Abstract
Objective
To compare the accuracy of CT colonography versus optical colonoscopy for neoplastic involvement at the surgical anastomosis one year after curative-intent colorectal cancer resection.
Design, Setting, Patients, and Interventions
201 patients (mean age, 58.6 years; 117M, 84F) underwent same-day contrast-enhanced CT colonography and colonoscopy approximately one year (mean, 12.1 months; median, 11.9 months) after colorectal cancer resection as part of a prospective, multicenter trial. All patients enrolled were without clinical evidence of disease and considered low-risk for recurrence (stage I-III).
Main Outcome Measures
Suspected neoplastic lesions within 5 cm of the colonic anastomosis were recorded at CT colonography, with subsequent colonoscopy performed for the same, with segmental unblinding of colonography findings. Anastomotic region biopsy or polypectomy was performed at endoscopist discretion.
Results
None of the 201 patients had intraluminal anastomotic cancer recurrence or advanced neoplasia (or metachronous cancers). CT colonography detected extramural peri-anastomotic recurrence in two patients (1.0%); neither was detected at colonoscopy. Only two patients (1.0%; 2/201) were called positive at CT colonography for intraluminal anastomotic non-diminutive lesions (7–8 mm polyps), which were confirmed at colonoscopy but non-neoplastic at histopathology. At optical colonoscopy, the anastomosis was deemed abnormal and/or biopsied in 10.0% (20/201), yielding only one non-diminutive benign neoplasm (7 mm tubular adenoma).
Limitations
The lack of luminal cancer recurrence in our lower-risk cohort precludes assessment of sensitivity for detection, rendering the study underpowered in this regard. Potential cost savings of combined CT/CT colonography over the standard CT/colonoscopy approach were not assessed.
Conclusions
Relevant intraluminal anastomotic pathology appears to be very uncommon one-year after colorectal cancer resection in lower-risk cohorts. Unlike colonoscopy, diagnostic contrast-enhanced CT colonography effectively evaluates both the intra- and extra-luminal aspects of the anastomosis.
Keywords: Colorectal cancer, CT colonography, Virtual colonoscopy, Colonoscopy
Introduction
Most current guidelines for colorectal cancer (CRC) surveillance one year after curative-intent resection recommend both optical colonoscopy (OC) to assess for recurrent or metachronous intra-luminal cancer and IV contrast-enhanced CT to evaluate for both distant metastatic and local extra-luminal recurrence.1–3 However, because only a small minority of local CRC recurrence is luminal in nature,1,4,5 the efficacy of lumen-only tests such as OC is limited, as reflected by the unique outlier recommendation by ASCO to delay post-operative OC for 3 years.6 Rather, local recurrence tends to be extramural and peri-anastomotic,7 leading some investigators to consider using CT colonography (CTC) technique at the time of diagnostic CT to simultaneously evaluate for both intra- and extra-luminal pathology, potentially sparing the patient of short-term OC if the colon can be cleared of relevant lesions.8–14 Cumulative results from mainly single-center, retrospective studies have been very promising,13 but likely require validation from a prospective multi-center trial setting. In particular, evaluation of the surgical anastomosis can be challenging at OC, with many potential false-positive results related to non-neoplastic findings, as well as potential false-negatives related to the lack of a detectable intraluminal component to most anastomotic and peri-anastomotic recurrences.4,7,10,13
We recently completed a prospective multicenter trial to assess the feasibility, patient preference, and diagnostic performance of combined CT-CTC for surveillance one year after CRC resection, relative to the standard combination of CT and OC. This report specifically focuses on the diagnostic performance of both OC and CTC for evaluating the colonic surgical anastomosis. Since standard CT is common to both arms, either approach can likely detect extraluminal peri-anastomotic recurrence, although the CT-CTC combination may have some advantages in this regard. Although prior retrospective studies have shown that CTC is highly sensitive for detecting anastomotic and peri-anastomotic cancer recurrence,13 the rate of potential CTC false-positives leading to unnecessary OC referral (with associated increased costs) is less certain.4 This is perhaps of greatest relevance in relatively low-risk post-surgical cohorts without clinical evidence of recurrent disease. Therefore, the purpose of this study was to compare the performance of CTC and OC in this prospective trial for colonic anastomotic evaluation one year after CRC resection.
