Abstract
Scope
The effect of diabetes on the pharmacokinetics, bioavailability and brain distribution of grape polyphenols and select metabolites was studied in the Zucker diabetic fatty (ZDF) rat model.
Methods and Results
(ZDF) rats and their lean controls (LN) were dosed with a Standardized Grape Polyphenol (SGP) Mixture consisting of grape seed extract, Concord grape juice and resveratrol (RES) by oral gavage for 10 days. An 8 hour pharmacokinetic study was performed. After 24 hours a second dose of SGP was administered and one hour later animals were sacrificed and brain tissue harvested. Plasma, urine and brain tissue were analyzed for grape polyphenols. ZDF rats exhibited significantly diminished Cmax for all catechin, epicatechin, quercetin and resveratrol conjugated metabolites. Bioavailability was significantly lower in ZDF rats for methylated flavan-3-ol, RES and quercetin metabolites. Significantly lower levels of metabolites of RES, quercetin, and flavan-3-ols were found in brains of ZDF rats. There was no significant difference between ZDF and LN in anthocyanins in plasma and no anthocyanins were detectable in brain extracts. ZDF rats showed significantly higher urinary excretion for all polyphenols.
Conclusions
Diabetes may alter the overall bioavailability of some polyphenols in plasma and brain in part due to higher urinary clearance.
Keywords: Bioavailability, Brain, Diabetes, Grape polyphenols, Pharmacokinetics
INTRODUCTION
Alzheimer’s disease (AD) is an age-related neurodegenerative disease that affected more than 5.4 million Americans in 2016 with an estimated cost of $236 billion [http://www.alz.org/documents_custom/2016-facts2016_infographic.pdf]. These costs are estimated to increase to over $1 trillion by 2050. Despite its severity and economic burden, there is currently no cure. Diabetes mellitus is a condition defined by hyperglycemia which leads to high risks of microvascular damage including retinopathy, nephropathy and neuropathy. Diabetes affects 29.1 million people in the United States in 2012 which accounted for 9.3% of the U.S. population [http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf]. Like AD, diabetes poses a huge economic burden on the society with $245 billion dollars medical costs estimated in 2012.
Epidemiological studies have suggested a strong link between diabetes and AD [1]. Sedentary life style and high fat consumption are believed to contribute to the development of metabolic syndrome which may eventually progress to diabetes. In a 27 year longitudinal population-based study, obesity and body mass index has been strongly associated with onset of dementia and AD [2]. The same group also highlighted body mass index as a risk factor for AD [3, 4]. Disturbances in insulin signaling in diabetes have been implicated in the development and progression of AD [5]. Using data from the Rotterdam study it was found after a 3 year follow up that insulin resistance increased the risk of developing AD by 40% [6]. In a recently published four year longitudinal study, researchers found that insulin resistance in a late-middle aged cohort is positively associated with brain atrophy in the regions that are affected by early AD. Their results suggested that higher insulin resistance predicted medial temporal lobe atrophy that corresponded to cognitive deficits [7]. Considering the link between diabetes and neurodegenerative processes, diabetic and metabolic syndrome patients are potential targets for AD preventive treatments. There have been a number of studies reviewed by Xiao and Högger [8] which have demonstrated the benefit of polyphenols for the prevention or management of diabetes. Higher anthocyanin intake is associated with a lower risk of type-2 diabetes in some studies. One possible mechanism by which polyphenols may modulate diabetes risk or severeity is by inhibition of α-glucosidase in the small intestin. This would decrease the amout of glucose absorbed and help maintain a lower blood glucose.
Grape products including purple grape juice, red wine, grape skin and seed extracts have been shown in rodent models to ameliorate or prevent diabetes and AD [9–16]. These polyphenol rich products provide distinct polyphenol profiles which include flavan-3-ols, flavonols, and stilbenoids such as resveratrol (RES). Conjugated phase II metabolites of the parent polyphenols, present in the extract or food, have been identified in plasma and brain tissue [17–20]. These metabolites including both methylated and glucuronidated conjugates of the polyphenols found in grape juice, grape seed extract (GSE) and red wine have been demonstrated to target distinct molecular mechanisms related to AD [18–20]. In order to target multiple mechanisms simultaneously a combination of grape juice, GSE and resveratrol was developed to deliver all relevant polyphenol metabolites to brain tissues simultaneously. This combination treatment has been shown in animal models to reduce total amyloid content in the brain [21].
While promising, the relevance of this procedure to at risk groups including type II diabetics or obese individuals is not clear. Impaired insulin signaling, a major defect of type II diabetes, is believed to alter expression of key metabolizing enzymes responsible for generation of bioactive polyphenol metabolites, including UDP-glucuronosyltransferase and sulfotransferases [22]. Additionally, diabetes may alter gastrointestinal mobility which can, in turn, impact polyphenol absorption and metabolism [23].
Another complication of diabetes is osmotic dieresis caused by hyperglycemia and electrolyte imbalance. Osmotic dieresis results in excessive urinary losses of water and electrolytes and if diabetes is untreated gradually progresses to kidney failure [24]. Polyphenol conjugated metabolites are rapidly excreted in urine. Excessive urinary losses in diabetics could potentially impact circulating polyphenol metabolite concentrations, and ultimate delivery to brain tissues may be significantly reduced. Therefore, while the diabetic condition may be a risk factor for AD onset and progression, it may also alter the bioavailability of polyphenols which might have therapeutic or preventive benefits. Preventive strategies may need to be altered to compensate for alterations in bioavailability associated with diabetes.
In order to better understand the impact of the diabetic condition on polyphenol bioavailability and metabolism, this study examined the pharmacokinetic behavior and brain deposition of grape derived polyphenol metabolites from a SGP mixture of Concord grape juice, GSE and RES in both lean (non-diabetic) and Zucker diabetic fatty (ZDF) rats.
MATERIALS AND METHODS
Chemicals and Materials
(+)-Catechin (C), (−)-epicatechin (EC) and quercetin -3-glucuronide (Q-3-glucr) authentic standards were purchased from Sigma-Aldrich (St. Louis, MO). All extraction and liquid chromatography solvents were certified ACS grade (extraction) and LC-MS grade (Analysis) and were obtained from J.T. Baker (Phillipsburg, NJ). GSE MegaNatural® AZ powder was provided by Polyphenolics (Madera, CA). Concord grape juice extract was prepared from 100% Concord grape juice (Welch’s) by loading onto C18 Solid Phase extraction cartridges (10 c.c.) and washed with 0.01% HCl in double distilled water to remove sugar. Polyphenols were then eluted with methanol (MeOH), 0.01% HCl. MeOH was then removed under vacuum and resulting concentrate was kept frozen until use. Res, resveratrol-3 glucuronide (RES-3-glucr), malvidin (Mv)-3-glucoside chloride and cyanidin (Cy)-3-glucoside chloride were purchased from ChromaDex (Irvine, CA).
SGP Material Analysis
The SGP dose consisted of 150 mg/kg body weight (BW) of GSE, 62 mg/kg BW of Concord grape juice and 297 mg/kg BW of trans-resveratrol all based on the total phenolic contents. Grape polyphenols were analyzed by HPLC/UV/MS methods as described previously [25]. Total polyphenol content in GSE was 94% wt/wt. The purity of resveratrol was 98% wt/wt and the polyphenol content of Concord grape juice was 8.8% wt/wt as determined by the Folin-Ciocalteu assay [26]. The detailed polyphenol profile in the overall SGP is shown in Supplemental Table S1.
