Abstract
The role of retinoic acid (All Trans Retinoic Acid; ATRA) in the development of hypervitaminosis A pathophysiology is not well understood or established in the literature. As well, the role of Citral (inhibitor of retinoid function; a non-toxic chemical that exists in two forms (diethyl; C1 or cis-trans dimethyl; C2).) in the reversal of pathophysiological implications is also not ascertained under an in vivo setting. Therefore, it is hypothesized that ovalbumin exposure will sensitize the body to supra-physiologic levels of retinoic acid leading to a negative pathophysiological impact and that Citrals 1 and 2 will reverse or ameliorate the related damage to the body’s pathophysiology. Even though ovalbumin and retinoic have been previously applied through intra-tracheal route in cancer prevention and immunological research, the objective of this study was to evaluate their interaction as a remedy for hypervitaminosis A. This IACUC approved in vivo study used Fischer 344 rats (n = 80 ;229 to 273g), which were randomly assigned to controls as well as ovalbumin and mold-sensitized treatment groups (0.80 mg/kg and 1X109 mold spores combined from 4 strains/100 μl intra-tracheal; all others were dosed by intra-peritoneal injection at days 1 and 7 with 80 mg/kg each of ATRA as well as 20 and 50 mg/kg each of Citrals 1 or 2 individually or in combination to represent all four chemicals and mold spores treatments.. Positive and negative controls for each treatment were also included in the study. Animals were housed in rat cages at the JSU Research Animal Core Facilities and were placed on a 12:12 light dark cycle. A standard rodent diet and water access were provided ad-libidum. Rat weights were recorded on day 1 and 21, all animals were sacrificed on day 21 and blood was collected and processed for hematological parameters. Results showed that even though C1 and C2 were not toxic individually, their combination at high dosing was lethal. Exposure of ovalbumin-sensitized rats to ATRA showed various levels of weight losses and negative hematological implications that were ameliorated by exposure to Citrals at various combinations with retinoic acid. Taken together, the study showed that there are variable pathophysiological responses from the interaction of ovalbumin, mold spores and retinoic acid and that Citrals were found to be individually effective in reversing health-related pathophysiologies. These findings warrants further investigations as to the actual role of these interactions in relation to acute pathophysiologic health implications and the possibility of reversing hypervitaminosis A-mediated health-related impacts.
Keywords: ATRA, RP, Citral, F344, Ovalbumin, Chronic Lung Pathology, Hpervitaminosis A
INTRODUCTION
The role of retinoic acid (All Trans Retinoic Acid; ATRA) in the development of hypervitaminosis A pathophysiology is not well understood or established in the literature. The current known clinical uses of some selected retinoids include the treatment of dermatologic diseases such as acne, psoriasis and eczema, photo-damaged skin, and specific forms of cancer [1, 2]. Biological effects of retinoids are generally exerted through a series of nuclear receptors that are ligand-inducible transcription factors belonging to the steroid/thyroid receptor superfamily such as RAR and RXR retinoid nuclear receptors. The impact of retinoic acid (All Trans Retinoic Acid; ATRA) and MLD exposure in the development of lung pathology and tissue remodeling were not well established in the literature. Equally, the role of citral (inhibitor of retinoid function) in the improvement of lung pathology was not ascertained under an in vivo setting. Retinoids represent the chemical derivatives of vitamin A or all-trans retinol and include retinol, retinaldehyde, and all forms of the final oxidized product retinoic acid (RA). Vitamin A is generally obtained from the diet in the form of retinyl esters that are linked to fatty acids such as palmitic acid (retinylpalmitate; RP) or in the form of carotenoids which are dimers of retinal; the oxidative aldehyde form of retinoid isomers [3, 4 and 5].
A recent challenge in the treatment of chronic lung disease includes restoration of alveolar surface area, respiratory and mechanical function of the lung parenchyma has led to a focus on retinoids as therapeutic agents [6, 7, and 8]. It has been well known that alveolar architecture depends on the anatomy of prenatal airways in mice, rats and humans. Airway branching, elongation, and cellular differentiation are influenced by retinoids, however, one of the important component of the alveolus is the septum which is composed of epithelial and endothelial cells, fibroblasts and some immune and neuro-endocrine cells. Retinoids are known as alveolar morphogens based on the fact that RA was shown to ameliorate emphysema in rats after the intra-tracheal instillation of elastase [9, 10, and 11]. Circulating retinoids as well as the endogenous stores furnish the required amounts of retinoids to body cells through the hydrolysis of RP [12, 13]. Retinoids are pleiotropic regulatory compounds that are capable of modulating the structure and function of a wide range of inflammatory, immune and structural body cells. They possess a hormone-like function that regulate epithelial cell proliferation, pattern formation in developing tissues, morphogenesis in the lung, and cellular differentiation [14]. Citral has been reported to exhibit activity as a Vitamin A antagonist by inhibiting the oxidation of retinal to retinoic acid. This suggests that Citral is able to block the endogenous RA signaling pathway [15]. Hypervitaminosis A is a condition representing retinoid toxicity, which may reflect its effects on the lungs as a damaging agent. To this end and in reference to the literature, the findings on the responses to ATRA both in vitro and in vivo appeared to be contadory [2, 3 and 16]. This study was undertaken to explore this contraversy.
