Abstract
Introduction:
Neurogenic bladder patients are at long-term risk of secondary upper urinary tract damage. Symptoms are unreliable and follow-up urodynamics is the only method of ascertaining safety of bladder pressures. This review examines the recommendations, shortcomings and utilization of existing guidelines. The evidence with regard to follow-up urodynamics in different settings relevant to neurogenic bladder is evaluated and an algorithm is proposed.
Methods:
A pubmed search was conducted for studies on follow-up urodynamics in patients with neurogenic bladder. Additional search was made of secondary sources including reviews and guidelines.
Results:
The need for follow-up urodynamics should be considered in all patients undergoing an initial assessment and weighed against the risks. Existing guidelines, while unanimous in their recommendation of its utilization, give scant details regarding its incorporation in clinical management. Follow-up urodynamics can document efficacy and identify the need for escalation of therapy in patients on intermittent catheterization and antimuscarinics. Patients with spinal injury, spinal dysraphism and anorectal malformations are at higher risk for upper tract damage. Follow-up urodynamics can help identify patients suitable for intravesical botulinum and mark those destined for failure. Patients undergoing augmentation cystoplasty may be candidates for less aggressive urodynamic follow-up.
Conclusions:
Neurogenic bladder is managed by a broad cross-section of physicians. Clear recommendations and a management algorithm are important for improving patient care. Follow-up urodynamics can identify patients at risk, prevent renal dysfunction and improve the quality of life. There is an urgent need for more evidence on this important subject.
INTRODUCTION
Patients with neurogenic bladder are at risk of long-term upper tract damage. This is despite the normal functional and anatomical state of the kidneys early in the course of disease. Not just those with spinal injury who obviously have normal upper tracts at inception, up to 90% of children with spinal dysraphism also have normal upper tract function when assessed in infancy.[1] However, a significant proportion of these patients may develop renal dysfunction with time.[2,3] Damage to the upper tracts has been shown to be secondary to an abnormal lower urinary tract.[3,4] Symptoms are a poor guide to lower urinary tract dysfunction, especially in a setting of neurogenic dysfunction.[5,6,7]
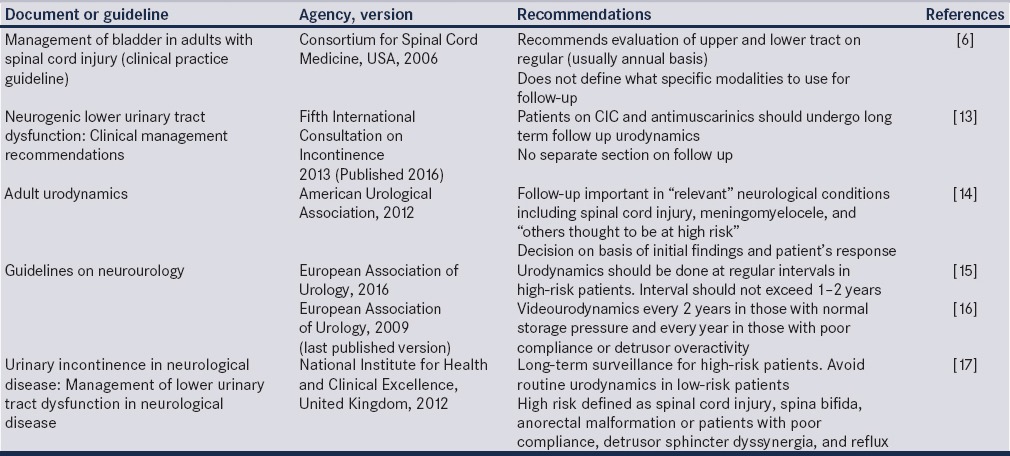
Given these facts, urodynamics remains underutilized in the management of patients with neurogenic bladder. In two recent series, a large proportion of patients did not receive even a single urodynamics in the course of their management.[8,9] The initial urodynamics is important in planning therapy.[7] However, it is clear that many patients with neurogenic bladder continue to deteriorate in follow-up. Despite clean intermittent catheterization (CIC), children with spinal dysraphism may show progressive renal dysfunction and develop new renal scars triggered by poor bladder function.[10,11] Achieving and maintaining safe storage pressures are critical in ensuring long-term safety of the upper tracts.[12] While existing guidelines recommend follow-up evaluation, there are scant details regarding the incorporation of these recommendations into actual clinical practice [Table 1]. This review examines the evidence for follow-up urodynamics in patients with neurogenic bladder and provides an algorithm for incorporating it into clinical decision-making.
Table 1.
Position of various guidelines on follow-up urodynamics
METHODS AND THEIR LIMITATIONS
Literature search was performed by cross-referencing “urodynamics,” “cystometry,” or “pressure-flow study” with forty different terms related to “neurogenic bladder.” Landmark reviews and clinical practice guidelines on neurogenic bladder were also used as secondary source documents. Of note, none of the articles referenced in recent reviews on follow-up dealt directly with the value of follow-up urodynamics in the management of neurogenic bladder patients.[18,19] A search of the evidence quoted in major clinical practice guidelines with regard to value of follow-up urodynamics in patients with neurogenic bladder showed little evidence on the subject.[6,13,14,15,16,17,20] Hence, conclusions need to be drawn from studies carried out with a different objective thus limiting the strength of conclusions.