Materials and methods
Study Design
Five institutions (Fox Chase Cancer Center, Mayo Clinic Rochester, Memorial Sloan-Kettering Cancer Center, University of Chicago, and University of Wisconsin) participated in this NIH-sponsored protocol (NCI grant 1R01CA155347-01), which was approved by the institutional review board at each center. A total of 231 patients (mean age, 58 years; age range, 25–89 years; 97 women, 141 men) recruited through oncology and GI clinics completed the trial. Written informed consent was obtained from all patients by study coordinators at each site. All consented patients had prior surgical CRC resection with curative intent and were scheduled to undergo both IV contrast-enhanced diagnostic CT and OC for routine post-surgical follow-up at one year, according to most standard clinical guidelines. We excluded patients with known metastases, clinically suspected recurrent or metastatic disease, or contraindications to IV contrast administration. Between November 2011 and March 2016, patients received IV contrast-enhanced diagnostic CT combined with CTC, followed by same-day optical colonoscopy.
All patients received a cathartic bowel preparation for CTC and OC, as well as dilute barium and water-soluble iodinated oral contrast for stool and fluid tagging, respectively, for CTC. Patients underwent CT imaging after insufflation of the colon using a low-pressure automated carbon dioxide delivery system. This included low-dose CT imaging of the abdomen and pelvis in the prone or decubitus position, followed by standard diagnostic supine imaging after IV contrast administration. An additional decubitus scan was obtained as needed to optimize luminal evaluation. This CT-CTC combination combines both studies into a single examination. CTC studies were interpreted using a combined 2D and 3D assessment using the dedicated CTC software in current use at each institution. Extracolonic CT evaluation was performed concurrently using the standard PACS at each institution,
In terms of lesion localization, we prospectively defined the “anastomotic region” as being within 5 cm of the surgical anastomosis. Focal lesions suspicious for polyps of cancer recurrence were prospectively identified by the interpreting radiologist at CTC, with emphasis on non-diminutive lesions (>5 mm). Potential lesions at CTC were characterized according to size, morphology (sessile, pedunculated, flat, or mass), and diagnostic confidence (3-point scale).15 All patients went on to same-day OC, performed by experience gastroenterologists. The endoscopists were initially blinded to the CTC findings, with sequential unblinding by colonic segment at the time of evaluation. For relevant potential lesions called at CTC but not initially seen at OC, the segment in question was immediately re-evaluated. This provides an enhanced reference standard for colorectal pathology.16 Polypectomy or biopsy was performed for all potentially suspicious lesions identified at OC (as determined by the endoscopist), with all specimens sent to surgical pathology for histopathologic assessment.
Statistical analyses
The final results on optical colonoscopy after unblinding served as the reference standard by which the results of CTC and the initial OC evaluation were compared. Of primary interest was advanced neoplasia, as well as any adenomatous polyps measuring ≥6 mm. Advanced neoplasia was defined as a large (≥10 mm) adenoma or cancer, or any neoplasm containing high-grade dysplasia or prominent villous component.17 Non-adenomatous lesions (such as inflammatory or hyperplastic polyps, pseudopolyps, granulation tissue, or normal mucosa) and diminutive lesions (≤5 mm) were of secondary interest. As noted above, we defined anastomotic lesions as occurring within 5 cm of the anastomosis. The polyp matching algorithm utilized has been described previously,16 and allows for a 50% margin of error in polyp size between OC and CTC to be considered a true positive match. The simulated colonoscopy referral rate for anastomotic findings was defined by any suspicious findings ≥6 mm at CTC that would have prompted OC referral for OC. If OC failed to demonstrate a CTC abnormality, or if biopsy of the perceived abnormal anastomosis (by CTC or OC) returned non-neoplastic disease, this was reported as a false positive.
Results
Of the total 231 patients who completed the trial, 30 patients were excluded from this study due to absence of a bowel-to-bowel surgical anastomosis. The remaining 201 patients represent the final study cohort (mean age, 58.6 years; 84 women, 117 men). Initial stage at diagnosis was stage I, II, and III in 40, 67, and 94 patients, respectively.