This paradox in the function of retinoids [17–24] as curing or damaging agents has prompted the execution of our study with the hypothesis that application of supraphysiologic levels of retinoids ATRA will cause lung pathologic damage similar to MLD exposure. The objective of the study, was to assess the role of exposing the F344 rat model to supra-physiologic levels of MLD+ATRA, MLD+C1, MLD+C2, and their comparison to untreated control and single treatments including MLD, ATRA, C1 and C2 as an attempt to provide insights into the role of citral in ameliorating such pathology with regards to the treatment of chronic lung disease in an in vivo setting.
METHODS
High purity All Trans Retinoic Acid (ATRA), Citral 1 (C1; diethyl acetal and Citral 2 (C2; cis and Trans dimethyl), DMSO, Isoflurane and PBS were purchased from Sigma Aldrich Company, St. Louis, MO. The spores from four MLD strains including were purchased from ATCC, USA.
Animals and housing
Fisher rats (F344; 260–324 g; N=80) were obtained from Harlan Laboratories (Frederic, MD). The animals were housed at the Jackson State University (JSU) Animal Core Facilities (Olaw class 2 level). Animals were acclimatized for a week and all protocols including handling, husbandry, anesthesia, euthanasia, and experimental protocols were approved by JSU-IACUC (protocol # 08-13-08) and were performed according to Olaw recommendations. Animals were kept under controlled environment at 12/12 light /dark cycles and were allowed unrestricted access to water and rodent chow. Each animal was kept in a separate rat cage that is well maintained by a technical staff supervised by a veterinarian.
Experimental design
A total of 80 F344 rats were used in this study. Five animals were not treated and designated as negative control. The remaining 75 animals were divided into fifteen different groups of 5 animals each and were exposed as treatment group through intra-peritoneal (iP) and intra-tracheal (iT) routes on day 1 using ovalbumin (80 mg/kg), all trans retinoic acid (ATRA; 80 mg/Kg), citral 1 (C1; 50 mg/Kg), Citral 2 (C2; 50 mg/Kg) by the intra-peritoneal route and mold (MLD; (Aspergillus, Penicillum, Stachybotrys and Cladosporium); sores were collected, mixed and treated with equal volumes of 95% ethanol and 5% vinegar for inactivation to sterility by subculture, centrifuged and re-suspended in PBS, a dose of 100 μL of 5.0X1012 by iT route was used. All animals were weighed on days 1 and 21 and were sacrificed (euthanized by inhalation of CO2 in special chamber) on day 21 following approved protocols and blood was collected for parameter analysis, which was done by the Mississippi State Veterinary lab; using an automated cell sorter. Dead animals were subjected to in-house post-mortem (Necropsy) procedures and entire lungs from all animals were collected in 10% formalin for histopathological analysis.
Statistical analysis
As based on the experimental design of the study, factorial analysis statistics for F-ratios associated with ANOVA was employed to support significance and interpretation of data using the standard software packages SPSS. Variance in mean differences (p<0.05) was determined by ANOVA and the Duncan ranking statistics and presented as mean ± SD.
