Abstract
Aims
Limited information is available on long-term antihypertensive and lipid-lowering therapy effects on hypertensive patients with atrial fibrillation/flutter (AF/AFL) compared to those without. AF/AFL at baseline or during the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) (mean follow-up 4.9 years) markedly increased risk of stroke, heart failure, CHD, and all-cause mortality. We aimed to determine if AF/AFL continued to impact outcomes during post-trial follow-up (mean 3.8 years).
Methods
Patients were randomized to chlorthalidone, amlodipine, or lisinopril, and to pravastatin vs. placebo in the lipid-lowering trial (LLT). Of 31,473 available subjects, AF/AFL occurred in 854; 383/14,371 chlorthalidone (2.7%), 247/8,565 amlodipine (2.9%), and 224/8,537 lisinopril (2.6%). Post-hoc analyses utilized administrative databases for post-trial data. Individuals with AF/AFL were compared to those without during post-trial. Outcomes were analyzed by treatment groups for the antihypertensive and LLT trials.
Results
Among 854 AF/AFL participants, 491 (57.5%) died: 220 in-trial, 271 post-trial. Ten-year all-cause mortality rates for those with in-trial AF/AFL were similar for chlorthalidone and lisinopril, but lower for amlodipine (68, 66, and 49 per 100 persons, respectively); adjusted HR for amlodipine vs. chlorthalidone was 0.68 (95% Cl, 0.54–0.87). Ten-year all-cause mortality rates were 57 vs. 65 per 100 persons (pravastatin vs. usual care); non-CVD mortality rates, 18 vs. 39 per 100 persons (pravastatin vs. usual care) (adjusted HR=0.46, 95% CI, 0.24–0.86).
Conclusion
Post-trial follow-up revealed continued deleterious AF/AFL effects. The amlodipine (ALLHAT) and pravastatin (ALLHAT-LLT) treatment groups showed lower all-cause and non-CVD mortality compared to the chlorthalidone and usual-care groups, respectively.
Keywords: Atrial fibrillation, heart failure, stroke, antihypertensive therapy
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia in older adults, with direct and indirect influences on cardiovascular disease (CVD) outcomes.1–5 We previously reported from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT),6 that baseline and incident AF were strong risk factors for heart failure (HF), stroke, fatal and non-fatal coronary heart diseases (CHD), and mortality.7 Also, randomization to any of the study drugs (the diuretic chlorthalidone, calcium-channel blocker [CCB] amlodipine, or angiotensin-converting enzyme inhibitor [ACE-I] lisinopril) did not significantly influence AF incidence or its impact on study outcomes.7
In the ALLHAT lipid component (ALLHAT-LLT),8 randomization to pravastatin versus usual treatment did not influence AF/AFL incidence nor the effect of AF/AFL on clinical outcomes.7,8
This study reports the impact of baseline and incident AF and atrial flutter (AF/AFL) during the active surveillance phase of ALLHAT (mean follow-up 4.9 years) plus 4 additional years of passive follow-up using national databases (total mean follow-up 8.8 years). We examine (1) whether baseline and incident AF/AFL continue to influence clinical outcomes post-trial, and (2) whether this influence is modified by the original randomized treatments.
METHODS
Details of ALLHAT’s design have been published.9 Eligible participants were >= 55 years old, with systolic blood pressure (BP)≥140 mmHg and/or diastolic BP≥90 mmHg, and/or taking 1–3 antihypertensive medications with a BP of ≤160/100 mmHg at randomization, with at least 1 additional CHD risk factor (including pre-existing CVD and/or cerebrovascular disease). Individuals with a history of symptomatic HF or left ventricular ejection fraction (EF)<35% were excluded. Active follow-up of ALLHAT participants ended March 31, 2002 (mean active follow-up, 4.9 years).
It was recognized at ALLHAT’s inception that post-trial follow-up would be important in assessing long-term antihypertensive treatment effects. Informed consent was sought for active study participation and passive post-study morbidity/mortality follow-up. The University of Texas Health Science Center Institutional Review Board approved the long-term follow-up study.
ALLHAT’s Extension Study protocol can be found at http://allhat.sph.uth.tmc.edu/. The pre-specified primary outcome was CVD mortality. Secondary outcomes included total and cause-specific mortality, CVD, CHD, stroke, and HF. During extended follow-up, hospitalizations and deaths were ascertained using administrative databases. Information on post-trial medications, BP, outpatient morbidity/treatment, and laboratory data were not collected.
Cause of death was determined by investigators during the trial.10 All-cause mortality data were available for the extended cohort (all non-Canadian participants) through National Death Index (NDI) and Social Security Administration databases for in-trial and post-trial periods. Death certificates were obtained for deaths discovered through administrative databases and used to confirm patient identification; NDIPlus provided ICD-10 coded causes-of-death. Details of the mortality outcome have been published.11
ECGs were recorded at baseline and biannually at clinical sites using standardized procedures and were coded by the ECG Reading Center (University of Minnesota, Minneapolis)8 for rhythm, with AF and AFL coded separately using Minnesota Code definitions.7 Per study protocol, paroxysmal AF data were not specifically collected apart from the baseline and biannual ECG readings.
