Summary
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a progressive, potentially fatal complication of conditioning for haematopoietic stem cell transplant (HSCT). The VOD/SOS pathophysiological cascade involves endothelial-cell activation and damage, and a prothrombotic-hypofibrinolytic state. Severe VOD/SOS (typically characterized by multi-organ dysfunction) may be associated with >80% mortality. Defibrotide is approved for treating severe hepatic VOD/SOS post-HSCT in the European Union, and for hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States. Previously, defibrotide (25 mg/kg/day in 4 divided doses for a recommended ≥21 days) was available through an expanded-access treatment protocol for patients with VOD/SOS. Data from this study were examined post-hoc to determine if the timing of defibrotide initiation post-VOD/SOS diagnosis affected Day +100 survival post-HSCT. Among 573 patients, defibrotide was started on the day of VOD/SOS diagnosis in approximately 30%, and within 7 days in >90%. The relationship between Day +100 survival and treatment initiation before/after specific days post-diagnosis showed superior survival when treatment was initiated closer to VOD/SOS diagnosis with a statistically significant trend over time for better outcomes with earlier treatment initiation (P < 0·001). These results suggest that initiation of defibrotide should not be delayed after diagnosis of VOD/SOS.
Keywords: defibrotide, veno-occlusive disease, sinusoidal obstruction syndrome, treatment initiation, survival
Introduction
Hepatic veno-occlusive disease, also called sinusoidal obstruction syndrome (VOD/SOS), is an unpredictable, potentially life-threatening complication of haematopoietic stem cell transplant (HSCT) conditioning (Bearman, 1995; Mohty et al, 2015) that also may occur as a result of primary chemotherapy, immuno-toxin conjugate therapy, or radiation (Fan & Crawford, 2014; Helmy, 2006). The hallmark clinical signs and symptoms of VOD/SOS include weight gain, hyperbilirubinaemia, ascites and painful hepatomegaly (Dignan et al, 2013; Mohty et al, 2016). The reported incidence of VOD/SOS has ranged widely (0–62%), varying by type of transplant, diagnostic criteria used and population risk factors (Mohty et al, 2016; Coppell et al, 2010). However, even with reduced-intensity conditioning, a VOD/SOS incidence of approximately 9% in allogeneic transplant patients has been reported (Tsirigotis et al, 2014) and of 11% in patients with acute lymphoblastic leukaemia receiving inotuzumab ozogamicin without HSCT (Kantarjian et al, 2016). Severe VOD/SOS, which is typically characterized by the presence of renal and/or pulmonary dysfunction (multi-organ dysfunction [MOD]) (Dignan et al, 2013; Mohty et al, 2016), may develop in approximately 20–40% of patients with VOD/SOS who received allogeneic HSCT (Carreras et al, 2011), and is associated with a mortality rate of >80% (Coppell et al, 2010).
VOD/SOS develops via a progressive cascade of pathophysiological events that generate a prothrombotic-hypofibrinolytic state (Fan & Crawford, 2014; Kumar et al, 2003; Carreras & Diaz-Ricart, 2011; Palomo et al, 2010; Richardson et al, 2013). The initial toxic injury occurs to sinusoidal endothelial cells and hepatocytes in zone 3 of the liver acinus, causing endothelial cell activation, which in turn both triggers and supports an inflammatory response (Bearman, 1995; Carreras & Diaz-Ricart, 2011). The injured sinusoidal endothelial cells round up and slough off the endothelial wall, compromising its integrity, and permitting extravasation of blood into the space of Disse, which leads to thrombosis and extraluminal compression of the sinusoidal vessels (Fan & Crawford, 2014; Carreras & Diaz-Ricart, 2011). Endothelial cell injury also leads to upregulation of prothrombotic pathways, resulting in platelet activation, aggregation and sinusoidal thrombosis (Fan & Crawford, 2014; DeLeve et al, 2002). These developments cause further deterioration of the vasculature (Carreras & Diaz-Ricart, 2011). Profound endothelial dysfunction may result, accompanied by cytokine release and inflammation, with subsequent post-sinusoidal portal hypertension and the potential for hepatorenal syndrome, which manifests as MOD, and may progress rapidly to advanced MOD and death (Bearman, 1995; DeLeve et al, 2002; Ho et al, 2007).