RESULTS
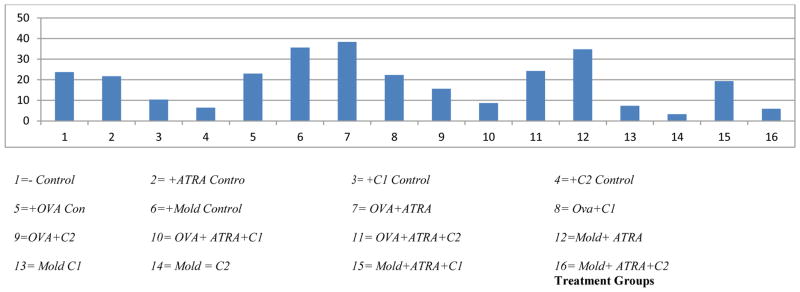
In examining the effect of different treatments on rat weight, the mean weight changes for the treatment groups were compared by means of a two-way ANOVA test. The multiple comparison tests suggest that 10 treatment groups are different in weight change. When each of the treatment groups were compared to the control, the following were significantly different: C1 control (3), C2 control (4), Mold control (6), OVA+ATRA (7), OVA + C2 (9), OVA + ATRA + C1 (10), Mold + ATRA (12), Mold + C1 (13), Mold + C2 (14), and Mold + ATRA + C2 (16). A group of five treatments (2, 5, 8, 11, and 15) were not significantly different within themselves or with the control (1). However, all of these five groups are significantly different from the above group of 10 treatments in that they have no effect on weight gain by the animals exposed to such treatments (table 6.1.2, the pair-wise group comparisons are shown by superscripted letters; treatment groups labeled with the same letters are not statistically significant). Within the 10 groups, four including Mold control (6), OVA+ATRA (7), Mold+ATRA (12), and Mold+C1 (13, showed significant positive weight gain, while the remaining six treatments including C1 (3), C2 (4), OVA+ C2 (9), OVA+ATRA+ C1 (10), Mold+C2 (14), and Mold+ATRA+C2 (16) showed negative impact on weight gain.
The comprehensive analysis of weight data revealed that 9 treatment groups including ATRA control (2), OVA control (5), Mold control (6), OVA+ATRA (7), OVA+ C1 (8), OVA+ATRA+C1 (11), Mold+ATRA (12), Mold+C1 (13), Mold+ATRA+C1, showed either a positive impact or no impact on weight gain as compared to the negative control. However, six treatments including C1 (3), C2 (4), OVA+ C2 (9), OVA+ATRA+ C1 (10), Mold+C2 (14), and Mold+ATRA+C2 (16) showed negative impact on weight gain. F344 rats exposed to C1 and C1 in combination with both OVA and ATRA as well as C2 and C2 in combination with OVA, Mold, and Mold+ATRA is showing the negative impact on weight gain due the impact of C1 and C2 treatments on these animals (Table 1 and Fig 1).
Table 1.
Comparison of weight data for the F344 rat model exposed to 16 different supra -physiologic individual, bi and tri-level treatment combinations (N=80)
| # | Treatment Type | N | Initial Weight (g) | Final Weight (g) | Weight Diff. (g) | Std. Diff. (g) | Weight Gain/Av Control % | Survival to term |
|---|---|---|---|---|---|---|---|---|
| 1 | − Control | 5 | 264.0±8.0 | 287.7±2.6 | 23.7 | 0.00 | 100.00 | +++++ |
| 2 | + ATRA Control | 5 | 229.7±9.5 | 251.3±17.6 | 21.7 | −2.00 | 91.55 | +++++ |
| 3 | + C1 Control | 5 | 244.6±10.3 | 255.0±4.6 | 10.4* | −13.30 | 44.00 | +++++ |
| 4 | + C2 Control | 5 | 261.4±8.4 | 267.9±9.8 | 6.5* | −17.12 | 27.50 | +++++ |
| 5 | + OVA Control | 5 | 272.6±3.8 | 305.6±3.0 | 23.0 | −0.70 | 97.05 | +++++ |
| 6 | + Mold Control | 5 | 256.9±11.5 | 292.5±3.09 | 35.6* | +11.90 | 150.21 | +++++ |
| 7 | OVA + ATRA | 5 | 264.6±17.9 | 303.0±7.9 | 38.4* | +14.70 | 162.03 | +++++ |
| 8 | OVA + C1 | 5 | 255.7±13.2 | 278.0±11.9 | 22.3 | −1.40 | 94.09 | +++++ |
| 9 | OVA + C2 | 5 | 253.4±2.8 | 269.0±2.06 | 15.6* | −8.10 | 65.82 | +++++ |
| 10 | OVA + ATRA + C1 | 5 | 251.0±14.9 | 259.7±10.5 | 8.7* | −15.00 | 36.70 | +++++ |
| 11 | OVA + ATRA + C2 | 5 | 260.6±4.7 | 284.9±7.2 | 24.3 | +0.60 | 102.53 | +++++ |
| 12 | Mold + ATRA | 5 | 262.6±13.8 | 297.4±10.3 | 34.8* | +11.10 | 146.84 | +++++ |
| 13 | Mold + C1 | 5 | 260.1±10.9 | 267.5±11.1 | 7.4* | −16.3 | 31.22 | +++++ |
| 14 | Mold + C2 | 5 | 257.7±10.4 | 261.0±11.5 | 3.3* | −20.4 | 13.90 | +++++ |
| 15 | Mold+ ATRA + C1 | 5 | 256.1±16.6 | 275.5±10.3 | 19.4 | −4.3 | 81.86 | +++++ |
| 16 | Mold+ ATRA + C2 | 5 | 251.8±11.5 | 257.7±12.4 | 5.9* | −17.8 | 24.89 | +++++ |
Statistically significant at p<0.05.
