Abstract
Somatostatin-expressing GABAergic neurons constitute a major class of inhibitory neurons in the mammalian cortex and are characterized by dense wiring into the local network and high basal firing activity that persists in the absence of synaptic input. This firing provides both GABA type A receptor (GABAAR)- and GABABR-mediated inhibition that operates at fast and slow timescales. The activity of somatostatin-expressing neurons is regulated by brain state, during learning and in rewarded behaviour. Here, we review recent advances in our understanding of how this class of cells can control network activity, with specific reference to how this is constrained by their anatomical and electrophysiological properties.
Inhibitory neurons in the cerebral cortex can be categorized into multiple molecularly and anatomically distinct classes that have very different and highly specialized roles in shaping network output. New transgenic mice that enable investigators to visualize and manipulate the activity of specific interneuron subtypes1–4 are markedly advancing our understanding of how specific neural circuits are built and how they regulate brain activity. Particular advances have been made in defining a role for somatostatin-expressing neurons (referred to in this article as SST neurons; also referred to as SOM neurons), a clearly defined subset of GABAergic interneurons that shares little or no overlap with other major classes of cortical inhibitory neurons, parvalbumin-expressing cells (referred to in this article as PV cells) and serotonin receptor 3A (5HT3AR)-expressing cells5–9. As a class, SST cells broadly encompass neurons that have been identified — using various anatomical and electrophysiological criteria — as so-called ‘Martinotti’ cells, bitufted cells, regular-spiking non-pyramidal cells or low-threshold spiking cells (for example, see REFS 6,9–11). In many brain areas, SST neurons represent approximately 30% of the total interneuron population8, and their cell bodies are distributed throughout the neocortex and the hippocampus. Notably, they are densely wired into local neuronal networks, as they are synaptically connected to most nearby pyramidal cells12–17.
One of the most notable properties of hippocampal and neocortical SST cells, as observed both in vitro and in vivo, is that they have high levels of spontaneous activity. This activity is enabled by intrinsic membrane conductances, persists in the absence of synaptic input and can be fine-tuned by synaptic inputs and neuromodulatory factors. This property was missed by many early studies in acute brain slices (in which experimental conditions were optimized to silence activity) and in vivo (as SST-neuron firing is profoundly suppressed by many common anaesthetics, including isoflurane and urethane). Moreover, up- and downregulation of the spontaneous and evoked activity of SST cells — for instance, as is associated with changes in brain state — is thought to influence information flow primarily through synapse-specific, fast, GABA type A receptor (GABAAR)-mediated inhibition as well as through more diffuse, slow, GABABR-mediated synapse silencing and membrane hyperpolarization of postsynaptic neurons18. Additional data suggest that SST neurons may undergo long-lasting changes in anatomy and function during experience-dependent plasticity of the neocortical network.
Here, we review recent advances in our understanding of how SST neurons regulate activity in the neocortex and hippocampus, focusing on their synapses, their local network properties and their controlled activity during sensation, movement and learning. We focus on recent studies that use molecular, rather than electrophysiological, classification schemes. The activity of SST neurons is regulated during different behavioural states and has a crucial role during learning. We discuss how fine-scale anatomical and electrophysiological analyses of the wiring of SST neurons into cortical networks are facilitating an increasingly complete account of how SST cells influence brain function, from local networks to behaviour.
Classifying cortical SST neurons
SST cells in the brain (unlike in the spinal cord19) are exclusively GABAergic and serve as prominent sources of inhibition in the neocortex and hippocampus — the areas in which they have been most comprehensively studied. Although these neurons are defined by their expression of the neuropeptide somatostatin, and somatostatin receptors are widely expressed in cortical tissues (particularly somatostatin receptor 4, which is highly expressed in CA1 pyramidal neurons and deep layers of the cortex), the specific conditions under which this peptide might be released have not yet been elucidated20. In general, activation of somatostatin receptors has an inhibitory effect, suppressing neuronal firing21–23. As somatostatin is a neuropeptide and is packaged into a different pool of vesicles to those containing GABA, it will be interesting to determine precisely when somatostatin-mediated inhibition might be engaged.
During mouse embryonic development, SST neurons arise from progenitors within the medial ganglionic eminence (MGE), migrating diffusely through the cortex during embryonic development to populate the telencephalon, including the neocortex and hippocampus24,25. The SST-cell lineage is more closely related to that of fast-spiking PV GABAergic neurons, which also derive from the MGE, than to 5HT3AR-expressing neurons, which derive from the caudal ganglionic eminence (CGE) during development26. In mice, the neurons that will become SST cells are born in approximately the second week of embryonic development25. In rodents and cats, somatostatin expression progressively increases during the prenatal and early postnatal period, and reaches a maximum number of cells and intensity of expression by the late postnatal period27–30. Although SST neurons remain, somatostatin protein levels dramatically decrease in adult rodents28,30. Dissociating SST-cell presence from levels of somatostatin expression is therefore crucial in determining whether this cell class is differentially affected in disease states. Indeed, the expression of somatostatin may be cyclic AMP-responsive element-binding protein (CREB)-dependent and thus regulated by activity31, and so reductions in the activity of SST neurons may be associated with decreases in the expression of somatostatin rather than an elimination of these cells. Using Cre-based strategies for irreversible cell labelling has been an important advance in the field.
SST neurons show substantial within-category diversity in terms of their molecular profiles, anatomical features (FIG. 1) and electrophysiology (see REF. 26 for a comprehensive table and REF. 32 for a summary of the electrophysiology). Indeed, the unambiguous classification of this broad subtype remains controversial. Neocortical SST neurons include Martinotti-type, low- threshold spiking cells or irregular-spiking neurons observed in layer 2/3 (L2/3) and L5a, stuttering non-pyramidal cells in L4 and L5b, and some fast-spiking neurons1,6,9,33. A recent high-throughput analysis of neocortical interneurons suggested that SST neurons (that had been identified by immunohistochemistry) in L2 and L5 could be divided into three primary types: Martinotti cells, which are defined by a multipolar appearance and a prominent axonal arbor in L1; neurons with a bitufted appearance; and a small fraction of neurons that are characterized by a basket cell anatomy and fast-spiking phenotype34. Cells in the lattermost, ‘basket cell’ category may be particularly abundant in some brain areas, such as frontal or entorhinal cortex33,35,36.