At post-operative surveillance approximately one year after CRC resection (mean, 12.1 months; median, 11.9 months), none of the 201 patients had an anastomotic (or metachronous) luminal cancer a large or advanced adenoma near the anastomosis at final evaluation. Only two patients (1.0%; 2/201) were called positive at CTC for a focal non-diminutive luminal lesion within the anastomotic region. In one patient, an 8-mm lesion called at CTC near the ileo-colic anastomosis was confirmed at OC and found to represent a small hyperplastic polyp adjacent to suture material (Figure 1). In another patient, a 7-mm lesion adjacent to a colo-colic anastomosis was found to represent normal mucosa at OC biopsy. In 22 cases (10.9%), benign-appearing CTC findings such as a possible diminutive lesion or nodular thickening along the anastomosis felt to be compatible with suture granuloma or expected postoperative appearance. In general, such findings would not have triggered OC referral. Therefore, the estimated OC referral rate for anastomotic findings at CTC was 1.0% (2/201).
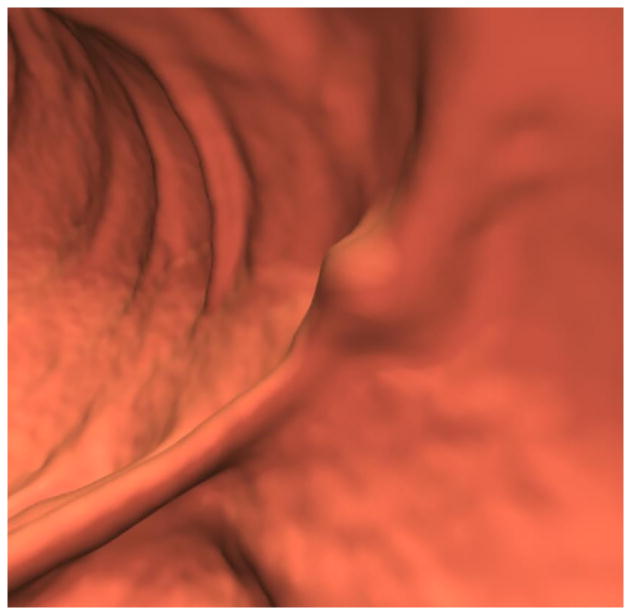
Figure 1. Sub-cm hyperplastic polyp at the colonic anastomosis (1.4 years after right hemicolectomy for CRC) in 70-year-old woman.

A and B, 3D (A) and 2D (B) CTC images show a focal 8-mm soft tissue polyp (arrow) at the anastomosis. The high attenuation seen at the edge of the lesion on 2D represents either oral contrast coating or calcification involving a suture granuloma. Given the OC findings, the latter is favored.
C, Image from same-day OC confirms a polyp at the anastomosis, with adjacent suture material. The lesion proved to be a hyperplastic polyp at surgical pathology.
At OC, the anastomosis was deemed abnormal and/or biopsied in 10.0% (20/201) of cases. A total of 25 lesions in 20 patients were biopsied (mean size, 4.9 mm; range, 2–10 mm). Of the 25 lesions, 16 were diminutive (≤5 mm), 8 were small (6–9 mm), and 1 was large (≥10 mm). Biopsy results from these 25 anastomotic lesions are summarized in Table 1. Only one non-diminutive benign neoplasm was found (a 7-mm tubular adenoma), which was not called at CTC, but no large adenomas or cancers were identified. Other pathology results in these cases included lymphoid tissue (n=6), granulation tissue with or with inflammation (n=5), normal mucosa (n=4), inflammatory polyp (n=4), hyperplastic polyp (n=3), and diminutive tubular adenoma (n=2). Only one large anastomotic lesion was called at OC, a 10-mm nodule at an ileo-colic anastomosis that proved to be normal mucosa at histology.
TABLE 1.