Animals
All animal studies were approved by the Purdue University Animal Care and Use Committee (Protocol 1111000160). Twelve 10-week old male ZDF rats and twelve 10-week old male Zucker lean (LN) rats were obtained from Charles Rivers Labs (Wilmington, MA).
Rats were placed on AIN-93M polyphenol free diet with corn oil replacing soybean oil (Dyets, Bethlehem, PA) with water ad libitum until 13 weeks of age. Diabetes was at an advanced stage in the ZDF rats at 13 weeks of age.
Study Design
The study design was modified from Ferruzzi et al. [17] with minor changes. At 11.5 weeks of age ZDF rats were randomly assigned to ZDF - control (CRTL) (n=4), ZDF - SGP (n=8), and 11.5 week old LN rats were assigned to LN CRTL (n=4) and LN - SGP (n=8).
The SPG groups were dosed orally with SPG for 10 days. The daily dose was divided in half and rats were gavaged twice a day at 8 hour intervals. The SGP was mixed with deionized water to a volume of 1.0 mL and delivered to rats as the first gavage. Residual material in the syringe was mixed with 0.5 mL deionized water and administered as a second gavage. CNTL groups received deionized water. Body weight and food intake were monitored every other day throughout the study. Rats were housed in metabolic cages and 24 hour urine samples were collected daily. Urinary excretion of polyphenols from SGP was determined based on the sum of polyphenol excretion on day 2, day 6 and day 10.
After 10 days of SGP treatment, a pharmacokinetic study was performed. Blood glucose after an 8 hour fast was measured by AlphaTRAK® glucose meter from Abbott Laboratories (32004-02, Abbott Park, IL) the day before SGP treatment began and on the pharmacokinetics day.
SGP Pharmacokinetics
Eight days after SGP treatment began, a jugular catheter was implanted. Rats were anesthetized with 3–5% of isoflurane and a polyethylene catheter was surgically implanted for blood sampling. Rats were injected with Buprenex (0.05 mg/kg) before regaining consciousness to alleviate pain. Catheters were kept patent by flushing with ~0.3 mL heparinized saline (100 units/mL) every 12 h. A pharmacokinetic study was performed on the tenth day of SGP treatment after an 8 hour fast. For this study a full day’s dose of SGP was given as a single dose. Food was returned 2 hours after dosing. The control group was dosed with deionized (DI) water. Heparinized blood was collected at 0, 0.25, 0.5, 1, 2, 4, 6, 8 hours from the jugular catheter. The blood was centrifuged at 6500 rpm for 10 min at 4 °C and plasma was collected and acidified with acidified saline (1% ascorbic acid wt/v) in 4:1 ratio, purged with nitrogen and stored at -80 °C until analysis. The day after pharmacokinetics, another dose was administered and rats were sacrificed 1 hour post dose. Rats were then perfused with ice-cold saline to remove residual blood from tissues. Brain tissues were harvested following perfusion and snap frozen in liquid nitrogen.
Polyphenol Metabolites Extraction in Plasma, Urine and Brain Tissues
C/EC, Q, RES metabolites and anthocyanins were extracted from plasma, urine and brain homogenates by SPE using 1 mL Waters Oasis HLB cartridges (Milford, MA) as described by Ho et al. [19]. In brief, acidified plasma, urine and methanolic brain extracts were loaded onto preconditioned SPE cartridges. The cartridges were washed with 1 mL of 1.5 M formic acid (v/v) followed by 1 mL of 5% aqueous methanol (v/v) for C/EC, Q, Res metabolites, and 2 mL of 2% formic acid (v/v) for anthocyanins. C/EC, Q, Res metabolites were eluted with 2 mL of 0.1% formic acid/methanol (v/v) and anthocyanins with 2% formic acid/methanol (v/v). Eluents were dried under vacuum at 37 C. Dried extracts were reconstituted with LC mobile phases and analyzed immediately. Plasma from two rats was combined for anthocyanin analysis due to their low concentrations.
Polyphenol Analysis of Plasma, urine and Brain Tissues by LC-MS or LC-MS/MS
Analyses of C/EC, Q, RES metabolites was performed on an Agilent 6460 triple quadrupole LC/MS equipped with an ESI source under multiple reaction monitoring modes (MRM). The method was previously described by Ho et al. [19]. Briefly, a Waters XTerra RP-C18 column (2.1 mm × 100 mm, 3.5 μm particle size) was employed for all analyses. For C/EC, Q and Res, binary mobile phases were A: 0.1% aqueous formic acid (v/v) and B: 0.1% formic acid in acetonitrile (v/v). The column was heated to 30°C and the system flow rate was 0.3 mL/min. The binary gradient to elute all polyphenol metabolites was: 10% B at 0 min, 40% B at 10 min, 95% B at 11 min and back to 10% B at 12 min to 18 min. Fragmentor voltage was set at 135V and collision energy was 17eV for all compounds. ESI source conditions were as followed: gas temperature was 350°C, drying gas flow was 11 l/min, nebulizer was 30 psi, sheath gas temp was 350°C, sheath gas flow was 11 L/min, capillary voltage was 3500V and nozzle voltage was 1000V. For anthocyanins, the binary mobile phases were A: 2% aqueous formic acid (v/v) and B: 0.1% formic acid in acetonitrile (v/v) and the column was heated up to 35°C. The gradient used was: 5% B at 0 min, 10% B at 10 min, 25% B at 30 min, 5% B at 31 min and continue on 5% B to 35 min. ESI source condition setting was the same as described above.
Flavan-3-ols and RES metabolites in urine samples were analyzed by Agilent 1100 LC-time of flight MS equipped with ESI source. LC condition was the same as described above used in LC-MS/MS. MS condition: gas temperature was 350°C, drying gas flow was 11 L/min, nebulizer was 30 psi, capillary voltage was 3500V, skimmer was 60V, fragmentor was 135V and the mass range was 100–1000 m/z. Quercetin metabolites and anthocyanins were analyzed by triple quadrupole as described above.
Data Analysis
Quantification of C/EC, RES and Q metabolites was by multi-level response curves generated from authentic standards of C, EC, RES-3-glucr and Q-3-glucr standards, respectively. Quantification of Cy-3-glucoside was calculated from a calibration curve constructed with an authentic standard while other anthocyanins were based on Mv-3-glucoside. All data are presented as mean ± SEM. Pharmacokinetic parameters included: area under the plasma concentration versus time (AUC0–8h) (calculated using the linear trapezoidal rule), and maximum plasma concentrations (Cmax) and the time of maximum concentration (Tmax) (determined directly from the pharmacokinetic curves of plasma concentration versus time). Statistical analyses were performed using SAS 9.3 statistical analysis program (Cary, NC). Group differences of average body weight gain, average food intake and the difference of fasting blood glucose before and after treatment were determined by one-way ANOVA with Tukey’s post-hoc test. Differences between ZDF and LN on pharmacokinetic parameters, urine concentration and brain concentrations were analyzed by Student’s t test. The significance was accepted at the level of α<0.05.