Figure 1.
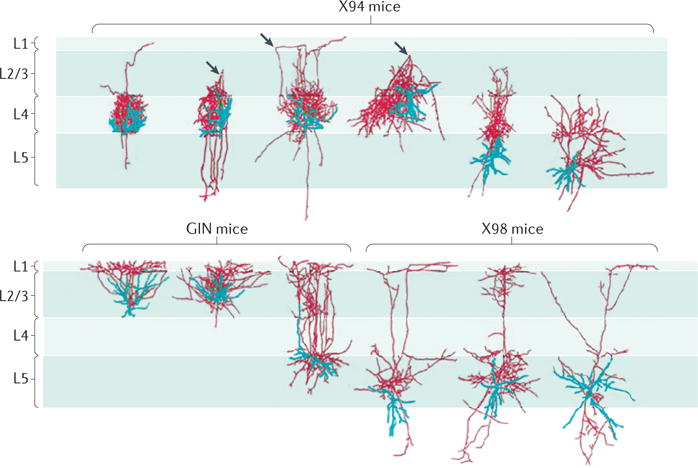
Three-dimensional morphological reconstructions of SST interneurons in the primary somatosensory cortex of different transgenic mouse lines. Somatostatin-expressing (SST) neuron diversity is highlighted by the differences in anatomical properties of cells within and across layers. Cell bodies and dendrites are shown in blue, and axons are shown in red. The three arrowheads in the top row point to a turning point of the axon, from the upper layers back to layer 4 (L4). The top panel shows neurons in the X94 line. The bottom panel shows neurons in the green fluorescent protein (GFP)-expressing inhibitory neuron (GIN) line and in the X98 line. Note that in the GIN and X98 lines, both L2/3 and L5 SST neurons have an axonal branch that ascends and prominently elaborates in L1, as well as substantial branching within the ‘home’ layer that contains the cell body. L4 SST neurons may also have an axonal branch that ascends to L1, although most of the axon is concentrated in L4. This selective axonal targeting to L1 is similar to that observed in hippocampal stratum oriens–lacunosum moleculare (OLM) neurons, in which the SST cell body lies in the stratum oriens, and the axon elaborates in the lacunosum moleculare. Dense, lamina-specific axons from SST neurons suggest an important role for these cells in regulating synapses that lie within this layer through either GABA type A receptor (GABAAR)- or GABABR-mediated mechanisms. Figure is republished with permission of Society for Neuroscience, from Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. Ma, Y., Hu, H., Berrebi, A. S., Mathers, P. H. & Agmon, A., J. Neurosci. 26 (19) 5069–5082 (2006); permission conveyed through Copyright Clearance Center, Inc.
Different subtypes of SST neurons can express other markers, such as calbindin, calretinin, neuropeptide Y (NPY), cholecystokinin and nitric oxide synthase5,6,9,32,37, and the distribution of these subtypes can also depend on the brain subregion. For example, a subset of SST neurons — Martinotti cells that reside in L2/3 and L5 but not L4 — express calbindin1. Somatostatin and NPY are often co-expressed, and these are also sometimes co-expressed with calbindin in the same cells6,38. Such differential co-expression underlines the fact that even SST cells that reside in the same brain area are not equivalent. A handful of different transgenic mouse lines selectively label only SST neurons in L4 and show a more local, lamina-restricted pattern of axonal arborization for these cells compared with Martinotti-type cells in L2/3 and L5 (REFS 1,14), consistent with molecular sub-specialization of neurons in granular cortical layers (BOX 1).
In supragranular layers of the neocortex (L1–L3), a fraction of SST cells also express the calcium-binding protein calretinin, and cells expressing this marker tend to have a stronger input from excitatory neurons within the same layer than do calretinin-negative neurons, which receive greater input from L4 (REFS 39,40). SST neurons in L5 are more likely to be calretinin-negative and show lower-threshold bursts compared with their counterparts in the granular layer (L4) and supragranular layers of the cerebral cortex1,41. Anatomical and molecular studies suggest that hippocampal SST neurons are even more diverse than neocortical cells and include bistratified cells and neurons in the stratum oriens that project to the lacunosum moleculare (known as OLM cells)42–46.
Patterns of evoked spiking, as assessed using whole-cell patch clamp recording techniques, were initially used to electrophysiologically define what we now classify as SST neurons. SST cells characteristically have a low threshold for action potential generation — and thus, before the use of molecular markers, many were often electrophysiologically classified as low-threshold spiking cells — and they show spike frequency adaptation with a gradually decreasing action potential height47,48. Importantly, use of a single action-potential waveform to classify SST neurons is not definitive, as some SST neurons (for example, low-threshold spiking SST neurons in the GFP-expressing inhibitory neuron (GIN) transgenic mouse line1,40) show a narrow action potential that is characteristic of fast-spiking PV interneurons. A substantial fraction (approximately 30%) of SST neurons in the prefrontal cortex show a fast-spiking phenotype, and the activity of this subtype can be differentiated from non-fast-spiking SST neurons35. In a small number of cases, this fast-spiking phenotype might result from off-target reporter expression in PV interneurons, as parvalbumin expression has been reported in a small fraction (approximately 10%) of SST neurons in the somatosensory cortex of juvenile SST–Cre transgenic mice13,33; however, this phenotype has been associated with SST neurons in lines besides the SST– Cre line1,34 and thus must be considered to be a real subgroup.
SST-neuron activity
Neocortical SST neurons in acute brain slices from mice can exhibit high levels of spontaneous (constitutive) 3–10 Hz activity that is largely independent of glutamatergic or GABAergic input onto the cells and that is markedly increased by neuromodulators such as acetylcholine and noradrenaline10,18,49,50. Targeted recordings in awake mice have confirmed that this activity is a characteristic feature of SST neurons49,51–53 (TABLE 1). Owing to how SST neurons are wired into local networks (that is, they receive strong inhibition and provide strong inhibition), this tonic activity facilitates fine-scale up- and downregulation of overall levels of inhibition in the neocortex and hippocampus.
Table 1.
