from last colonoscopy to CRC was 32.7 months in the Finnish compared to 31.0 months in the non-Finnish (p > 0.05) and was therefore unaffected by the recommended colonoscopy interval. Differences in population incidence of CRC could not explain the lower point estimates for CRC in the Finnish series. Ten-year overall survival after CRC was similar for the Finnish and non-Finnish series (88% and 91%, respectively; p > 0.05).
Conclusions
The hypothesis that the high incidence of CRC in path_MLH1 carriers was caused by a higher incidence in the Finnish series was not valid. We discuss whether the results were influenced by methodological shortcomings in our study or whether the assumption that a shorter interval between colonoscopies leads to a lower CRC incidence may be wrong. This second possibility is intriguing, because it suggests the dogma that CRC in path_MLH1 carriers develops from polyps that can be detected at colonoscopy and removed to prevent CRC may be erroneous. In view of the excellent 10-year overall survival in the Finnish and non-Finnish series we remain strong advocates of current surveillance practices for those with LS pending studies that will inform new recommendations on the best surveillance interval.
Electronic supplementary material
The online version of this article (10.1186/s13053-017-0078-5) contains supplementary material, which is available to authorized users.
Keywords: Lynch syndrome, Hereditary non-polyposis colorectal cancer, Colorectal cancer, Microsatellite instability
Background
Lynch syndrome (LS) is an autosomal dominantly inherited cancer syndrome predisposing to colorectal cancer (CRC) and several extra-colonic malignancies [1]. It is the most common hereditary cause of CRC, accounting for about 3% of the disease. LS is caused by constitutional pathogenic variants of any of four DNA mismatch repair (MMR) genes (MLH1, MSH2, PMS2 and MSH6) or by a deletion in the EPCAM gene which leads to MSH2 inactivation. In mutation carriers, a somatic mutation affecting the second allele leads to defective MMR activity.
Based on the international Prospective Lynch Syndrome Database (PLSD) we have demonstrated that stringent current screening guidelines do not protect fully against the development of colorectal cancer (CRC) in path_MMR carriers. This observation is despite undertaking colonoscopies with polypectomies every 3 years or even more frequently [2]. This finding is in contrast to the declared goal of the guidelines [1] that follow-up by colonoscopy and polypectomy aims to prevent CRC – an outcome that we expected to be true when issuing these guidelines. Nonetheless, LS patients undergoing surveillance often survive their first cancer and many develop subsequent cancers, again with a good prognosis, albeit a CRC mortality rate of approximately 10%.
There are many possible reasons why CRC continued to occur in our previously reported series despite follow-up with colonoscopy and polypectomy. These reasons include too great an interval between colonoscopies (Table 1). A high rate of interval CRCs in LS patients having surveillance with screening intervals of over 3 years previously prompted those involved in revising guidelines to recommend shorter surveillance intervals [1, 3–11]. This change was based upon the assumption that a major cause of interval cancers was a fast transition from visible adenomatous polyp to cancer as a consequence of the increased mutation rate associated with MMR deficiency [12]. A 3-year interval between colonoscopies has been shown to reduce the CRC incidence and mortality in LS in a comparative prospective study [3]. However, previous relevant studies [3–7, 13–18] (summarized in Table 2) have provided no definite empirically observed evidence to support what interval between colonoscopies might best prevent CRC in LS, and how protective such an intervention might be.
Table 1.
National surveillance protocols for colorectal and endometrial cancer
| Center | Series cencored | Colonoscopy | Gynecological examination | Reference no. and additional details | |||
|---|---|---|---|---|---|---|---|
| Interval | From-to | Interval | From-to | Modalities in addition to clinical examination | |||
| Finland | 2014 | 3 years | 1985–2014 | 1 year | 1995-2014 | EB, ultrasound, CA12-5 | [3, 4, 8] |
| Denmark | 2014 | 2 years | 1991–2014 | 2 years | 1991-2014 | US | [9] |
| Germany | 2014 | 1 year | 1995–2014 | 1 year | 1995-2014 | US | [10, 17] |
| Italy | 2013 | 1-2 years (1 year when age > 40 years; adenoma) | 1990–2013 | 2 years (1 years when age > 35 yrs.) | 1990-2013 | US, Pap smear | [11] |
| UK (Manchester) | 2014 | 2 years | 1994–2014 | 1 year | 1990-2014 | Hysteroscopy, US, CA12-5 | [6] |
| Sweden | 2014 | 2 years | 1990–2000 | 1 year | 1992-2014 | US, CA 12-5.Some patients: EB | [25, 26] |
| 18 months | 2000–2014 | ||||||
| Australia | 2014 | 1 year | 1990–2014 | 1 year | 1990-2005 | US, EB, CA12-5 |
https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp106_0.pdf
https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp106_0.pdf |
| 2005-2014 | Risk reducing surgery only | ||||||
| Spain | 2013 | 1-2 years (1 year when age > 40 years) | 1999–2013 | 1 year | 1999-2013 | US | Unpublished |
| The Netherlands | 2013 | 2-3 years | 1987–1996 | 1-2 years | 1994-2005 | US | [7] |
| 2 years | 1997–2013 | 1-2 years | 2005-2013 | US, EB | |||
| UK (Cardiff) | 2013 | 3 years | 1991–1994 | 1 year | 1998-2010 | US, CA12-5(3 or 4 monthly) | Unpublished Colonoscopy 96% compliance with interval. |
| 2 years | 1994–2013 | Gyn: only 27% of eligible women had gyn. Cancer screening. Since 2010 not systematic. | |||||
| UK (Newcastle) | 2014 | 2 years | 1995–2014 | No fixed | Unpublished | ||
| Norway | 2013 | 3 years | 1989–1996 | 2 years | 1989-2013 | US, CA12-5 | [5] |
| 2 years (one year when adenoma) | 1996–2013 | ||||||
US Transvaginal ultrasound
EB Endometrial biopsy
Table 2.