RESULTS
Body Weight, Food Intake and Fasting Blood Glucose
Body weight and food intake of all groups were measured every other day. Body weight gain was defined by the body weight difference between the first day of treatment and the day of jugular implant surgery. The average weight of the lean rats at the start of the treatment was 289.2 ± 3.6g. The average starting weight of the ZDF rats was 337.6g. There were no significant differences in weight gain in any of the groups over the course of the SGP treatment (Supplemental Figure S1). There was no significant difference in food intake between lean control and lean SPG rats. Food intake for both ZDF groups was significantly greater than the corresponding lean groups. The food intake of the ZDF-SPG group was significantly lower than the ZDF-CNTL group (See Supplemental Figure S2). Fasting blood glucose was measured before and after 10 days of SGP treatment. LN-SGP rats had average fasting blood glucose of 107 ± 2 mg/dL before SGP treatment and 112 ± 3 mg/dL after 10 days of SGP treatment. ZDF-SGP rats, had average fasting glucose level of 234 ± 16 mg/dL prior to SGP and 300 ± 41 mg/dL after 10 days of SGP treatment. LN-CNTL rats had fasting blood glucose of 114 ± 2 before and 117 ± 2 mg/dL after 10 days of water treatment. ZDF-CNTL rats had fasting blood glucose at 263 ± 31 before and 346 ± 20 mg/dL after water treatment. The difference of fasting blood glucose before and after treatment was not significantly different in the lean groups. Fasting blood glucose was significantly higher after 10 days of treatment in both the CNTL and SPG treated ZDF rats. However there was no difference between the post treatment glucose levels in the CNTL and SPG treated groups indicating that there is no effect of SPG treatment of fasting blood glucose in either LN or ZDF rats. (See Supplemental Figure S3.)
Characterization of Major Polyphenols in Plasma and Brain Tissues
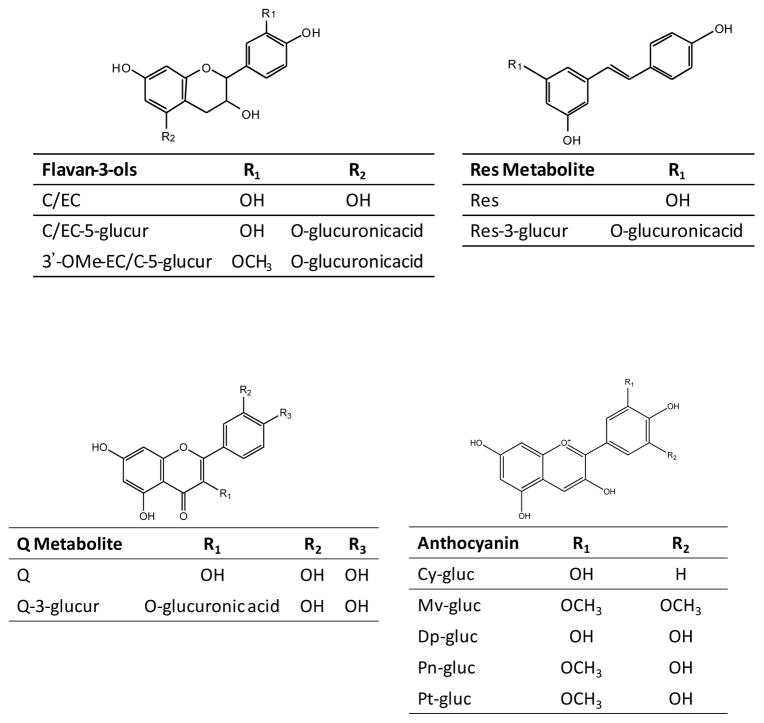
The structures of the SPG derived polyphenol metabolites detected and tentatively identified in plasma and brain tissue are illustrated in Figure 1. Four major flavan-3-ol metabolites found in both plasma and brain tissue of SGP treated rats were C-5-glucr, EC-5-glucr, 3′-OMeC-5-glucr, and 3′-OMeEC-5-glucr. Identification of flavan-3-ol metabolites is based on Blount et al. [27, 28]. The main resveratrol metabolite, tentatively identified as RES-3-glucur, was also found in both plasma and brain tissue. (For flavan-3-ol chromatograms see Supplemental Figure S4).
Figure 1.
Chemical structures of major C/EC, Res, and Q metabolite and anthocyanin derivatives detected in plasma and brain extracts from rats gavaged 10 days with SGP.
Quercetin metabolites found in both plasma and brain extracts were Q-3-glucr and MeO-Q-glucr. A major peak shown in the brain extract was tentatively assigned as a Q-glucr based on MRM response and in-line spectra (data not shown). However, assignment of glucuronidation site was not possible as isolation and NMR analysis would be required for exact assignment. The methylation site was also not determined (Supplemental Figure S5).
No flavan-3-ol or quercetin metabolites were detected in plasma or brain extracts from control rats.
Major anthocyanins detected in plasma of SPG treated rats were Mv, petunidin (Pt), peonidin (Pn), (Cy) and delphinidin (Dp) glucosides with structures depicted in Figure 1. MRM chromatograms of anthocyanins from plasma were shown in Supplemental Figure S6. All anthocyanins were determined by comparison with authentic standards. There were no detectable levels of anthocyanidin conjugated metabolites in the brain tissues of SGP treated rats or control animals.
Plasma Pharmacokinetics of SGP polyphenol metabolites in ZDF versus LN Rats
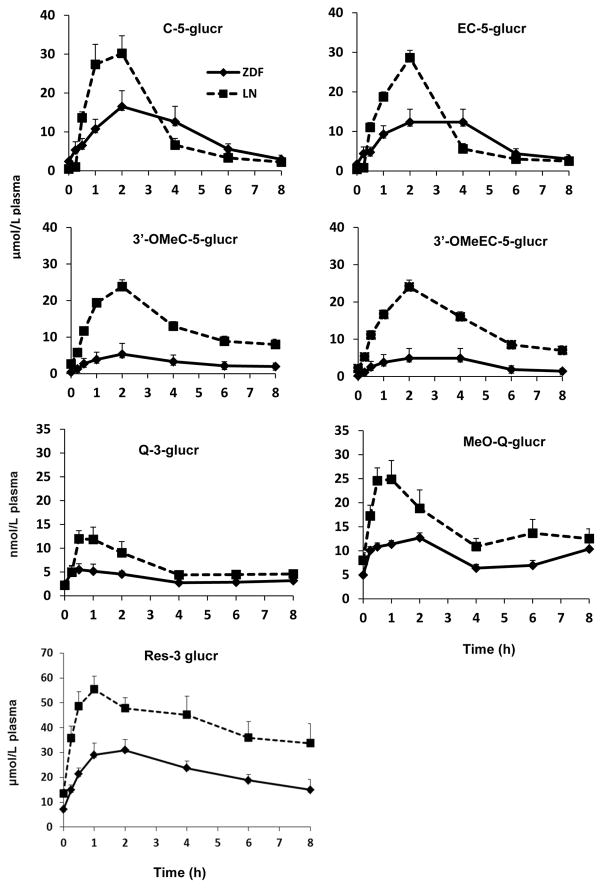
Eight-hour plasma pharmacokinetic curves of flavan-3-ol metabolites in ZDF and LN rats after 10 days of SGP treatment are shown in Figure 2. Plasma levels of four major flavan-3-ol metabolites, C-5-glucr, EC-5-glucr and their methylated metabolites peaked between 1.5 to 2.5 hours for ZDF rats and their LN counterparts. This peak was followed by a drop at 4 hours for LN rats returning close to baseline by 8 hours post gavage. ZDF rats exhibited a slower rate of elimination and return to baseline levels. ZDF rats exhibited a significantly diminished Cmax compared to their LN respectively for C-5-glucr (p=0.0104), EC-5-glucr (p=0.0077), and their methylated metabolites (p<0.0001) (Table 1).
