Significance
N6-methyladenosine (m6A) modification has been found to constitute an important regulatory mechanism in RNA biology. Unlike mammals and yeast, no component of the m6A cellular machinery has been described in plants at present. Although the influence of the m6A cellular machinery has been suspected to occur in the plant virus cycle, it has never been proved. Here we have identified a plant protein with m6A demethylase activity (atALKBH9B) and demonstrate that this protein removes m6A modification from RNA in vitro. Remarkably, we found that m6A abundance on the viral genome of alfalfa mosaic virus is influenced by atALKBH9B activity and regulates viral infection. This study extends the vast repertoire that plants exploit to control cytoplasmic-replicating RNA viruses.
Keywords: m6A, demethylase, ALKBH9B, plant virus, coat protein
Abstract
N6-methyladenosine (m6A) is an internal, reversible nucleotide modification that constitutes an important regulatory mechanism in RNA biology. Unlike mammals and yeast, no component of the m6A cellular machinery has been described in plants at present. m6A has been identified in the genomic RNAs of diverse mammalian viruses and, additionally, viral infection was found to be modulated by the abundance of m6A in viral RNAs. Here we show that the Arabidopsis thaliana protein atALKBH9B (At2g17970) is a demethylase that removes m6A from single-stranded RNA molecules in vitro. atALKBH9B accumulates in cytoplasmic granules, which colocalize with siRNA bodies and associate with P bodies, suggesting that atALKBH9B m6A demethylase activity could be linked to mRNA silencing and/or mRNA decay processes. Moreover, we identified the presence of m6A in the genomes of two members of the Bromoviridae family, alfalfa mosaic virus (AMV) and cucumber mosaic virus (CMV). The demethylation activity of atALKBH9B affected the infectivity of AMV but not of CMV, correlating with the ability of atALKBH9B to interact (or not) with their coat proteins. Suppression of atALKBH9B increased the relative abundance of m6A in the AMV genome, impairing the systemic invasion of the plant, while not having any effect on CMV infection. Our findings suggest that, as recently found in animal viruses, m6A modification may represent a plant regulatory strategy to control cytoplasmic-replicating RNA viruses.
N6-methyladenosine (m6A) is an internal, reversible nucleotide modification present in RNAs of mammals, insects, plants, yeast, and animal viruses that participates in RNA biology through diverse mechanisms such as regulation of mRNA stability (1, 2), translation (3, 4), nuclear export (5), exon splicing (6), and protein/RNA interactions (7).
In mammals, RNA m6A methylation is catalyzed by a polyprotein complex composed of METTL3, METTL14, WTAP, KIAA1429, and several cofactors not yet identified (8–10). Removal of the m6A is catalyzed by two RNA demethylases belonging to the AlkB family of nonheme Fe(II)/α-ketoglutarate (α-KG)-dependent dioxygenases, FTO, and ALKBH5 (5, 11). In addition, several proteins (YTHDF1, YTHDF2, YTHDF3, YTHDC, eiF3, and HNRNPC) bind to the m6A-modified mRNAs to control their stability and translation (1, 2, 12, 13).
The internal m6A modification has also been found in viral RNAs (vRNAs) of animal viruses that replicate either in the nucleus or in the cytoplasm, representing a mechanism for the regulation of the viral life cycle (14–19). Silencing of METTL3/14 decreased HIV-1 replication, whereas depletion of ALKBH5 enhanced the export of vRNAs from the nucleus and protein expression, which consequently increased viral replication (15, 16). However, hepatitis C virus (HCV) and Zika virus (ZIKV) infection were positively and negatively regulated by knockdown of METTL3/14 and ALKBH5 or FTO, respectively (17, 19). Further, depletion of YTHDF proteins promoted ZIKA and HCV vRNA expression (17, 19), while in the case of HIV-1, positive (18) and negative (15) effects on HIV-1 vRNA expression have been reported. Furthermore, the host machinery that controls m6A modification detects viral infection and regulates gene expression by modulating the m6A levels of host mRNAs (16, 17).