ADHERENCE TO CLINICAL GUIDELINES
Adherence to guidelines in clinical practice shows considerable variation but often falls short of recommendations. A large Canadian study examined the utilization of urodynamics in 1551 patients with spinal cord injury over a 10-year period and found that only 10% patients received the recommended follow-up with one-third not receiving even a single follow-up study.[9] Studies from UK showed improving compliance with guidelines in recent years, but most institutions were still not performing regular follow-up urodynamics.[21,22] A survey from France showed that routine follow-up urodynamics was performed by 56% of urologists and 83% of physiatrists, most often on an annual basis.[23] However, only 12% urologists in the Netherlands performed routine follow-up urodynamics despite European Association of Urology (EAU) guidelines recommendations.[24]
LITERATURE ON FOLLOW-UP URODYNAMICS
Early and appropriate urodynamics-based management of spinal cord injury patients can prevent the long-term development of poor compliance requiring augmentation cystoplasty surgery. None of the 246 patients with spinal cord injury from a Switzerland center needed augmentation cystoplasty during a mean follow-up of 17 years. Of note, therapy was initiated early and patients were followed carefully by a protocol that included urodynamics.[25] In contrast, children with meningomyelocele often show poor compliance, and thus, this group needs a different and more aggressive follow-up approach.[26]
Follow-up urodynamics in patients on clean intermittent catheterization and antimuscarinics
Antimuscarinics and CIC remain the mainstay of therapy in patients with florid neurogenic bladder such as spina bifida or spinal cord injury.[8,27,28,29] In contrast, patients with demyelinating conditions such as multiple sclerosis are more likely to be voiding spontaneously.[30]
Follow-up urodynamics demonstrates the efficiency of antimuscarinincs in improving capacity, volume at first involuntary contraction, and its strength. In a study of adults with spinal injury or multiple sclerosis, both solifenacin and oxybutynin showed dose-dependent efficacy.[31] However, not all patients responded to antimuscarinics.[28,32]
Improvement in urodynamic parameters was also noted in myelodysplasia children who underwent follow-up evaluation.[33] Oxybutynin eliminated or reduced the contraction pressure of phasic detrusor contractions and reduced storage pressures. However, 8% of patients developed upper tract changes indicating the importance of lower tract surveillance. These results were better than historical controls treated expectantly. The authors emphasized the need for proactive urodynamics-based management.
Follow-up urodynamics documenting failure to resolve storage pressure abnormalities in patients on antimuscarinics may guide the need for careful addition of mirabegron.[34]
Given that storage pressures are critical for the upper urinary tract, urodynamics can be used to objectively document the reduction in unsafe pressures in patients on antimuscarinics and can help identify patients who need more aggressive management. Alternatively, antimuscarinics may be tapered down or withdrawn under careful urodynamic monitoring in some patients.
Follow-up urodynamics in patients on intravesical botulinum toxin
Follow-up urodynamics has been used both to identify refractory patients suitable for intravesical botulinum injection and for assessing the response to that therapy. In a landmark, large multicentric randomized trial of 416 patients with multiple sclerosis (n = 227) and spinal cord injury (n = 189), Ginsberg et al. showed that botulinum toxin A injections reduced the contraction pressure of involuntary detrusor contractions by 33 cm H2O and abolished the contractions in two-third patients for a median duration of 9 months.[35]
Urodynamics has been almost universally utilized to assess the efficacy of botulinum toxin in children with spina bifida. All 12 studies included in a recent review utilized urodynamics to follow the outcome of botulinum toxin injections.[36] Although detrusor pressures reduced following injection in all eight studies, pressures often remained unsafe. A compliance of 20 ml/cm H2O was attained in only two of the studies.[36] Patients with neurogenic detrusor overactivity respond better than patients with poor compliance.[37] In a study of 37 children with neurogenic bladder, 17 children failed to respond to botulinum toxin A injection. The mean preinjection compliance of nonresponders was significantly lower than responders, 8.6 ml/cm of H2O as compared with 25.1 ml/cm of H2O (P = 0.039).[38] Not all initial responders continue to respond to injections in this setting and hence continued urodynamic surveillance of these patients remains important.[39]
It is clear that patients with poorly compliant bladder receiving botulinum toxin injection need follow-up urodynamics to identify nonresponders for alternate therapies.[26]
Follow-up urodynamics in patients undergoing augmentation cystoplasty
Follow-up urodynamics almost universally shows a marked reduction in storage pressures and adequate cystometric capacity following augmentation cystoplasty. In a series of 26 patients followed 8 years, the maximum detrusor pressure fell from 81 to 20 cm H2O while the capacity increased from 201 to 615 ml.[40] Similar results were noted in a series of children with neurogenic bladder due to meningomyelocele with filling pressure reducing from 41 to 11 cm H2O.[41] In contrast, Vainrib et al. showed that 11% of adult meningomyelocele patients had unsatisfactory compliance at a mean follow-up of 10.4 years. It is unclear whether these patients had symptoms or clinical markers of poor performance.[42]
The almost universal success of augmentation cystoplasty in achieving a good bladder capacity and reduction of storage pressures calls into question the need for routine use of long-term urodynamics following augmentation cystoplasty. It might be judicious to perform one follow-up study at 6 months and reserve long-term follow-up for the small subgroup having unsatisfactory findings on this study or other clinical clues to an unfavorable outcome.
Follow-up urodynamics after bladder neck reconstruction for neurogenic bladder patients with incontinence
A major concern in patients offered isolated surgery for enhancing outlet resistance is the possibility of unrecognized poor bladder storage function causing renal deterioration. Patients with profound neurogenic incontinence present a technical problem during urodynamics. Severe incontinence precludes bladder filling and this may mask poor storage. Maneuvers such as peripenile compression while filling (in males) or tucking a balloon catheter against the bladder neck (in females) are imperfect but useful adjuncts to unmask such problems.
In a series of 82 patients undergoing isolated bladder outlet reconstruction or closure, 10 patients (12%) needed a subsequent augmentation over a mean follow-up of 60 months. These patients had poor compliance and reduced capacity.[43] In contrast, 45% patients in another study required augmentation at 2.6 years following outlet surgery.[44]
There seems to be a significant risk of postoperative elevated bladder pressures following isolated bladder neck reconstruction. Careful preoperative urodynamics cannot eliminate this risk and this risk may persist even at 5 years. Hence, all patients undergoing isolated bladder neck reconstruction should be followed with long-term urodynamics.