Diagnosis of VOD/SOS has traditionally been based on Baltimore (Jones et al, 1987) or modified Seattle criteria (McDonald et al, 1993; Corbacioglu et al, 2012). However, these criteria were developed in an era when the risk/benefit ratio of available treatments was unfavourable (Mohty et al, 2016). More recently, an expert committee of the European Society for Blood and Marrow Transplantation (EBMT) challenged specific aspects of these criteria on the grounds that they may exclude or delay identification of some patients with VOD/SOS (Mohty et al, 2015). The EBMT emphasis on early intervention in management of VOD/SOS was in part prompted by the recent availability of effective therapy for this syndrome (Mohty et al, 2016). Defibrotide is now approved in the United States for treatment of adult and paediatric patients with hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT (http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208114lbl.pdf, accessed 30 August 2016), and is also approved in the European Union for treatment of severe hepatic VOD/SOS post-HSCT (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002393/WC500153150.pdf, accessed 30 August 2016). In a phase 3, historically controlled, clinical trial (N = 134), defibrotide treatment in patients with established hepatic VOD/SOS and MOD was associated with a statistically significant 23% improvement in survival rates (P = 0·0109, propensity-adjusted analysis) at Day +100 post-HSCT (Richardson et al, 2016). In vitro data suggest that defibrotide may decrease activation of endothelial cells, thereby stabilizing and protecting them, and promoting the restoration of thrombofibrinolytic balance, as well as having anti-inflammatory effects (Palomo et al, 2010; Richardson et al, 2013; Pescador et al, 2013).
Monitoring for VOD/SOS, early diagnosis and timely treatment are crucial for post-HSCT patients. Determining the optimal time to initiate defibrotide treatment and its potential impact on outcomes is of high clinical interest. A post-hoc analysis from a defibrotide expanded-access treatment (T-IND) protocol for hepatic VOD/SOS (NCT00628498) was performed to investigate this issue.
Methods
The T-IND study was the largest prospective evaluation of defibrotide (25 mg/kg/day in 4 divided doses for at least 21 days) for the treatment of confirmed VOD/SOS, with and without MOD, in patients post-HSCT or post-chemotherapy. This exploratory analysis included all patients enrolled from 14 December 2007 to 31 December 2013, and data were reported to 5 December 2014. Study methods were previously reported (Richardson et al, 2015a); briefly, patients were initially eligible if they had VOD/SOS by Baltimore criteria (≤21 days post-HSCT, bilirubin ≥34.2 μmol/l and two or more of the following: hepatomegaly, ascites, weight gain ≥5%) (Jones et al, 1987) with associated MOD following HSCT. Following amendments to the protocol, patients were eligible if they were diagnosed with VOD/SOS by biopsy (Amendment 1 in December 2007), with or without MOD or following HSCT or chemotherapy (Amendment 2 in August 2009), or met Baltimore or modified Seattle criteria (≤20 days post-HSCT, with two or more of the following: bilirubin ≥ 34.2 μmol/l, hepatomegaly, or right upper quadrant pain, using a weight gain criterion of ≥5%) (McDonald et al, 1993; Corbacioglu et al, 2012) (Amendment 5 in August 2012). Key exclusion criteria were clinically significant bleeding or the need for ≥2 vasopressors. Concomitant medications that could increase the risk of bleeding must have been discontinued within 12 h of defibrotide administration.
The T-IND protocol recommended treatment with defibrotide at a dose of 25 mg/kg/day in 2-h intravenous infusions every 6 h for at least 21 days. The protocol-specified recommendation for treatment initiation stated: “The patient should receive their first dose of defibrotide as soon as the patient meets eligibility requirements.” Patients were followed for 100 days after HSCT or the start of non-transplant–associated chemo/radiotherapy, and the primary efficacy evaluation was survival rate at Day +100.
This post-hoc analysis examined Day +100 survival in HSCT patients based on time from VOD/SOS diagnosis to initiation of defibrotide. Two analyses of Day +100 survival rate were performed:
▪Analysis by treatment initiation for the entire HSCT population, comparing Day +100 survival rates before and after initiation on each of the following days: 1, 2, 3, 4, 7, and 14, from diagnosis date (using Fisher’s exact test, calculated for patients with known survival status)
▪Analysis of trend in Day +100 survival rates for only those patients with treatment initiated on a particular day or period: 0, 1, 2, 3, 4, 5, 6, 7, 8–14, and ≥15 days from diagnosis date using the Cochran-Armitage test for trend
P-values < 0.05 were considered statistically significant.