Figure 2.
Plasma pharmacokinetic curves of major flavan-3-ol, quercetin and resveratrol metabolites after intragastric gavage of SGP in ZDF rats (◆) and LN rats (■). No metabolites were detected in any control rats. Data was represented as mean ± SEM.
Table 1.
Pharmacokinetic Parameters of flavan-3-ol, quercetin and resveratrol metabolites of rats treated for 10 days.
| Parameters | ||||
|---|---|---|---|---|
|
| ||||
| Treatment | AUC0–8h (μmol/L* h) | Cmax (μmol/L) | Tmax (h) | |
| C-5-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 76.28 ± 16.74 | 18.54 ± 3.58 | 2.31 ± 0.53 | |
| LN-SGP | 93.36 ± 11.04 | 35.89 ± 4.65* | 1.50 ± 0.19╪ | |
|
| ||||
| EC-5-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 59.70 ± 13.53 | 15.51 ± 2.76 | 2.13 ± 0.44 | |
| LN-SGP | 81.20 ± 11.28 | 31.33 ± 4.28* | 1.56 ± 0.22 | |
|
| ||||
| 3′-OMeC-5-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 25.09 ± 13.47 | 5.37 ± 2.94 | 1.75 ± 0.16 | |
| LN-SGP | 108.09 ± 6.65* | 24.75 ± 1.62* | 1.75 ± 0.16 | |
|
| ||||
| 3′-OMeEC-5-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 22.95 ± 12.40 | 4.90 ± 2.62 | 2.00 ± 0.00 | |
| LN-SGP | 110.26 ± 5.94* | 24.06 ± 1.84* | 1.88 ± 0.13 | |
|
| ||||
| Q-3-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | .028 ± .002 | .008 ± .001 | 0.56 ± 0.14 | |
| LN-SGP | .051 ± .007* | .015 ± .002* | 1.25 ± 0.43 | |
|
| ||||
| MeO-Q-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | .071 ± .003 | .014 ± .006 | 2.50 ± 0.80 | |
| LN-SGP | .123 ± .019* | .028 ± .004* | 0.84 ± 0.19* | |
|
| ||||
| Res-3-glucr | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 180.78 ± 20.54 | 34.36 ± 3.48 | 2.63 ± 0.84 | |
| LN-SGP | 328.85 ± 40.53* | 60.36 ± 6.32* | 2.19 ± 0.92 | |
Significant difference from ZDF-SGP and LN-SGP groups. p < 0.05
difference between ZDF-SGP and LN-SGP groups 0.05 < p < 0.1
Two major flavonol metabolites derived from Concord grape juice were Q-3-glucr and its methylated glucuronide. Plasma level peaked ~ 1 hour for Q-3-glucr resulting a Cmax of 8.11 ± 1.42 nmol/L for ZDF rats significantly lower than 15.41 ± 2.06 nmol/L for their LN counterparts (Figure 2; Table 1). LN rats had MeO-Q-glucr peak before 1h whereas ZDF rats peaked between 2 to 4 h. However, both groups had the same residual plasma levels at 8 h post gavage. ZDF rats reached a Cmax at 14.29 ± 0.57 nmol/L which was 49% lower than their LN (Table 1). This reduction was significant for Q-3-glucr (p=0.0112) and for MeO-Q-glucr (p=0.0062). No flavonol metabolites were detected in either of the control groups.
RES-3-glucr plasma levels (Figure 2) reached a Cmax of 34.36 ± 3.48 μmol/L for ZDF rats. As with other phenolics, this level was significantly lower than Cmax of 60.36 ± 6.32 μmol/L observed in LN counterparts with a p=0.0029 (Table 1). After peaking, plasma levels in both groups gradully declined but were not completely eliminated by 8 h post gavage.
Bioavalability, defined as AUC0–8h in this study, of 3′-OMeC-5-glucr (p<0.0001), 3′-OMeEC-5-glucr (p<0.0001) and were significantly lower in ZDF rats than in LN (Table 1). In general, AUC0–8h of 3′-OMeC-5-glucr, 3′-OMeEC-5-glucr and RES-3-glucr were ~77%, 79% and 45% decreased relative to the LN. Q-3-glucr (p=0.0193), MeO-Q-glucr (p=0.0368) and RES-3-glucr(p=0.0057) were also significantly lower in ZDF rats than LN (Table 1). Although C-5-glucr and EC-5-glucr did not show significant differences from their LN counterparts (p=0.0969 and p= 0.0621), the concentrations still showed a diminished trend of 18% and 26% relative to the LN rats.
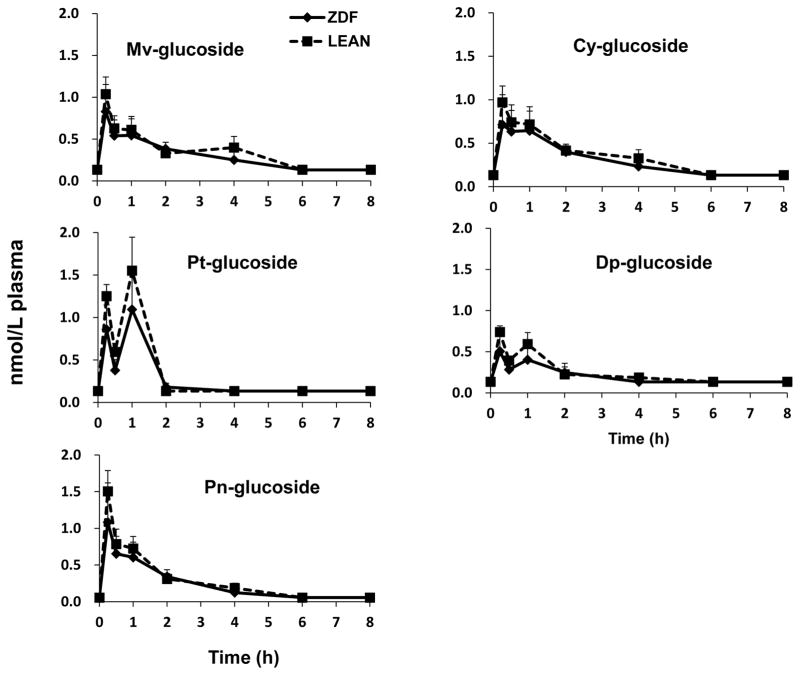
Anthocyanin plasma profiles displayed a rapid rise between 0.25 to 1 hours after SGP administration in both LN and ZDF rats (Figure 3). After reaching peak levels in plasma, Mv-3-glucoside, Pn-glucoside and Cy-3-glucoside concentrations gradually returned to baseline within 8 hours. However, Dp-glucoside and Pt-glucosides exhibited an obvious second peak around 1 hour possibly resulting from enterohepatic circulation [29]. There were no significant difference found between ZDF and their LN counterparts in AUC0–8h, Cmax or Tmax for anthocyanins (Table 2).