Follow-up urodynamics after neurosurgical interventions in patients with neurogenic bladder
Some patients with relatively nonobtrusive neurosurgical problems are offered neurosurgical intervention with the expectation that lower urinary tract symptoms (among other problems) might resolve. In a recent systematic review, follow-up urodynamics was used to analyze patients who underwent detethering surgery and showed urodynamic improvement in 11%–55%.[45] Symptoms are not a good guide in this postoperative setting.[46] Urodynamics may also identify patients doing poorly before deterioration.[47]
Follow-up urodynamics as an investigational tool for evaluating newer modalities of treatment
Follow-up urodynamics remains an important outcome measure to evaluate new treatments. A recent study examining the benefit of sectioning of the filum terminale in occult tethered cord utilized a scoring system based on urodynamics before and after treatment to assess efficacy to demonstrate lack of benefit.[48] In another study, researchers performed an age-matched comparison of follow-up urodynamics in children who had previously undergone in utero meningomyelocele closure with those that underwent postnatal surgery to show that the investigative surgery was not beneficial.[49]
Follow-up urodynamics was used to assess the impact of intraurethral botulinum toxin in spinal cord injury patients on a partial voiding regimen and showed persistent need for intermittent catheterization in most studies.[50] Follow-up urodynamics is also used to evaluate the impact of novel pharmacological approaches. The measure showed the lack of efficacy of selective alpha-blocker in children with neurogenic bladder.[51]
LITERATURE ON THE IMPACT OF FOLLOW-UP URODYNAMICS
Only two studies directly examine the impact of follow-up urodynamics on clinical decision-making. Linsenmeyer and Linsenmeyer studied 96 consecutive adult spinal cord injury patients who had sustained their injury at least 2 years before evaluation.[52] 48% of these patients needed some form of intervention based on follow-up urodynamics, most often escalation of antimuscarinics (69%). Of note, none of these patients had any new urological symptoms. Interventions were needed in patients who were injured for up to 5, 6–10, 11–15, and 16 years and above in 47%, 39%, 52%, and 50%, respectively. Interventions continued to be required despite long-term follow-up implying that ongoing urodynamic surveillance may be critical. Urodynamics also helped exclude a urological etiology in some patients with autonomic dysreflexia leading to nonurological interventions such as aggressive constipation management. The authors concluded that annual urodynamics was useful in the management of these group patients.
Nosseir et al. retrospectively studied eighty adult spinal cord injury patients who had undergone at least one urodynamic evaluation in a year for five consecutive years following their injury.[53] Fifty-one of the patients were on CIC and seven were on indwelling catheters. Changes to treatment were required in 77 patients including surgical intervention in 15 (sphincterotomy 8, Brindley stimulator 3, augmentation cystoplasty 3, Kock's pouch 1), botulinum toxin injection in 12 and changes to antimuscarinic therapy in 22. None of the patients developed any sign of renal damage in the period when assessed by ultrasonography, urine examination, and serum biochemistry. The authors concluded that urodynamics had an important role to play in the preservation of the upper tracts.
An interesting finding was the need for modification of therapy in patients on indwelling catheters in both the studies. These patients are usually not subjected to urodynamic follow-up with the expectation that a catheter would lower the intravesical pressure thus protecting the upper tracts. However, 40% of patients who had an indwelling catheter (urethral or suprapubic) required interventions often change in medication based on their urodynamic findings.[52] Hence, these patients must also be followed similar to the patients on more optimum therapies.
In a study of pediatric spinal cord injury patients follow-up showed improvement in bladder function and morphology with urodynamics-based initiation of antimuscarinics and CIC.[54] Another study of children with meningomyelocele showed that 8% of children showed renal damage and that at least one poor urodynamic parameter was noted in each these patients. The authors recommended follow-up urodynamics though the actual benefit of the follow-up studies was unclear.[55]
In another study of 100 spinal injury patients whose treatment was guided by urodynamics, 15% developed upper tract changes. It was unclear whether these were patients who had established renal dysfunction at enrollment. Of note, detrusor pressure was over 40 cm H2O in 64% at final evaluation suggesting need for a more aggressive management approach.[28]
Follow-up urodynamics can confirm improvement in bladder storage volume and pressure and this has been correlated with improved estimated renal plasma flow on nuclear renogram.[56]
RISK OF FOLLOW-UP URODYNAMICS
Altered urinary tract morphology and function, poor perineal hygiene, and the use of catheters can all impact the morbidity of repeated testing. Urodynamics is an invasive evaluation associated with discomfort and occasionally bleeding, infection, or autonomic dysreflexia.[16] Urinary tract infection is not uncommon following urodynamics. In a study of 72 patients with spinal cord injury undergoing urodynamics, seven patients developed urinary tract infection, of which five were symptomatic.[57] Of note, the presence of asymptomatic bacteriuria did not have an impact on the development of urinary infection. Patients on CIC will often have bacteriuria and it is difficult and perhaps undesirable to sterilize the urine in this setting. Instead, antimicrobial prophylaxis given empirically is effective and should be preferred.[58] Anecdotally, the author has found children with upper tract dilatation and renal insufficiency to be specifically vulnerable.
Autonomic dysreflexia is a major concern in patients with high lesions above T6 spinal level. Lack of autonomic dysreflexia at earlier urodynamics is by no means protective. All quadriplegics must have blood pressure monitoring and in those with a previous history of autonomic dysreflexia, it is preferable to use prophylaxis with terazosin (5 mg 30 min before the test) or even anesthesia.[7,59] Clearly, one must balance the need for testing against the dangers.
Radiation exposure is an important consideration. The cumulative impact of radiation in those offered video studies, as recommended by the EAU guideline, could be considerable. In a study of the radiation dose due to a single videourodynamic study in 64 children, the mean exposure time was 1.8 min and the mean total radiation exposure was 10 mGy potentially adding up to a substantial proportion of permissible lifetime exposure with repeated testing.[60,61] Hence, the decision to use videourodynamics must be a deliberate, documented decision with a strategy for minimizing radiation exposure.
One must be careful while performing urodynamics in patients after augmentation cystoplasty. Reduced bladder sensation and a thin-walled potentially weaker augmented bladder can result in bladder rupture during filling.[62] Latex allergy can be an important issue in some of these patients who are undergoing repeated procedures.[63] While minor reactions are not uncommon, rare occurrence of anaphylaxis has been recorded.
SURROGATES FOR FOLLOW-UP URODYNAMIC FINDINGS
A reliable surrogate for bladder function would be very useful and could potentially help avoid repeated urodynamic testing. Symptoms are a poor surrogate. Studies show a striking lack of correlation between symptoms and urodynamic findings in patients with neurogenic bladder.[52] Symptom-based assessment would have missed 69% of the target patients in another group of spinal cord injury adults.[53] In patients with a bladder diverticulum, gross reflux and severe sphincteric incompetence normal storage pressures may be misleading and storage symptoms can be a clue to storage abnormalities.
Incontinence episodes recorded on a CIC diary could potentially be a useful marker. Persistence of incontinence in a patient with documented elevated storage pressure might indicate the need for intervention. However, patients may have unsafe storage pressures despite achieving continence.[32] The CIC diary can be used to titrate the dose of antimuscarinics, notwithstanding the caveats mentioned. It also gives vital information regarding 24-h urine volume and average CIC volumes, both of which have impact on clinical care.