Figure 1.
represents treatment type and initial and final weights of the animals at the beginning and ending of the study in the unit of grams, weight difference, percent weight gain divided by average of control and survival rate after treatment
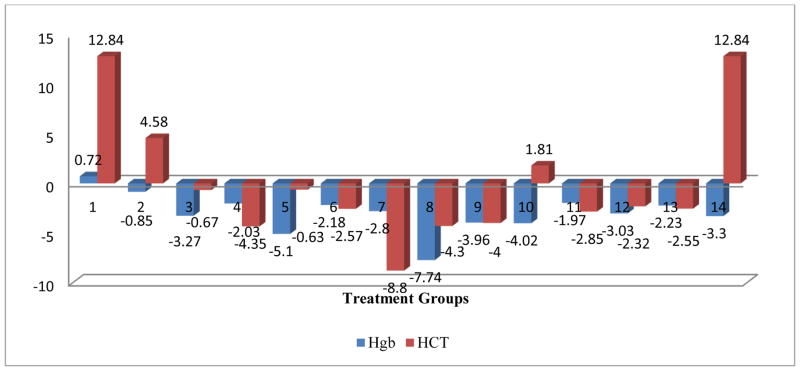
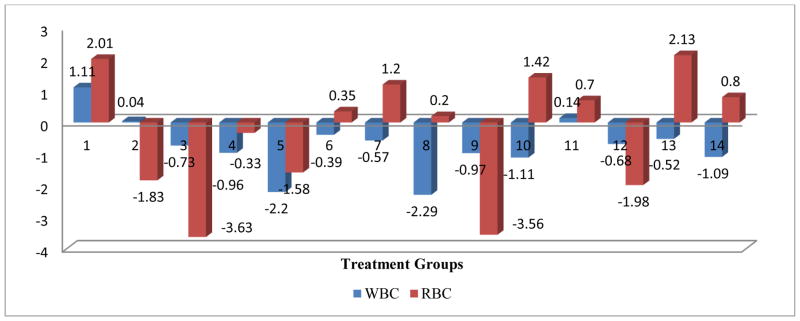
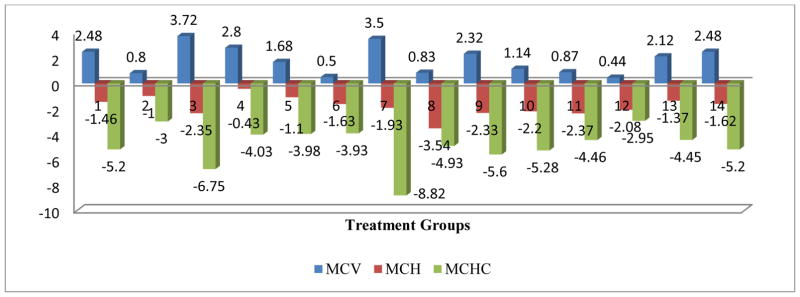
The comprehensive analysis of the impact of various treatments on the RBC parameters, the OVA control and the Mold+ATRA+C2 treatments showed a significant negative impact on the RBC parameter. All treatments had a significant negative impact on the HCT parameter, and except for the Mold control, all other treatment has a significant negative impact on the Hb parameter. Only Mold +ATRA had a significant negative impact on MCH parameter and Only Mold control and Mold+C1 had a significant negative impact on MCHC parameter. The OVA control, OVA+C1, Mold+ATRA, Mold+ATRA+C2 showed a significant negative impact on the MCV parameter (Figs 2–6).
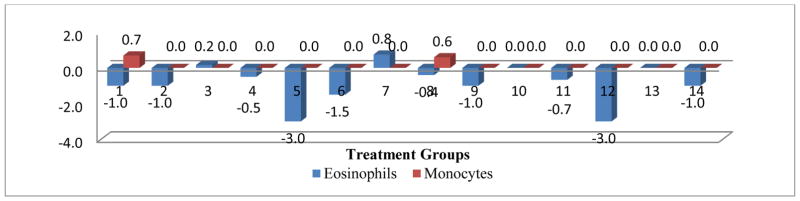
Figure 2.
Mean change in eosinophils and monocytes post treatment centered by pre-treatment mean
Figure 6.