Results
The date of initial treatment with defibrotide was available for 573 HSCT patients in the T-IND programme, including 351 (61·3%) with MOD. Baseline characteristics of the total HSCT group and subgroup with MOD were similar. The mean age was 20·6 years overall, and 21·1 and 19·7 in the groups with and without MOD, respectively (Table I). In the paediatric group of 319 patients (55·7%) aged younger than 16 years, 92 (28·8%) were younger than 1 year; 159 (49·8%) were aged 2–11 years; and 68 (21·3%) were 12–16 years.
Table I.
Baseline demographic and clinical characteristics of HSCT patients who received defibrotide.
| Variable | All HSCT Patients (N = 573) |
HSCT Patients Without MOD (n = 222) |
HSCT Patients With MOD (n = 351) |
|---|---|---|---|
|
| |||
| Gender, n (%) | |||
| Male | 324 (56·5) | 140 (63·1) | 184 (52·4) |
| Female | 249 (43·5) | 82 (36·9) | 167 (47·6) |
|
| |||
| Race, n (%) | |||
| White | 374 (65·3) | 137 (61·7) | 237 (67·5) |
| Non-white | 199 (34·7) | 85 (38·3) | 114 (32·5) |
|
| |||
| Age at HSCT, years | |||
| Mean (SD) | 20·6 (19·9) | 19·7 (20·3) | 21·1 (19·6) |
| Median (range) | 14·0 (0·1–69·0) | 13·5 (0·1–69·0) | 15 (0·1–69·0) |
|
| |||
| Age category at HSCT, n (%) | |||
| <16 years | 319 (55·7) | 130 (58·6) | 189 (53·8) |
| ≥16 years | 254 (44·3) | 92 (41·4) | 162 (46·2) |
|
| |||
| Weight (kg) | |||
| Mean (SD) | 47·8 (31·3) | 45·2 (30·8) | 49·4 (31·5) |
| Median (range) | 50·4 (3·0–134·5) | 47·7 (3·0–118·6) | 52·8 (3·2–134·5) |
|
| |||
| GVHD prophylaxis, n (%) | |||
| None | 81 (14·1) | 37 (16·7) | 44 (12·5) |
| Ciclosporin | 189 (33·0) | 71 (32·0) | 118 (33·6) |
| Methotrexate | 188 (32·8) | 77 (34·7) | 111 (31·6) |
| Sirolimus | 64 (11·2) | 17 (7·7) | 47 (13·4) |
| Tacrolimus | 264 (46·1) | 98 (44·1) | 166 (47·3) |
| Other | 184 (32·1) | 72 (32·4) | 112 (31·9) |
|
| |||
| Type of HSCT, n (%) | |||
| Allograft | 503 (87·8) | 186 (83·8) | 317 (90·3) |
| Autograft | 68 (11·9) | 34 (15·3) | 34 (9·7) |
| Unknown | 2 (0·3) | 2 (0·9) | 0 (0·0) |
GVHD, graft-versus-host disease; HSCT, haematopoietic stem cell transplantation; MOD, multi-organ dysfunction; SD, standard deviation.
Approximately half of the 573 HSCT patients had a primary diagnosis of acute leukaemia (165 [28·9%] with acute myeloid leukaemia and 118 [20·7%] with acute lymphocytic leukaemia). Other primary diseases in ≥5% of patients were neuroblastoma in 43 patients (7·5%), myelodysplastic syndrome in 33 patients (5·8%), and non-Hodgkin lymphoma in 29 patients (5·1%).
The vast majority of these patients received allogeneic HSCT: 503/573 (87·8%). Of the remaining 70 patients, 68 (11·9%) received autologous HSCT, and the transplant type was unknown for 2 patients (0·3%).
Overall, 31·9% of patients received defibrotide on the day of diagnosis, and it was started in 93·0% of patients by day 7 post-diagnosis. Between those dates, defibrotide treatment was started by day 1 in 59·7% of patients, by day 2 in 73·6%, by day 3 in 81·8%, by day 4 in 87·3%, by day 5 in 90·1% and by day 6 in 91·1%.