Ultrasonographic measurement of bladder wall thickness has also been studied as a surrogate marker for urodynamic findings. However, although bladder wall thickness is higher in patients with poor compliance, meaningful cut-offs are elusive and standardization lacking.[64,65] Ultrasonography can only identify upper tract dilatation once its already occurred, a situation that follow-up urodynamics can avoid.
The degree of functional disability was correlated with urodynamics in a study of 134 adults with spinal dysraphism. Being wheelchair-bound increased the odds of finding unsafe storage pressures on urodynamics (odds ratio 5.36).[45] The authors suggested that in patients who are asymptomatic and not wheelchair-bound, urodynamics might not be necessary.
Aside from CIC diary, the author uses surrogate markers sparingly when there are technical issues that render the urodynamic findings less reliable.
URODYNAMIC PARAMETERS ASSESSED IN FOLLOW-UP
Follow-up urodynamics must include all parameters assessed at the initial study specifically those that had a demonstrable abnormality. One must record storage and voiding phase parameters including bladder sensation, capacity, compliance, presence of detrusor overactivity, the Pdet.max and Pdet. Qmax, and the flow rate and pattern. In patients on a complete CIC protocol, the voiding phase parameters may lose some of their relevance while a tube-free uroflow may be relevant in patients who are voiding spontaneously.
Improvement in the bladder volume at first involuntary detrusor contraction and the height of that contraction may be useful to assess the impact of medication. The detrusor leak point pressure, a term given to the pressure at which fluid starts leaking by the side of the urethral catheter at the time of filling in the absence of a detrusor contraction, is a useful parameter to assess safety of bladder pressures, with certain caveats (vide infra).
At urodynamics, multiple cycles of testing should always be performed. Patients on an indwelling catheter may show a significant increase in the volume at first involuntary detrusor contraction and cystometric capacity.[66] While such changes were not noted in patients on CIC by these authors, it is good practice to perform multiple cycles to ensure that any findings noted are consistently seen.[66,67]
Scoring systems have been sporadically used to identify patients at risk for upper tract damage. One study combined bladder volume, compliance, detrusor activity, and sphincter coordination into a score that could follow the impact of detethering and predicting retethering.[68] Others have shown that the 6-month score had prognostic value.[69] However, in the absence of clinical criteria, these scorings have limited utility.
Most authors recommend videourodynamics as the study of choice.[70] However, the evidence in this regard remains weak.[71] Surveys in Canada and the Netherlands have shown that few urologists use videourodynamics.[24,72,73] Video studies should be considered in patients with anatomical changes to the upper tracts but whether every follow-up should incorporate video is debatable.[74] While the American Urological Association guideline on urodynamics states that urologists “may perform” fluoroscopy during urodynamics, this seems to refer to the initial evaluation rather than follow-up.[16] Ambulatory studies have not been found to be useful in this setting.[75]
INCORPORATING FOLLOW UP URODYNAMICS INTO CLINICAL MANAGEMENT
Follow-up urodynamics must be considered in all patients offered an initial study, especially if the lower tract was unsafe or there are current clinical or investigative features showing deterioration.[76] Typically, these are patients with pontine and infrapontine lesions.[77] High-risk patients may be better served by an aggressive follow-up approach.[78] Ensuring optimum lower urinary tract function has been associated with an improved quality of life.[79]
The urodynamic question almost invariably pertains to safety of the lower tract or the etiology of ongoing incontinence episodes. Unfortunately, no specific cut-off storage pressure reliably defines safe storage despite the oft-quoted figure of 40 cm of H2O.[80] Children may damage their upper tracts at lower pressures, especially in the presence of secondary vesicoureteral reflux. In fact, in the face of large volume bilateral reflux, any rise in storage pressure is suspicious. Storage pressures must be safe at the usual CIC volumes while storage volumes should be matched up to the age-adjusted expected urine volume such that five daily catheterizations are adequate to evacuate the bladder effectively.
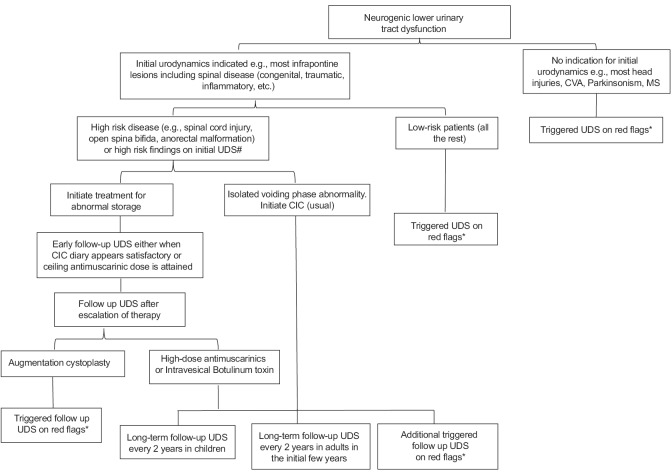
Not just the actual pressure recorded, but the pattern of pressure rise and the usual CIC volume are equally critical in determining safety of the lower urinary tract [Figure 1]. Figure 2 summarizes these considerations in an algorithm. There is no unanimity regarding how long urodynamic follow-up should continue. In infants with spinal dysraphism, annual urodynamics until the age of 6 years or until growth is complete, followed by triggered testing has been suggested.[74,81] However, the need for surgery in over one-fourth of adults treated earlier for spina bifida may indicate need for routine long-term evaluation.[8] A recent review noted the need for regular urodynamics and the possibility of changing patterns with time while acknowledging the lack of adequate evidence.[82] Others have suggested long-term urodynamics (without defining an end-point) for patients on intermittent catheterization and antimuscarinics.[15,70] The National Institute for Health and Clinical Excellence guideline suggests that long-term repeated urodynamics might not be necessary after augmentation cystoplasty.[19,40] A recent Center for Disease Control protocol is examining routine urodynamics at 3 months and thereafter annually for all children with spina bifida with additional studies at 6 months if the pressures are unsafe.[83] The author practices routine urodynamics in children until the age of 18 years and for the first 5 years in adults followed by triggered testing alone.
Figure 1.