Mean change in Hgb and HCT post treatment centered by pre-treatment mean
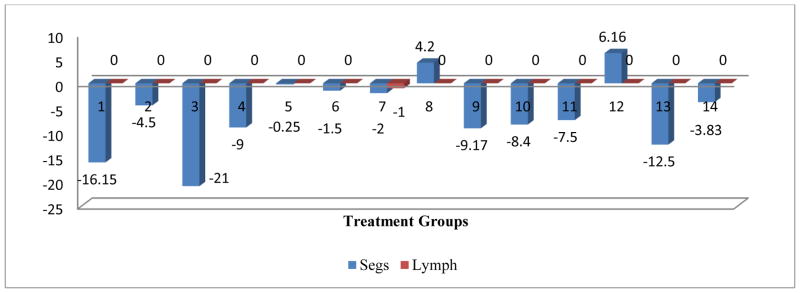
The comprehensive analysis of the impact of various treatments on the WBC parameters showed that OVA+ C1, Mold control, and Mold +ATRA+C1 had a significant negative impact on the WBC parameter. No significant impact was shown for all treatment on the monocyte and lymph parameters. Even though OVA treatment did not impact eosinophils, C1 and C1 in combinations with OVA both produced a significant negative impact on eosinophils. The OVA control, OVA+C1, C1control, C2 control, Mold+C1 and Mold +ATRA treatments showed significant negative impact on the Segs parameter (Figs 2–6).
DISCUSSION
The comprehensive analysis of weight data revealed that 9 treatment groups including ATRA control (2), OVA control (5), Mold control (6), OVA+ATRA (7), OVA+ C1 (8), OVA+ATRA+C1 (11), Mold+ATRA (12), Mold+C1 (13), Mold+ATRA+C1, showed either a positive impact or no impact on weight gain as compared to the negative control. However, six treatments including C1 (3), C2 (4), OVA+ C2 (9), OVA+ATRA+ C1 (10), Mold+C2 (14), and Mold+ATRA+C2 (16) showed negative impact on weight gain. F344 rats exposed to C1 and C1 in combination with both OVA and ATRA as well as C2 and C2 in combination with OVA, Mold, and Mold+ATRA is showing the negative impact on weight gain due the drastic impact of C1 and C2 treatments on these animals The reversal of weight loss by individual treatments or lack of reversal was dependent on the type of combination, nature of the parameter and the presence of OVA or mold in the combination (Table 1 and Fig 1).
The comprehensive analysis of the impact of various treatments on the RBC parameters, the OVA control and the Mold+ATRA+C2 treatments showed a significant negative impact on the RBC parameter. All treatments had a significant negative impact on the HCT parameter, and except for the Mold Control, all other treatments had a significant negative impact on the HgB parameter. Only Mold +ATRA had a significant negative impact on MCH parameter and Only Mold Control and Mold+C1 had a significant negative impact on MCHC parameter. The OVA Control, OVA+C1, Mold+ATRA, Mold+ATRA+C2 showed a significant negative impact on the MCV parameter (Figs 2–6).
The comprehensive analysis of the impact of various treatments on the WBC parameters showed that OVA+ C1, Mold Control, and Mold +ATRA+C1 had a significant negative impact on the WBC parameter. No significant impact was shown for all treatment on the monocyte and lymphocyte parameters. Even though OVA treatment did not impact eosinophils, C1 and C1 in combinations with OVA both produced a significant negative impact on eosinophils. The OVA control, OVA+C1, C1Control, C2 Control, Mold+C1 and Mold +ATRA treatments showed significant negative impact on the Segs parameter (Figs 2–6).. Treatment of animals with OVA triggers increase in eosinophils, neutrophils, lymphocytes, and monocytes [25, 26, 27, 28, 29]. Hangfang and Wang [30] showed that total cell count and proportion of inflammatory cells in BALF in two treatment groups were significantly reduced (Figs 2–6).
The literature, has documented changes occurring with the treatment of these animals with the aforesaid treatment combinations [31] have demonstrated treatment of animals with citral is associated with increase red blood cells. Nicholls et al. [32] have demonstrated treatment of animals with ATRA stimulated the syndrome of diffuse alveolar hemorrhage associated with pulmonary capillaritis. DiRenzo et al. [29] demonstrated that citral inhibits the synthesis of retinoic acid but their mechanism remains unknown. Hangfang and Wang [30] have shown that ATRA treatment of animals caused airway inflammation in asthmatic rats. The total cell count and proportion of inflammatory cells in BALF in two treatment groups were significantly reduced. Massaro and Massaro [33] demonstrated that ATRA treatment of animals could improve improved diffusion capacity and RBC and WBC balances (Figs 2–6).