Results of all OC biopsies performed at the anastomosis for abnormal findings.
| Pathology | No. of Polyps (No. of Patients) | |||
|---|---|---|---|---|
| ≤ 5 mm | 6–9 mm | ≥ 10 mm | Total | |
| Advanced Neoplasia* | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Tubular adenomas | 2 (2) | 1 (1) | 0 (0) | 3 (2) |
| Non-neoplastic** | 14 (13) | 7 (4) | 1 (1) | 22 (18) |
Advanced neoplasia includes invasive cancer and advanced adenomas, which are defined by large size (≥10 mm), high-grade dysplasia, and/or prominent villous component
Non-neoplastic lesions included lymphoid tissue (n=6), granulation tissue with or with inflammation (n=5), normal mucosa (n=4), inflammatory polyp (n=4), hyperplastic polyp (n=3)
Beyond a strictly luminal evaluation, CT/CTC detected abnormal extramural peri-anastomotic soft tissue deposits in two (1.0%) patients that were ultimately confirmed to represent biopsy-proven local cancer recurrence (Figure 2). In two other patients (1.0%), peritoneal-based soft tissue nodules more distant from the anastomosis also were subsequently shown to represent biopsy-proven early peritoneal carcinomatosis. Neither the peri-anastomotic recurrences nor the cases of early peritoneal carcinomatosis were detected at OC.
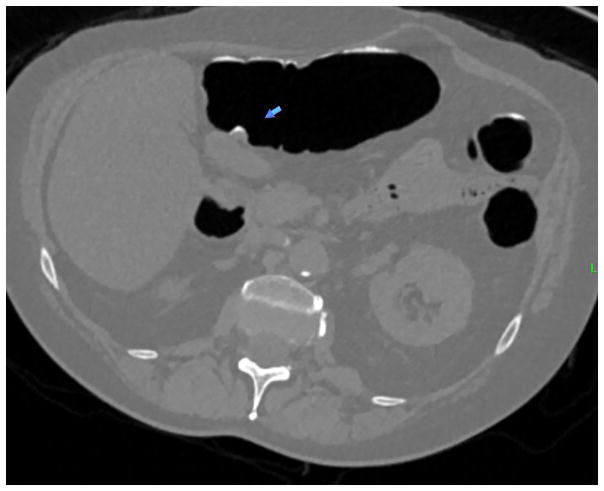
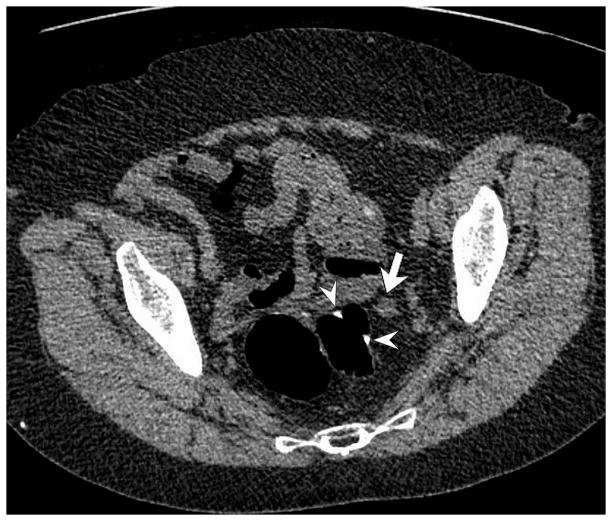
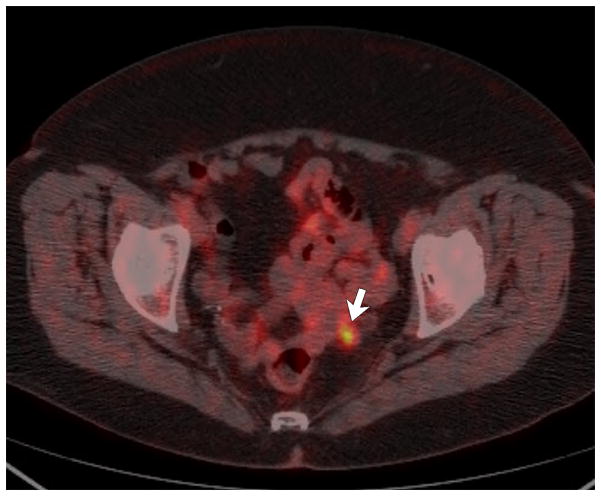
Figure 2. Peri-anastomotic cancer recurrence (12 months after resection of T4aN0M0 sigmoid cancer) in 76-year-old woman with slightly elevated CEA level (7 ng/mL).