Studies assessing the effect of colonoscopy surveillance interval in LS
| Study | Ref. | Subjects | Inclusion criteria | Setting | CS Interval | Findings |
|---|---|---|---|---|---|---|
| Järvinen, 2000 | [3] | 252 | Amsterdam criteria and LS, gene tested | Prospective, controlled non-randomized trial | 3 years | CRC reduced 62% in 15 years compared to not screened.Mortality reduced 65% vs. no CS surveillance. Adherence 93%. |
| Dove-Edwin, 2005 | [13] | 290 | Amsterdam criteria | Retrospective | 3 years | Estimated 72% reduction of CRC mortality when screened. Adherence not reported. |
| Mecklin, 2007 | [4] | 420 | LS, gene tested | Prospective, observational | 3 years | Estimated risk for CRC 22% for women and 35% for men before age 60. No increase in CRC mortality compared to non-carriers. Adherence 98%. |
| Järvinen, 2009 | [14] | 242 | LS, gene tested | Prospective, controlled non-randomized observational | 3 years | CRC incidence 12.4% in 11·5 years, no increase in CRC mortality compared to non-carriers. Adherence 96%. |
| De vos tot Nederveen Cappel, 2002 | [15] | 857 | Amsterdam criteria or gene tested | Retrospective | 2-3 years | 10.5% cumulative CRC risk in 10 years under surveillance. Lower tumor stage if CS interval < 2 years. |
| Stormorken, 2007 | [5] | 601 | Amsterdam criteria or gene tested | Prospective, observational | 2-3 years | CRC incidence of LS carriers not increased compared to non-carriers. Adherence not reported. |
| Newton, 2015 | [6] | 227 | LS, gene tested | Retrospective | 2-3 years | CRC incidence 25% at age 70. Adherence 87%. |
| Stupart, 2009 | [16] | 178 | LS, MLH1 mutation | Prospective, controlled non-randomized observational | 1-2 years | CRC incidence 11% in 5 years compared to 27% if no surveillance. Adherence not reported. |
| Vasen, 2010 | [7] | 745 | LS, gene tested | Retrospective | 1-2 years | CRC cumulative risk 6% in 7·2 years. Adherence not reported. |
| Engel, 2010 | [17] | 622 | LS, gene tested | Prospective, controlled non-randomized observational | 1-2 years | CRC cumulative risk at age 60 23%, early stages. Adherence 81% to 15 months. |
| Stuckless, 2012 | [18] | 152 | LS, MSH2 mutation | Retrospective | 1-2 years | CRC reduced 71% in 10 years, interval cancers 27% in males and 15% in females. Adherence 44%. |
LS Lynch syndrome
CRC colorectal cancer
CS colonoscopy
Among LS patients in our previous reports from PLSD, path_MLH1 carriers had the highest incidence of CRC despite surveillance colonoscopy, making them the best cohort to examine why CRC continued to occur. The first hypothesis considered was that the large number of carriers reported in PLSD who were from Finland represented a potential confounder. The high overall incidence of CRC observed in path_MLH1 carriers in PLSD might have arisen because Finland, unlike other countries, had not shortened the recommended interval between colonoscopies from the original 3 year interval advocated many years ago.
Here we report whether or not the high CRC incidence in path_MLH1 carriers despite colonoscopy surveillance was caused by a high incidence in Finland and investigate time to CRC cancer since last colonoscopy and overall survival.
Methods
The Prospective Lynch syndrome database
PLSD contains data stored as an Oracle© relational database. Details on data storage and manipulation have been described in detail earlier [2]. Patients who were subject to prospective follow-up including colonoscopy were reported from LS registries in 13 centers in Europe and Australia. In some cases screening for early detection of endometrial and ovarian cancer were also implemented. Details on the guidelines followed at each contributing center are given in Table 1.
Study design
The design was a prospective, case-based, open observational study of path_MLH1 carriers subjected to colonoscopy comparing two groups with different recommended intervals between colonoscopies. The prospective observations recorded included follow-up from first prospectively planned and carried out colonoscopy onwards. Patients with any cancer prior to, or at the age of first colonoscopy (prevalent cancers) were excluded, as were subjects with less than 1 year of prospective observation. The following observations were used: age at first colonoscopy, gender, age at last observation, months from last completed colonoscopy to diagnosis of CRC, age at any cancer together with the ICD diagnosis of the cancer, and age at death.