DiRenzo et al. [29] and [28] demonstrated the inhibitory effects of citral in retinoic acid synthesis in which levels of HCT and MHC were elevated. Further, these treatments have also been associated with decrease in levels of MCHC depending of the treatment conditions [29, 31]. In the literature, it has been revealed that lymphocytes and neutrophils or Segs are increased in animals treated with OVA [25, 34, 35, 36, 37, 38 and 39]. Significant mean differences in Hb, RBCs and HCT were attributed to lung hemorrhage, bronchoconstriction, inflammation and damage to lung tissues [25 and 34].
The observation that there was no increase in levels of WBC parameters is an indication of the extent of stimulation of the immune system [40]. The Segs, Lymph, Eosino, and Mono are white blood components essential in the determination of the activities in which each of these components play during positive activation of the immune system and could also be reflected as a systemic inflammatory response; which is not the case in the findings of this study [39, and 40]. Hence, all the treatments used in this study did not reflect any acute immune stimulation; rather all impacts are reflective of a local inflammatory situation (Figs 2–6).
Verhoeff et al. [41] have demonstrated that humans sensitized to mold develop respiratory complications and bronchial constrictions. Kuhn and Grannum [42] notes that humans as well as animals that are exposed to molds develop respiratory complications including asthma. Etzel and Rylander [35] showed that children as well as adults that are exposed to indoor mold manifest symptoms of respiratory complications. Edmondson et al. [43] have demonstrated the allergic pulmonary effects of mold ranging from upper airway inflammation to asthma as a health impact of such exposure.
CONCLUSIONS
The objective of the study was to assess the impact of exposing the F344 rat model to supra-physiologic levels of Ova, MLD, ATRA, C1, C2, and their combinations in comparison to untreated control and single treatments. The comprehensive analysis of weight data revealed that nine treatment groups including showed negative impact on weight gain. Mold control showed the highest positive significant weight gain in comparison to the negative control. In regards to bi-level treatments, ATRA and OVA combination treatment had a highly synergistic positive impact on weight gain). OVA and C1 combination treatment reversed the negative impact of C1; the combination treatment ha d an antagonistic positive impact on weight gain, which is in contrast to the effects of OVA and C2 combination treatment that has not fully reversed the negative impact of C2 treatment on weight gain producing a lower (weaker reversal) antagonistic positive impact on weight gain in comparison to the control. Weight gain analysis for tri-level treatments showed that, OVA+ATRA+C1 combination did not reverse the adverse effects of C1 on weight gain; OVA and ATRA did not protect the model from weight loss imp acted by exposure to C1 treatment. In contrast OVA+ATRA+C2 combination completely reversed the negative effects of C2 on weight gain. With respect to the effects single treatments on RBC parameters, Ova control showed significant positive impact on HCT with no negative impact on any of the other RBC parameters. All treatments had a significant negative impact on the HCT and the Hb parameters. In reference to tri-level treatments on blood parameters, OVA+ATRA+C1 combination reversed the negative effects of individual treatments on the WBC parameters. Mold as a single treatment showed significant negative impact on WBCs and Segs. Citral has positive health implications in regards to hypervitaminosos A.
Figure 3.
Mean change in WBC and RBC post treatment centered by pre-treatment mean.
Figure 4.
Mean change in MCV, MCH and MCHC post treatment centered by pre-treatment mean
Figure 5.
Mean change in Segs and lymph post treatment
Acknowledgments
This research is supported by NIH/NCRR RCMI grant # G12-MD007581.