Basal firing frequencies of SST neurons
| Brain area | Preparation* | Firing rate in Hz ± s.e.m. (range of firing rates in Hz) | Refs |
|---|---|---|---|
| L2/3 of S1 | Acute slice | 3.1 ± 0.1 (2–3.9) | 10 |
| L2/3 of S1 | Acute slice | 2.4 ± 0.6 (0–13) | 18 |
| L5 of S1 | Acute slice | 4.5 (0.7–8.5) | 18 |
| Hippocampus OLM | Acute slice | 4.3 ± 1 | 58 |
| L2/3 of S1 | In vivo, anaesthetized | 0 | 52 |
| L2/3 of V1 | In vivo, anaesthetized | 1.3 ± 0.5 | 49 |
| L2/3 of V1 | In vivo, anaesthetized | 2.7 ± 0.4 | 51 |
| L2/3 of S1 | In vivo, quiet awake | 6.3 ± 0.6 (0–15) | 52 |
| L2/3 of S1 | In vivo, whisking in air | 2.1 ± 0.4 | 52 |
| L2/3 of S1 | In vivo, with whisker touch | 1.7 ± 0.9 | 52 |
| L2/3 of V1 | In vivo, awake | 7.2 ± 8 | 53 |
| L2/3 of V1 | In vivo, awake | 7 ± 2 | 51 |
| L2/3 of V1 | In vivo, running | 11.6 ± 10.8 | 53 |
| L2/3 of V1 | In vivo, awake, visual stimuli | 26 ± 2 | 51 |
L2/3, layer 2/3; L5, layer 5; OLM, stratum oriens–lacunosum moleculare; S1, primary sensory cortex; SST neurons, somatostatin-expressing neurons; V1, primary visual cortex.
All experiments were carried out in mice or in slices derived from mice.
The membrane potentials of pyramidal cells and other GABAergic interneurons show bistability — that is, they fluctuate between a so-called ‘upstate’ (more depolarized) and a ‘downstate’ (more hyperpolarized) that together reflect overall changes in network activity in the cortex. Although the membrane potential of SST cells shows no such fluctuation52, SST neurons may play a crucial part in regulating the duration of upstates in the cortex. Recent studies in acute brain slices show that they fire more at upstate onset36 and, as they are a source of GABABR-mediated activation18, might facilitate upstate termination54.
Several studies in acute brain slices and in vivo have reported that cholinergic and noradrenergic inputs can enhance SST-neuron activity. In acute brain slices from mice, application of cholinergic and noradrenergic agonists increases spontaneous activity of neocortical SST neurons10,49,50,55. In vivo, optogenetic activation of cholinergic afferents from the nucleus basalis also increases the firing of SST cells in the primary visual cortex (V1)49 and, in the hippocampus, both in acute brain slices and in vivo, cholinergic input excites SST OLM neurons56–58.
Notably, neuromodulators that reduce SST-neuron activity have not yet been identified. Instead, reductions in SST-neuron activity may primarily come from inhibitory synaptic input from other GABAergic neuron subtypes, which have strong connections on to SST cells15,34,59,60. Indeed, multiple recent studies have defined a conserved anatomical circuit motif for synaptic inhibition of SST neurons. Vasoactive intestinal peptide-expressing interneurons (referred to in this article as VIP neurons) form synapses on to SST neurons, and excitation of VIP neurons suppresses SST-cell activity through GABAAR activation15,59,61. For example, in mouse S1, motor inputs from primary motor cortex activate VIP neurons, which in turn reduce the firing of SST neurons59, suggesting a mechanism for the potent suppression of SST-cell firing in superficial layers during whisking52 (FIG. 2a). VIP-neuron-mediated inhibition of SST neurons has also been observed in the auditory cortex and in the prefrontal cortex during motivated behaviour35,61.
Figure 2.
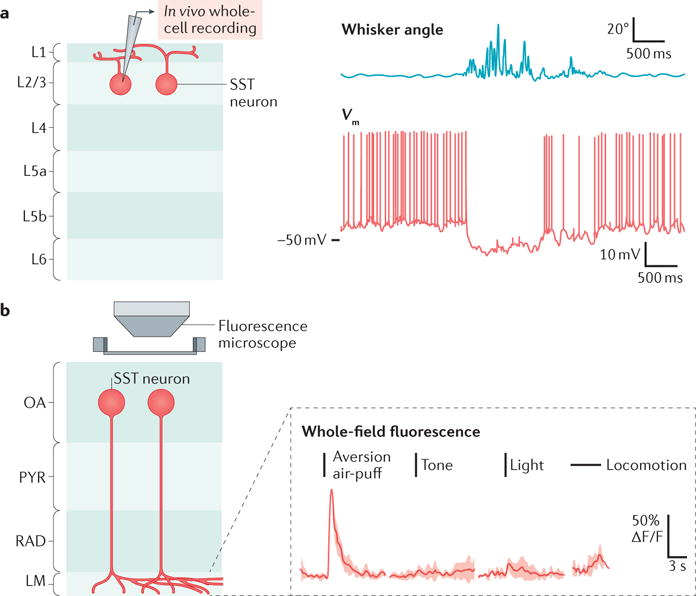
Regulation of SST-neuron activity during movement and sensation. The activity of somatostatin-expressing (SST) neurons can be up- or downregulated during different activities and behaviours, and in response to different stimuli. a | Schematic of the configuration (left panel) used for in vivo whole-cell recording (right panel; red trace) from a layer 2/3 (L2/3) SST neuron from mouse barrel cortex, during periods of quiet resting or whisking activity (right panel; blue trace). Whisking is associated with hyperpolarization of the SST neuron. b | Schematic of the calcium imaging configuration (left panel) used for the whole-field measurement of calcium transients in different cell types in different layers of the hippocampus — in this case, SST neurons with cells bodies in the stratum oriens–alveus (OA) that project to the stratum lacunosum moleculare (LM), known as OLM cells. Example trials (right panel) of Ca2+ transients in the LM during sensory stimuli and locomotion show that SST neurons increase their activity during presentation of the unconditioned stimulus (an aversive air-puff), but not to other sensory stimuli or during locomotion. PYR, stratum pyramidale; RAD, stratum radiatum; Vm, membrane potential. Part a is adapted from REF. 52, Nature Publishing Group. Part b is from Lovett-Barron, M. et al. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863 (2014); reprinted with permission from AAAS.