A and B, 2D low-dose unenhanced prone (A) and post-contrast supine (B) CTC images show an irregular 1 cm soft tissue nodule (arrows) adjacent to the colonic anastomosis (arrowheads). Note the peripheral enhancement of the nodule on the contrast-enhanced view, as well as collapse of the anastomosis. The anastomosis was deemed normal at OC (not shown).
C, Fused image from PET/CT obtained after the CT/CTC study shows that the lesion is hypermetabolic, which proved to be local recurrence.
Discussion
Numerous randomized controlled trials have repeatedly shown that more intensive follow-up after CRC resection with CT and CEA levels is associated with reduced overall mortality, a shorter interval to detecting recurrence, increased detection of asymptomatic recurrence, a higher rate of repeated curative-intent surgery, and improved survival after recurrence.1 In particular, use of CT follow-up has been associated with improved overall survival compared with no imaging.1,5,18–20 More recently, the FACS (follow-up after colorectal surgery) RCT showed that more intensive surveillance was associated with increased rates of curative-intent surgery, which was the main outcome measure of this trial due to lack of statistical power to assess overall survival.21 In contrast, short-term colonoscopy follow-up within a year of CRC resection has not demonstrated a survival benefit.5,22–25 Given the rarity of isolated luminal anastomotic recurrence, the primary rationale for surveillance colonoscopy in this setting is presumably for the detection of metachronous (or previously missed synchronous) cancers – and advanced adenomas. Older studies have shown that, although much less frequent than extraluminal cancer recurrence, metachronous or missed synchronous luminal cancers can be found within two years of surgery.2 However, if the colon has been adequately cleared for synchronous neoplasia at the time of cancer diagnosis, there may be little role for colonoscopy one year after CRC resection, which is reflected in the ASCO guidelines that call for OC three years after surgery, and are at odds with the standard NCCN and USMSTF guidelines familiar to most colorectal surgeons, gastroenterologists, and oncologists.6 The need for surveillance OC at one year is further mitigated if colonography technique (CTC) is performed concurrently with standard CT to exclude advanced neoplasia.
CTC has been shown to be comparable to OC for the detection of advanced adenomas and CRC in the screening setting.16,17,26 Comparable detection of advanced neoplasia by CTC and OC has also been demonstrated in symptomatic patients.27 A meta-analysis reported an overall sensitivity for CRC of 96.1% for CTC and 94.7% for OC.28 However, as most of the published experience with CTC performance derives from more experienced “centers of excellence”, relatively little is known regarding the generalizability of these results at the community practice level. This issue of CTC generalizability may be even more pertinent to post-operative surveillance in CRC patients, where the examination is more technically challenging. Nonetheless, the inter-observer variability for detecting relevant colorectal pathology (eg, advanced neoplasia) among academic radiologists at screening CTC appears to be considerably smaller than the wider variability seen amongst gastroenterologists at OC.29,30
In the setting of prior CRC resection, one study reported that CTC correctly identified all 51 local cancer recurrences; only one metachronous cancer was seen.14 A recent meta-analysis looking at additional CTC studies showed excellent pooled sensitivity for detecting both local cancer recurrence (95%) and metachronous cancers (100%).13 Specificity for anastomotic cancer in this meta-analysis was reported to be 100%. However, specificity for anastomotic cancer recurrence may not reflect the actual OC referral rate from CTC related to abnormal focal findings at the anastomosis. For example, a large study of CTC with OC correlation in 548 patients after CRC resection found only a single case of anastomotic cancer recurrence, but all 22 of the other focal luminal anastomotic lesions were non-neoplastic.10 In our study, many benign and non-neoplastic anastomotic lesions were identified and biopsied at OC. The relatively high rate of anastomotic biopsies yielding non-neoplastic findings suggests that there may be a need for greater recognition of the variable endoscopic appearance of the normal post-operative anastomosis by gastroenterologists. However, in comparison, less than 1% of cases would have been referred to OC from CTC related to an anastomotic finding. Considering that no luminal cancers were found at short-term follow up in our cohort (whether anastomotic, metachronous, or previously missed), and that all extraluminal recurrences were detected by CT/CTC and none by OC, the need for short-term OC surveillance, at least of the anastomosis, could be questioned.