Statistical analyses used STATA software (version 12) (2011; Stata Corporation, College Station, TX). To compare baseline characteristics of participants with baseline or incident AF/AFL to those without, contingency tables and Z-tests were used. Analyses of treatment effects on risks for outcomes were performed using Cox regression. Follow-up included randomized trial (mean follow-up, 4.9 years) and subsequent extension period (mean follow-up, 3.8 years). AF/AFL mortality rates were calculated using Kaplan-Meier method. Adjusted mortality rates and adjusted hazard ratios (HRs) were calculated using Cox regression and baseline characteristics. Time-dependent Cox regression was used to estimate HRs associated with treatment interventions separately for in-trial and post-trial periods. Treatment HRs for mortality were calculated with baseline or incident AF/AFL development as a time-dependent variable, and tests for interactions conducted to determine whether treatment effects differed. Given the many multivariate, subgroup, and interaction analyses performed, statistical significance at the 0.05 level should be interpreted cautiously. Unless otherwise indicated, post-trial HRs described in this report are adjusted.
RESULTS
A total of 33,357 ALLHAT participants were randomly assigned to chlorthalidone, amlodipine, and lisinopril. For this analysis, 31,473 participants remain after removing 1331 with either no ECG or for whom AF/AFL incidence or prevalence could not be determined, and 553 Canadian participants for whom post-trial mortality was unobtainable. During the trial, 383/14,371 chlorthalidone participants (2.7%); 247/8565 amlodipine participants (2.9%); and 224/8537 lisinopril participants (2.6%) had baseline or incident AF/AFL (total, 854 events: 803 AF and 51 AFL). Of the 803 AF events, 315 were baseline (prevalent) and 488 incident. Of the 51 AFL events, 16 were baseline (prevalent) and 35 incident. Of those with baseline or incident AF/AFL, 491/854 (57.5%) were dead at the end of the extended follow-up period (through December, 2006): 220 in-trial and 271 post-trial deaths (Figure 1 [A]).
Figure 1.
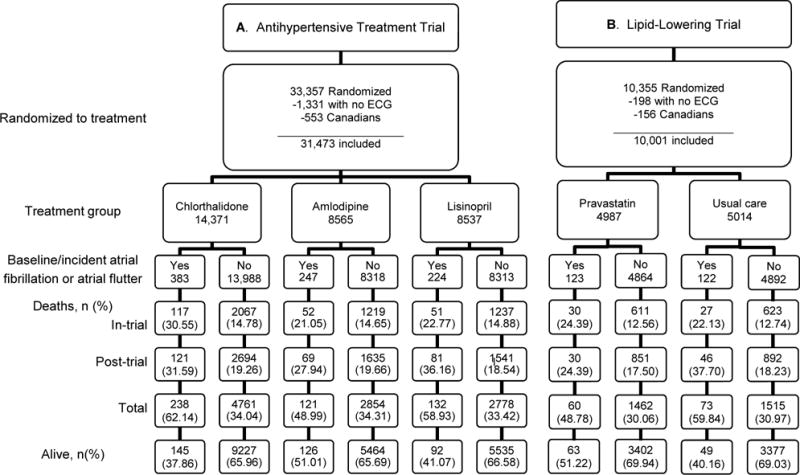
Consort diagrams
Baseline characteristics are provided in Table 1. Participants with baseline or incident AF/AFL were older, more often male or non-Black, more likely to have a history of CVD, CHD, LVH, and had lower estimated glomerular filtration rates (eGFR).
Table 1.
Baseline characteristics of cases with either baseline atrial fibrillation/flutter or in-trial atrial fibrillation/flutter vs. cases with neither
| Antihypertensive Treatment Amlodipine/Lisinopril/Chlorthalidone |
Lipid Treatment Pravastatin vs. Usual Care |
|||||
|---|---|---|---|---|---|---|
| Baseline characteristics n (%) |
Atrial fibrillation n=854 |
No atrial fibrillation n=30,619 |
P-value | Atrial fibrillation N=245 |
No atrial fibrillation N=9756 |
P-value |
| Age range in years | <0.001 | <0.001 | ||||
| 55–69 | 347 (40.63) | 19978 (65.25) | 99 (40.41) | 6641 (68.07) | ||
| 70–79 | 398 (46.60) | 8732 (28.52) | 116 (47.35) | 2587 (26.52) | ||
| ≥ 80 | 109 (12.76) | 1909 (6.23) | 30 (12.24) | 528 (5.41) | ||
| Male | 602 (70.49) | 16257 (53.09) | <0.001 | 180 (73.47) | 4958 (50.82) | <0.001 |
| Black | 167 (19.56) | 10988 (35.89) | <0.001 | 62 (25.31) | 3750 (38.44) | <0.001 |
| Diabetes | 276 (32.32) | 11022 (36.00) | 0.027 | 77 (31.43) | 3416 (35.01) | 0.245 |
| Current smoker | 124 (14.52) | 6768 (22.10) | <0.001 | 32 (13.06) | 2282 (23.39) | <0.001 |
| History of CHD | 317 (37.34) | 7601 (25.02) | <0.001 | 56 (22.86) | 1361 (13.95) | <0.001 |
| LVH | 64 (8.16) | 1416 (5.19) | <0.001 | 18 (8.00) | 450 (5.12) | 0.054 |
| ASCVD | 574 (67.21) | 15654 (51.13) | <0.001 | 150 (61.22) | 4330 (44.38) | <0.001 |
| Aspirin use | 340 (39.81) | 10983 (35.87) | 0.018 | 92 (37.55) | 2988 (30.73) | 0.022 |
| Treated for hypertension | 792 (92.74) | 27646 (90.29) | 0.017 | 228 (93.06) | 8766 (89.85) | 0.099 |
| BMI ≥ 30 kg/m2 | 382 (44.99) | 12865 (42.14) | 0.097 | 119 (48.77) | 4211 (43.25) | 0.086 |
| GFR < 60 ml/min | 191 (22.74) | 5171 (17.51) | <0.001 | 52 (21.67) | 1450 (15.14) | 0.006 |
| HDL < 35 mg/dl | 241 (28.93) | 5621 (19.12) | <0.001 | 64 (26.67) | 1400 (14.64) | <0.001 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body-mass-index; CHD, coronary heart disease; GFR, glomerular filtration rate ; HDL, high-density lipoprotein; LVH, left-ventricular hypertrophy;
The average numbers of antihypertensive medications for those with AF/AFL were 1.96, 1.84, and 1.94 for the chlorthalidone, amlodipine, and lisinopril treatment groups, respectively. For those without AF/AFL, the average numbers of antihypertensive medications were 1.62, 1.66, and 1.80 for the chlorthalidone, amlodipine, and lisinopril treatment groups, respectively (data not shown).