In the post-HSCT population-wide analysis of treatment initiation before or after days 0, 1, 2, 3, 4, 5, 6, 7 and 14 following VOD/SOS or VOD/SOS with MOD diagnosis, earlier initiation of defibrotide was associated with higher survival rates (Table II). Differences in survival rates before and after each of the temporal cut-off points ranged from 8·8% for patients with defibrotide initiated ≤1 or >1 day after diagnosis to 22·1% for patients with defibrotide initiated ≤2 or >2 days after diagnosis – this was statistically significant at all cut-off points assessed except day 14, although only 2·8% of patients began treatment post-day 14. For the VOD/SOS with MOD subgroup, Day +100 survival differences before and after each cut-off point ranged from 12·8% for patients with defibrotide initiated ≤1 or >1 day after diagnosis to 25·6% for patients with defibrotide initiated ≤2 or >2 days after diagnosis, and were statistically significant at all cut-off points except day 14; only 3·1% of patients with MOD began treatment after day 14.
Table II.
Day +100 survival by defibrotide initiation day.
| Initiation Period | HSCT VOD/SOS (N = 573) |
HSCT VOD/SOS With MOD
(n = 351) |
||||
|---|---|---|---|---|---|---|
| Alive n (%) |
Dead n (%) |
Unknown n (%) |
Alive n (%) |
Dead n (%) |
Unknown n (%) |
|
| ≤1 daya | 183 (53·5) | 142 (41·5) | 17 (5·0) | 103 (50·2) | 93 (45·4) | 9 (4·4) |
| >1 day | 105 (45·5) | 116 (50·2) | 10 (4·3) | 56 (38·4) | 85 (58·2) | 5 (3·4) |
| Difference (95% CI)b | 8·8% (0·2–17·3%) | 12·8% (2·0–23·4%) | ||||
| P-valuec | 0·045 | 0·021 | ||||
| ≤2 days | 235 (55·7) | 166 (39·3) | 21 (5·0) | 132 (52·2) | 111 (43·9) | 10 (4·0) |
| >2 days | 53 (35·1) | 92 (60·9) | 6 (4·0) | 27 (27·6) | 67 (68·4) | 4 (4·1) |
| Difference (95% CI)b | 22·1% (12·6–31·2%) | 25·6% (13·8–36·9%) | ||||
| P-valuec | <0·001 | <0·001 | ||||
| ≤3 days | 251 (53·5) | 193 (41·2) | 25 (5·3) | 138 (49·5) | 129 (46·2) | 12 (4·3) |
| >3 days | 37 (35·6) | 65 (62·5) | 2 (1·9) | 21 (29·2) | 49 (68·1) | 2 (2·8) |
| Difference (95% CI)b | 20·3% (9·6–30·8%) | 21·7% (8·6–34·5%) | ||||
| P-valuec | <0·001 | 0·001 | ||||
| ≤4 days | 263 (52·6) | 212 (42·4) | 25 (5·0) | 146 (48·7) | 142 (47·3) | 12 (4·0) |
| >4 days | 25 (34·2) | 46 (63·0) | 2 (2·7) | 13 (25·5) | 36 (70·6) | 2 (3·9) |
| Difference (95% CI)b | 20·2% (7·7–32·4%) | 24·2% (9·1–38·9%) | ||||
| P-valuec | 0·002 | 0·002 | ||||
| ≤7 days | 275 (51·6) | 232 (43·5) | 26 (4·9) | 152 (47·4) | 156 (48·6) | 13 (4·0) |
| >7 days | 13 (32·5) | 26 (65·0) | 1 (2·5) | 7 (23·3) | 22 (73·3) | 1 (3·3) |
| Difference (95% CI)b | 20·9% (4·5–37·1%) | 25·2% (6·1–43·8%) | ||||
| P-valuec | 0·013 | 0·011 | ||||
| ≤14 days | 282 (50·6) | 249 (44·7) | 26 (4·7) | 156 (45·9) | 171 (50·3) | 13 (3·8) |
| >14 days | 6 (37·5) | 9 (56·3) | 1 (6·3) | 3 (27·3) | 7 (63·6) | 1 (9·1) |
| Difference (95% CI)b | 13·1% (−12·9 to 39·5%) | 17·7% (−14·6 to 51·5%) | ||||
| P-valuec | 0·433 | 0·344 | ||||
Among all HSCT VOD/SOS patients, 13 with a recorded negative dosing delay were adjusted to have 0 days dosing delay; 1 patient with –293 days dosing delay was excluded from this analysis. In the MOD subgroup, 12 patients with a recorded negative dosing delay were adjusted to have 0 days dosing delay.