In V1, SST-neuron firing increases when animals engage in running behaviour53 (but see calcium imaging data in REF. 62), indicating that this brain area also has circuitry for movement-related regulation of SST-cell activity. Indeed, the VIP-neuron–SST-neuron pathway has also been identified in this brain area15. This trisynaptic motif (that is, from excitatory neuron to VIP neuron, to an SST neuron, to another postsynaptic excitatory neuron) represents a ‘computational primitive’: a repeating, ubiquitous motif found in cortical circuits. Taken together, these experimental findings indicate that specific patterns of synaptic input both within the neocortical circuit and from more-distant brain areas can regulate activity of SST cells.
Evidence suggests that this VIP-neuron–SST-neuron pathway might also be activated by thalamic input during sensory stimulation. Specifically, in vivo recordings from mouse S1 have shown that the high basal firing activity of SST neurons in superficial layers is briefly suppressed by a single whisker deflection52. Thus, even if SST cells receive direct thalamic excitation in L2/3, it is likely to be overridden by this recurrent inhibition in the circuit. Although it is unknown whether SST neurons in L4 are suppressed by sensory input, they do not receive strong excitatory input from the thalamus63,64. It will be of interest to determine whether the wiring principles for SST neurons are conserved across cortical layers. Current data indicate that the identity and local connectivity of L4 SST cells may in fact be different to that of L2/3 SST cells (see Supplementary information S1 (table)). The local circuits that control SST-neuron activity may also be different in V1, as V1 SST cells are not suppressed by visual stimulation51.
Regulation of the activity of SST neurons has also been observed in the hippocampus. In particular, the basal, theta-frequency firing of OLM cells42 can be increased by cholinergic activation from subcortical afferents58. Interestingly, hippocampal SST neural activity can be regulated by sleep: OLM cells show less activity during sleep states42. Overall, the high set point of SST-cell firing raises interesting questions about which behavioural and pharmacological conditions might raise or lower their firing to gate information flow and plasticity in the local network.
Network inhibition
SST cells are powerful regulators of local neuronal activity at different timescales, as they release GABA to activate fast synaptic GABAARs12,14–16,63, as well as slowly but persistently acting metabotropic GABABRs18. Typically, fast GABAAR transmission will induce effects on a timescale of tens of milliseconds, whereas GABABR activation can persist for seconds. The effect of SST-cell-mediated activation of GABABRs is profound: GABA released from SST neurons can activate presynaptic GABABRs on pyramidal cells, silencing connections between pyramidal cells18. Thus, GABA released from active SST cells may contribute to the high GABA tone in the cerebral cortex and affect the effective connectivity of nearby excitatory neurons, regardless of whether they have a direct synaptic connection with SST neurons.
Direct studies of the synaptic connectivity of cortical SST neurons indicate that they are densely, and perhaps nonspecifically, wired into the cortical network12,13 and are thus capable of providing strong inhibition to many different cell types within and across layers12,13,34. However, they typically share few synaptic connections with each other, indicating some specificity in wiring15,60.
The anatomical properties of SST neurons are distinct from those of other interneurons and suggest how these cells might function in a laminated, organized network, either in the neocortex or the hippocampus. SST cells have spatially constrained dendrites and long-range axonal arbors that project in characteristic patterns across the cortical column, as characterized in rat and mouse tissue. Subsets of SST neurons in the neocortex9,14,65,66 or the hippocampus42,67 have axonal arbors that elaborate extensively, specifically in L1 or the lacunosum moleculare, respectively. Martinotti SST cells have axonal arbors that can extend both locally and across layers; for example, L5b Martinotti cells arborize in L5a and L1 (REF. 14), or L4 (REF. 9). By contrast, L2/3 Martinotti neurons arborize mainly in L1 and to a lesser degree in L2/3, and L4 Martinotti cells mostly target L4, with only a small number of branches targeting L1 (REF. 9). An unusual subset of the SST neurons in CA1 of the hippocampus has long-range projections to the medial septum, which is an extrahippocampal area68–70.
Almost all studies show that SST neurons in the neocortex exhibit a high connection probability, receiving input from pyramidal neurons (Supplementary information S1 (table); approximately 30% of pyramidal cells form synapses on to a nearby SST neuron13,16,17,63) and synapsing on to adjacent pyramidal neurons (approaching 100% by some estimates12,14,15,63). Although SST neurons rarely form chemical synapses with each other15,60, they do form synapses with PV neurons14,15,71 and may be reciprocally connected with inhibitory VIP neurons15. They can also form electrical synapses with each other10,47,72.
Local circuits
The local circuits in which SST cells participate are specified according to brain area or subregion. For example, the relative difference in connectivities between SST cells and PV cells, and between SST cells and pyramidal cells, is different between L2/3 and L4. Specifically, there are more SST-cell–PV-cell connections than SST-cell–pyramidal-cell connections in L4 (REF. 14), and the reverse is true in L2/3 (Supplementary information S1 (table)). Thus, in vitro, the net impact of silencing SST neurons in L4 seems to be an increase in overall inhibition through local PV GABAergic neurons14. Data that support the existence of this powerful circuit — whereby activation of SST neurons inhibits PV cell firing — have been collected from V1 of anaesthetized mice71. However, in other brain areas, such as S1, silencing of SST neurons in L2/3 increases firing rates of nearby pyramidal cells, both in acute brain slices and in awake animals14,52. This suggests that SST-cell-mediated inhibition of PV cells is less effective in this layer. This difference might also be attributable to the presence of a sensory stimulus, which can alter broad-scale network activation.
In CA1 of the hippocampus, a subset of bistratified SST cells specifically innervates the dendritic zones of pyramidal neurons that receive input from CA3 (REF. 73). This is in contrast to OLM SST neurons, which modulate input from the entorhinal cortex67. A separate population of SST interneurons exists in the hilar region of the dentate gyrus. These cells arborize in the outer molecular layer, in which their projections are aligned with inputs from the entorhinal cortex and have been proposed to be important for feedback inhibition of granule cells74. Thus, differences in SST-cell type within subregions of the hippocampus will have discrete effects on network function.