Inclusion criteria
All patients included had been subject to prospective follow-up with colonoscopy because of their increased risk for CRC. All were confirmed carriers of path_MLH1 variants by genetic testing. All path_MLH1 variants were checked against the LOVD database (http://chromium.lovd.nl/LOVD2/colon_cancer/). Please see our previous report for a more detailed description of inclusion criteria [2].
Time between colonoscopies in the different centers
All subjects were offered planned regular surveillance colonoscopy and polyp removal according to the guidelines followed at each reporting center (Table 1). The recommended colonoscopy interval in the Finnish national registry protocol was 3 years throughout the study period. From 1997 onwards, all the other centers recommended surveillance intervals of 2 years or less. Thus, only observations from 1997 onwards were included in the present study. Each center kept track of their activities through medical files and/or research registries, and all were able to contribute complete reports for all patients included with no missing values. Whilst these methods do not conform to the stringency of a clinical trial they nonetheless represent the best that can be achieved by a collaborative group of highly interested expert clinicians working in diverse health service systems.
Surveillance for extra-colonic cancers
Patients in the contributing centers were informed of general cancer awareness but the surveillance for extra-colonic cancers varied across the centers (Table 1).
Cancer treatment
All cancers were treated according to local standards. Treatment modalities were not considered.
Events scored
All infiltrating CRCs were scored using the first three positions in the ICD-9 system. Age at each cancer was recorded. Age at death was recorded.
Annual and cumulative incidence rates
Each patient was observed from age at inclusion to age at last observation or first cancer. For each patient, the number of years observed in each five-year group from 25 years of age onwards was counted. All first cancers were scored according to the age at diagnosis. Annual incidence rates (AIR) for age groups were calculated by dividing the number of cancers observed by the total number of observation years. Each patient was counted once only, irrespective of how many synchronous cancers the patient might have had as first cancers. Later cancers were not considered. Cumulative incidence, denoted by Q, was computed starting at age 25, assuming zero incidence before age 25, using the formula Q(age) = Q(age-1) + [1-Q(age-1)]·AIR(age) where AIR(age) is the annual incidence for the corresponding 5 year interval. The hazard rate H = −ln[1-AIR] was used with standard error estimated as SEH = SEAIR/(1-AIR). The standard error, denoted by SEQ, was computed in two steps and 95% confidence intervals were estimated. Follow-up continued after the occurrence of first cancers, and all patients were either reported to be alive or validated to be alive in a population register when censored. See our previous report [2] for a more detailed description of these methods. Differences in time-to-events were also calculated by the Kaplan-Meier algorithm and Mantel-Cox p-values when appropriate.
Survival
Crude survival was calculated by the Kaplan-Meier algorithm and Mantel-Cox p-values as time from first cancer to last observation/death. Cancer stages at diagnosis and causes of death were not considered in this report.
Results
There were 7924 observation years in the final analysis from 430 males and 514 females (n = 944). The Finnish series included 4625 and the non-Finnish series 3299 observation years after first colonoscopies. Ages at inclusion were similar for both series (35.2 years and 36.1 years, respectively; p > 0.05). Baseline characteristics of the study population and the classification of path_MLH1 variants are presented in Table 3.
Table 3.
Baseline characteristics of the study population
| All subjects | 3-year interval (Finnish) | 1-2-year interval (non-Finnish) | ||
|---|---|---|---|---|
| Observation years | 7928 | 4625 | 3299 | |
| Number of subjects | 944 | 505 | 439 | |
| Gender | Male | 430 | 246 (48.7%) | 184 (41.9%) |
| Female | 514 | 259 (51.3%) | 255 (58.1%) | |
| Age at inclusion | (Mean, SD) | 35.5 (11.7) | 35.2 (12.1) | 36.1 (11.0) |
| Follow-up time | (Mean, SD) | 8.4 (5.7) | 9.2 (5.9) | 7.5 (5.2) |
SD standard deviation
Classification of path_MLH1 variants
Seven hundred and six (75%) of the patients carried path_MLH1 variants found in LOVD, and 238 of variants (25%) were not found in LOVD. Among the 706 found in LOVD, 691 (98%) were pathogenic (class 5) and 15 (2%) were probably pathogenic (class 4). Among the 505 in the Finnish series 406 (80%) and four (0.7%) had class 5 and class 4 variants, Among the non-Finnish series 285 (65%) had class 5 and 11 (2.5%) class 4 variants in LOVD, Among the 238 variants not reported to LOVD 95 were detected in the Finnish series and 143 in the non-Finnish series. All of them were eventually classified as class 5 and 4 based on the combined assessment of two of the co-authors (IF, JS) following the updated InSight rules [19].
Cumulative incidences of cancers
A total of 101 CRCs and 83 extra-colonic cancers were diagnosed during the follow-up period. Despite less frequent colonoscopy, the cumulative incidences of CRC were modestly lower for the Finnish series compared to the non-Finnish series in all age groups (39.2%; 95% confidence interval 29.4–48.9 vs 53%; 39.6–68) but not significantly so. Regarding extra-colonic cancers, similar observations were made (39.7%; 28.2–51.2 vs 57.2%; 41–73.5, respectively). The cumulative incidence rates categorized by 10-year intervals from age 25 are shown in Table 4 and Fig. 1a and b. Additional file 1: Table S1 presents the annual incidence rates of cancers categorized by five-year intervals from 25 to 70 years.