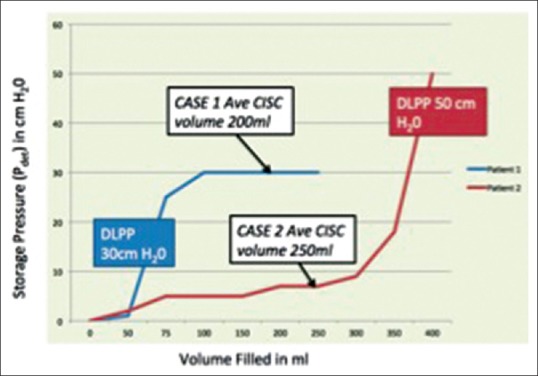
Impact of pattern of storage pressure in two hypothetical children 8 years old on clean intermittent catheterization and antimuscarinics with reduced compliance and a history of urinary incontinence. Child 1 has an average clean intermittent catheterization volume of 200 ml, a detrusor leak point of 30 cm H2O and high storage pressures at 100 ml fill volume. Child 2 has an average clean intermittent catheterization volume of 250 ml, a detrusor leak point of 50 cm H2O but storage pressures remain low till about 350 ml. Interpretation: The second child is likely to be safe in the long run but the first child needs escalation of therapy
Figure 2.
Algorithm for follow-up urodynamics in the management of neurogenic lower urinary tract dysfunction *Red flags – incontinence, need for additional catheterizations in clean intermittent catheterization regimen, recurrent urinary infection, worsening of upper tract morphology or function, stone formation. #High risk findings on initial urodynamics – poor compliance, high pressure neurogenic detrusor overactivity, detrusor sphincter dyssynergia with high voiding pressures
Patients with neurogenic bladder cross path with a broad cross-section of physicians from various departments. Clear urodynamic-based goals and an unambiguous algorithm could greatly improve our ability to manage this complex problem.
CONCLUSIONS
Follow-up urodynamics is critical in the management of neurogenic bladder patients. It is useful for monitoring response to therapies, safety of the lower urinary tract, and the identification of patients needing escalation of their management. Patients need periodic urodynamic evaluation and triggered testing guided by red flags in patient care. Select patients may be eligible for less aggressive follow-up. Existing guidelines while generally recommending follow-up urodynamics give little detail regarding the incorporation of the test in clinical practice. An algorithm based on limited evidence must suffice until more evidence is available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bauer SB. Neurogenic bladder: Etiology and assessment. Pediatr Nephrol. 2008;23:541–51. doi: 10.1007/s00467-008-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre M, Guida E, Bisio G, Scarsi P, Piatelli G, Cama A, et al. Risk factors for renal function impairment in a series of 502 patients born with spinal dysraphisms. J Pediatr Urol. 2011;7:39–43. doi: 10.1016/j.jpurol.2010.02.210. [DOI] [PubMed] [Google Scholar]
- 3.Filler G, Gharib M, Casier S, Lödige P, Ehrich JH, Dave S. Prevention of chronic kidney disease in spina bifida. Int Urol Nephrol. 2012;44:817–27. doi: 10.1007/s11255-010-9894-5. [DOI] [PubMed] [Google Scholar]
- 4.Joseph DB. Current approaches to the urologic care of children with spina bifida. Curr Urol Rep. 2008;9:151–7. doi: 10.1007/s11934-008-0027-y. [DOI] [PubMed] [Google Scholar]
- 5.Rosier PF, Kuo HC, de Gennaro M, Kakizaki H, Hashim H, van Meel TD, et al. Urodynamic testing. In: Abrams P, Khoury S, Cardozo L, Wein A, editors. Incontinence: 5th International Consultation on Incontinence, Paris, 2012. Arnhem, Netherlands: ICUD-EAU; 2013. pp. 429–506. [Google Scholar]
- 6.Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: A clinical practice guideline for health-care providers. J Spinal Cord Med. 2006;29:527–73. [PMC free article] [PubMed] [Google Scholar]
- 7.Danforth TL, Ginsberg DA. Neurogenic lower urinary tract dysfunction: How, when, and with which patients do we use urodynamics? Urol Clin North Am. 2014;41:445–52, ix. doi: 10.1016/j.ucl.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu JS, Greiman A, Casey JT, Mukherjee S, Kielb SJ. A snapshot of the adult spina bifida patient – High incidence of urologic procedures. Cent European J Urol. 2016;69:72–7. doi: 10.5173/ceju.2016.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welk B, Liu K, Shariff SZ. The use of urologic investigations among patients with traumatic spinal cord injuries. Res Rep Urol. 2016;8:27–34. doi: 10.2147/RRU.S99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo J, Palazzi K, Dwek J, Kaplan G, Chiang G. Early clean intermittent catheterization may not prevent dimercaptosuccinic acid renal scan abnormalities in children with spinal dysraphism. J Pediatr Urol. 2014;10:274–7. doi: 10.1016/j.jpurol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Larijani FJ, Moghtaderi M, Hajizadeh N, Assadi F. Preventing kidney injury in children with neurogenic bladder dysfunction. Int J Prev Med. 2013;4:1359–64. [PMC free article] [PubMed] [Google Scholar]
- 12.Drake M, Apostolidis A, Emmanuel A, Gajewski J, Harrison SC, Heesakkers J, et al. Neurologic urinary and faecal incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 5th ed. Arnhem, Netherlands: ICUD-EAU; 2013. pp. 827–1000. [Google Scholar]
- 13.Drake MJ, Apostolidis A, Cocci A, Emmanuel A, Gajewski JB, Harrison SC, et al. Neurogenic lower urinary tract dysfunction: Clinical management recommendations of the Neurologic Incontinence committee of the fifth International Consultation on Incontinence 2013. Neurourol Urodyn. 2016;35:657–65. doi: 10.1002/nau.23027. [DOI] [PubMed] [Google Scholar]
- 14.Winters JC, Dmochowski RR, Goldman HB, Herndon CD, Kobashi KC, Kraus SR, et al. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2464–72. doi: 10.1016/j.juro.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 15.Blok B, Pannek J, Castro-Diaz D, del Popolo G, Groen J, Hamid R, et al. EAU Guidelines on Neuro-urology. 2016. [Last accessed on 2016 Oct 16]. Available from: http://www.uroweb.org/guideline/neuro-urology . [DOI] [PubMed]
- 16.Stöhrer M, Blok B, Castro-Diaz D, Chartier-Kastler E, Del Popolo G, Kramer G, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009;56:81–8. doi: 10.1016/j.eururo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence (NICE), Urinary Incontinence in Neurological Disease: Management of Lower Urinary Tract Dysfunction in Neurological Disease. 2012. [Last accessed 2016 Oct 16]. Available from: http://www.nice.org.uk/CG148 .