In contrast to mammals, very few studies on the function of m6A modification have been reported in plants. Transcriptome-wide profiles in Arabidopsis thaliana detected the m6A modification in over two-thirds of the mRNAs (20). A METTL3 homolog in Arabidopsis (MTA) has been identified that plays a critical role in plant development (21, 22). In addition, the Arabidopsis FIP37 protein, a plant homolog of WTAP, interacts in vitro and in vivo with MTA and is essential to mediate m6A mRNA modification of key shoot meristem genes (21, 23). However, no demethylase or YTHFD plant activities have been described at present. The Arabidopsis genome contains 13 homologs of Escherichia coli AlkB (atALKBH1-10B) (24). Although their functional characterization has not been reported, a subcellular localization study showed that all of these proteins display a nucleocytoplasmic localization pattern except atALKBH1D, which localizes to the chloroplast as well, and atALKBH9B, which is exclusively cytoplasmic (24). Interestingly, an AlkB domain has been identified in the ORF of the replicase genes from diverse plant viruses. These domains were found to be functional in removing m1A and m3C modifications from RNA and DNA in vitro, suggesting that the replicase proteins play a role in reversing methylation modifications in the viral genomes (25).
In this work, we identified a plant protein with m6A demethylase activity (atALKBH9B) in Arabidopsis and showed that the protein interacts with the coat protein (CP) of alfalfa mosaic virus (AMV) in the cell cytoplasm. The AMV genome consists of three single-stranded RNAs of positive sense polarity. RNAs 1 and 2 encode the replicase subunits, whereas RNA 3 encodes the movement protein and serves as a template for the synthesis of nonreplicating subgenomic RNA 4 (sgRNA 4), which encodes the CP (26). The AMV CP localizes to both the nucleus/nucleous and the cytoplasm (27), although viral replication occurs in the cytoplasm, most probably associated with the tonoplast membrane (28). We found that an Arabidopsis knockout mutation of atALKBH9B negatively affects virus accumulation and systemic invasion, correlating with increased levels of m6A of the vRNAs. Our results show that the viral genome methylation state plays a key role in the life cycle of a plant virus.
Results
Arabidopsis atALKBH9B Interacts with the CP and the Viral RNA of AMV.
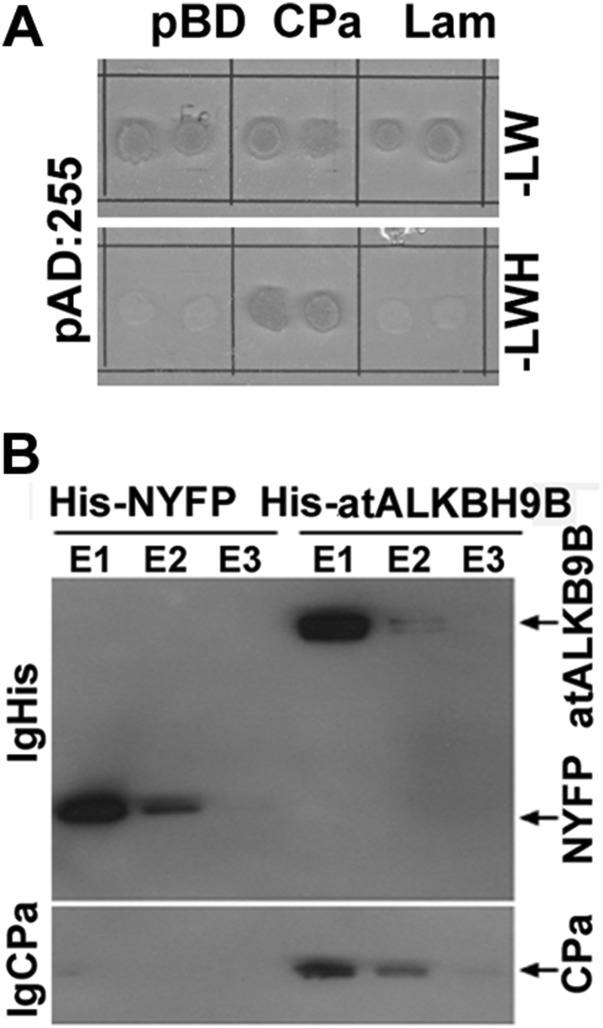
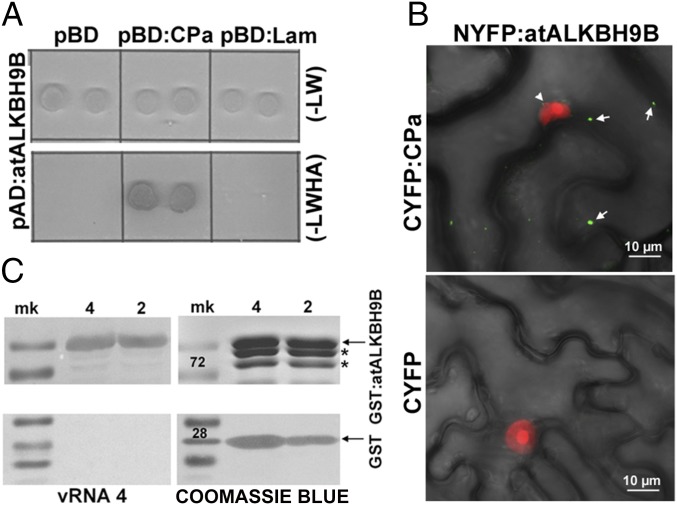
The CP of AMV is a multifunctional protein that participates in replication, translation, viral movement, and encapsidation (26, 29), which most probably implies that this protein interacts with host factors involved in diverse cellular functions (30, 31). A yeast two-hybrid (Y2H) screen, using the CP as bait and an Arabidopsis leaf-specific cDNA library as the prey, was performed to identify host proteins that interact with the AMV CP. Several clones containing part of the atALKBH9B gene (at2g17970) ORF were found to grow on interaction minimal synthetic selective medium (Fig. S1A). To validate the original Y2H screening, the