It is well established that the great majority of local cancer recurrence following CRC resection is predominately extraluminal.4,7,14 CT is an effective tool for the initial detection of these peri-anastomotic and other recurrences, the likelihood of which may be heightened by a rising CEA level. PET/CT can be quite useful for distinguishing abnormal soft tissue related to cancer recurrence from that due to fibrosis or other benign post-operative changes.7 Adding CTC technique to the standard CT surveillance likely further improves assessment of the anastomosis and peri-anastomotic region by dint of luminal distention and cleansing relative to CT without colonography technique. However, we are unable to assess this additive value directly in our study, as patients did not have a concurrent CT without colonography technique.
We acknowledge limitations to our study. The lack of luminal cancer recurrence in our low-risk cohort renders our trial underpowered in this regard, precludes assessment of the sensitivity of CTC for detection. However, as discussed above, numerous prior single-center studies have demonstrated a high sensitivity of CTC for CRC detection in this setting. Furthermore, our study showed improved specificity for CTC over OC with a much lower false-positive rate related to calling non-neoplastic anastomotic findings. Despite its prospective, multicenter design, our study cohort was relatively small due to unanticipated recruitment challenges, which included lower-than-expected adherence to the surveillance guidelines. In addition, we did not focus on luminal lesion detection beyond the anastomotic region, which will be the focus of a separate report. However, no metachronous cancer was seen at surveillance in this cohort, which we feel largely reflects current practice and diminishes the role of OC in this setting. Finally, we did not report the potential cost savings associated with this approach of combined CT/CTC versus the standard of CT and OC, but we plan to study this. Of note, the recent meta-analysis suggested a substantial cost savings of the CT/CTC approach.13
In conclusion, our findings are concordant with prior studies demonstrating that clinically-relevant intraluminal anastomotic pathology is rare one-year after CRC resection in lower-risk cohorts. Primary CTC evaluation performed in conjunction with standard abdominopelvic CT for metastatic surveillance appears to be effective in evaluating both the intraluminal and extraluminal aspects of the anastomosis, with relatively few false-positives leading to unnecessary OC. When combined with prior studies demonstrating high sensitivity of CT/CTC for anastomotic cancer recurrence, our findings suggest that CTC evaluation is superior to OC for evaluation of the colonic anastomosis at one-year post-operative surveillance.
Acknowledgments
This research is supported in part by the National Institutes of Health (NCI grant 1R01CA155347-01)
Footnotes
Please note that the DCR Editor has granted our request for 10 authors; All authors listed: 1) Provided substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) Drafted the article or revising it critically for important intellectual content; and 3) Gave final approval of the version to be published.