- 18.Averbeck MA, Madersbacher H. Follow-up of the neuro-urological patient: A systematic review. BJU Int. 2015;115(Suppl 6):39–46. doi: 10.1111/bju.13084. [DOI] [PubMed] [Google Scholar]
- 19.Frimberger D, Cheng E, Kropp BP. The current management of the neurogenic bladder in children with spina bifida. Pediatr Clin North Am. 2012;59:757–67. doi: 10.1016/j.pcl.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Middleton J, Ramakrishnan K, Cameron I. Management of the Neurogenic Bladder for Adults with Spinal Cord Injuries. Agency for Clinical Innovation Guideline. 2014. [Last accessed 2016 Oct 16]. Available from https://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0010/155179/Management-Neurogenic-Bladder.pdf .
- 21.Bycroft J, Hamid R, Bywater H, Patki P, Craggs M, Shah J. Variation in urological practice amongst spinal injuries units in the UK and Eire. Neurourol Urodyn. 2004;23:252–6. doi: 10.1002/nau.20005. [DOI] [PubMed] [Google Scholar]
- 22.Burki JR, Omar I, Shah PJ, Hamid R. Long-term urological management in spinal injury units in the U.K. and Eire: A follow-up study. Spinal Cord. 2014;52:640–5. doi: 10.1038/sc.2014.90. [DOI] [PubMed] [Google Scholar]
- 23.Denys P, Soler JM, Fatton B, Rischmann P, Yelnik A, Aegerter P, et al. Highlighting differences in the management of neurogenic bladder existing between urologists and physiatrists: A survey conducted among 383 specialists. Presse Med. 2012;41(12 Pt 1):e599–608. doi: 10.1016/j.lpm.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Rikken B, Blok BF. Management of neurogenic bladder patients in the Netherlands: Do urologists follow guidelines? Neurourol Urodyn. 2008;27:758–62. doi: 10.1002/nau.20582. [DOI] [PubMed] [Google Scholar]
- 25.Schöps TF, Schneider MP, Steffen F, Ineichen BV, Mehnert U, Kessler TM. Neurogenic lower urinary tract dysfunction (NLUTD) in patients with spinal cord injury: Long-term urodynamic findings. BJU Int. 2015;115(Suppl 6):33–8. doi: 10.1111/bju.13085. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg DA. Botulinum Toxin Use in the Lower Urinary Tract in Adults and Children. AUA Update Series. 2015;34:145–55. [Google Scholar]
- 27.Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85–99. doi: 10.2147/RRU.S29644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna R, Sandhu AS, Doddamani D. Urodynamic management of neurogenic bladder in spinal cord injury. Med J Armed Forces India. 2009;65:300–4. doi: 10.1016/S0377-1237(09)80086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li WJ, Oh SJ. Management of lower urinary tract dysfunction in patients with neurological disorders. Korean J Urol. 2012;53:583–92. doi: 10.4111/kju.2012.53.9.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Çetinel B, Tarcan T, Demirkesen O, Özyurt C, Sen I, Erdogan S, et al. Management of lower urinary tract dysfunction in multiple sclerosis: A systematic review and Turkish consensus report. Neurourol Urodyn. 2013;32:1047–57. doi: 10.1002/nau.22374. [DOI] [PubMed] [Google Scholar]
- 31.Amarenco G, Sutory M, Zachoval R, Agarwal M, Del Popolo G, Tretter R, et al. Solifenacin is effective and well tolerated in patients with neurogenic detrusor overactivity: Results from the double-blind, randomized, active- and placebo-controlled SONIC urodynamic study. Neurourol Urodyn. 2015;36:414–21. doi: 10.1002/nau.22945. [DOI] [PubMed] [Google Scholar]
- 32.Hadiji N, Previnaire JG, Benbouzid R, Robain G, Leblond C, Mieusset R, et al. Are oxybutynin and trospium efficacious in the treatment of detrusor overactivity in spinal cord injury patients? Spinal Cord. 2014;52:701–5. doi: 10.1038/sc.2014.113. [DOI] [PubMed] [Google Scholar]
- 33.Kasabian NG, Bauer SB, Dyro FM, Colodny AH, Mandell J, Retik AB. The prophylactic value of clean intermittent catheterization and anticholinergic medication in newborns and infants with myelodysplasia at risk of developing urinary tract deterioration. Am J Dis Child. 1992;146:840–3. doi: 10.1001/archpedi.1992.02160190072024. [DOI] [PubMed] [Google Scholar]
- 34.Wada N, Okazaki S, Kobayashi S, Hashizume K, Kita M, Matsumoto S, et al. Efficacy of combination therapy with mirabegron for anticholinergic-resistant neurogenic bladder: Videourodynamic evaluation] Hinyokika Kiyo. 2015;61:7–11. [PubMed] [Google Scholar]
- 35.Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxinA for urinary incontinence from neurogenic detrusor overactivity. J Urol. 2012;187:2131–9. doi: 10.1016/j.juro.2012.01.125. [DOI] [PubMed] [Google Scholar]
- 36.Hascoet J, Manunta A, Brochard C, Arnaud A, Damphousse M, Menard H, et al. Outcomes of intra-detrusor injections of botulinum toxin in patients with spina bifida: A systematic review. Neurourol Urodyn. 2016;27:186–89. doi: 10.1002/nau.23025. [DOI] [PubMed] [Google Scholar]
- 37.Tiryaki S, Yagmur I, Parlar Y, Ozel K, Akyildiz C, Avanoglu A, et al. Botulinum injection is useless on fibrotic neuropathic bladders. J Pediatr Urol. 2015;11:27.e1–4. doi: 10.1016/j.jpurol.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Kim SW, Choi JH, Lee YS, Han SW, Im YJ. Preoperative urodynamic factors predicting outcome of botulinum toxin-A intradetrusor injection in children with neurogenic detrusor overactivity. Urology. 2014;84:1480–4. doi: 10.1016/j.urology.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Altaweel W, Jednack R, Bilodeau C, Corcos J. Repeated intradetrusor botulinum toxin type A in children with neurogenic bladder due to myelomeningocele. J Urol. 2006;175(3 Pt 1):1102–5. doi: 10.1016/S0022-5347(05)00400-3. [DOI] [PubMed] [Google Scholar]