References
- 1.McGowan SE. Contributions of Retinoids to the Generation and Repair of the pulmonary alveolus. Chest. 2002;121:206S–208S. doi: 10.1378/chest.121.5_suppl.206s. [DOI] [PubMed] [Google Scholar]
- 2.Belloni PN, Garvin L, C-Ping Mao, Bailey-Healy I, Leaffer D. Effects of All-Trans-Retinoic Acid in Promoting Alveolar Repair. Chest. 2000;117:235S–241S. doi: 10.1378/chest.117.5_suppl_1.235s. [DOI] [PubMed] [Google Scholar]
- 3.Massaro GD, Massaro D. Retinoic Acid Treatment Abrogates Elastase-induced Pulmonary Emphysema. Nature Med. 1997;3:675–703. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 4.Chambone PA. A Decade of Molecular Biology of Retinoic Acid Receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 5.Chytil F. Retinoids in Lung Development. FASEB J. 1996;10:986–992. doi: 10.1096/fasebj.10.9.8801181. [DOI] [PubMed] [Google Scholar]
- 6.McGowan SE, Doro MM, Jackson SK. Endogenous Retinoids Increase Perinatal Elastin Gene Expression in Rat Lung Fibroblasts and Fetal Explants. Am JPhysiol Lung Cell Mol Physiol. 1997;273:L410–L416. doi: 10.1152/ajplung.1997.273.2.L410. [DOI] [PubMed] [Google Scholar]
- 7.Massaro GD, Massaro D. Retinoic Acid Treatment Partially reverses Failed Septation in Rats and Mice. AM J Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 8.Morabia A, Menkes M, Comstock G, et al. Serum Retinol and Airway Obstruction. AM J Epidemiol. 1990;132:77–82. doi: 10.1093/oxfordjournals.aje.a115645. [DOI] [PubMed] [Google Scholar]
- 9.Paiva S, Goday I, Vannucchi H, et al. Assessment of Vitamin A Status in Chronic Obstructive Pulmonary Disease Patients and Healty Smokers. Am J Clin Nutr. 1996;64:929–934. doi: 10.1093/ajcn/64.6.928. [DOI] [PubMed] [Google Scholar]
- 10.Napoli J. Retinoic Acid Biosynthesis and Metabolism. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 11.Haq R, Pfahl M, Chytil F. Retinoic Acid Affects the Expression of Nuclear Retinoic Acid Receptors in Tissues of Retinol Deficient Rats. Proc Natl Acad Sci USA. 1991;88:8272–8276. doi: 10.1073/pnas.88.18.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zachman R. Role of Vitamin A in Lung Development. J Nut. 1995;125(Suppl):1934S–1638S. doi: 10.1093/jn/125.suppl_6.1634S. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Harvey C, McGowan S. Retinoic Acid increase Elastin in Rat Lung Fibroblasts Cultures. Am J Physiol. 1993;265:L430–L437. doi: 10.1152/ajplung.1993.265.5.L430. [DOI] [PubMed] [Google Scholar]
- 14.Okabe T, Yorifuji H, Yamada E, et al. Isolation and characterization of Vitamin A Storing Lung Cells. Exp Cell Res. 1984;154:125–135. doi: 10.1016/0014-4827(84)90673-6. [DOI] [PubMed] [Google Scholar]
- 15.Schuh TJ, Kraft J, Hall BL. V-erbA and Citral Reduce the Teratogenic Effects of all-trans Retinoic Acid and Retinol, Respectively, in Xenopus Embryogenesis. Development. 1993;119:785–798. doi: 10.1242/dev.119.3.785. [DOI] [PubMed] [Google Scholar]
- 16.Tanumihardjo SA, Penniston KL. The Acute and Chronic Toxic Effects of Vitamin A. American Journal of Clinical Nutrition. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Massaro GD, Massaro D. Postnatal Treatment with Retinoic Acid Increases the Number of Pulmonary Alveoli in Rats. Am J Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 18.Swee M, Parks W, Pierce R. Developmental regulation of Elastin Production. J Biol Chem. 1995;270:14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- 19.Austead G. Steroids, Retinoids and Wound Healing. Adv Wound Care. 1998;11:277–285. [PubMed] [Google Scholar]
- 20.Hunt T. Vitamin A and Wound Healing. J Am Acad Dermatol. 1986;15:817–821. doi: 10.1016/s0190-9622(86)70238-7. [DOI] [PubMed] [Google Scholar]
- 21.Tepper J, Pfeiffer J, Aldrich M, et al. Can Retinoic Acid Ameliorate the Physiologic and Morphologic effects of elastase instillation in the Rat. Chest. 117(suppl 1):242S–244S. doi: 10.1378/chest.117.5_suppl_1.242s. [DOI] [PubMed] [Google Scholar]
- 22.Maple S, Mendelssohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- 23.Mao JT, Goldin JG, Ermand J, et al. A pilot study of all-trans retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718–723. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- 24.Fan H, Hongfang J, Wang H. Effects of all-trans retinoic acids on airway inflammation in asthmatic rats and mechanism. Journal of Huazhong University of Science and Technology. 1997;24(3):229–232. doi: 10.1007/BF02831997. [DOI] [PubMed] [Google Scholar]