Table 4.
Cumulative incidences from 25 years of age and 95% confidence intervals for colorectal cancer and extra-colonic cancer
| 3-year interval (Finnish) | 1-2-year interval (non-Finnish) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Current age | Age stop | Obs years | #Ca | Cumulative incidence (%) | 95% CI | Obs years | #Ca | Cumulative incidence (%) | 95% CI |
| Colorectal cancer | |||||||||
| All | |||||||||
| 25 | 40 | 1982 | 19 | 12.9 | [7.4–18.3] | 1299 | 17 | 16.4 | [9.2–23.7] |
| 25 | 50 | 3301 | 40 | 25.4 | [18.6–32.3] | 2424 | 36 | 30.0 | [21.7–38.3] |
| 25 | 60 | 4070 | 47 | 31.7 | [24.0–39.4] | 2935 | 48 | 44.9 | [34.6–55.2] |
| 25 | 70 | 4330 | 51 | 39.2 | [29.4–48.9] | 3094 | 50 | 53.8 | [39.6–68.0] |
| Male | |||||||||
| 25 | 40 | 1026 | 12 | 15.7 | [7.5–23.9] | 610 | 9 | 18.5 | [7.2–29.7] |
| 25 | 50 | 1682 | 25 | 30.8 | [20.7–40.9] | 1110 | 21 | 37.0 | [24.3–49.8] |
| 25 | 60 | 2075 | 29 | 36.9 | [26.1–47.7] | 1288 | 28 | 57.8 | [41.5–74.0] |
| 25 | 70 | 2189 | 30 | 41.1 | [28.2–54.0] | 1365 | 28 | 57.8 | [41.5–74.0] |
| Female | |||||||||
| 25 | 40 | 956 | 7 | 9.6 | [2.8–16.4] | 689 | 8 | 14.8 | [5.3–24.4] |
| 25 | 50 | 1623 | 15 | 19.4 | [10.6–28.3] | 1314 | 15 | 24.1 | [13.4–34.8] |
| 25 | 60 | 2010 | 18 | 26.2 | [15.2–37.2] | 1646 | 20 | 35.2 | [22.0–48.3] |
| 25 | 70 | 2156 | 21 | 36.4 | [22.1–50.6] | 1729 | 22 | 55.3 | [30.5–80.0] |
| Extra-colonic cancer | |||||||||
| All | |||||||||
| 25 | 40 | 1982 | 4 | 2.7 | [0.1–5.3] | 1299 | 3 | 2.9 | [0.0–6.1] |
| 25 | 50 | 3301 | 24 | 16.9 | [10.7–23.2] | 2424 | 22 | 17.8 | [11.0–24.6] |
| 25 | 60 | 4070 | 39 | 32.2 | [23.5–40.9] | 2934 | 35 | 35.3 | [24.8–45.7] |
| 25 | 70 | 4330 | 42 | 39.7 | [28.2–51.2] | 3094 | 41 | 57.2 | [41.0–73.5] |
Obs years observation years at age group
#Ca number of cancers during observation
CI confidence interval
Fig. 1.
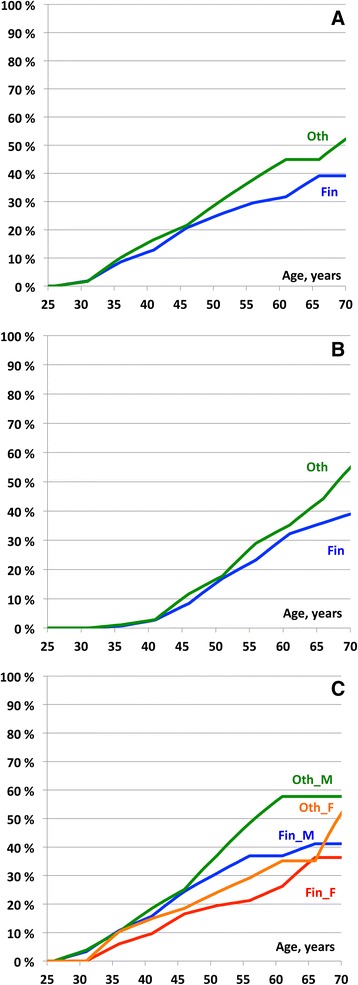
a Calculated cumulative incidence for colorectal cancer in the 3-year and non-Finnish series. Fin = Finnish series (blue line). Oth = non-Finnish series (green line). b Calculated cumulative incidence for extra-colonic cancer in the 3-year and non-Finnish series. Fin = Finnish series (blue line). Oth = non-Finnish series (green line). c Calculated cumulative incidence for colorectal cancer by gender in the 3-year and non-Finnish series. Fin_M = Finnish series, males (blue line). Fin_F = Finnish series, females (red line). Oth_M = non-Finnish series, males (green line). Oth_F = non-Finnish series, females (orange line)
The similar age of inclusion allowed us to make comparisons of time from inclusion to diagnosis of CRC using the Kaplan-Meier algorithm. In the Finnish series, 3 and 11% of carriers were diagnosed with CRC after 5 and 10 years of follow-up, respectively, compared to 5 and 14%, respectively in the non-Finnish series (p > 0.05). The differences were modestly larger in males than in females (Fig. 1c). The percentages of carriers developing extra-colonic cancers were also similar: 3 and 9% at 5 and 10 years after inclusion in the Finnish series compared to 3 and 11% in the non-Finnish series (p > 0.05). In sum, none of the differences were significant and all point estimates were similar or lower in the Finnish series.