Mortality and fatal/non-fatal outcomes adjusted for baseline characteristics are shown in Table 2 and Figure 2. HRs were significantly increased for all-cause mortality, CHD, stroke, HF, other CVD, and non-CVD., and for combined mortality/morbidity outcomes for stroke, HF, CHD, and combined CVD, for those with AF/AFL compared to those without. There were several statistically significant treatment-by-baseline/incident AF/AFL interactions: for all-cause mortality amlodipine (HR=1.43) compared to chlorthalidone (HR=2.11) [p-value for interaction: 0.001], stroke mortality with amlodipine (HR=3.99) compared to chlorthalidone (HR=1.45) [p-value for interaction: 0.022], and non-CVD mortality with amlodipine (HR=0.93) compared to chlorthalidone (HR=1.72) [p-value for interaction: 0.002]. Further analyses examining CHD risk for those with AF/AFL vs. without were performed to determine if results would differ by sex or race (Black vs. Non-Black); no significant interactions were found (data not shown).
Table 2.
Unadjusted and adjusted hazard ratios by treatment group for cases with either baseline or in-trial atrial fibrillation/flutter vs. cases with neither*
| Chlorthalidone | Amlodipine | Lisinopril | Combined | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Mortality | ||||||||
| All-cause | ||||||||
| Unadjusted | 2.84(2.50,3.24) | <0.001 | 1.83(1.53,2.20) | <0.001† | 2.42(2.03,2.88) | <0.001 | 2.41(2.20,2.64) | <0.001 |
| Adjusted | 2.11(1.84,2.43) | <0.001 | 1.43(1.17,1.75) | <0.001† | 1.75(1.45,2.13) | <0.001 | 1.81(1.64,2.00) | <0.001 |
| CHD | ||||||||
| Unadjusted | 3.70(3.11,4.41) | <0.001 | 2.58(2.04,3.27) | <0.001† | 3.04(2.39,3.87) | <0.001 | 3.16(2.80,3.57) | <0.001 |
| Adjusted | 2.52(2.09,3.05) | <0.001 | 2.03(1.57,2.63) | <0.001 | 2.07(1.58,2.71) | <0.001 | 2.26(1.98,2.58) | <0.001 |
| Stroke | ||||||||
| Unadjusted | 2.31(1.30,4.12) | 0.004 | 4.82(3.00,7.74) | <0.001 | 4.31(2.63,7.07) | <0.001 | 3.67(2.74,4.92) | <0.001 |
| Adjusted | 1.45(0.77,2.76) | 0.252 | 3.99(2.39,6.67) | <0.001† | 3.52(2.09,5.93) | <0.001 | 2.74(2.00,3.75) | <0.001 |
| Heart Failure | ||||||||
| Unadjusted | 6.27(4.07,9.65) | <0.001 | 3.45(1.75,6.80) | <0.001 | 5.33(2.86,9.94) | <0.001 | 5.10(3.73,6.98) | <0.001 |
| Adjusted | 4.07(2.54,6.53) | <0.001 | 2.75(1.32,5.73) | 0.007 | 3.50(1.79,6.82) | <0.001 | 3.50(2.50,4.91) | <0.001 |
| Other CVD | ||||||||
| Unadjusted | 3.92(2.78,5.53) | <0.001 | 2.70(1.72,4.25) | <0.001 | 3.10(1.90,5.06) | <0.001 | 3.30(2.60,4.19) | <0.001 |
| Adjusted | 3.30(2.28,4.77) | <0.001 | 2.06(1.23,3.44) | 0.006 | 1.88(1.05,3.39) | 0.035 | 2.49(1.91,3.25) | <0.001 |
| Non-CVD | ||||||||
| Unadjusted | 2.16(1.76,2.65) | <0.001 | 1.22(0.90,1.65) | 0.197† | 1.88(1.43,2.46) | <0.001 | 1.79(1.55,2.06) | <0.001 |
| Adjusted | 1.72(1.39,2.13) | <0.001 | 0.93(0.66,1.31) | 0.683† | 1.39(1.04,1.88) | 0.028 | 1.39(1.19,1.62) | <0.001 |
| Combined | ||||||||
| Mortality/Morbidity | ||||||||
| Stroke | ||||||||
| Unadjusted | 2.76(2.06,3.70) | <0.001 | 2.27(1.56,3.30) | <0.001 | 2.07(1.39,3.06) | <0.001 | 2.41(1.97,2.94) | <0.001 |
| Adjusted | 2.34(1.71,3.21) | <0.001 | 2.15(1.44,3.20) | <0.001 | 1.95(1.29,2.95) | 0.002 | 2.15(1.74,2.66) | <0.001 |
| Heart Failure | ||||||||
| Unadjusted | 3.22(2.48,4.18) | <0.001 | 3.21(2.38,4.35) | <0.001 | 2.53(1.79,3.58) | <0.001 | 3.01(2.54,3.58) | <0.001 |
| Adjusted | 2.35(1.77,3.12) | <0.001 | 2.73(1.96,3.81) | <0.001 | 2.30(1.59,3.31) | <0.001 | 2.45(2.03,2.95) | <0.001 |
| CHD | ||||||||
| Unadjusted | 1.97(1.49,2.59) | <0.001 | 1.31(0.88,1.95) | 0.184 | 1.66(1.15,2.39) | 0.007 | 1.68(1.39,2.04) | <0.001 |
| Adjusted | 1.45(1.08,1.94) | 0.014 | 1.05(0.69,1.62) | 0.807 | 1.32(0.89,1.96) | 0.164 | 1.30(1.06,1.60) | 0.012 |
| Combined CVD | ||||||||
| Unadjusted | 2.82(2.37,3.36) | <0.001 | 2.15(1.71,2.71) | <0.001 | 2.10(1.66,2.65) | <0.001‡ | 2.40(2.13,2.71) | <0.001 |
| Adjusted | 2.23(1.85,2.69) | <0.001 | 1.78(1.38,2.29) | <0.001 | 1.83(1.42,2.35) | <0.001 | 1.97(1.73,2.24) | <0.001 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL, high-density lipoprotein; HR, hazard ratio; LVH, left-ventricular hypertrophy.