- 40.Quek ML, Ginsberg DA. Long-term urodynamics followup of bladder augmentation for neurogenic bladder. J Urol. 2003;169:195–8. doi: 10.1016/S0022-5347(05)64066-9. [DOI] [PubMed] [Google Scholar]
- 41.Zaragoza Torres RI, Galarza-Flores ME, Gómez-Castellanos JC, Barrera-de León JC. Urodynamic changes after bladder augmentation surgery in paediatric patients with myelomeningocele due to neurogenic bladder. Cir Cir. 2016;84:115–20. doi: 10.1016/j.circir.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Vainrib M, Reyblat P, Ginsberg DA. Differences in urodynamic study variables in adult patients with neurogenic bladder and myelomeningocele before and after augmentation enterocystoplasty. Neurourol Urodyn. 2013;32:250–3. doi: 10.1002/nau.22304. [DOI] [PubMed] [Google Scholar]
- 43.Snodgrass W, Granberg C. Clinical indications for augmentation in children with neurogenic urinary incontinence following bladder outlet procedures: Results of a 14-year observational study. J Pediatr Urol. 2016;12:46.e1–8. doi: 10.1016/j.jpurol.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Whittam B, Szymanski K, Misseri R, Carroll A, Kaefer M, Rink R, et al. Long-term fate of the bladder after isolated bladder neck procedure. J Pediatr Urol. 2014;10:886–91. doi: 10.1016/j.jpurol.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Veenboer PW, Bosch JL, Rosier PF, Dik P, van Asbeck FW, de Jong TP, et al. Cross-sectional study of determinants of upper and lower urinary tract outcomes in adults with spinal dysraphism – New recommendations for urodynamic followup guidelines? J Urol. 2014;192:477–82. doi: 10.1016/j.juro.2014.02.2566. [DOI] [PubMed] [Google Scholar]
- 46.Yener S, Thomas DT, Hicdonmez T, Dagcinar A, Bayri Y, Kaynak A, et al. The effect of untethering on urologic symptoms and urodynamic parameters in children with primary tethered cord syndrome. Urology. 2015;85:221–6. doi: 10.1016/j.urology.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Vernet O, Farmer JP, Houle AM, Montes JL. Impact of urodynamic studies on the surgical management of spinal cord tethering. J Neurosurg. 1996;85:555–9. doi: 10.3171/jns.1996.85.4.0555. [DOI] [PubMed] [Google Scholar]
- 48.Steinbok P, MacNeily AE, Hengel AR, Afshar K, Landgraf JM, Hader W, et al. Filum section for urinary incontinence in children with occult tethered cord syndrome: A randomized, controlled pilot study. J Urol. 2016;195(4 Pt 2):1183–8. doi: 10.1016/j.juro.2015.09.082. [DOI] [PubMed] [Google Scholar]
- 49.Lee NG, Gomez P, Uberoi V, Kokorowski PJ, Khoshbin S, Bauer SB, et al. In utero closure of myelomeningocele does not improve lower urinary tract function. J Urol. 2012;188(4 Suppl):1567–71. doi: 10.1016/j.juro.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Mehta S, Hill D, Foley N, Hsieh J, Ethans K, Potter P, et al. A meta-analysis of botulinum toxin sphincteric injections in the treatment of incomplete voiding after spinal cord injury. Arch Phys Med Rehabil. 2012;93:597–603. doi: 10.1016/j.apmr.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 51.Kroll P, Gajewska E, Zachwieja J, Sobieska M, Mankowski P. An evaluation of the efficacy of selective alpha-blockers in the treatment of children with neurogenic bladder dysfunction – Preliminary findings. Int J Environ Res Public Health. 2016;13:pii: E321. doi: 10.3390/ijerph13030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linsenmeyer TA, Linsenmeyer MA. Impact of annual urodynamic evaluations on guiding bladder management in individuals with spinal cord injuries. J Spinal Cord Med. 2013;36:420–6. doi: 10.1179/2045772313Y.0000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007;26:228–33. doi: 10.1002/nau.20319. [DOI] [PubMed] [Google Scholar]
- 54.Chao R, Mayo ME. Long-term urodynamic follow up in pediatric spinal cord injury. Paraplegia. 1994;32:806–9. doi: 10.1038/sc.1994.127. [DOI] [PubMed] [Google Scholar]
- 55.Kurzrock EA, Polse S. Renal deterioration in myelodysplastic children: Urodynamic evaluation and clinical correlates. J Urol. 1998;159:1657–61. doi: 10.1097/00005392-199805000-00084. [DOI] [PubMed] [Google Scholar]
- 56.Shin JC, Lee Y, Yang H, Kim DH. Clinical significance of urodynamic study parameters in maintenance of renal function in spinal cord injury patients. Ann Rehabil Med. 2014;38:353–9. doi: 10.5535/arm.2014.38.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pannek J, Nehiba M. Morbidity of urodynamic testing in patients with spinal cord injury: Is antibiotic prophylaxis necessary? Spinal Cord. 2007;45:771–4. doi: 10.1038/sj.sc.3102114. [DOI] [PubMed] [Google Scholar]
- 58.Latthe PM, Foon R, Toozs-Hobson P. Prophylactic antibiotics in urodynamics: A systematic review of effectiveness and safety. Neurourol Urodyn. 2008;27:167–73. doi: 10.1002/nau.20501. [DOI] [PubMed] [Google Scholar]
- 59.Chancellor MB, Erhard MJ, Hirsch IH, Stass WE., Jr Prospective evaluation of terazosin for the treatment of autonomic dysreflexia. J Urol. 1994;151:111–3. doi: 10.1016/s0022-5347(17)34884-x. [DOI] [PubMed] [Google Scholar]
- 60.Ngo TC, Clark CJ, Wynne C, Kennedy WA., 2nd Radiation exposure during pediatric videourodynamics. J Urol. 2011;186(4 Suppl):1672–6. doi: 10.1016/j.juro.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Kellerer AM, Nekolla E. Consideration on a limit for lifetime occupational radiation exposure. Radiat Environ Biophys. 1998;37:81–5. doi: 10.1007/s004110050098. [DOI] [PubMed] [Google Scholar]