Perhaps because of their elaborate axonal arbor in L1, neocortical SST neurons are presumed to form synapses primarily with the apical tufts of pyramidal cells75,76; however, this simplification is not well supported by experimental data. Anatomical reconstructions indicate that L5 SST cells contact both apical and basal dendrites of pyramidal neurons, and approximately 20% of contacts occur within 50 μm of the pyramidal cell soma9,65,77. Martinotti cells in L2 have a markedly denser axonal arbor in L1 than do L5 Martinotti cells. And despite their elaborate axonal arbor in L1, SST neurons do not necessarily restrict their input to pyramidal apical dendrites in this layer: approximately one-half of contacts from L5 SST neurons are on the basal dendrites of L5 pyramidal neurons77. The fact that SST cells have dense axonal branches in L1 raises the interesting possibility that SST neurons might modulate afferent inputs both directly and indirectly, through GABAAR-mediated inhibition and GABABR-mediated silencing of presynaptic terminals of input neurons. The subcellular location of synapses from SST neurons on to other inhibitory cell types is an important variable in understanding their influence. For example, the proportion of SST-cell synapses on to PV neurons is more than twofold higher among their distal dendrites than on the somatic compartment78. The close proximity of these inputs to the excitatory synapses on to PV cells suggests that SST-cell-initiated, GABABR-mediated suppression of glutamatergic input to PV cells18 may be important.
In both the neocortex and the hippocampus, inhibitory SST-cell synapses on to pyramidal cells are primarily associated with the dendritic shaft (approximately 71%), although a notable proportion of contacts (approximately 22%) are found on spine heads9,79. The effect of this synapse distribution has only just begun to be explored. SST terminals that synapse on to spine heads that are also innervated by pyramidal cells may regulate Ca2+ entry through postsynaptic NMDARs in the same dendritic compartment79. In addition, GABA that is released from SST axonal terminals near pyramidal-cell– pyramidal-cell synapses elicits presynaptic inhibition through GABABRs18.
Neocortical SST neurons seem to receive their excitatory synaptic input primarily from local and distant cortical areas13,15,17,34,66,80 rather than from subcortical areas. The high degree of convergence from local pyramidal neurons to SST neurons in the neocortex is undermined by the notable weakness of these excitatory synapses, which are difficult to detect from their single presynaptic spikes even under conditions that optimize neurotransmitter release11,17,66,81–83. In vivo paired recordings have confirmed this characteristic feature of local pyramidal inputs on SST neurons13. It is important that the low probability of neurotransmitter release from excitatory inputs to SST neurons is not mistaken for an absence of input. Optogenetic studies that aim to map longer-range neural circuits should be sensitive to this property of SST-cell inputs.
Although the prevalence of chemical synapses between SST neurons is extremely low, more than half of the proximal SST-neuron pairs in the neocortex are electrically coupled by gap junctions72,84,85. These electrical synapses are mediated by the connexon connexin 36 (REF. 86) and typically occur on the proximal dendrites of SST neurons85. Electrical coupling of SST neurons can provide depolarizing input that can synchronize firing — that is, a presynaptic spike can result in a postsynaptic depolarization of several millivolts — and this may be an important source of excitatory drive to these neurons. Indeed, synchronized firing in pairs of SST neurons has been observed under some experimental conditions, typically when SST cells are depolarized and firing rates are very high10,72,87. Because the frequency at which pairs of SST cells are coupled by gap junctions is very high, SST neurons can act as an electrically coupled network that can extend for hundreds of microns across the brain.
Disynaptic inhibition
The high connection probability between SST neurons and pyramidal neurons, and vice versa (Supplementary information S1 (table)), suggests that SST cells could, under some conditions, provide precisely timed feedback inhibition through fast GABAAR-mediated inhibition. This was initially proposed based on observations that high-frequency firing of a single pyramidal cell can drive disynaptic inhibition of local pyramidal neurons through Martinotti neurons — this is a ubiquitous anatomical motif that is observed both in superficial and deep layers of the neocortex17,66. However, the disynaptic Martinotti loop, which has been characterized in acute brain slices, can only be detected when the presynaptic pyramidal cell exhibits extremely high firing frequencies (approximately 70 Hz) and only following spikes late in the train17,66. It is thus important to note that the pyramidal cell firing frequencies that are required to activate this disynaptic loop have never been observed in vivo. Thus, how such disynaptic Martinotti inhibition might operate during sensation, perception and behaviour remains an open question.
Given the low probability of neurotransmitter release at excitatory synapses on to SST neurons, what are the conditions that enable chemical synaptic transmission to excite these neurons? The particular electrophysiological properties of these cells — including high input resistance and a hyperpolarized spike threshold (approximately −43 mV, versus −39 mV or −36 mV in pyramidal or PV neurons in L2/3 of somatosensory cortex, respectively)10 — suggest that SST cells may be sensitive to even small depolarizing inputs. Thus, the coincident firing of only a few excitatory cells in the network might be sufficient to initiate a spike in SST neurons88. Of course, electrical coupling between nearby SST neurons can also drive firing, synchronizing their inhibition on to excitatory pyramidal cells and PV inhibitory neurons.
Activity during complex behaviours
Genetic methods to target, record and control the activity of SST neurons in mice have revealed intriguing evidence for their role in decision making, synaptic plasticity and learning. Results from several different studies using targeted whole-cell recordings, Ca2+ imaging or optogenetically mediated cell identification indicate that the high basal firing of SST neurons is regulated upwards or downwards during complex behaviours (FIG. 2b), such as during fear learning, auditory discrimination or rewarded foraging35,57,61. SST cells generally suppress excitatory transmission (although they can also inhibit other GABAergic neurons, leading to more complex network effects14,15), and their activity is reduced during movement and active sensation. Because of these properties, it is tempting to speculate that the reductions in SST-neuron activity associated with complex behaviours might facilitate synaptic plasticity and learning by enhancing excitatory transmission, particularly through specific inputs that arise near synapses from SST cells18,79.
Indeed, the functional properties of SST neurons during more complex behavioural tasks can further distinguish this class of neurons from other GABAergic cells. In the anterior cingulate cortex, the high basal firing of a narrow-spiking subset of SST neurons is suppressed at the time when the animal enters an area associated with water reward (unlike the firing of fast-spiking PV neurons)35. Moreover, reductions in SST-neuron firing activity in superficial layers of the visual cortex have been observed during performance of a visually presented active avoidance task. These reductions persisted through several days of training75, thereby implicating SST cells in learning.