Adjusted for age, gender, race, diabetes, current smoking, history of CHD, LVH, ASCVD, aspirin use, anti-hypertensive medications, BMI, GFR and HDL
P-value for interaction between amlodipine vs. chlorthalidone and atrial fibrillation HR for outcome is < 0.05
P-value for interaction between lisinopril vs. chlorthalidone and atrial fibrillation HR for outcome is < 0.05
Figure 2.
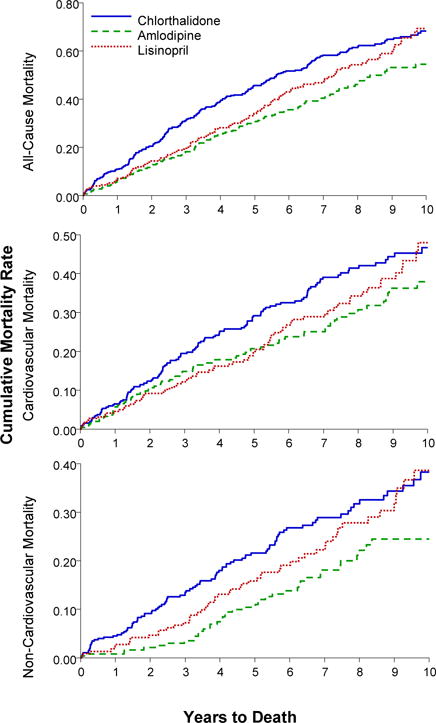
Kaplan-Meier curves of cumulative incidence of all-cause mortality, cardiovascular death, and non-cardiovascular death for cases with baseline atrial fibrillation/atrial flutter or in-trial atrial fibrillation/atrial flutter, for all chlorthalidone, amlodipine, and lisinopril groups
Ten-year mortality rates of those with AF/AFL were similar for chlorthalidone and lisinopril but lower for amlodipine, with 10-year all-cause mortality rates per 100 persons of 68 for chlorthalidone, 49 for amlodipine, and 66 for lisinopril. Compared to chlorthalidone, the adjusted HR for amlodipine was 0.68 (95% CI, 0.54–0.87) and for lisinopril, 0.83 (95% CI, 0.66–1.05) (Table 3).
Table 3.
Mortality rates and hazard ratios for amlodipine vs. chlorthalidone and lisinopril vs. chlorthalidone treatment groups for cases with either baseline or in-trial atrial fibrillation/flutter
| Mortality | Chlorthalidone (n=383) |
Amlodipine (n=247) |
Lisinopril (n=224) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||||
|
| |||||||||||
| n, 10-yr Rate/100 Persons |
n, 10-yr Rate/100 Persons |
HR (95% CI) | P | HR (95% CI) | P | n, 10-yr Rate/100 Persons |
HR (95% CI) | P | HR (95% CI) | P | |
| All-cause* | 236 67.91 | 115 49.45 | 0.67(0.54,0.83) | <0.001 | 0.68(0.54,0.87) | 0.002 | 129 66.04 | 0.84(0.68,1.04) | 0.108 | 0.83(0.66,1.05) | 0.123 |
| CVD | 132 44.77 | 70 32.49 | 0.73(0.55,0.97) | 0.030 | 0.78(0.57,1.06) | 0.113 | 70 41.92 | 0.81(0.60,1.08) | 0.146 | 0.81(0.59,1.11) | 0.184 |
| Non-CVD | 96 37.46 | 40 18.94 | 0.58(0.41,0.83) | 0.003 | 0.55(0.37,0.83) | 0.004 | 53 36.43 | 0.86(0.62,1.20) | 0.375 | 0.83(0.58,1.19) | 0.316 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; yr, year.
All-cause includes CVD, non-CVD, and unknown.
Ten-year CVD and non-CVD mortality was similar for chlorthalidone and lisinopril but lower for amlodipine, with 10-year CVD mortality rates per 100 persons of 45 (chlorthalidone), 32 (amlodipine), and 42 (lisinopril). Compared to chlorthalidone, the adjusted HR for amlodipine was 0.78 (95% CI, 0.57–1.06) and for lisinopril, 0.81, (95% CI, 0.59–1.11). The 10-year non-CVD mortality rates were 37 (chlorthalidone), 19 (amlodipine), and 36 (lisinopril). Compared to chlorthalidone, the adjusted HR for amlodipine was 0.55 (95% CI, 0.37–0.83) and for lisinopril, 0.83 (95% CI, 0.58–1.19) (Table 3).