Figure 3.
Plasma pharmacokinetic curves of anthocyanins after intragastric gavage of SGP in ZDF rats (◆) and LN rats (■). No metabolites were detected in any control rats. Data was represented as mean ± SEM.
Table 2.
Pharmacokinetic Parameters of anthocyanins of rats treated for 10 days.
| Parameters | ||||
|---|---|---|---|---|
|
| ||||
| Treatment | AUC0–8h (nmol/L* h) | Cmax (nmol/L) | Tmax (h) | |
| Mv-3-glucoside | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 2.31 ± 0.53 | 0.90 ± 0.28 | 0.83 ± 0.58 | |
| LN-SGP | 2.66 ± 0.50 | 1.04 ± 0.20 | 0.25 ± 0.00 | |
|
| ||||
| Pt-glucoside | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 1.59 ± 0.13 | 0.62 ± 0.09 | 0.83 ± 0.58 | |
| LN-SGP | 1.90 ± 0.33 | 0.74 ± 0.08 | 0.25 ± 0.00 | |
|
| ||||
| Dp-glucoside | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 2.13 ± 0.39 | 1.26 ± 0.48 | 0.75 ± 0.25 | |
| LN-SGP | 2.58 ± 0.29 | 1.72 ± 0.24 | 0.75 ± 0.25 | |
|
| ||||
| Pn-glucoside | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 2.38 ± 0.63 | 0.81 ± 0.27 | 0.25 ± 0.00 | |
| LN-SGP | 2.75 ± 0.55 | 0.97 ± 0.19 | 0.25 ± 0.00 | |
|
| ||||
| Cy-3-glucoside | ZDF-CNTL | N.D. | N.D. | N.D. |
| LN-CNTL | N.D. | N.D. | N.D. | |
| ZDF-SGP | 1.91 ± 0.53 | 1.18 ± 0.45 | 0.83 ± 0.58 | |
| LN-SGP | 2.23 ± 0.49 | 1.50 ± 0.28 | 0.25 ± 0.00 | |
Significant difference from ZDF-SGP and LN-SGP groups. p < 0.05
ZDF = Zucker diabetic fatty rat
LN =Zucker lean rat
CNTL = control
Brain Distribution of SGP Polyphenols in ZDF versus LN Rats
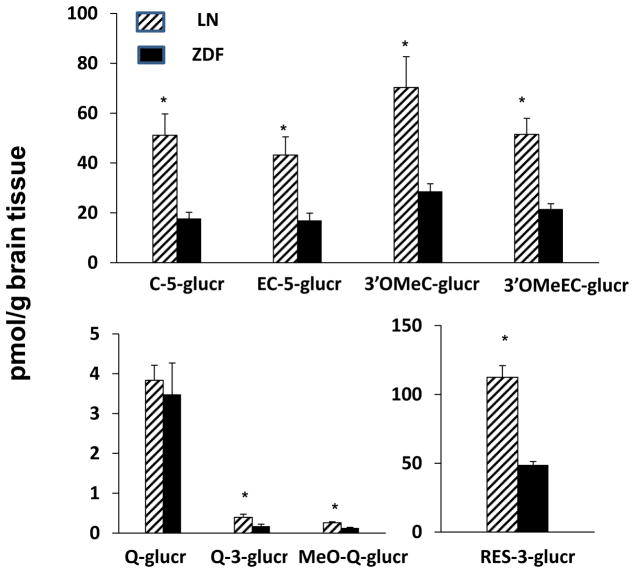
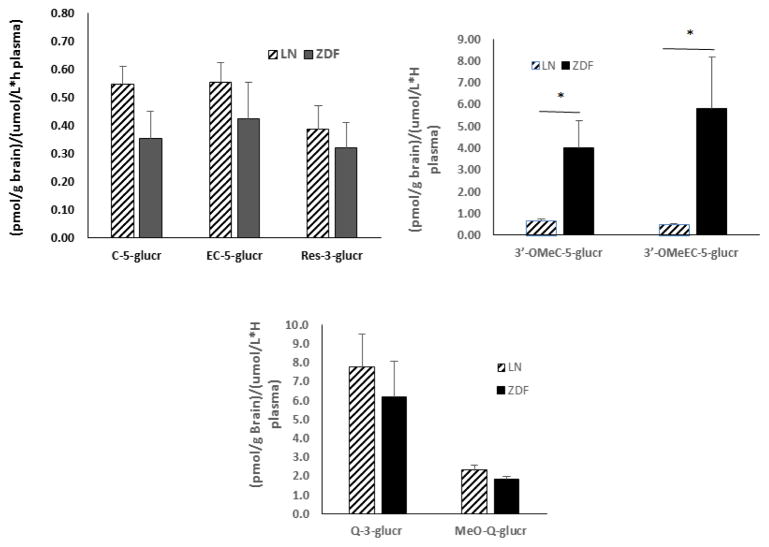
There were no polyphenolic compounds detected in any control rats. There were no anthocyanins found in brain tissues of SGP treated ZDF or LN rats. Deposition levels of all other polyhphenols were in the pmol/g of tissue range after dosing SGP for 10 days. ZDF rats exibited significantly lower brain concentrations in flavan-3-ol, flavonol and resveratrol metabolites relative to LN counterparts. ZDF rats showed significantly lower brain concentrations in C-5-glucr (p=0.0056), EC-5-glucr (p=0.0082), 3′-OMeC-5-glucr (p=0.0114), 3′-OMeEC-5-glucr (p=0.002) and Res-3-glucr (p=0.0088) relative to LN animals. Similarly, brain concentrations of MeO-Q-glucr (p=0.0009) and Q-3-glucr (p=0.0267) were significantly lower for ZDF rats relative to their LN counterparts (Figure 4). The ratio of brain concentrations of flavan-3-ol, flavonol and resveratrol metabolites to plasma AUC is shown in Figure 5. There were no significant differences in the ratios for flavan-3-ol or resveratrol glucuronides or Q metabolites for LN and ZDF rats. However, ratios were significantly greater for the ZDF rats than the LN rats for 3′-OMeC-5-glucr (p=0.0304) and 3′-OMeEC-5-glucr (p=0.0417).
Figure 4.
Brain deposition of major flavan-3-ol, quercetin and resveratrol metabolites after intragastric gavage of SGP in ZDF rats (■) and LN rats (▨).
Figure 5.
Ratio of major brain flavan-3-ol, quercetin and resveratrol metabolites (pmol/g brain tissue) to plasma AUC (umol/L*h plasma) in ZDF (■)and Lean (▨)Rats.