Correlation with population incidence of CRC
Next, we explored the correlation between observed CRC incidences in path_MLH1 LS patients and the corresponding population incidences of CRC. The relative incidence of CRC in the Finnish population compared to the other countries was 0.80 [the age-standardized incidence rate in Finland was 35.0 per 100,000 versus a mean of 43.6 per 100,000 for the other reporting countries (http://eco.iarc.fr/EUCAN/; downloaded October 2015)]. By comparison, the observed ratio of cumulative risk for CRC at 70 years in the Finnish series of path_MLH1 carriers compared to the others was similar: 0.72 (39.2% in the Finnish series versus the average of 54.8% in other countries combined).
Time since last colonoscopy to CRC and survival
The mean time from last colonoscopy to CRC did not differ between the Finnish and non-Finnish series irrespective of the interval between colonoscopies. It was 32.7 (SD 13.6) months for the Finnish series and 31.0 (SD 23.4) months for the non-Finnish series (p > 0.05). Times since last colonoscopy to CRC in the two series by 6 months periods are shown in Table 5. Of note, the rates of interval cancers occurring before the next planned 2-yearly or 3-yearly colonoscopy were similar in both series at 56.9% and 50% in the Finnish and the others respectively (Table 5).
Table 5.
Months since last colonoscopy with no cancer to colorectal cancer diagnosed
| 3-year interval (Finnish) | 1-2-year interval (non-Finnish) | |||||
|---|---|---|---|---|---|---|
| Months since last colonoscopy | Number CRC | Cumulative number CRC | Cumulative % | Number CRC | Cumulative number CRC | Cumulative % |
| <6 | 0 | 0 | 0% | 0 | 0 | 0% |
| 7–11 | 2 | 2 | 3.9% | 5 | 5 | 10% |
| 12–17 | 3 | 5 | 9.8% | 13 | 18 | 36% |
| 18–23 | 4 | 9 | 17.6% | 7 | 25 | 50% |
| 24–29 | 17 | 26 | 51% | 6 | 31 | 62% |
| 30–35 | 3 | 29 | 56.9% | 4 | 35 | 70% |
| 36–41 | 14 | 43 | 84.3% | 3 | 37 | 74% |
| 42–47 | 3 | 46 | 90.2% | 3 | 40 | 80% |
| 48–120 | 5 | 51 | 100% | 10 | 50 | 100% |
CRC colorectal cancer
Moreover, there were no differences in the survival of CRC in MLH1 carriers in the two series. Five- and ten-year overall survival after CRC was 90% (95% confidence interval: 78-96%) and 88% (75-95%), respectively, in the Finnish series compared to 96% (85-99%) and 91% (78-97%) in the 1-2-year interval series (p > 0.05). There were not enough deaths for meaningful stratification by time since last colonoscopy with respect to survival. Finally, there were no differences in survival of extracolonic cancers. Five- and ten-year survival after extra-colonic cancer was 79% (64-89%) and 79% (64-89%), respectively, in the Finnish series compared to 82% (68-92%) and 82% (69-92%) in the 1-2-year interval series (p > 0.05).
Discussion
This study showed that the high incidence of CRC observed in our combined international series of path_MLH1 carriers was not caused by a higher incidence in the Finnish series compared to the others. In contrast to what we expected, the CRC incidence in the Finnish series was lower than in the others, but not significantly so.
First, we considered the possibility that the trend towards a lower CRC incidence in the Finnish series could reflect the lower population incidence of CRC in Finland. In fact, the observed ratio of cumulative risk for CRC at 70 years showed a similar pattern in the Finnish and non-Finnish series. Disease expression differences between Finnish and non-Finnish path_MLH1 carriers could reflect population-specific environmental and behavioural factors or a lower penetrance of the Finnish founder path_MLH1 variants compared to other path_MLH1 variants but this study shows that these factors cannot have a major impact on the observed high cumulative risk of CRC in path_MLH1 carriers in the total PLSD cohort of path_MLH1 patients.
Secondly, we examined whether or not the Finnish patients were actually colonoscoped less frequently than the others. We found that the time between last colonoscopy and CRC was similar for both series irrespective of the recommendations in place. Also, it was evident that a proportion of CRCs in both series could be classified as interval cancers (Table 5). In particular, the rates of interval cancers that occurred before the planned next 2-yearly or 3-yearly colonoscopy were 56.9% and 50% in the Finnish series and the others, respectively (Table 5). While the organizational aspects of surveillance at the non-Finnish centers were heterogenous and details that might impact our observations were not readily available, the organization of surveillance in the Finnish series displays two key features that are critical for the interpretation of our findings: there are special and dedicated booking and call back systems in place to follow all identified LS carriers and the reported adherence to colonoscopy intervals has been consistently very high throughout the years [4, 8]. Thus, in the Finnish series non-compliance/adherence with local surveillance guidelines cannot explain the occurrence of all cancers and the pattern of time from last colonoscopy to CRC suggests that only a limited proportion of CRCs would have been prevented by reducing the interval from 3 to 2 years.