A total of 10,355 ALLHAT-LLT participants were randomly assigned to pravastatin and usual care (UC). For this analysis, 10,001 participants remained after removing 198 with no ECG or for whom AF/AFL incidence or prevalence could not be determined, and after excluding 156 Canadian participants for whom post-trial mortality was unobtainable (Figure 1 [B]). During the trial (through March 2002), 123/4987 pravastatin participants (2.5%) and 122/5014 UC participants (2.4%) had baseline or incident AF/AFL (245 total AF/AFL events). Of participants with baseline or incident AF/AFL, 54.3% (133/245) were dead at the end of extended follow-up: 57 in-trial deaths, and 76 post-trial.
Baseline characteristics for those with and without in-trial baseline or incident AF/AFL for ALLHAT-LLT are provided in Table 1. Those with baseline or incident AF/AFL were older, more often male or non-Black, more likely to have a history of CVD and CHD, and had lower eGFR.
Adjusted mortality outcomes are shown in Table 4. Kaplan-Meier mortality (all-cause, CVD, and non-CVD) curves are shown in Figures 2 and 3. HRs for mortality showed significantly increased risk of death due to all causes, CHD, stroke, HF, other CVD, and non-CVD for those with versus without AF/AFL. HRs for fatal/non-fatal outcomes showed significantly increased risk for HF and combined CVD. There were no statistically significant treatment-by AF/AFL interactions in either of the adjusted analyses in Table 4 except for non-CV mortality in pravastatin (adjusted HR=0.95) compared to UC (adjusted HR=2.29).
Table 4.
Unadjusted and adjusted mortality rates and hazard ratios by treatment group for cases with either baseline or in-trial atrial fibrillation/flutter vs. cases with neither* in the lipid lowering trial
| Usual Care | Pravastatin | Usual Care/Pravastatin combined | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
|
|
||||||
| Mortality | ||||||
| All-cause | ||||||
| Unadjusted | 2.81(2.22,3.55) | <0.001 | 2.19(1.69,2.83) | <0.001 | 2.48(2.09,2.96) | <0.001 |
| Adjusted | 2.08(1.61,2.70) | <0.001 | 1.52(1.15,2.00) | 0.003 | 1.74(1.44,2.10) | <0.001 |
| CHD | ||||||
| Unadjusted | 2.76(1.93,3.93) | <0.001 | 3.18(2.28,4.43) | <0.001 | 2.96(2.32,3.77) | <0.001 |
| Adjusted | 1.95(1.31,2.89) | 0.001 | 2.20(1.54,3.13) | <0.001 | 2.02(1.56,2.63) | <0.001 |
| Stroke | ||||||
| Unadjusted | 4.38(2.03,9.44) | <0.001 | 2.75(1.12 – 6.76) | 0.028 | 3.52(1.96 – 6.31) | <0.001 |
| Adjusted | 3.21(1.36,7.53) | 0.008 | 1.75(0.69 – 4.41) | 0.237 | 2.38(1.28 – 4.44) | 0.006 |
| Heart Failure | ||||||
| Unadjusted | 5.26(2.28,12.13) | <0.001 | 4.07(1.64,10.13) | 0.003 | 4.64(2.51,8.60) | <0.001 |
| Adjusted | 4.41(1.83,10.61) | 0.001 | 2.40(0.85,6.78) | 0.097 | 3.22(1.66,6.24) | 0.001 |
| Other CVD | ||||||
| Unadjusted | 4.05(2.25,7.29) | <0.001 | 3.84(2.13,6.91) | <0.001 | 3.93(2.60,5.96) | <0.001 |
| Adjusted | 3.21(1.70,6.06) | <0.001 | 2.96(1.58,5.55) | 0.001 | 3.00(1.93,4.67) | <0.001 |
| Non-CVD | ||||||
| Unadjusted | 2.94(2.14,4.04) | <0.001 | 1.34(0.86,2.09) | 0.194† | 2.10(1.62,2.72) | <0.001 |
| Adjusted | 2.29(1.62,3.24) | <0.001 | 0.95(0.59,1.53) | 0.844† | 1.53(1.16,2.01) | 0.003 |
| Combined | ||||||
| Mortality/Morbidity | ||||||
| Stroke | ||||||
| Unadjusted | 1.82(1.00,3.31) | 0.052 | 1.77(0.94,3.33) | 0.076 | 1.80(1.17,2.78) | 0.008 |
| Adjusted | 1.48(0.75,2.89) | 0.256 | 1.13(0.56,2.30) | 0.736 | 1.27(0.78,2.07) | 0.338 |
| Heart Failure | ||||||
| Unadjusted | 2.96(1.82,4.82) | <0.001 | 2.51(1.49,4.21) | 0.001 | 2.74(1.92,3.90) | <0.001 |
| Adjusted | 2.69(1.58,4.56) | <0.001 | 1.46(0.81,2.64) | 0.203 | 1.97(1.33,2.91) | 0.001 |
| CHD | ||||||
| Unadjusted | 1.22(0.65,2.28) | 0.534 | 1.90(1.13,3.18) | 0.015 | 1.54(1.03,2.29) | 0.033 |
| Adjusted | 0.79(0.37,1.67) | 0.531 | 1.40(0.82,2.40) | 0.223 | 1.10(0.71,1.69) | 0.682 |
| Combined CVD | ||||||
| Unadjusted | 2.12(1.50,2.98) | <0.001 | 2.43(1.75,3.38) | <0.001 | 2.27(1.79,2.87) | <0.001 |
| Adjusted | 1.79(1.24,2.57) | 0.002 | 1.57(1.10,2.25) | 0.013 | 1.66(1.29,2.14) | <0.001 |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Adjusted for age, gender, race, diabetes, current smoking, history of CHD, LVH, ASCVD, aspirin use, anti-hypertensive medications, BMI, GFR and HDL
P-value for interaction between pravastatin vs. usual care and atrial fibrillation HR for outcome is <0.05
Figure 3.