Urinary Excretion of SGP Polyphenols in ZDF versus LN Rats
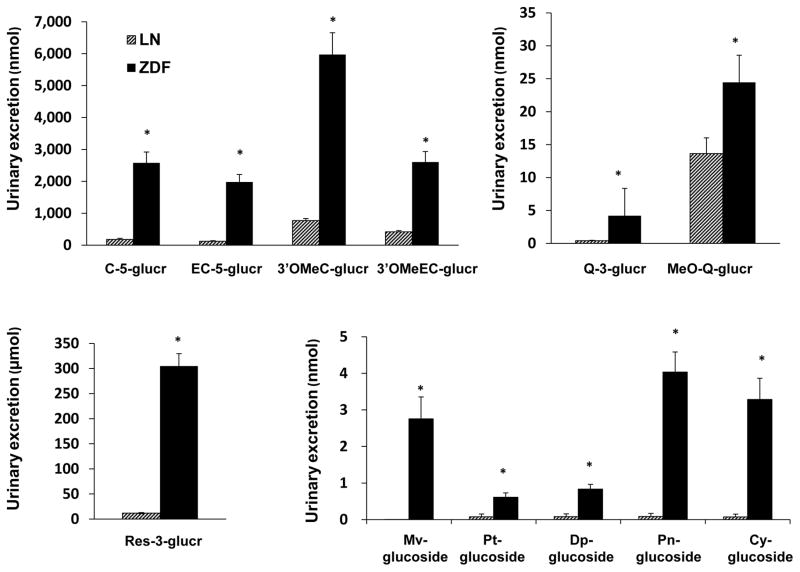
Urinary output from ZDF rats was significantly (p < 0.05) higher with average of 67.39±5.43 mL/day compared to 4.43±0.32 mL/day for LN rats. Urinary excretion of polyphenols from SGP was determined based on the sum of polyphenol excretion on day 2, day 6 and day 10. There were no polyphenols found in baseline urine samples for any rats and no polyphenols were found in the urine or either of the control groups on subsequent days. Total urinary polyphenol losses of days 2, 6 and 10 are shown in Figure 6. ZDF rats had a significantly higher urinary excretion than LN rats for C-5-glucr (p=0.003), EC-5-glucr (p<0.0001) and 3′-OMeC-3-glucr (p=0.0002) and 3′OMe-EC-5-glucr (p=0.0002), RES-3-glucur (p=0.003), Q-3-glucr (p=0.002) and MeO-Q-glucr (p=0.04). Anthocyanin excretion was also significantly higher in ZDF rats compared to LN rats for Mv-glucoside (p=0.01), Pt-glucoside (p=0.02), Dp-glucoside (p=0.001), Pn-glucoside (p=0.002) and Cy-glucoside (p=0.006).
Figure 6.
Urinary excretion of SGP polyphenols and metabolites in ZDF rats (■) and LN rats (▨).
Discussion
There are a number of pathological conditions which arise in diabetes which could potentially affect bioavailability. Delayed gastric emptying is one of the gastrointestinal complications of diabetes. This condition can progress to a clinical disorder, gastroparesis [30]. Diabetes can cause altered motor function of the small intestine which may or may not be accompanied by malabsorption and changes in key transport and metabolizing systems in the intestine that impact polyphenols [31]. Diabetes has been shown in animal models to cause endothelial dysfunction in the mesenteric arteries which may affect blood supply to the intestine [32]. Alterations in the microbiome have been associated with diabetes [33]. Since polyphenolic compounds are subject to metabolism both by mammalian systems [31] and by the microbiome [34, 35], diabetes may alter the profile of metabolites available for absorption. Changes in kidney function may also influence bioavailability. Hyperglycemia results in osmotic diuresis which may result not only in the urinary loss of glucose but in the loss of other molecules. Diabetes of long duration may lead to decreased kidney function and decreased clearance of some substances. Since diabetes carries and increased risk of AD [1, 5] and since in previous studies we have shown the potential benefits of SPG for the prevention of AD [9–11, 18–20] it may be beneficial for diabetics to increase consumption of grape polyphenols. In this study we used an animal model to investigate the effect of diabetes on the bioavailability of grape polyphenols and the accumulation of their primary phase II metabolites in the brain. The presence of microbial metabolites was not targeted in the current study and remains a focus for future investigations.
The 10 day SPG regimen did not significantly alter the food consumption of weight gain of the rats indicating that the SPG was well tolerated at these doses. The fact that there was no difference in fasting blood glucose between ZDF-SPG rats and ZDF-CNTL rats indicates that 10 days of SPG treatment did not affect the severity of the diabetes although other studies in humans have shown some benefit of grape polyphenols in diabetes [36].
The metabolite profiling of polyphenols from SGP was consistent with our previous findings [17, 18–20, 28, 37] in Sprague-Dawley rats treated individually with GSE, red wine or resveratrol suggesting that similar metabolites are found in both Sprague-Dawley and ZDF rodent models (Tables 1 and 2). Furthermore, the consistency metabolite profiles after SGP dosing with the metabolite profiles after dosing of the components individually suggests that combining the components into SGP treatment does not alter the individual polyphenol bioavailability and metabolism when they were ingested simultaneously as a mixture. Combining grape polyphenols from different grape products may be more beneficial in prevention of AD than use of single grape products since the different polyphenols act through different mechanisms [21].
The plasma AUCs, and Cmax of the flavan-3-ols, quercetin and resveratrol were all lower in the diabetic groups than in the non-diabetic groups. All of these differences except for C-5-glucr and EC-5-glucr AUCs were significant (p < 0.05). Since the urinary excretion of all of these compounds was significantly greater in the diabetic than the normal, this is probably a major factor in the differences in AUC and Cmax. For the anthocyanins the AUCs and the Cmax were all slightly lower but the differences were not significant in spite of the fact that the urinary losses of all of the anthocyanins were significantly greater in the diabetic group than the non-diabetic group. For both diabetic and non-diabetic animals the bioavailability of flavan-3-ol, quercetin and resveratrol metabolites is higher than anthocyanin metabolites. The high urinary losses of polyphenols may be attributable to several kidney pathologies related to diabetes. Some of the loss may be attributable to osmotic diuresis. The diabetic rats were not treated with insulin or drugs to maintain normoglycemia and the hyperglycemia resulted in loss of glucose and high urinary volume. The high volume loss may result in the loss of other small molecules. Diabetes also results in other kidney pathologies such as thickening of basement membranes, mesangial expansion and loss of podocytes. These structural changes result in albuminuria and proteinuria. It is highly likely that these structural changes are also contributing to the loss of other small molecules and may be a factor in the loss of SPG polyphenols. Alterations in gastrointestinal motility in diabetes might be expected to alter the kinetic profile of the SPG polyphenols. Decreased gastrointestinal motility has been demonstrated in type I and II diabetes models. In a type I rodent model, gastrointestinal mobility delay was associated with loss of myenteric nitric oxide synthase expression which negatively influenced the function of myenteric plexus [38]. The Tmax values were in most cases later in the diabetic animals but the differences only reached significance in MeO-Q-glur, but here was a trend (0.05 <p< 0.1) for C-5glucr, Mv-3-glucoside, Pt-glucoside, and Cy-3-glucoside. Another contributing factor differences in urinary and plasma metabolite profiles might be altered microbial metabolism in the ZDF rat. This is an area that requires further investigation.