Our study has a number of strengths including: (i) a large number of observation years; (ii) a focus on path_MLH1 carriers showing a high cumulative risk thereby avoiding mixing of carriers of path_MMR variants in different MMR genes and (iii) the robustness of the dataset collated based upon prospectively observed outcomes. On the other hand, several limitations need to be highlighted: (i) in spite of the number of observation years included our sample size is still limited. Stochastic variation cannot be completely ruled out and our observations do not exclude a significant impact of differences in population prevalence of CRC on LS expressivity; and (ii) only a detailed analysis of the interval, quality and reported findings of the colonoscopies will allow a refined assessment of the impact of this confounder. However, such an analysis will not change the critical observation that time between colonoscopy and CRC is essentially the same in both series.
The prospective health outcome observations presented here challenge the dogma that the performance of very frequent colonoscopies, many of them performed in expert centers, has a strong primary preventive effect on CRC incidence in LS. It is notable that despite surveillance, the expert centers with good recall systems in place that have contributed their data to this study have not succeeded in preventing CRC as has been achieved in non-LS familial CRC [20]. Consequently, our observations set the foundations for a more detailed analysis of the determinants of this lack of success. In this regard, there is biological evidence that CRC in LS may arise directly without a precursor polyp although the relative contribution of this distinct natural history to the totality of CRCs observed is a matter of controversy [21–24]. Thus, it is possible that even colonoscopies achieving the best quality standards would not be able to prevent all CRCs in LS.
Whether shorter colonoscopy interval could result in lower CRC stage needs to be addressed in future studies with cancer stage included. We are in the process of expanding our database in this regard.
Conclusions
In summary, we tested whether or not the high incidence of CRC in path_MLH1 carriers observed in our previous reports was caused by a distinct high incidence in the Finnish series in which 3-yearly colonoscopy was recommended. In the present report we show that the cumulative risk of CRC is high for path_MLH1 carriers undergoing colonoscopic surveillance irrespective of the specific characteristics of their country of origin and the associated factors that may have an impact on the expression of the disease. The contribution of more observation years from countries with different population CRC incidences will be needed to formally test the hypothesis that population CRC incidence correlates with LS expression. The lack of a difference in the time between last colonoscopy and CRC in the two series investigated in this study challenges current beliefs regarding colonoscopy intervals in LS surveillance. Our findings mandate a detailed study that will eventually inform policy makers. Finally, in view of the excellent 10-year overall survival in the Finnish and non-Finnish series we remain strong advocates of current surveillance practices for those with LS pending studies that will inform new recommendations on the best surveillance interval.
Acknowledgements
Not applicable.
Funding
The Finnish contribution: The Finnish Cancer Foundation, The Sigrid Juselius Foundation, Mary and Georg C. Ehrnrooth foundation, Jane and Aatos Erkko foundation and State Research Funding. The Spanish contribution: Spanish Ministry of Economy and Competitiveness, the Carlos III Health Institute, the Scientific Foundation Asociación Española Contra el Cáncer and the Government of Catalonia. The Welsh Contribution: Wales Gene Park.
The study sponsors did not have a role in planning the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. We have published a website www.lscarisk.org on which cancer risks for all published data can be reviewed and calculated in graphic form.
Abbreviations
- AIR
Annual incidence rate
- CI
Confidence interval
- CRC
Colorectal cancer
- ICD9
International Classification of Disease, 1975 revision.
- InSiGHT
International Society for Gastrointestinal Hereditary Tumours
- LOVD
Leiden Open Variation Database
- LS
Lynch Syndrome
- MMR
Mismatch repair
- path_MLH1
Pathogenic (disease-causing) variant of the MLH1 gene
- path_MSH2
Pathogenic (disease-causing) variant of the MSH2 gene
- path_MSH6
Pathogenic (disease-causing) variant of the MSH6 gene
- path_PMS2
Pathogenic (disease-causing) variant of the PMS2 gene
Additional file
Annual incidence rates (AIR) and 95% confidence intervals for colorectal cancer and extra-colonic cancer. (DOCX 15 kb)
Authors’ contributions
TS, PM, GDE, JRS, GC, JB, GM and IMF wrote the paper. PM: Designed the study, managed the database and computed the results. EH, SN, EAR and KT calculated the confidence intervals to the cumulative incidences. All others: Participated in study design, interpreting of results, commenting on the manuscript and approved the final manuscript.
Ethics approval and consent to participate
All reporting centers obtained informed consent for genetic testing and surveillance procedures. De-identified data was exported for the current study. No named registry needing approval was established for the current study.
Consent for publication
Not applicable.
Competing interests
Toni Seppälä: a co-owner (20%) of Healthfund Finland Oy (educational and health care services in Finland, not related to patients or scope of this manuscript). Travel costs to a scientific meeting by Medtronic Finland.