Alive and dead. 95% CI calculated using exact method.
Fisher’s exact test, alive and dead.
CI, confidence interval; HSCT, haematopoietic stem cell transplantation; MOD, multi-organ dysfunction; VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome.
In the post-HSCT with VOD/SOS cohort, the analysis of the relationship between Day +100 survival and treatment initiation day based on specific days or periods (0, 1, 2, 3, 4, 5, 6, 7, 8–14 and ≥15 days) found that there was a statistically significant trend over time (Cochran-Armitage test) for improved Day +100 survival with earlier treatment initiation (P < 0·001) (Fig 1). Similar improvement with earlier treatment initiation was shown for the subgroup of patients with MOD (P < 0·001).
Fig 1. Day +100 survival by day of dosing (P<0.001 by Cochran-Armitage test for trend)a.
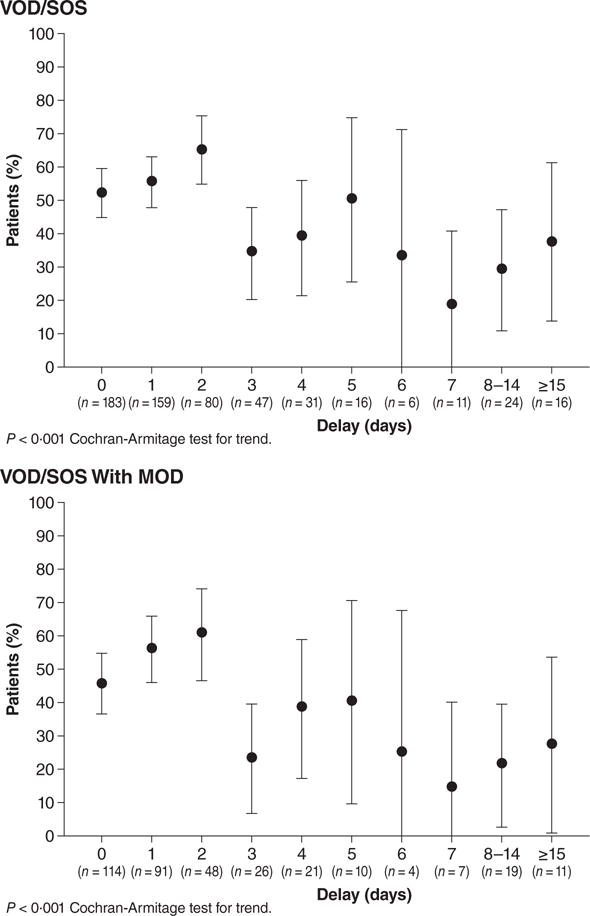
aBars around point estimates denote 95% confidence intervals.
Note: Among all HSCT VOD/SOS patients, 13 with a recorded negative dosing delay were adjusted to have 0 days dosing delay; 1 patient with −293 days dosing delay was excluded from this analysis. In the subgroup with MOD, 12 patients with a recorded negative dosing delay were adjusted to have 0 days dosing delay.
HSCT, haematopoietic stem cell transplantation; MOD, multi-organ dysfunction; VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome.
The overall Day +100 survival rate of 45% in post-HSCT patients with VOD/SOS and MOD (n = 387) in this expanded-access programme (Richardson et al, 2015b) compares favourably with survival rates in the literature for patients with severe VOD/SOS receiving only supportive care (usually <25% survival) (Coppell et al, 2010). Safety data from the expanded-access programme further show that defibrotide was generally well tolerated, and drug-related toxicities were consistent with prior studies ((http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208114lbl.pdf, accessed 30 August 2016; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002393/WC500153150.pdf, accessed 30 August 2016)).
Discussion
The results of this exploratory post-hoc analysis show that in the overall post-HSCT with VOD/SOS population and in the subgroup with MOD, earlier initiation of defibrotide treatment from time of diagnosis of VOD/SOS was associated with significantly greater Day +100 survival compared to later treatment initiation. Further, in this study, there was no clinically apparent plateau where greater delay would not reduce survival.