Certain behavioural conditions have been associated with altered SST-neuron activity. In a trained, rewarded task, SST-neuron activity in the prefrontal cortex slowly decreases during the animal’s movement to the target, but not during movement to non-target (that is, non-rewarded) locations35. By contrast, presentation of an unconditioned stimulus (a shock) is associated with an increase in SST-neuron activity in the hippocampus. The activity of SST OLM axons in the hippocampal lacunosum moleculare is also increased during exposure to an aversive stimulus57 (FIG. 2b). OLM-neuron-mediated inhibition of CA1 dendrites is required for correctly encoding fear memories, so this increase in SST-neuron activity may suppress sensory-related entorhinal inputs to this area, decoupling irrelevant sensory cues from signals of an aversive stimulus. Consistent with this hypothesis, specific silencing of SST neurons increased activity of CA1 pyramidal cells and reduced freezing behaviour in response to contextual cues that had previously been associated with an aversive stimulus (that is, a foot shock)57.
In the mouse motor cortex, SST neurons have an important role in suppressing dendritic Ca2+ spikes, which is an effect that might be mediated through postsynaptic GABABR activation89. During motor learning, SST cells in M1 regulate task-related spine dynamics of L5 pyramidal cells76 and the branch specificity of dendritic Ca2+ spikes in these cells90. Diphtheria-toxin-mediated ablation of M1 SST cells lowers performance of a motor-training task90, and optogenetic silencing of these cells prevents task-induced increases in spine size76. Consistent with the idea that SST-cell firing might gate plasticity in neocortical circuits, SST-cell firing in L2/3 of mouse V1 is also suppressed during associative visual learning75. Overall, regulated SST activity has been associated with circuit plasticity in both the neocortex and hippocampus.
It may not be surprising that interrupting the activity of a large population of GABAergic neurons can influence network output. However, given the specialized synaptic and anatomical properties of SST neurons, it is now possible to consider their specific role in channelling network activity or in controlling information flow to support learning. SST-neuron-mediated regulation of learning may occur through short-term changes in their activity91 that directly or indirectly influence excitatory neurons, or through long-lasting changes in SST-neuron anatomy, network activity or GABA synthesis76,92. Because SST cells profoundly suppress presynaptic release properties of (at least) excitatory inputs through GABABR activation on a timescale that can span hundreds of milliseconds, it is possible that many of their effects are mediated through reductions in excitatory transmission that, in many cases, effectively silence synapses.
In addition to the observations described above that SST-neuron activity is acutely regulated during certain behaviours or training, the influence of SST cells can undergo long-lasting increases or decreases. For example, the number of axonal boutons and the axonal arborization of SST neurons in the neocortex and hippocampus have been reported to change with training. For instance, SST axonal boutons in L1 of M1 are lost during learning of a novel motor task76. Long-lasting increases in SST-neuron firing have also been observed under some training conditions. For example, spontaneous firing activity of SST cells in the hippocampus increases after eyeblink conditioning. This is a property that might be attributed to the specific class of SST cell evaluated in this area91.
SST cells in disease
Neurological and psychiatric disorders have been associated with alterations in the gene expression, neural activity or anatomy of SST neurons. Along with PV interneurons, SST neurons have been implicated in schizophrenia, as individuals with schizophrenia have been reported to show decreased mRNA expression of somatostatin and mislocalization of SST neurons93–95. These findings are consistent with a neurodevelopmental origin for the disease, as MGE-derived interneurons (including PV cells) appear to be particularly affected.
Seizure disorders, which are characterized by recurrent elevated activity in neural networks, particularly of the hippocampus and neocortex, have also been associated with abnormal function of SST cells. SST neurons in the lacunosum moleculare of mice exhibited increases in axonal sprouting 2 months after pilocarpine administration in an epileptogenesis model96. In various models of epilepsy, SST neurons in the hilus have been reported to receive a stronger excitatory input97 and show increased axonal sprouting98,99 compared with healthy animals, which may change their ability to synchronize network inhibition100. As seizure disorders can initiate many changes across local networks, some of which might seek to restore a balance of excitation and inhibition, the increased output from SST neurons in epileptic tissue might be pathological. In contrast to models of temporal lobe seizures, in which SST output is enhanced, SST-neuron activity in the neocortex is reduced in a genetic form of epilepsy called Dravet syndrome101. Targeted interventions that aim to selectively increase the activity of specific interneuron subtypes such as SST cells may have some therapeutic advantage in seizure disorders102, and activation of somatostatin neuropeptide receptors has been proposed as another potential avenue for anticonvulsant therapies103.
Conclusions and future directions
A key property of SST neurons in active neuronal networks, both ex vivo and in the awake animal, is their high rate of basal firing activity, which persists in the absence of direct excitatory transmission. This tonic firing can be regulated by synaptic input and neuromodulators under different brain states or behavioural demands.
The way in which diverse neural circuits influence and transduce the effects of SST neurons, as well as the specific role of neuromodulators in regulating their activity — especially during learning — are exciting areas for future investigations. Their tonic activity and their dense axonal arborizations suggest that they may be an important source of extracellular GABA, activating both synaptic and extrasynaptic GABAARs and GABABRs. Recent studies have largely failed to examine whether the effects of SST-cell activity are mediated through fast, GABAAR transmission, which would require precisely timed activation, or slow, GABABR suppression, which might silence presynaptic inputs18 or suppress postsynaptic excitability89 at longer timescales. Because excitatory inputs to SST neurons are weak and difficult to activate without high-frequency, repetitive firing (which does not occur frequently in vivo), the role of these neurons in providing precisely timed inhibition remains unresolved.
The restricted, laminar axonal elaboration of some SST cells suggests that these cells may selectively suppress inputs that arrive through L1 in the neocortex and the lacunosum moleculare in the hippocampus. In addition, it is likely that the relative position of SST-cell synapses in relation to other inputs — both in terms of their general location along the dendritic tree and also their fine-scale position with respect to other synapses at the spine or dendritic shaft — is crucial for their heterosynaptic modulatory role. High-resolution anatomical reconstructions of neural circuits will help to constrain hypotheses about how broadly SST neurons can influence network activity.