John Burn: a patent for high speed low cost tumor profiling pending to John Burn and QuantuMDx.
All others: None declared.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13053-017-0078-5) contains supplementary material, which is available to authorized users.
Contributor Information
Toni Seppälä, Phone: +358443398858, Email: toni.seppala@fimnet.fi, Email: toni.t.seppala@hus.fi.
Kirsi Pylvänäinen, Email: kirsi.pylvanainen@ksshp.fi.
Dafydd Gareth Evans, Email: gareth.evans@cmft.nhs.uk.
Heikki Järvinen, Email: heikki.jj.jarvinen@gmail.com.
Laura Renkonen-Sinisalo, Email: laura.renkonen-sinisalo@hus.fi.
Inge Bernstein, Email: i.bernstein@rn.dk.
Elke Holinski-Feder, Email: elkeholinski-feder@t-online.de.
Paola Sala, Email: salapaola54@gmail.com.
Annika Lindblom, Email: annika.lindblom@ki.se.
Finlay Macrae, Email: finlay.macrae@mh.org.au.
Ignacio Blanco, Email: ignacio.blanco.guillermo@gmail.com.
Rolf Sijmons, Email: r.h.sijmons@medgen.umcg.nl.
Jacqueline Jeffries, Email: jeffriesjl@cardiff.ac.uk.
Hans Vasen, Email: hfavasen@stoet.nl.
John Burn, Email: john.burn@newcastle.ac.uk.
Sigve Nakken, Email: sigven@ifi.uio.no.
Eivind Hovig, Email: ehovig@ifi.uio.no.
Einar Andreas Rødland, Email: einarro@ifi.uio.no.
Kukatharmini Tharmaratnam, Email: k.tharmaratnam1@lancaster.ac.uk.
Wouter H. de Vos tot Nederveen Cappel, Email: w.h.de.vos@isala.nl
James Hill, Email: james.hill@cmft.nhs.uk.
Juul Wijnen, Email: j.wijnen@lumc.nl.
Mark Jenkins, Email: m.jenkins@unimelb.edu.au.
Maurizio Genuardi, Email: maurizio.genuardi@unicatt.it.
Kate Green, Email: kate.green@cmft.nhs.uk.
Fiona Lalloo, Email: fiona.lalloo@cmft.nhs.uk.
Lone Sunde, Email: lonsunde@rm.dk.
Miriam Mints, Email: miriam.mints@ki.se.
Lucio Bertario, Email: lucio.bertario@gmail.com.
Marta Pineda, Email: mpineda@iconcologia.net.
Matilde Navarro, Email: mnavarrogarcia@iconcologia.net.
Monika Morak, Email: monika.morak@gmx.de.
Ian M. Frayling, Email: fraylingim@cf.ac.uk
John-Paul Plazzer, Email: johnpaul.plazzer@gmail.com.
Julian R. Sampson, Email: sampson@cardiff.ac.uk
Gabriel Capella, Email: gcapella@ico.scs.es.
Gabriela Möslein, Email: gabriela.moeslein@helios-kliniken.de.
Jukka-Pekka Mecklin, Email: jukka-pekka.mecklin@ksshp.fi.
Pål Møller, Email: moller.pal@gmail.com.
in collaboration with The Mallorca Group, http://mallorca-group.eu.
References
- 1.Vasen HFA, Blanco I, Aktán-Collán K, Gopie JP, Alonso A, Aretz S, et al. Revised guidelines for the clinical management of lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812–823. doi: 10.1136/gutjnl-2012-304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective lynch syndrome database. Gut. 2017;66:464–472. doi: 10.1136/gutjnl-2015-309675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/S0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 4.Mecklin J-P, Aarnio M, Läärä E, Kairaluoma MV, Pylvänäinen K, Peltomäki P, et al. Development of colorectal tumors in colonoscopic surveillance in lynch syndrome. Gastroenterology. 2007;133:1093–1098. doi: 10.1053/j.gastro.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Stormorken AT, Clark N, Grindedal E, Mæhle L, Møller P. Prevention of colorectal cancer by colonoscopic surveillance in families with hereditary colorectal cancer. Scand J Gastroenterol. 2007;42:611–617. doi: 10.1080/00365520601010230. [DOI] [PubMed] [Google Scholar]
- 6.Newton K, Green K, Lalloo F, Evans DG, Hill J. Colonoscopy screening compliance and outcomes in patients with lynch syndrome. Color Dis. 2015;17:38–46. doi: 10.1111/codi.12778. [DOI] [PubMed] [Google Scholar]
- 7.Vasen HFA, Abdirahman M, Brohet R, Langers AMJ, Kleibeuker JH, van Kouwen M, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with lynch syndrome. Gastroenterology. 2010;138:2300–2306. doi: 10.1053/j.gastro.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 8.Pylvänäinen K, Kairaluoma M, Mecklin J-P. Compliance and satisfaction with long-term surveillance in Finnish HNPCC families. Familial Cancer. 2006;5:175–178. doi: 10.1007/s10689-005-5442-3. [DOI] [PubMed] [Google Scholar]
- 9.Olsen KR, Bojesen SE, Gerdes A-MM, Lindorff-Larsen K, Bernstein IT. Cost-effectiveness of surveillance programs for families at high and moderate risk of hereditary non-polyposis colorectal cancer. Int J Technol Assess Health Care. 2007;23:89–95. doi: 10.1017/S0266462307051616. [DOI] [PubMed] [Google Scholar]