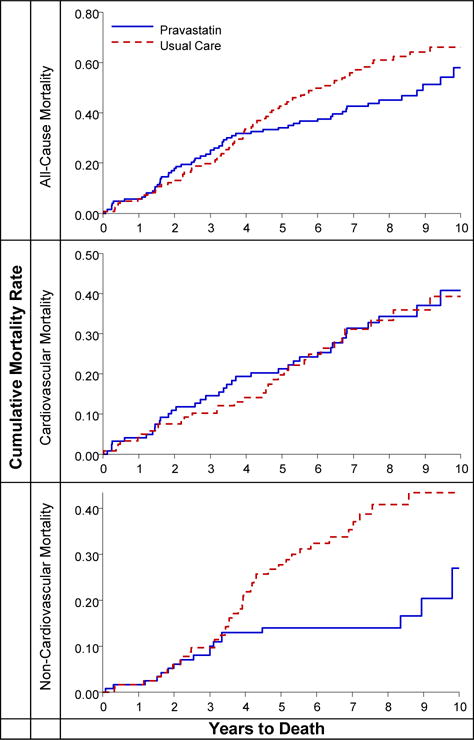
Kaplan-Meier curve of cumulative incidence of all cause-mortality, cardiovascular death, and non-cardiovascular death for cases with either baseline atrial fibrillation/atrial flutter or incident baseline atrial fibrillation/atrial flutter, for the pravastatin and usual care groups
Mortality rates for those with AF/AFL were not significantly lower for pravastatin compared to UC, with10-year all-cause rates per 100 persons of 57 for pravastatin and 65 for UC. Ten-year CVD mortality rates were 38 (pravastatin) and 31 for (UC), and, for non-CVD mortality, 18 (pravastatin) and 39 (UC) with an adjusted HR (pravastatin compared to UC) of 0.46 (95% CI, 0.24–0.86, P=0.016) (Table 5).
Table 5.
Mortality rates and hazard ratios for pravastatin vs. usual care treatment groups for cases with either baseline or in-trial atrial fibrillation/flutter
| Mortality cause | Usual care (n=122) | Pravastatin (n=123) | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
|
| ||||||
| N (10-yr rate/100 persons) | N (10-yr rate/100 persons) | HR (95% CI) | P | HR (95% CI) | P | |
| All-cause | 73 (65.38) | 58 (57.43) | 0.76 (0.54,1.07) | 0.114 | 0.80 (0.54,1.19) | 0.270 |
| CVD | 32 (31.43) | 37 (37.78) | 1.06 (0.66,1.70) | 0.820 | 1.08 (0.62,1.87) | 0.782 |
| Non-CVD | 40 (38.55) | 18 (17.76) | 0.46 (0.27,0.79) | 0.005 | 0.46 (0.24,0.86) | 0.016 |
Abbreviations: CI: confidence interval; CVD: cardiovascular disease.
DISCUSSION
The first part of this paper sought to establish whether or not the significant impact of prevalent or incident AF/AFL during the ALLHAT trial continued during the post-trial period. The answer is decidedly positive. Participants with AF/AFL continued to suffer 2- to 3-fold higher CVD risk compared to participants without. These findings are consistent with reports from multiple studies in diverse populations demonstrating deleterious influences of long-term AF/AFL on CVD and stroke.3, 12, 13 ALLHAT clearly demonstrates these outcomes in a high-risk hypertensive population.
Our second objective was to examine whether use of 3 primary antihypertensive study medications exerted an effect on CVD outcomes post-trial. The use of amlodipine was associated with a lower risk of all-cause, CVD, and non-CVD mortality compared to chlorthalidone. This finding stands in contrast to the lack of modifying effect of any of the antihypertensive medications on outcomes during the trial. There is little literature to support a reliable long-term primary role of amlodipine for AF prevention or suppression, despite a suggestion that it might have an anti-fibrotic effect when used long-term.14–16 Amlodipine’s primary use has been as an antihypertensive agent.10, 14
Similarly, diuretics have not been assumed to have a primary role in arrhythmia prevention, except as related to anti-aldosterone properties. The effectiveness of diuretics as antihypertensive and anti-HF agents might be expected to indirectly influence AF-related outcomes, whereas the side effects of hypokalemia would have an opposite effect.17, 18 The stronger effect of chlorthalidone on CVD outcomes compared to amlodipine or lisinopril began early, generally within 2 years of the trial’s onset, suggesting an early negative effect rather than a positive effect of amlodipine.10
A large meta-analysis of antihypertensive trials, including ALLHAT, assessing HF outcomes found diuretics better than ACE-Is and CCBs in reducing HF risk, presumed due to hypertension control.19 We found no significant differences between these drugs on AF-related outcomes in the completed trial, and no obvious explanation for amlodipine’s improved outcomes compared to chlorthalidone and lisinopril post-trial.