There appear to be benefits to therapeutic intervention for VOD/SOS as early as possible following diagnosis, when the disease state may be more favourable for response (Mohty et al, 2016); prompt use of defibrotide in particular also has been emphasized in prior publications (Dignan et al, 2013; Mohty et al, 2016). In the present study, nearly a third of patients were treated on the day of diagnosis and almost three-quarters received treatment within the first 2 days post-diagnosis. In addition, mortality rates among these patients were below 50%, an outcome that also supports the T-IND protocol recommendation, that treatment begin as soon as the patient met eligibility requirements.
In the T-IND programme, patients were to receive defibrotide as soon as eligibility requirements were met; however, 28% received defibrotide on day 1 post-diagnosis, 14% on day 2, 8% on day 3, 5% on day 4, 3% on day 5, 1% on day 6, 2% on day 7 and 7% of patients received defibrotide even later. Factors contributing to any treatment delay were not captured consistently, but may have included ineligibility to receive defibrotide immediately as a result of active bleeding, haemodynamic instability, receipt of multiple vasopressors (a protocol-specified contraindication to defibrotide treatment based on theoretical safety concerns for infusional-related hypotension), delayed availability of defibrotide at the medical centre, delays while awaiting test results to confirm VOD/SOS, and/or diagnostic uncertainty.
The benefit of treatment with defibrotide for VOD/SOS may be associated with its ability to stabilize and protect endothelial cells via multiple pathways, as demonstrated in vitro, and thus counteract the pathogenic cascade of thrombotic/hypofibrinolytic processes of VOD/SOS within the endothelium that drive the development of hepatorenal syndrome and MOD (Carreras & Diaz-Ricart, 2011; Palomo et al, 2010; Richardson et al, 2013; Palomo et al, 2016). Preclinical data suggest that defibrotide’s actions include increasing tissue plasminogen activator and thrombomodulin expression, promoting plasmin activity and angiogenesis, while inhibiting von Willebrand factor and plasminogen activator inhibitor-1 expression and fibrin deposition, and reducing inflammatory and oxidative factors and processes (Pescador et al, 2013; Palomo et al, 2016; Echart et al, 2009; Benimetskaya et al, 2008). In vitro data have shown that defibrotide exerts these actions through binding and interaction with endothelial cell membranes, and internalization by endothelial cells (Palomo et al, 2016). Because toxic injury to sinusoids is believed to be the initial pathogenic mechanism of VOD/SOS, defibrotide’s actions in protecting and stabilizing endothelial cells from damage may provide a rationale for using it as early as possible post-diagnosis. Similarly, delayed diagnosis due to difficulty in definitively establishing VOD/SOS, potentially due to the highly dynamic nature of its signs and symptoms (Mohty et al, 2016), may also result in treatment being initiated later in disease progression. Conversely, even in cases where treatment delay is unavoidable, the lack of a clear clinical cut-off for benefit in this analysis suggests that there is no point beyond which defibrotide initiation would not be warranted.
Few other analyses of the impact of timing of initiation of defibrotide on outcomes of VOD/SOS have been published. One retrospective study reported that early treatment with defibrotide post-diagnosis in patients with VOD/SOS and MOD was associated with a better outcome (Corbacioglu et al, 2004). For the 34 (76%) patients with a complete response (CR; resolution of VOD/SOS- and MOD-related symptoms and bilirubin <34.2 μmol/l), the average delay from VOD/SOS diagnosis to start of defibrotide therapy was 1 day vs. 5·5 days in patients without CR (n = 11; P < 0·01); this difference also was observed in the subgroup with VOD/SOS and MOD (1·3 days for those with CR vs. 5·5 days for those without CR; P < 0·01). A maximum delay of 1 day to initiate treatment vs. more than 1-day delay was the only significant predictor of CR identified (Corbacioglu et al, 2004).
Conclusions
This post-hoc analysis found that earlier initiation of defibrotide post-diagnosis was associated with increased Day +100 survival in the overall post-HSCT with VOD/SOS population and in the subgroup with MOD. No specific day post-diagnosis appeared to provide a viable cut-off resulting in better outcome, but earlier treatment initiation consistently provided more favourable clinical benefit in this population.