full-length atALKBH9B ORF was fused to the activation domain (pAD plasmid) and transformed into yeast cells expressing the AMV CP fused to the binding domain (pBD plasmid). After growth at 28 °C for 5 d on interaction selective medium, we found that the AMV CP specifically interacted with atALKBH9B but not with the empty pBD vector or the one expressing the Gal4 binding domain fused with lamin (pBD:LAM) (Fig. 1A). To corroborate this interaction, histidine-tagged atALKBH9B (His-atALKBH9B) and the N-terminal fragment of YFP (His-NYFP) were expressed in E. coli and purified by Ni-NTA agarose chromatography. Before the elution step, Ni-NTA columns containing his-tagged proteins were incubated with purified AMV virions. Western blot analysis using anti-CP antibody of eluates after incubation with viral particles showed that virions interacted with atALKBH9B but not with NYFP (Fig. S1B). To confirm that the CP–atALKBH9B interaction occurs in planta we used bimolecular complementation (BiFC) analysis. Confocal laser scanning microscopy (CLSM) images showed that reconstituted YFP fluorescence formed discrete granules in cells of Nicotiana benthamiana infiltrated with atALKBH9B plus AMV CP but not when the host protein was coinfiltrated with the C-terminal fragment of YFP alone (CYFP) (Fig. 1B). Finally, a Northwestern blot assay demonstrated that atALKBH9B interacts with viral RNA, showing that the protein has RNA binding activity (Fig. 1C).
Fig. S1.
(A) Identification of clones interacting with the AMV CP by yeast two-hybrid screen of an Arabidopsis cDNA library. After sequencing, these clones corresponded to atALKB9B ORF. (B) Immunoblot analysis of coimmunoprecipitated products after incubation of AMV virions and the histidine-tagged proteins indicated Above the figure. Eluted (E1–E3) proteins were detected with antibodies against the His-tag (IgHis) and the CP of AMV (IgCP). The CP was only detected when virions were incubated with His-atALKBH9B.
Fig. 1.
atALKBH9B interacts with the CP of AMV in vitro and in vivo and with the viral RNA. (A) Yeast two-hybrid analysis of the interaction between AMV CP and atALKBH9B. Interacting colonies were identified by growth after 5 d on medium lacking leucine, tryptophan, histidine, and adenine (−LWHA). (B) BiFC images of epidermal cells coinfiltrated with the indicated constructs. YFP reconstitution was found to form discrete granules in the cytoplasm (arrows). Fibrillarin fused to the mCherry protein was used to identify the cell nuclei (arrowhead). Pictures are the overlapped images of green, red, and transmitted channels. (C) Analysis of the RNA binding activity of atALKBH9B by Northwestern blot assay. Duplicate membranes with purified GST:atALKBH9B (Upper) or GST proteins (Lower) (4 and 2 µg) were incubated with viral RNA 4 (Left) to show the RNA binding activity or stained with Coomassie blue (Right) to confirm the presence of the proteins. Positions of full-length GST and GST:atALKBH9B proteins are indicated by arrows. Asterisks denote truncated GST:atALKBH9B.
Arabidopsis atALKBH9B Activity Modulates AMV Infection.