References
- 1.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy Surveillance After Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2016;150:758–68. doi: 10.1053/j.gastro.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin. 2006;56:160–167. doi: 10.3322/canjclin.56.3.160. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Pickhardt PJ, Taylor AJ. Characteristics of advanced adenomas detected at CT colonographic screening: Implications for appropriate polyp size thresholds for polypectomy versus surveillance. AJR Am J Roentgenol. 2007;188:940–944. doi: 10.2214/AJR.06.0764. [DOI] [PubMed] [Google Scholar]
- 4.Choi YJ, Park SH, Lee SS, et al. CT colonography for follow-up after surgery for colorectal cancer. AJR Am J Roentgenol. 2007;189:283–289. doi: 10.2214/AJR.07.2305. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:1–8. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 7.Shyn PB, Madan R, Wu C, Erturk SM, Silverman SG. PET/CT pattern analysis for surgical staple line recurrence in patients with colorectal cancer. AJR Am J Roentgenol. 2010;194:414–421. doi: 10.2214/AJR.09.2892. [DOI] [PubMed] [Google Scholar]
- 8.Amitai MM, Fidder H, Avidan B, et al. Contrast-enhanced CT colonography with 64-slice MDCT compared to endoscopic colonoscopy in the follow-up of patients after colorectal cancer resection. Clin Imaging. 2009;33:433–438. doi: 10.1016/j.clinimag.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JG, Johnson CD, Krueger WR, et al. Contrast-enhanced CT colonography in recurrent colorectal carcinoma: feasibility of simultaneous evaluation for metastatic disease, local recurrence, and metachronous neoplasia in colorectal carcinoma. AJR Am J Roentgenol. 2002;178:283–290. doi: 10.2214/ajr.178.2.1780283. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Park SH, Pickhardt PJ, et al. CT colonography for combined colonic and extracolonic surveillance after curative resection of colorectal cancer. Radiology. 2010;257:697–704. doi: 10.1148/radiol.10100385. [DOI] [PubMed] [Google Scholar]
- 11.Laghi A, Iannaccone R, Bria E, et al. Contrast-enhanced computed tomographic colonography in the follow-up of colorectal cancer patients: a feasibility study. European Radiol. 2003;13:883–889. doi: 10.1007/s00330-002-1696-4. [DOI] [PubMed] [Google Scholar]
- 12.Neri E, Vagli P, Turini F, et al. Post-surgical follow-up of colorectal cancer: role of contrast-enhanced CT colonography. Abdom Imaging. 2010;35:669–675. doi: 10.1007/s00261-009-9596-6. [DOI] [PubMed] [Google Scholar]
- 13.Porte F, Uppara M, Malietzis G, et al. CT colonography for surveillance of patients with colorectal cancer: Systematic review and meta-analysis of diagnostic efficacy. European Radiol. 2017;27:51–60. doi: 10.1007/s00330-016-4319-1. [DOI] [PubMed] [Google Scholar]
- 14.You YT, Chang Chien CR, Wang JY, et al. Evaluation of contrast-enhanced computed tomographic colonography in detection of local recurrent colorectal cancer. World J Gastroenterol. 2006;12:123–126. doi: 10.3748/wjg.v12.i1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Choi JR, Nugent PA, Schindler WR. The effect of diagnostic confidence on the probability of optical colonoscopic confirmation of potential polyps detected on CT colonography: Prospective assessment in 1,339 asymptomatic adults. AJR Am J Roentgenol. 2004;183:1661–1665. doi: 10.2214/ajr.183.6.01831661. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 17.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 18.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database of Systematic Reviews. 2007 doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Pita-Fernandez S, Alhayek-Ai M, Gonzalez-Martin C, Lopez-Calvino B, Seoane-Pillado T, Pertega-Diaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Annals of Oncology. 2015;26:644–656. doi: 10.1093/annonc/mdu543. [DOI] [PubMed] [Google Scholar]
- 21.Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey SD, Howlader N, Etzioni R, Brown ML, Warren JL, Newcomb P. Surveillance endoscopy does not improve survival for patients with local and regional stage colorectal cancer. Cancer. 2007;109:2222–2228. doi: 10.1002/cncr.22673. [DOI] [PubMed] [Google Scholar]
- 23.Schoemaker D, Black R, Giles L, Toouli A. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7–14. doi: 10.1016/s0016-5085(98)70626-2. [DOI] [PubMed] [Google Scholar]
- 24.Tjandra JJ, Chan MKY. Follow-up after curative resection of colorectal cancer: A meta-analysis. Dis Col Rectum. 2007;50:1783–1799. doi: 10.1007/s10350-007-9030-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Cui Y, Huang WS, et al. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointestinal Endoscopy. 2009;69:609–615. doi: 10.1016/j.gie.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. New England Journal of Medicine. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkin W, Dadswell E, Wooldrage K, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1194–1202. doi: 10.1016/S0140-6736(12)62186-2. [DOI] [PubMed] [Google Scholar]
- 28.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pooler BD, Kim DH, Hassan C, Rinaldi A, Burnside ES, Pickhardt PJ. Variation in diagnostic performance among radiologists at screening CT colonography. Radiology. 2013;268:127–134. doi: 10.1148/radiol.13121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. New England Journal of Medicine. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]