For SPG to be effective in prevention of AD the polyphenols must reach the brain. In this study all of the flavan-3-ol, quercetin and resveratrol metabolites were found in the brains of both the diabetic and nondiabetic animals. Except for Q-glucr the amounts were significantly lower in the brains of the diabetic animals. This may be a result of the lower plasma metabolite levels in the diabetic animals. For all of the compounds except the methylated flavan-3-ol metabolites, there was no significant difference between the brain/plasma ratios for LN and ZDF rats indicating that for these compounds the lower levels in the brain were driven by the lower plasma levels. For the methylated flavan-3-ols the higher brain/plasma ratio in the ZDF rats was higher than the LN rats indicating that the diabetic brain may be more permeable to these compounds or have differential turnover of these metabolites compared to normal brain. Additional experiments to dissect the mechanisms involved are warranted.
No anthocyanins were found in the brain of either diabetic or non-diabetic rats. This is in contrast to other studies done by us in Sprague dawley rats in which we did find anthocyanins in rat brains after 10 days of SPG treatment [16]. We have also found anthocyanins in the brains of pigs fed bilberry extract for 3 weeks [39]. Others have also previously reported anthocyanins in brain tissues [37, 40–44]. It may be that there are differences in permeability of the blood brain barrier to different polyphenols in different strains of rats. In our previous study in Sprague Dawley rats level of falvan-3-ol metabolites in the brain were on the order of 10 times higher than in the non-diabetic Zucker rats whereas quercetin metabolits in the brain are on the same order of magnetude in both Sprague-Dawley rats and Zucker non-diabetic rats. Anthocyanins in the Sprague-Dawley rats are near the lower limit of detection. It may be that the anthocyanin levels in the Zucker rats were below the limit of detection (estimated at 0.42 pmol/g tissue for Mv-glucoside and 0.82 pmol/g tissue for Cy-glucoside).
The results of these studies raise a number of questions which need further study. First of all was the lower bioavailablity in the diabetic animals soley a consequense of the urinary losses or were other components of diabetic pathology also contributing? It is plausible that changes in the microbiota as a result of the diabetic state would have impact on the extent to which lower molcular weight phenolic metabolites are generated. The total flux of metabolites could change and the levels observed in the urine of Phase II metabolites only represents a portion of the full picture. As the profiles of key microbial phenolic metabolites was not obtained this remains to be explored. Another possible contributing factor is the severity of the disease. The ZDF rats in this study were 13 weeks old. At that age they are severly diabetic but not yet suffering from kidney failure and loosing weight. The bioavailality and brain distribution may vary with the severity of the disease.
Other potential contributing factors to differnces in plasma and brain polyphenol profiles in LN and ZDF rats are alterations of liver function and mitochondrial activity in ZDF rats. Polyphenols are transformed by metbolizing enzymes in the liver. In many human studies and animal models, the metabolism of polyphenol compounds has been shown to be altered by obesity and diabetes [45]. ZDF rats have been shown to have decreases in some CPY [46,47] and UGT[48] enzymes. Mitochondrial disfunction has been demonstrated by Raza et al [49] in both liver and brain. Further bioavailablity studies using well controlled and poorly controlled subjects and subjects with different degrees of severity of diabetes could contribute to answering these questions. The differences in bioavailabilty between diabetics and non-diabetics has implications not only for developing a stadegy for the prevention of AD but also for all other drugs used by diabetics. For human diabetic patients drugs are prescribed without consideration of how their diabetes might alter the bioavailabiltiy of the drug and consequently patients may be getting an inadequate dose.
Conclusion
The goal of this study was to investigate the effect of diabetes on the bioavailability and metabolism of polyphenols associated with AD modifying potential. Overall, ZDF rats had diminished plasma bioavailability and brain deposition levels relative to their LN counterparts. ZDF rats showed total urinary excretion on all polyphenols from SGP which inversely correlated with plasma concentration and was probably a major contributing factor to the decreased plasma levels. Data from this study support the hypothesis that diabetic condition may have a profound impact on the absorption, metabolism and excretion of grape polyphenols. The mechanism behind these observations merits further investigation including possible alteration of transport and intestinal function as well as major phase II metabolizing enzymes that involve in grape polyphenols transformation. The future direction will be to analyze the level of the same metabolites from gastrointestinal contents as well as the intestines. This will further confirm whether the diminished absorption observed here is due to differences in intestinal transport or processing of polyphenols.
Supplementary Material
Acknowledgments
These studies were supported by the U.S. National Institutes of Health [grant PO1 AT004511: National Center for Complementary and Alternative Medicine (NCCAM) Bioavailability Core to M.F.]. This study was also supported in part by Grant Number P50 AT008661-01 from the NCCIH and the ODS. The authors thank Pamela Lachcik for animal care and technical support.
Abbreviations
- 3′-OMeC-5-glucr
3′-O-methylcatechin-5-glucuronide
- 3′-OMeEC-5-glucr
3′-O-methylepicatechin-5-glucuronide
- AD
Alzheimer’s disease
- AUC0–8h
area under the curve from 0 to 8h
- BW
body weight
- C
catechin
- C-5-glucr
catechin-5-glucuronide
- CNTL
control
- Cmax
maximum plasma concentration
- Cy
cyanidin
- Dp
delphinidin
- EC
epicatechin
- EC-5-glucr
epicatechin-5-glucuronide
- G
glycoside (either glucoside or galactoside)
- GI
gastrointestinal
- GSE
grape seed extract
- LN
lean
- MeOH
methanol
- MeO-Q-glucr
methylquercetin glucuronide
- Mv
malvidin
- PAC
proanthocyanidin
- Pn
peonidin
- Pt
petunidin
- Q
quercetin
- Q-3-glucr
quercetin-3-glucuronide
- RES
Resveratrol
- RES-3-glucr
Resveratrol-3-glucuronide
- SGP
Standardized Grape Polyphenol
- Tmax
time at maximum concentration
- ZDF
Zucker diabetic fatty
Footnotes
Author Contributions
E. M. Janle designed and directed the research, T-Y Chen performed the research, M.G. Ferruzzi and B. Cooper supervised the chemical analysis, Q-L Wu, J.E. Simon and S. T. Talcott prepared and analyzed the treatment materials, G.Todd was the undergraduate student who did the glucose study, E.M. Janle, T-Y Chen and M.G. Ferruzzi wrote the paper, G.M. Pasinetti was the PI of the PO1 grant.
Conflict of Interest
None of the authors have any conflict of interest.