The use of a lipid-lowering agent to modify CVD outcomes in people with AF/AFL was examined. By lowering lipids, persons with AF/AFL related to atherosclerotic CVD could possibly have a lower risk of long-term CVD outcomes. However, assignment to pravastatin in the ALLHAT-LLT did not influence AF incidence.7 Here, only in non-CVD mortality did pravastatin have a favorable effect over UC. Mortality in those with AF is believed to be largely arrythmogenic or HF-related,19–26 neither of which is strongly associated with lipid levels. Furthermore, the possibility that statins might have a beneficial anti-inflammatory effect on the genesis and persistence of AF/AFL has not been consistently borne out in multiple studies and meta-analyses.20,21
The conditions influencing the atria which facilitate the development of persistent or recurrent AF/AFL, anatomical and pathophysiologic, are the subject of ongoing investigations. Results that cannot be readily attributed to accepted mechanisms or do not conform to prior experiences are not unusual.27,28
The strengths of this study include the large high-risk hypertensive diverse ALLHAT population, including minorities and diabetics. Weaknesses of this study include lack of post-trial data regarding antihypertensive treatment. Also, ALLHAT was not designed to assess the influence of AF on outcomes; observations here are post-hoc.6 Treatment group comparisons of outcomes following incident AF, about two-thirds of the combined AF/AFL cases, are not protected by randomization, and could be confounded by unmeasured characteristics, earlier morbid events, or AF with different clinical characteristics.
Nevertheless, ALLHAT provided the opportunity to observe the major influences of prevalent and incident AF on study outcomes. Our findings strongly suggest the need for future comparative effectiveness trials such as ALLHAT, involving high-risk, diverse hypertensive populations, which include plans to assess the influence of AF on study outcomes and to determine the optimal antihypertensive and/or lipid-lowering agents in preventing or reducing the major cardiovascular consequences of atrial fibrillation.
Influence of Prevalent and Incident Atrial Fibrillation on Post-Trial Major Events in ALLHAT: Implications.
As the US population ages, atrial fibrillation (AF) will continue to increase. Its cost is immense: clinically, AF has been directly linked to subsequent stroke, heart failure, CHD, and mortality; economically, AF represents a steeply growing burden on society. Patients with AF incur twice as many hospitalizations and four times as many cardiovascular hospitalizations as those without (Curr Med Res Opin 2005;21(10):1693–1699). AF increases the risk of stroke by five-fold (www.stroke.org); these patients frequently require acute hospitalization plus long-term care and ongoing therapy. Hypertension is a major risk factor of AF. ALLHAT confirmed that high-risk hypertensive patients with AF experience increased CVD, stroke, and mortality compared to those without. We have long known that treatment of hypertension reduces stroke risk (SHEP results, ALLHAT stroke results in Black population); ALLHAT data suggest that adequate control of hypertension is paramount to reduction of atrial fibrillation and its severe morbidity and mortality outcomes.
Acknowledgments
The authors thank Ellen Breckenridge and Kara Elam, The University of Texas School of Public Health, for editorial assistance in the preparation of this manuscript.
Funding/Support
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health, Bethesda, MD [NO1-HC-35130, HHSN268201100036C]. The ALLHAT investigators acknowledge contributions of study medications supplied by Pfizer, Inc. (amlodipine, doxazosin), AstraZeneca (atenolol, lisinopril) and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration: www.clinicaltrials.gov NCT00000542
Role of Sponsor
This work reflects the views of the authors and does not necessarily reflect the positions of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the U.S. Department of Health and Human Services.
Author Responsibility
All authors have read the final version and have approved submission of this manuscript. All have made substantial contributions to the concept and design of the research, acquisition of data, or analysis and interpretation of data; and/or contributed substantially to drafting and revising the paper for important intellectual content.
Financial conflict of interest statement
William Cushman reports grants from Eli Lilly, Boerhinger Ingelheim, and Merck. Drs. Davis and Piller report grants from NHLBI. All other authors have no financial interests to disclose. Jeffrey Cutler is a contractor for NHLBI; no specific funding was allocated for this work. The views expressed in this manuscript are those of the authors and do not necessarily represent those of NHLBI.
Declaration of Helsinki
The study complies with the Declaration of Helsinki. All clinical sites had local ethics board approvals; post-trial follow-up was approved by the ethics board at the University of Texas Health Science Center at Houston. Written informed consent was obtained from all subjects or their legally authorized representatives.