Acknowledgments
This study was funded by Jazz Pharmaceuticals, Inc. Under the direction of the authors, Larry Deblinger, employee of The Curry Rockefeller Group, LLC, provided medical writing assistance. The Curry Rockefeller Group, LLC, also provided editorial assistance in formatting, proofreading, copy-editing and fact checking for this publication. Jazz Pharmaceuticals, Inc, provided funding to The Curry Rockefeller Group, LLC, for support in writing and editing this publication.
Footnotes
Conflict of interest statement
Paul G. Richardson has served on an advisory committee and as a consultant to Jazz Pharmaceuticals and Gentium, and has received research funding from Gentium; Angela R. Smith has no relevant financial relationships to disclose; Brandon M. Triplett has no relevant financial relationships to disclose; Nancy A. Kernan has received research funding from Gentium; Nancy A. Kernan’s research was supported by National Cancer Institute of the National Institutes of Health under award number P30 CA008748; the content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health; Stephan A. Grupp has served as a consultant to Jazz Pharmaceuticals; Joseph H. Antin has served on an advisory board for Jazz Pharmaceuticals and for Gentium; Leslie Lehmann has no relevant financial relationships to disclose; Maja Miloslavsky and Robin Hume are employees of Jazz Pharmaceuticals, who in the course of their employment have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; Alison L. Hannah served as a consultant to Jazz Pharmaceuticals; Bijan Nejadnik is a former employee of Jazz Pharmaceuticals, who in the course of his employment had received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; Robert J. Soiffer has served on an advisory board for Gentium and Jazz Pharmaceuticals.
Author contributions
PGR, RJS, RH and ALH were responsible for the study conception and design. All authors contributed to the provision of study materials or patients. PGR, ARS, BMT, NAK, SAG, JHA, LL, MM, RH, ALH, BN and RJS were involved in the collection and assembly of data. All authors participated in the study data analysis and interpretation and manuscript writing, and provided their final approval of this manuscript.
References
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–3020. [PubMed] [Google Scholar]
- Benimetskaya L, Wu S, Voskresenskiy AM, Echart C, Zhou JF, Shin J, Iacobelli M, Richardson P, Ayyanar K, Stein CA. Angiogenesis alteration by defibrotide: implications for its mechanism of action in severe hepatic veno-occlusive disease. Blood. 2008;112:4343–4352. doi: 10.1182/blood-2008-04-149682. [DOI] [PubMed] [Google Scholar]
- Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplantation. 2011;46:1495–1502. doi: 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biology of Blood and Marrow Transplantation. 2011;17:1713–1720. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, Guinan E, Vogelsang G, Krishnan A, Giralt S, Revta C, Carreau NA, Iacobelli M, Carreras E, Ruutu T, Barbui T, Antin JH, Niederwieser D. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biology of Blood and Marrow Transplantation. 2010;16:157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D, Straham B, Gross-Wieltsch U, Sykora KW, Ridolfi-Lüthy A, Basu O, Gruhn B, Güngör T, Mihatsch W, Schulz AS. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplantation. 2004;33:189–195. doi: 10.1038/sj.bmt.1704329. [DOI] [PubMed] [Google Scholar]
- Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, Boelens JJ, Hewitt A, Schrum J, Schulz AS, Müller I, Stein J, Wynn R, Greil J, Sykora KW, Matthes-Martin S, Führer M, O’Meara A, Toporski J, Sedlacek P, Schlegel PG, Ehlert K, Fasth A, Winiarski J, Arvidson J, Mauz-Körholz C, Ozsahin H, Schrauder A, Bader P, Massaro J, D’Agostino R, Hoyle M, Iacobelli M, Debatin KM, Peters C, Dini G. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomized controlled trial. Lancet. 2012;379:1301–1309. doi: 10.1016/S0140-6736(11)61938-7. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Seminars in Liver Disease. 2002;22:27–41. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, Veys P, Potter MN; Haemato-oncology Task Force of the British Committee for Standards in Haematology; the British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. British Journal of Haematology. 2013;163:444–457. doi: 10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- Echart CL, Graziadio B, Somaini S, Ferro LI, Richardson PG, Fareed J, Iacobelli M. The fibrinolytic mechanism of defibrotide: effect of defibrotide on plasmin activity. Blood Coagulation and Fibrinolysis. 2009;20:627–634. doi: 10.1097/MBC.0b013e32832da1e3. [DOI] [PubMed] [Google Scholar]
- Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occluisve disease). Journal of Clinical and Experimental Hepatology. 2014;4:332–346. doi: 10.1016/j.jceh.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Alimentary Pharmacology and Therapeutics. 2006;23:11–25. doi: 10.1111/j.1365-2036.2006.02742.x. [DOI] [PubMed] [Google Scholar]
- Ho VT, Linden E, Revta C, Richardson PG. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: review and update on the use of defibrotide. Seminars in Thrombosis and Hemostasis. 2007;33:373–388. doi: 10.1055/s-2007-976173. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, Gökbuget N, O’Brien S, Wang K, Wang T, Paccagnella ML, Sleight B, Vandendries E, Advani AS. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. The New England Journal of Medicine. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clinic Proceedings. 2003;78:589–598. doi: 10.4065/78.5.589. [DOI] [PubMed] [Google Scholar]
- McDonald G, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Annals of Internal Medicine. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Ciceri F, Corbacioglu S, Dalle JH, Duarte RF, Fukuda T, Huynh A, Masszi T, Michallet M, Nagler A, NiChonghaile M, Pagluica T, Peters C, Petersen FB, Richardson PG, Ruutu T, Savani BN, Wallhult E, Yakoub-Agha I, Carreras E. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives—a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplantation. 2015;50:781–789. doi: 10.1038/bmt.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Ciceri F, Corbacioglu S, Dalle JH, Dignan F, Fukuda T, Huynh A, Masszi T, Michallet M, Nagler A, NiChonghaile M, Okamoto S, Pagliuca A, Peters C, Petersen FB, Richardson PG, Ruutu T, Savani BN, Wallhult E, Yakoub-Agha I, Duarte RF, Carreras E. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplantation. 2016;51:906–912. doi: 10.1038/bmt.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo M, Rovira M, Escolar G, Carreras E, Diaz-Ricart M. Protective effect of defibrotide on the prothrombotic and proinflammatory effect of autologous HSCT on endothelial cells from both macrovascular and microvascular locations. Platelets (Nottingham Platelet Conference, Platelets Annual Meeting Abstracts) 2010;21:411. [Google Scholar]
- Palomo M, Mir E, Rovira M, Escolar G, Carreras E, Diaz-Ricart M. What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance. Blood. 2016;127:1719–1727. doi: 10.1182/blood-2015-10-676114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vascular Pharmacology. 2013;59:1–10. doi: 10.1016/j.vph.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Corbacioglu S, Ho VT, Kernan NA, Lehmann L, Maguire C, Maglio M, Hoyle M, Sardella M, Giralt S, Holler E, Carreras E, Niederwieser D, Soiffer R. Drug safety evaluation of defibrotide. Expert Opinion on Drug Safety. 2013;12:123–136. doi: 10.1517/14740338.2012.749855. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Smith AR, Triplett BM, Kernan N, Grupp S, Antin J, Lehmann L, Miloslavsky M, Hume R, Hannah A, Nejadnik B, Soiffer R. Pediatric and adult subgroup results from an ongoing defibrotide expanded access program in the US for patients with hepatic veno-occlusive disease. Pediatric Blood & Cancer (SIOP Annual Meeting Abstracts) 2015a;62:S146–S147. [Google Scholar]
- Richardson PG, Krishnan A, Giralt S, Soiffer RJ. Defibrotide for the treatment of severe hepatic veno-occlusive disease/sinusoidal obstruction syndrome: evidence for clinical benefit. Expert Opinion on Orphan Drugs. 2015b;3:1491–1501. [Google Scholar]
- Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, Arai S, Grupp SA, Guinan EC, Martin PL, Steinbach G, Krishnan A, Nemecek ER, Giralt S, Rodriguez T, Duerst R, Doyle J, Antin JH, Smith A, Lehmann L, Champlin R, Gillio A, Bajwa R, D’Agostino RB, Sr, Massaro J, Warren D, Miloslavsky M, Hume RL, Iacobelli M, Nejadnik B, Hannah AL, Soiffer RJ. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–1665. doi: 10.1182/blood-2015-10-676924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirigotis PD, Resnick IB, Avni B, Grisariu S, Stepensky P, Or R, Shapira MY. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplantation. 2014;49:1389–1392. doi: 10.1038/bmt.2014.168. [DOI] [PubMed] [Google Scholar]