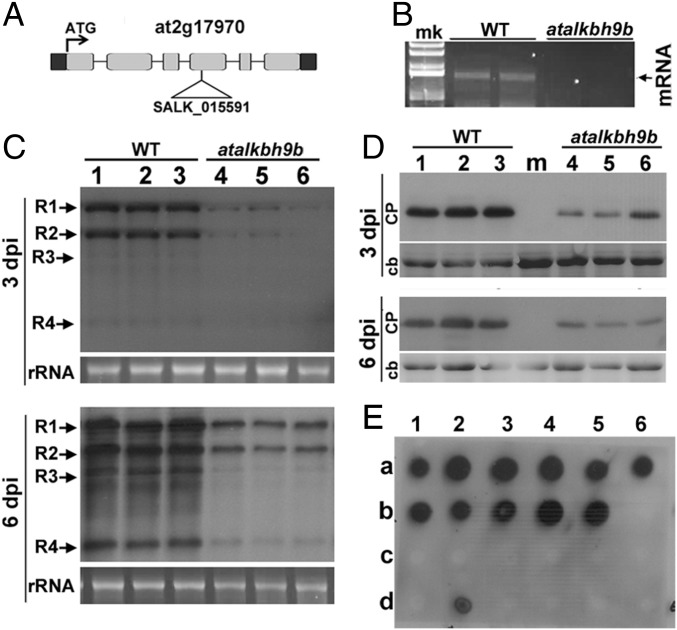
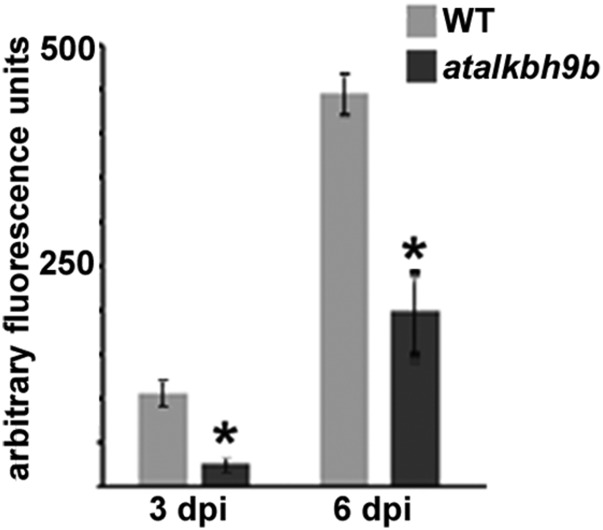
To investigate possible roles for atALKBH9B in virus infection, we searched for T-DNA insertions in atALKBH9B. We identified a homozygous T3 line in the Nottingham Arabidopsis Stock Centre (N671317; SALK_015591, ecotype Col-0) with a T-DNA insertion in exon 4 (Fig. 2A). Absence of atALKBH9B mRNA expression was confirmed by RT-PCR with gene-specific primers (Fig. 2B). To determine whether reduced levels of atALKBH9B affect virus infection, WT Col-0 and atalkbh9b plants were inoculated with AMV viral particles. Total RNA and proteins were extracted and vRNA and CP accumulation were analyzed at 3 and 6 d postinoculation (dpi) by Northern and Western blots using a digoxigenin-labeled probe to detect the vRNAs (DigAMV) and a specific anti-CP antibody, respectively. We found that levels of both vRNAs and CP were clearly reduced in atalkbh9b compared with WT plants (Fig. 2 C and D and Fig. S2), indicating that viral accumulation is impaired in atalkbh9b plants. To analyze AMV systemic movement, total RNA extracted from upper noninoculated floral stems were blotted onto nylon membranes, and vRNAs were detected with DigAMV. Fig. 2E shows that only 11% of atALKBH9B plants were systemically infected at 14 dpi, while this percentage reached 100% in WT plants. Overall, these results indicate that atALKBH9B positively regulates AMV infection.
Fig. 2.
AMV infection is impaired in atalkbh9b plants. (A) Annotated genomic atALKBH9B gene structure showing the exons (gray boxes) and location of the T-DNA insertion (SALK_015591). (B) Agarose gel electrophoresis of RT-PCR products produced with specific primers to amplify the full-length mRNA of the atalkbh9b gene from WT and atalkbh9b plants. The position of the mRNA is indicated on the Right. (C and D) Representative Northern and Western blots from inoculated leaves at 3 and 6 dpi of three WT and atalkbh9b plants. Positions of the vRNAs and CP are indicated on the Left. Ethidium bromide and Coomassie blue staining of ribosomal RNAs and total protein extracts (rRNA and cb, respectively) were used as RNA and protein loading controls. (E) Dot-blot hybridization of upper noninoculated floral stems to determine the extent of viral systemic movement. Dots in rows a and b correspond to WT plants; dots in c and d correspond to atalkbh9b plants. Samples b6 and d6 are healthy WT and atalkbh9b plants used as negative controls.