Understanding the effects of spikes of specific subtypes of SST neurons and also of their ensemble activity, which may be synchronized by gap junction coupling, in modulating the flow of information across the network will be essential in developing a framework to explain the role of these neurons in cognition and behaviour. For example, it remains unclear which conditions in vivo enable SST-neuron spikes to be strongly synchronized across the population. Is the precise timing of SST-cell spikes crucial for GABAAR-mediated inhibition of pyramidal cells? Determining the conditions that distinguish between tonic basal firing and time-locked, input-specific activation of SST neurons during normal cortical activity will be of great interest.
Detailed anatomical, electrophysiological and molecular analysis of SST neurons indicates that they can be further distinguished into different subsets according to their brain area and laminar location, anatomy and molecular expression39,40, and this classification is an additional challenge for future studies. Studies that look at the effects of broad-scale inhibition of SST neurons have not distinguished between specific roles of these potentially diverse subtypes. For example, a subpopulation of narrow-spiking SST cells in the prefrontal cortex showed suppressed activity under some behavioural conditions, but this subpopulation is notably different from most (broader-spike) SST neurons that have been characterized in sensory cortex1. Synaptic sources of both excitation and inhibition of the SST class of interneuron should be defined, and these are likely to differ depending on specific SST-cell subtypes across neocortical layers and other brain areas. Finally, most current studies have focused on rodents — in particular, mice — because of the accessibility of new tools for identifying and manipulating SST activity. It will be important to determine whether the principles that have been identified in rodents are conserved across neural circuits in other species.
The evolution of new tools to identify and control the activity of specific subclasses of GABAergic neurons is bringing new clarity to our understanding of how neural circuits regulate the flow of information from different incoming streams. Analysis of the broad class of neurons that express somatostatin has been particularly exciting, and has implicated their regulated activity in cognition and behaviour. A synthesis of the effects of SST neurons on local and brain-wide activity is within reach.
Box 1. Transgenic mice.
An important advance in the field of research into somatostatin-expressing (SST) neurons was the generation of transgenic mice that express fluorophores selectively in this subset of interneurons. Serendipitously, a subset of SST cells that are localized mainly to the superficial layers of the cortex (that is, layers 2–4 (L2–L4) and upper L5) and to the stratum radiatum of the hippocampus was found to be labelled in a transgenic reporter line of mice known as green fluorescent protein (GFP)-expressing inhibitory neuron (GIN) mice. In these mice, the expression of GFP is controlled by expression of the GABA-synthesizing enzyme glutamate decarboxylase 1 (GAD67). Martinotti cells in the brain of these mice are GFP-labelled2. These animals, as well as certain other strains that express the Cre recombinase under the control of the somatostatin gene promoter (namely, SST–Cre mice), have enabled targeted whole-cell recordings for a comprehensive analysis of the firing properties and local connectivity of SST neurons (see the table). Specifically, viral delivery of Cre-dependent transgenes to SST–Cre mice, and the breeding of SST–Cre mice with reporter mouse strains have allowed molecular manipulation of this cell class and its activity3,4.
Current studies that use SST–Cre transgenic mice to investigate the role of SST neurons in perception and behaviour cannot easily differentiate between potential classes of SST neurons — this represents an important caveat in interpreting this experimental data. The molecular identification and experimental control of different subsets of SST neurons represent important challenges for the future.
|
| |||
| Strain | Promoter | Cell location | Refs |
|
| |||
| GIN | Gad1 (GAD67) | L2–L4 and upper L5 of the neocortex; the stratum oriens, stratum radiatum and hilus of the hippocampus | 2 |
|
| |||
| X94 | Gad1 (GAD67) | L4 and L5b of the neocortex | 1 |
|
| |||
| X98 | Gad1 (GAD67) | L5b and L6 of the neocortex | 1 |
|
| |||
| SST–Cre | Somatostatin | L1–L6 of the cortex; the stratum oriens, stratum radiatum and hilus of the hippocampus | 3 |
|
| |||
| SST–Cre | Somatostatin | Stratum oriens of the hippocampus (other areas not examined) | 57 |
|
| |||
Acknowledgments
The authors acknowledge members of the Barth laboratory for helpful comments on the manuscript, and US National Institutes of Health (NIH) grant number NS088958 and the McKnight Foundation (A.L.B.) for support.
Footnotes
Competing interests statement
The authors declare no competing interests.
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliva AA, et al. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovett-Barron M, et al. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci. 2012;15:423–430. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- 5.Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 7.Kubota Y, et al. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes A, et al. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 12.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pala A, Petersen CC. In vivo measurement of cell-type-specific synaptic connectivity and synaptic transmission in layer 2/3 mouse barrel cortex. Neuron. 2015;85:68–75. doi: 10.1016/j.neuron.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy RB, Reyes AD. Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex. J Neurosci. 2012;32:5609–5619. doi: 10.1523/JNEUROSCI.5158-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urban-Ciecko J, Fanselow EE, Barth AL. Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Curr Biol. 2015;25:722–731. doi: 10.1016/j.cub.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bologna E, Leroux P. Identification of multiple somatostatin receptors in the rat somatosensory cortex during development. J Comp Neurol. 2000;420:466–480. doi: 10.1002/(sici)1096-9861(20000515)420:4<466::aid-cne5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Moore SD, Madamba SG, Joels M, Siggins GR. Somatostatin augments the M-current in hippocampal neurons. Science. 1988;239:278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer P, Madamba SG, Siggins GR. Somatostatin increases a voltage-insensitive K+ conductance in rat CA1 hippocampal neurons. J Neurophysiol. 1998;79:1230–1238. doi: 10.1152/jn.1998.79.3.1230. [DOI] [PubMed] [Google Scholar]
- 23.Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 1997;17:4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butt SJ, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bendotti C, et al. Developmental expression of somatostatin in mouse brain. II. In situ hybridization. Brain Res Dev Brain Res. 1990;53:26–39. doi: 10.1016/0165-3806(90)90121-e. [DOI] [PubMed] [Google Scholar]
- 28.Forloni G, Hohmann C, Coyle JT. Developmental expression of somatostatin in mouse brain. I. Immunocytochemical studies. Brain Res Dev Brain Res. 1990;53:6–25. doi: 10.1016/0165-3806(90)90120-n. [DOI] [PubMed] [Google Scholar]
- 29.Hogan D, Berman NE. The development of somatostatin immunoreactive neurons in cat visual cortical areas. Brain Res Dev Brain Res. 1993;71:221–238. doi: 10.1016/0165-3806(93)90174-9. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos GC, Cavanagh ME, Antonopoulos J, Michaloudi H, Parnavelas JG. Postnatal development of somatostatin-containing neurons in the visual cortex of normal and dark-reared rats. Exp Brain Res. 1993;92:473–478. doi: 10.1007/BF00229035. [DOI] [PubMed] [Google Scholar]