- 10.Plaschke J, Engel C, Krüger S, Holinski-Feder E, Pagenstecher C, Mangold E, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German hereditary nonpolyposis colorectal cancer consortium. J Clin Oncol. 2004;22:4486–4494. doi: 10.1200/JCO.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Bertario L, Russo A, Sala P, Eboli M, Radice P, Presciuttini S, et al. Survival of patients with hereditary colorectal cancer: comparison of HNPCC and colorectal cancer in FAP patients with sporadic colorectal cancer. Int J Cancer. 1999;80:183–187. doi: 10.1002/(SICI)1097-0215(19990118)80:2<183::AID-IJC4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HF, Nagengast FM, Khan PM. Interval cancers in hereditary non-polyposis colorectal cancer (lynch syndrome) Lancet. 1995;345:1183–1184. doi: 10.1016/S0140-6736(95)91016-6. [DOI] [PubMed] [Google Scholar]
- 13.Dove-Edwin I, Sasieni P, Adams J, Thomas HJW. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331:1047. doi: 10.1136/bmj.38606.794560.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järvinen HJ, Renkonen-Sinisalo L, Aktán-Collán K, Peltomäki P, Aaltonen LA, Mecklin J-P. Ten years after mutation testing for lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 15.de Vos Tot Nederveen Cappel WH, Nagengast FM, Griffioen G, Menko FH, Taal BG, Kleibeuker JH, et al. Surveillance for hereditary nonpolyposis colorectal cancer: a long-term study on 114 families. Dis Colon rectum. 2002;45:1588–1594. doi: 10.1007/s10350-004-7244-3. [DOI] [PubMed] [Google Scholar]
- 16.Stupart DA, Goldberg PA, Algar U, Ramesar R. Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Color Dis. 2009;11:126–130. doi: 10.1111/j.1463-1318.2008.01702.x. [DOI] [PubMed] [Google Scholar]
- 17.Engel C, Rahner N, Schulmann K, Feder EH, Goecke TO. Schackert HK, et al. Efficacy of annual Colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. YJCGH. 2010;8:174–182. doi: 10.1016/j.cgh.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Stuckless S, Green JS, Morgenstern M, Kennedy C, Green RC, Woods MO, et al. Impact of colonoscopic screening in male and female lynch syndrome carriers with an MSH2 mutation. Clin Genet. 2012;82:439–445. doi: 10.1111/j.1399-0004.2011.01802.x. [DOI] [PubMed] [Google Scholar]
- 19.Thompson BA, Spurdle AB, Plazzer J-P, Greenblatt MS, Akagi K, Al-Mulla F, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. 2014;46:107–115. doi: 10.1038/ng.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesher D, Dove-Edwin I, Sasieni P, Vasen H, Bernstein I, Royer-Pokora B, et al. A pooled analysis of the outcome of prospective colonoscopic surveillance for familial colorectal cancer. Int J Cancer. 2014;134:939–947. doi: 10.1002/ijc.28397. [DOI] [PubMed] [Google Scholar]
- 21.Kloor M, Huth C, Voigt AY, Benner A, Schirmacher P, Knebel-Doeberitz von M, et al. Prevalence of mismatch repair-deficient crypt foci in lynch syndrome: a pathological study. Lancet Oncol. 2012;13:598–606. doi: 10.1016/S1470-2045(12)70109-2. [DOI] [PubMed] [Google Scholar]
- 22.Kloor M, Michel S, Buckowitz B, Rüschoff J, Büttner R, Holinski-Feder E, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121:454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 23.Ahadova A, Knebel-Doeberitz von M, Bläker H, Kloor M. CTNNB1-mutant colorectal carcinomas with immediate invasive growth: a model of interval cancers in lynch syndrome. Familial Cancer. 2016;15:579–586. doi: 10.1007/s10689-016-9899-z. [DOI] [PubMed] [Google Scholar]
- 24.de Jong AE, Morreau H, Van Puijenbroek M, Eilers PHC, Wijnen J, Nagengast FM, et al. The role of mismatch repair gene defects in the development of adenomas in patients with HNPCC. Gastroenterology. 2004;126:42–48. doi: 10.1053/j.gastro.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren G, Liljegren A, Jaramillo E, Rubio C, Lindblom A. Adenoma prevalence and cancer risk in familial non-polyposis colorectal cancer. Gut. 2002;50:228–234. doi: 10.1136/gut.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsberg A, Kjellström L, Andreasson A, Jaramillo E, Rubio CA, Björck E, et al. Colonoscopy findings in high-risk individuals compared to an average-risk control population. Scand J Gastroenterol. 2015;50:1–9. doi: 10.3109/00365521.2014.966317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. We have published a website www.lscarisk.org on which cancer risks for all published data can be reviewed and calculated in graphic form.