- 62.Blok BF, Al Zahrani A, Capolicchio JP, Bilodeau C, Corcos J. Post-augmentation bladder perforation during urodynamic investigation. Neurourol Urodyn. 2007;26:540–2. doi: 10.1002/nau.20394. [DOI] [PubMed] [Google Scholar]
- 63.Zerin JM, McLaughlin K, Kerchner S. Latex allergy in patients with myelomeningocele presenting for imaging studies of the urinary tract. Pediatr Radiol. 1996;26:450–4. doi: 10.1007/BF01377199. [DOI] [PubMed] [Google Scholar]
- 64.Silva JA, Gonsalves Mde C, de Melo RT, Carrerette FB, Damião R. Association between the bladder wall thickness and urodynamic findings in patients with spinal cord injury. World J Urol. 2015;33:131–5. doi: 10.1007/s00345-014-1265-x. [DOI] [PubMed] [Google Scholar]
- 65.Oelke M. International consultation on incontinence-research society (ICI-RS) report on non-invasive urodynamics: The need of standardization of ultrasound bladder and detrusor wall thickness measurements to quantify bladder wall hypertrophy. Neurourol Urodyn. 2010;29:634–9. doi: 10.1002/nau.20834. [DOI] [PubMed] [Google Scholar]
- 66.Yildiz N, Alkan H, Sarsan A, Alkan S. The effects of repeated filling cystometries on cystometric variables in spinal cord-injured patients with overactive detrusor, who utilize different type of urine drainage methods. Spinal Cord. 2015;53:625–9. doi: 10.1038/sc.2015.23. [DOI] [PubMed] [Google Scholar]
- 67.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 68.Meyrat BJ, Tercier S, Lutz N, Rilliet B, Forcada-Guex M, Vernet O. Introduction of a urodynamic score to detect pre- and postoperative neurological deficits in children with a primary tethered cord. Childs Nerv Syst. 2003;19:716–21. doi: 10.1007/s00381-003-0829-7. [DOI] [PubMed] [Google Scholar]
- 69.Kim SW, Ha JY, Lee YS, Lee HY, Im YJ, Han SW. Six-month postoperative urodynamic score: A potential predictor of long-term bladder function after detethering surgery in patients with tethered cord syndrome. J Urol. 2014;192:221–7. doi: 10.1016/j.juro.2014.02.2549. [DOI] [PubMed] [Google Scholar]
- 70.Kuo HC, Chen SL, Chou CL, Chuang YC, Huang YH, Juan YS, et al. Clinical guidelines for the diagnosis and management of neurogenic lower urinary tract dysfunction. Tzu Chi Med J. 2014;26:103–13. [Google Scholar]
- 71.Anding R, Rosier P, Smith P, Gammie A, Giarenis I, Rantell A, et al. When should video be added to conventional urodynamics in adults and is it justified by the evidence? ICI-RS 2014. Neurourol Urodyn. 2016;35:324–9. doi: 10.1002/nau.22865. [DOI] [PubMed] [Google Scholar]
- 72.Blok BF, Karsenty G, Corcos J. Urological surveillance and management of patients with neurogenic bladder: Results of a survey among practicing urologists in Canada. Can J Urol. 2006;13:3239–43. [PubMed] [Google Scholar]
- 73.Veenboer PW, Ruud Bosch JL, de Kort LM. Assessment of bladder and kidney functioning in adult spina bifida patients by Dutch urologists: A survey. Neurourol Urodyn. 2014;33:289–95. doi: 10.1002/nau.22413. [DOI] [PubMed] [Google Scholar]
- 74.de Kort LM, Bower WF, Swithinbank LV, Marschall-Kehrel D, de Jong TP, Bauer SB. The management of adolescents with neurogenic urinary tract and bowel dysfunction. Neurourol Urodyn. 2012;31:1170–4. doi: 10.1002/nau.22206. [DOI] [PubMed] [Google Scholar]
- 75.Vírseda-Chamorro M, Salinas-Casado J, de la Marta-García M, Esteban-Fuertes M, Méndez S. Comparison of ambulatory versus video urodynamics in patients with spinal cord injury. Spinal Cord. 2014;52:551–5. doi: 10.1038/sc.2014.9. [DOI] [PubMed] [Google Scholar]
- 76.Abrams P, Agarwal M, Drake M, El-Masri W, Fulford S, Reid S, et al. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 2008;101:989–94. doi: 10.1111/j.1464-410X.2008.07457.x. [DOI] [PubMed] [Google Scholar]
- 77.Powell CR. Not all neurogenic bladders are the same: A proposal for a new neurogenic bladder classification system. Transl Androl Urol. 2016;5:12–21. doi: 10.3978/j.issn.2223-4683.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Snow-Lisy DC, Yerkes EB, Cheng EY. Update on urological management of spina bifida from prenatal diagnosis to adulthood. J Urol. 2015;194:288–96. doi: 10.1016/j.juro.2015.03.107. [DOI] [PubMed] [Google Scholar]
- 79.Pannek J, Kullik B. Does optimizing bladder management equal optimizing quality of life? Correlation between health-related quality of life and urodynamic parameters in patients with spinal cord lesions. Urology. 2009;74:263–6. doi: 10.1016/j.urology.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 80.Tarcan T, Demirkesen O, Plata M, Castro-Diaz D. ICS teaching module: Detrusor leak point pressures in patients with relevant neurological abnormalities. Neurourol Urodyn. 2017;36:259–62. doi: 10.1002/nau.22947. [DOI] [PubMed] [Google Scholar]
- 81.Guerra L, Leonard M, Castagnetti M. Best practice in the assessment of bladder function in infants. Ther Adv Urol. 2014;6:148–64. doi: 10.1177/1756287214528745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol. 2012;187:391–7. doi: 10.1016/j.juro.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Routh JC, Cheng EY, Austin JC, Baum MA, Gargollo PC, Grady RW, et al. Design and methodological considerations of the Centers for Disease Control and prevention urologic and renal protocol for the newborn and young child with spina bifida. J Urol. 2016;196:1728–34. doi: 10.1016/j.juro.2016.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]