References
- 1.Barbagallo M, Dominguez LJ. Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes. 2014;5:889–93. doi: 10.4239/wjd.v5.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitmer RA. The epidemiology of adiposity and dementia. Curr Alzheimer Res. 2007;4:117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer’s disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, et al. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 1987;75:1982–7. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willette AA, Xu G, Johnson SC, Birdsill AC, et al. Insulin Resistance, Brain Atrophy, and Cognitive Performance in Late Middle-Aged Adults. Diabetes Care. 2013;36:443–9. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao JB, Högger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr Med Chem. 2015;22:23–38. doi: 10.2174/0929867321666140706130807. [DOI] [PubMed] [Google Scholar]
- 9.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Ho L, Humala N, Dickstein DL, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. Faseb J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Ho L, Ono K, Rosensweig C, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chis IC, Ungureanu MI, Marton A, Simedrea R, et al. Antioxidant effects of a grape seed extract in a rat model of diabetes mellitus. Diab Vasc Dis Res. 2009;6:200–204. doi: 10.1177/1479164109336692. [DOI] [PubMed] [Google Scholar]
- 13.Wang YJ, Thomas P, Zhong JH, Bi FF, et al. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox Res. 2009;15:3–14. doi: 10.1007/s12640-009-9000-x. [DOI] [PubMed] [Google Scholar]
- 14.Drel VR, Sybirna N. Protective effects of polyphenolics in red wine on diabetes associated oxidative/nitrative stress in streptozotocin-diabetic rats. Cell Biol Int. 2012;34:1147–1153. doi: 10.1042/CBI20100201. [DOI] [PubMed] [Google Scholar]
- 15.Huang PH, Tsai HY, Wang CH, Chen YH, et al. Moderate intake of red wine improves ischemia-induced neovascularization in diabetic mice--roles of endothelial progenitor cells and nitric oxide. Atherosclerosis. 2012;212:426–435. doi: 10.1016/j.atherosclerosis.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Tang C, Ferruzzi MG, Gong B, et al. Role of standardized grape polyphenol preparation as a novel treatment to improve synaptic plasticity through attenuation of features of metabolic syndrome in a mouse model. Mol Nutr Food Res. 2013;57:2091–102. doi: 10.1002/mnfr.201300230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferruzzi MG, Lobo JK, Janle EM, Cooper B. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer’s disease. J Alzheimers Dis. 2009;18:113–124. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho L, Ferruzzi MG, Janle EM, Wang J, et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. Faseb J. 2013;27:769–81. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Ferruzzi MG, Ho L, Blount J, et al. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J Neurosci. 2012;32:5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Bi W, Cheng A, Freire D. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00042. Article 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113:88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samsom M, Vermeijden JR, Smout AJ, Van Doorn E, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–3122. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 24.Oh YK, Joo KW, Lee JW, Jeon US, et al. Altered renal sodium transporter expression in an animal model of type 2 diabetes mellitus. J, Korean Med, Sci. 2007;22:1034–1041. doi: 10.3346/jkms.2007.22.6.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Simon JE, Welch C, Wightman JD, et al. Survey of polyphenol constituents in grapes and grape-derived products. J Agric Food Chem. 2011;59:10586–93. doi: 10.1021/jf202438d. [DOI] [PubMed] [Google Scholar]
- 26.Waterhouse AL. Determination of Total Phenolics. In: Wrolstad RE, editor. Current Protocols in Food Analytical Chemistry. Wiley; 2001. pp. I1.1.1–I1.1.8. [Google Scholar]
- 27.Blount JW, Ferruzzi M, Raftery D, Pasinetti GM, Dixon RA. Enzymatic synthesis of substituted epicatechins for bioavativity studies in neurological disorders. Biochem Biophys Res Commun. 2012;417:457–61. doi: 10.1016/j.bbrc.2011.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blount JW, Redan BW, Ferruzzi MG, Reuhs BL, Cooper BR, Harwood JS, Shulaev V, Pasinetti G, Dixon RA. Synthesis and quantitative analysis of plasma-targeted metabolites of catechin and epicatechin. J Agric Food Chem. 2015;63:2233–40. doi: 10.1021/jf505922b. [DOI] [PubMed] [Google Scholar]
- 29.Kay CD. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutrition Research Reviews. 2006;19:137–146. doi: 10.1079/NRR2005116. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JE, Jones KL, Rayner CK, Horowitz M. Pathophysiology and pharmacotherapy of gastroparesis: current and future perspectives. Expert Opin Pharmacother. 2013;14:1171–1186. doi: 10.1517/14656566.2013.795948. [DOI] [PubMed] [Google Scholar]
- 31.Redan B, Novotny J, Ferruzzi MG. Altered Transport and Metabolism of Phenolic Compounds in Obesity and Diabetes: Implications for Functional Food Development and Assessment. Adv Nutr. 2016;7:1090–1104. doi: 10.3945/an.116.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahlberg N, Qin CX, Anthonisz J, Jap E. Adverse vascular remodelling is more sensitive than endothelial dysfunction to hyperglycaemia in diabetic rat mesenteric arteries. Pharmacol Res. 2016;111:325–335. doi: 10.1016/j.phrs.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 34.Braune A, Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7:216–34. doi: 10.1080/19490976.2016.1158395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Ho L, Faith J, Ono K, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res. 2015;59:1025–40. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Keogh JB, Clifton PM. Polyphenols and Glycemic Control. Nutrients. 2016;8:E17. doi: 10.3390/nu8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen T-Y, Lobo J, Weaver C, Simon JE, et al. Bioavailability and Brain Distribution of Anthocyanin and Quercetin Metabolites from Grape Juice and Wine. Faseb J. 2011;25:771.6. [Google Scholar]
- 38.Demedts I, Masaoka T, Kindt S, De Hertogh G, et al. Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J Neurogastroenterol Motil. 2013;19:161–170. doi: 10.5056/jnm.2013.19.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen TY, Kritchevsky J, Hargett K, Feller K. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol Nutr Food Res. 2015;59:2432–47. doi: 10.1002/mnfr.201500224. [DOI] [PubMed] [Google Scholar]
- 40.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, et al. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 41.Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agric Food Chem. 2005;53:7029–7034. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- 42.Talavera S, Felgines C, Texier O, Besson C, et al. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J Agric Food Chem. 2005;53:3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- 43.Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, et al. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- 44.Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J Agric Food Chem. 2010;58:3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- 45.Redan BW, Buhman KK, Novotny JA, Ferruzzi MG. Altered Transport and Metabolism of Phenolic Compounds in Obesity and Diabetes: Implications for Functional Food Development and Assessment. Adv Nutr. 2016;7:1090–1104. doi: 10.3945/an.116.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SY, Kim CH, Lee JY, Jeon JS, Kim MJ, Song HC, Kim HC, Oh SJ, Kyum KK. Hepatic expression of cytochrome P450 in Zucker diabetic fatty rats. Food Chem Toxicol. 2016;96:244–253. doi: 10.1016/j.fct.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Suh YH, Kim Y, Bang JH, Choi KS, et al. Analysis of gene expression profiles in insulin-sensitive tissues from pre-diabetic and diabetic Zucker diabetic fatty rats. J Mol Endocrinol. 2005;34:299–315. doi: 10.1677/jme.1.01679. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Rougée LR, Bedwell DW, Cramer JW, et al. Difference in the Pharmacokinetics and Hepatic Metabolism of Antidiabetic Drugs in Zucker Diabetic Fatty and Sprague-Dawley Rats. Drug Metab Dispos. 2016;44:1184–92. doi: 10.1124/dmd.116.070623. [DOI] [PubMed] [Google Scholar]
- 49.Raza H, John A, Howarth FC. Increased oxidative stress and mitochondrial dysfunction in Zucker diabetic rat liver and brain. Cell Physiol Biochem. 2015;35:1241–1251. doi: 10.1159/000373947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.