References
- 1.Kannel WB, Abbott RD, Savage DD, McNamara PN. Epidemiologic features of chronic atrial fibrillation: The Framingham Study. N Eng J Med. 1982;306:1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 3.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2155–2161. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. National implications of rhythm management and stroke prevention; the Anticoagulation and Risk Factor in in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Estes NA, 3rd, Sacco RL, Al-Khatib SM, Ellinor PT, Bezanson J, Alonso A, Antzelevitch C, Brockman RG, Chen PS, Chugh SS, Curtis AB, DiMarco JP, Ellenbogen KA, Epstein AE, Ezekowitz MD, Fayad P, Gage BF, Go AS, Hlatky MA, Hylek EM, Jerosch-Herold M, Konstam MA, Lee R, Packer DL, Po SS, Prystowsky EN, Redline S, Rosenberg Y, Van Wagoner DR, Wood KA, Yue L, Benjamin EJ. American Heart Association atrial fibrillation research summit: a conference report from the American Heart Association. Circulation. 2011 Jul 19;124(3):363–72. doi: 10.1161/CIR.0b013e318224b037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertens. 1996;9:342–60. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 7.Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, Williard A, ALLHAT Collaborative Research Group Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial) JACC. 2009;54(22):2023–31. doi: 10.1016/j.jacc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Papademetriou V, Piller LB, Ford CE, Gordon D, Hartney TJ, Geraci TS, Reisin E, Sumner BM, Wong ND, Nwachuku C, Narayan P, Haywood J, Habib G, ALLHAT Collaborative Research Group Characteristics and lipid distribution of a large high-risk hypertensive population. The lipid-lowering component of the ALLHAT. J Clin Invest. 2003;5(6):377–395. doi: 10.1111/j.1524-6175.2003.03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Am J Hypertens. 1996;9(41):342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 10.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes of high-risk hypertension patients randomized in angiotensin-converting enzyme inhibitor or calcium-channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 11.Cushman WC, Davis BR, Pressel SL, Cutler JA, Einhorn PT, Ford CE, Oparil S, Probstfield JL, Whelton PK, Wright JT, Jr, Alderman MH, Basile JN, Black HR, Grimm RH, Jr, Hamilton BP, Haywood LJ, Ong ST, Piller LB, Simpson LM, Stanford C, Weiss RJ, ALLHAT Collaborative Research Group Mortality and morbidity during and after the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. J Clin Hypertens (Greenwich) 2012;14(1):20–31. doi: 10.1111/j1751-7176.2011.00568x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, Carluccio E, Sardone MG, Porcellati C. Atrial fibrillation in hypertension predictors and outcome. Hypertens. 2003;41:218–223. doi: 10.1161/01.hyp.0000052830.02773.e4. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community-based study over two decades. Eur Heart J. 2006;27:936–041. doi: 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]
- 14.Udelson JE, DeAbate CA, Berk M, Neuberg G, Packer M, Vijay NK, Gorwitt J, Smith WB, Kukin ML, LeJemtel T, Levine TB, Konstam MA. Effects of amlodipine on exercise tolerance quality of life and left ventricular function. Am Heart J. 2000;139:503–510. doi: 10.1016/s0002-8703(00)90095-4. [DOI] [PubMed] [Google Scholar]
- 15.Fogari R, Zoppi A, Mugellini A, Corradi L, Lazzari P, Preti P, Derosa G. Comparative evaluation of effect of valsartari/amiodipine and atenolol/amiodipine combinations on atrial fibrillation. J Cardiovasc Pharmcol. 2008 Mar;51(3):217–22. doi: 10.1097/FJC.0b013e318160b42a. [DOI] [PubMed] [Google Scholar]
- 16.Popov SV, Kandinskiĭ ML, Kozlov BN, Antonchenko IV, Evtushenko AV, Dzhavadova GK, Vecherskiĭ IuIu, Akhmedov ShD, Afanas’ev SA, Shipulin VM. Effect of calcium antagonists verapamil and amiodipine on the risk of development of atrial fibrillation after coronary artery bypass grafting. Kardiologiia. 2003;43(7):37–30. Russian. [PubMed] [Google Scholar]
- 17.Jolobe OM. Agents with antialdosterone properties should be the preferred diuretics for reducing hypertension related atrial fibrillation. Eur J Intern Med. 2010 Feb;21(1):55. doi: 10.1016/j.ejim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Anne W, Willems R, Van der Merwe N, Van de Werf F, Ector H, Heidbüchel H. Atrial fibrillation after radiofrequency ablation of atrial flutter: preventive effect of angiotensin converting enzyme inhibitors. angiotensin II receptor blockers and diuretics. Heart. 2004 Sep;90(9):1022–30. doi: 10.1136/hrt.2003.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension. Arch Inter Med. 2011;171(5):384–394. doi: 10.1001/archinternmed.2010.427. [DOI] [PubMed] [Google Scholar]
- 20.Rahimi K, Emberson J, McGale P, Majoni W, Merhi A, Asselbergs FW, Krane V, Macfarlane PW, PROSPER Executive Effect of statins on atrial fibrillation, collaboration meta-analysis on published and unpublished evidence from randomized controlled trials. BMI. 2011:343. doi: 10.1136/bmj.d1250. [DOI] [PubMed] [Google Scholar]
- 21.Bang CN, Greve AM, Abdulla J, Køber L, Gislason GH, Wachtell K. The preventive effect of statin therapy on new onset of recurrent atrial fibrillation in patients not undergoing invasive cardiac intervention. A systemic review and meta-analysis. International Journal of Cardiology. 2013;167(3):624–630. doi: 10.1016/j.ijcard.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 22.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37(2):371–378. doi: 10.1016/s0735-1097(00)01107-4. doi: S0735-1097(00)01107-4 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Wachtell K, Hornestam B, Lehto M, Slotwiner DJ, Gerdts E, Olsen MH, Aurup P, Dahlöf B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Rokkedal J, Devereux RB. Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: The Losartan Intervention For End Point Reduction inHypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):705–711. doi: 10.1016/j.jacc.2004.06.080. doi: S0735-1097(04)02399-X [pii] [DOI] [PubMed] [Google Scholar]
- 24.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Internal Medicine. 2014;174(1):107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao TF, Huang YC, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen TJ, Hsieh MH, Lip GY, Chen SA. Acute myocardial infarction in patients with atrial fibrillation with a CHA2DS2-VASc score of 0 or 1: a nationwide cohort study. Heart Rhythm. 2014;11(11):1941–1947. doi: 10.1016/j.hrthm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Reinier K, Marijon E, Uy-Evanado A, Teodorescu C, Narayanan K, Chugh H, Gunson K, Jui J, Chugh SS. The association between atrial fibrillation and sudden cardiac death: the relevance of heart failure. JACC Heart Fail. 2014;2(3):221–227. doi: 10.1016/j.jchf.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Huxley RR1, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, Markl M, Ng J, Shah SJ. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015 Jul 28;4:279–291. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]