- 31.Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- 32.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 33.Hu H, Cavendish JZ, Agmon A. Not all that glitters is gold: off-target recombination in the somatostatin-IRES-Cre mouse line labels a subset of fast-spiking interneurons. Front Neural Circuits. 2013;7:195. doi: 10.3389/fncir.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 2015;350:aac9462. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kvitsiani D, et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neske GT, Patrick SL, Connors BW. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci. 2015;35:1089–1105. doi: 10.1523/JNEUROSCI.2279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dun NJ, Dun SL, Wong RK, Forstermann U. Colocalization of nitric oxide synthase and somatostatin immunoreactivity in rat dentate hilar neurons. Proc Natl Acad Sci USA. 1994;91:2955–2959. doi: 10.1073/pnas.91.8.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat. 2007;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci. 2009;29:70–85. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J Physiol. 2004;558:465–478. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katona L, et al. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron. 2014;82:872–886. doi: 10.1016/j.neuron.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baude A, et al. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 44.Chittajallu R, et al. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT3AR expression. Nat Neurosci. 2013;16:1598–1607. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katona I, Acsady L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- 46.Klausberger T, et al. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 47.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin-and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- 49.Chen N, Sugihara H, Sur M. An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat Neurosci. 2015;18:892–902. doi: 10.1038/nn.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 51.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gentet LJ, et al. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 53.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ. Distinct roles of GABAB1a- and GABAB1b-containing GABAB receptors in spontaneous and evoked termination of persistent cortical activity. J Physiol. 2013;591:835–843. doi: 10.1113/jphysiol.2012.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence JJ, Statland JM, Grinspan ZM, McBain CJ. Cell type-specific dependence of muscarinic signalling in mouse hippocampal stratum oriens interneurones. J Physiol. 2006;570:595–610. doi: 10.1113/jphysiol.2005.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovett-Barron M, et al. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leao RN, et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15:1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Hu H, Agmon A. Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype. J Neurosci. 2012;32:983–988. doi: 10.1523/JNEUROSCI.5007-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pi HJ, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu Y, et al. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- 64.Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Packer AM, McConnell DJ, Fino E, Yuste R. Axo-dendritic overlap and laminar projection can explain interneuron connectivity to pyramidal cells. Cereb Cortex. 2013;23:2790–2802. doi: 10.1093/cercor/bhs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 67.McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gulyas A, Hajos N, Katona I, Freund T. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- 69.Jinno S, et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jinno S, Kosaka T. Immunocytochemical characterization of hippocamposeptal projecting GABAergic nonprincipal neurons in the mouse brain: a retrograde labeling study. Brain Res. 2002;945:219–231. doi: 10.1016/s0006-8993(02)02804-4. [DOI] [PubMed] [Google Scholar]
- 71.Cottam JC, Smith SL, Hausser M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J Neurosci. 2013;33:19567–19578. doi: 10.1523/JNEUROSCI.2624-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu H, Agmon A. Properties of precise firing synchrony between synaptically coupled cortical interneurons depend on their mode of coupling. J Neurophysiol. 2015;114:624–637. doi: 10.1152/jn.00304.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buhl E, et al. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J Neurophysiol. 1994;71:1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- 74.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 75.Makino H, Komiyama T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci. 2015;18:1116–1122. doi: 10.1038/nn.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen SX, Kim AN, Peters AJ, Komiyama T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci. 2015;18:1109–1115. doi: 10.1038/nn.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill SL, Wang Y, Riachi I, Schurmann F, Markram H. Statistical connectivity provides a sufficient foundation for specific functional connectivity in neocortical neural microcircuits. Proc Natl Acad Sci USA. 2012;109:E2885–E2894. doi: 10.1073/pnas.1202128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hioki H, et al. Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J Neurosci. 2013;33:544–555. doi: 10.1523/JNEUROSCI.2255-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiu CQ, et al. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinnischtzke AK, Simons DJ, Fanselow EE. Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex. Cereb Cortex. 2014;24:2237–2248. doi: 10.1093/cercor/bht085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fanselow EE, Connors BW. The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UP-DOWN states of mouse neocortex. J Neurophysiol. 2010;104:596–606. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- 84.Amitai Y, et al. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- 86.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 87.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 88.Berger TK, Silberberg G, Perin R, Markram H. Brief bursts self-inhibit and correlate the pyramidal network. PLoS Biol. 2010;8:e1000473. doi: 10.1371/journal.pbio.1000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palmer LM, et al. The cellular basis of GABAB-mediated interhemispheric inhibition. Science. 2012;335:989–993. doi: 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- 90.Cichon J, Gan WB. Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature. 2015;520:180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKay BM, Oh MM, Disterhoft JF. Learning increases intrinsic excitability of hippocampal interneurons. J Neurosci. 2013;33:5499–5506. doi: 10.1523/JNEUROSCI.4068-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cybulska-Klosowicz A, et al. Interneurons containing somatostatin are affected by learning-induced cortical plasticity. Neuroscience. 2013;254:18–25. doi: 10.1016/j.neuroscience.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 93.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Volk DW, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peng Z, et al. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halabisky B, Parada I, Buckmaster PS, Prince DA. Excitatory input onto hilar somatostatin interneurons is increased in a chronic model of epilepsy. J Neurophysiol. 2010;104:2214–2223. doi: 10.1152/jn.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:2057–2064. doi: 10.1111/j.1528-1167.2011.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W, et al. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grosser S, Queenan BN, Lalchandani RR, Vicini S. Hilar somatostatin interneurons contribute to synchronized GABA activity in an in vitro epilepsy model. PLoS ONE. 2014;9:e86250. doi: 10.1371/journal.pone.0086250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA. 2014;111:E3139–E3148. doi: 10.1073/pnas.1411131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dobolyi A, et al. Receptors of peptides as therapeutic targets in epilepsy research. Curr Med Chem. 2014;21:764–787. doi: 10.2174/0929867320666131119154018. [DOI] [PubMed] [Google Scholar]