- 25.Wynn EA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 26.Beeman CS, Berndt W, Kronmillera JE, Nguyen T. Blockade of the initiation of murine odontogeness in vitro by citral, an inhibitor of endogenous retinoic acid synthesis. Archives of Oral Biology. 1995;4:7. doi: 10.1016/0003-9969(95)00015-h. [DOI] [PubMed] [Google Scholar]
- 27.Farah IO, Holt-Gray C, Cameron JA, Tucci M, Cason Z, Benghuzzi HA. Impact of Paired Combinations of Retinoic Acid (ATRA) and Ovalbumin on F344 Rat Lung Tissues and Improvement of Related Pathology by Citral. Biomed Sci Instrum. 2014;50:423–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Nenegola E, Giavini E, DiRenzo FY, Broccia MI. Citral, an inhibitor of retinoic acid synthesis, attenuates the frequency and severity of bronchial arch abnormalities induced by triazole-derivative fluconazole in rat’s embryos culture in vitro. Reproductive Toxicology. 2007;24(3):326–332. doi: 10.1016/j.reprotox.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 29.DiRenzo F, Broccia ML, Giavini E, Menegola E. Citral, an inhibitor of retinoic acid synthesis, attenuates the frequency and severity of bronchial arch abnormalities induced by triazole-derivative fluconazole in rat embryos cultured in vitro. Reproductive Toxicology. 2007;24(3–4):326–32. doi: 10.1016/j.reprotox.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Hongfang FH, Wang H. Effects of all-trans retinoic acids on airway inflammation in asthmatic rats and mechanism. Journal of Huazhong University of Science and Technology. 1997;24(3):229–232. doi: 10.1007/BF02831997. [DOI] [PubMed] [Google Scholar]
- 31.Liang X, Zheng-Yu F, Xiao-Nan T, Ming B, Gang F. Effect and Mechanism of Ligustrazine on Th1/Th2 Cytokines in a Rat Asthma Model. American Journal of Chinese Medicine. 2007;35(6):1011–1020. doi: 10.1142/S0192415X07005478. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls MR, Terada L, Tuder RM, Prindiville SA, Schwarz MI. Diffuse alveolar hemorrhage with underlying pulmonary capillarities in the retinoic acid syndrome. Am J Respir Crit Care Med. 1998;158:1302–1305. doi: 10.1164/ajrccm.158.4.9709085. [DOI] [PubMed] [Google Scholar]
- 33.Massaro GD, Massaro D. Retinoic Acid Treatment Partially reverses Failed Septation in Rats and Mice. AM J Physiol. 2003;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 34.Kakazu T, Chihara J, Saito A, Nakajima S. Effect of RANTES on eosinophil adhesion to plates coated with recombinant soluble intercellular adhesion molecule-1 and expression of beta 2-integrin adhesion molecules on eosinophils. Int Arch Allergy Immunol. 1995;108:9–11. doi: 10.1159/000237190. [DOI] [PubMed] [Google Scholar]
- 35.Etzel R, Rylande R. Indoor mold and children’s health. Environmental Health Perspect. 1999;3:463. doi: 10.1289/ehp.107-1566224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn DM, Ghannoum DMA. Indoor mold, toxigenic fungi, and Stachbortrys chartarum: Infectious disease perspective. Clinical Microbiology Reviews. 2003;16(1):144–172. doi: 10.1128/CMR.16.1.144-172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd CM, Gonzalo JA, Tguyen N, Delaney T, Tian J, Oettgen H, Coyle AJ, Gutierrez-Ramos JC. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. Journal of Immunology. 2001;166:2033–2040. doi: 10.4049/jimmunol.166.3.2033. [DOI] [PubMed] [Google Scholar]
- 38.Travlos GS, Ress NB, Orzech DP, et al. Carcinogenesis studies of microencapsulated citral in rats and mice. Society of Toxicology. 2003;71:198–206. doi: 10.1093/toxsci/71.2.198. [DOI] [PubMed] [Google Scholar]
- 39.Farah I, Holt-Gray C, Brown R. Impact of supra-physiologic retinoids on ovalbumin-sensitized F344 lung tissue and reversal of related pathology by citral. Pub Med. 2011;47:195–200. [PMC free article] [PubMed] [Google Scholar]
- 40.Ilayperuma I. Effects of Intraperitoneal Administration of Citral on Male Reproductive Organs in the Rat. Galle Medical Journal. 2008;13(1):29–32. [Google Scholar]
- 41.Verhoeff AP, van Strein RT, Wijnen JH, Bruneskreel B. Damp housing and childhood respiratory symptoms: The role of sensitization to dust mites and molds. American Journal of Epidemiology. 1995;141(2):103–110. doi: 10.1093/oxfordjournals.aje.a117398. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn DMD, Ghannoum MA. Indoor mold, toxigenic fungi, and Stachbortrys chartarum: Infectious disease perspective. Clinical Microbiology Reviews. 2003;16(1):144–172. doi: 10.1128/CMR.16.1.144-172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edmondson DA, Nordness ME, Zacharisen MC, Kurup VP, Fink JN. Allergy and “toxic mold syndrome. Ann Allergy Asthma Immunol Feb. 2005;94(2):234–9. doi: 10.1016/S1081-1206(10)61301-4. [DOI] [PubMed] [Google Scholar]