Fig. S2.
Graphic showing the average of vRNAs accumulation in WT and atalkbh9b mutant. SD values are shown. Asterisks indicate significant differences from the WT (*P < 0.05) using the t test (n = 3).
atALKBH9B Localizes to Cytoplasmic Bodies.
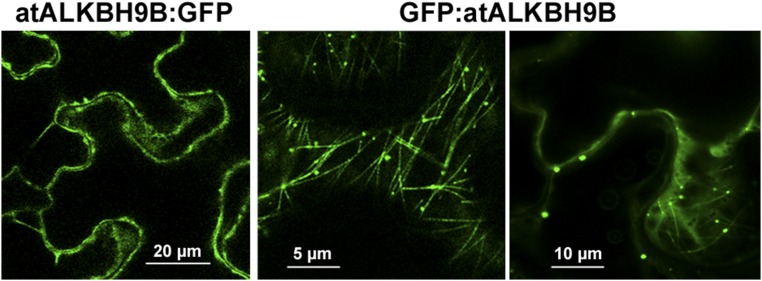
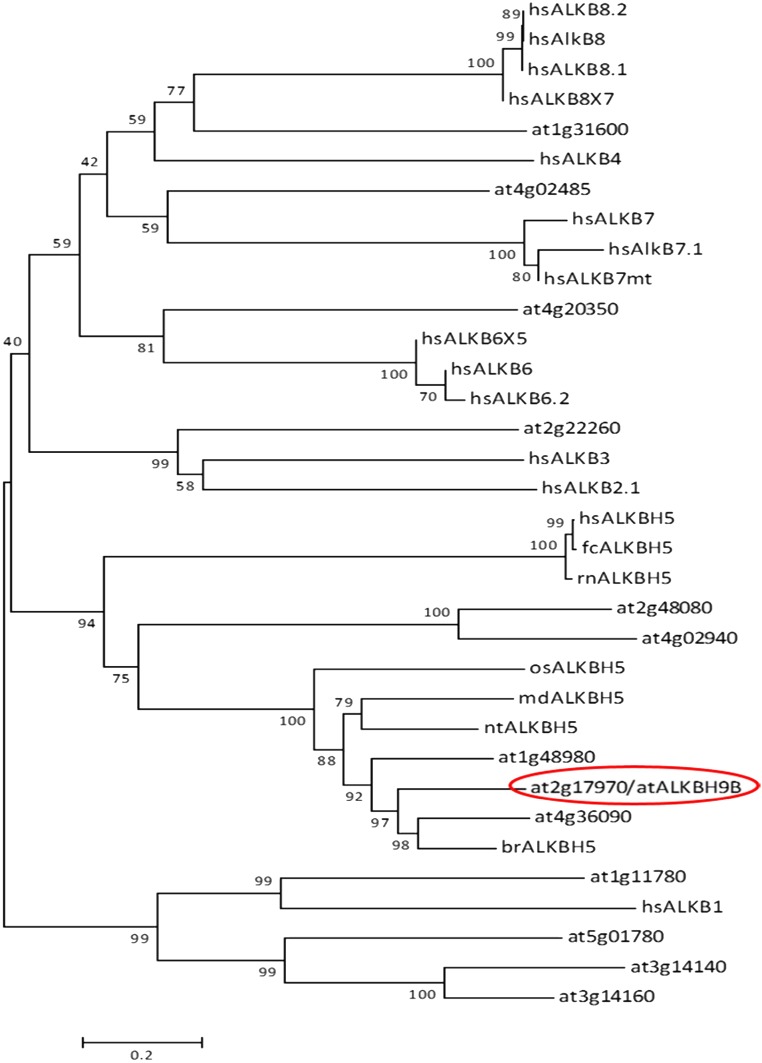
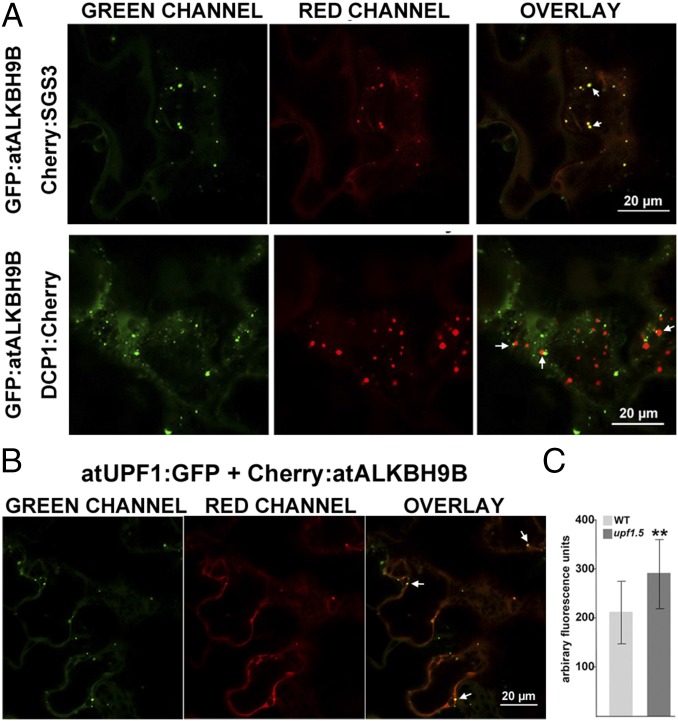
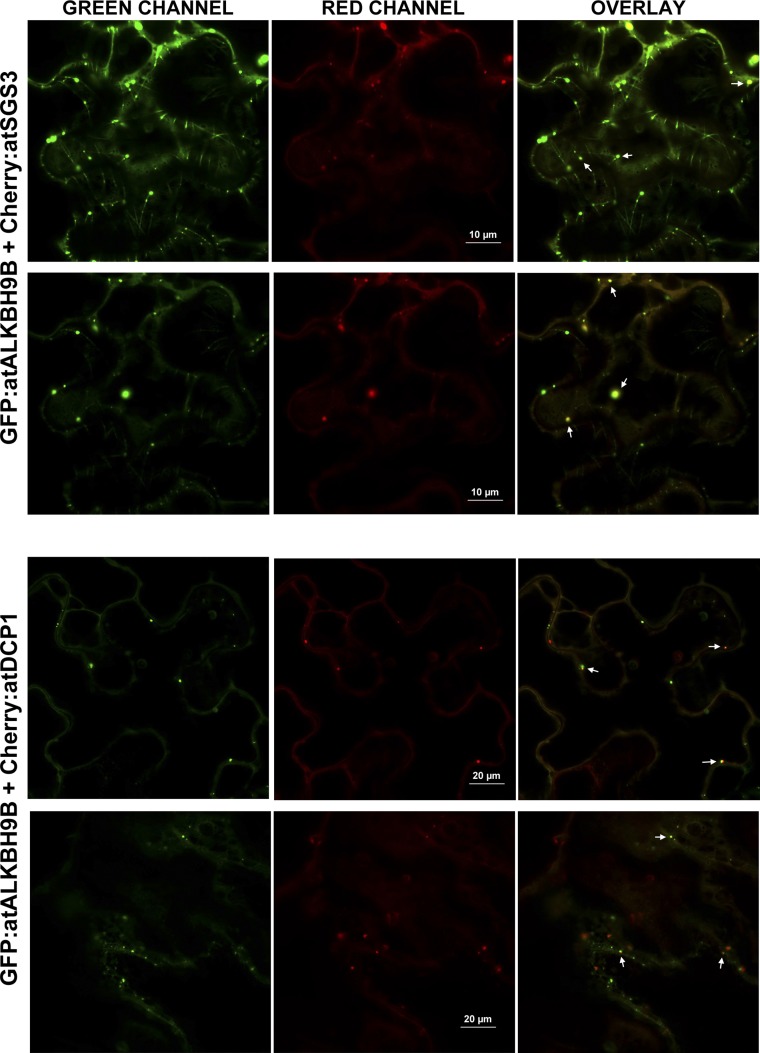
atALKBH9B is one of the 13 homologs of E. coli AlkB, and it is the only one that is uniquely located in the cytoplasm (24). To determine more precisely the atALKBH9B subcellular localization, we transiently expressed translational fusions with GFP (GFP:atALKBH9B and atALKBH9B:GFP) by agroinfiltration in N. benthamiana leaves. CLSM images showed that atALKBH9B:GFP was localized as a diffuse pattern throughout the cytoplasm, while GFP:atALKBH9B was accumulated in small cytoplasmic granules and filaments (Fig. S3). We next performed a sequence similarity analysis, which showed that five Arabidopsis AlkB homologs, including atALKBH9B, grouped in the same branch with human ALKBH5 (Fig. S4). Hence, these proteins might be orthologous to the human protein and other prospective m6A demethylases. It has been proposed that m6A methylation functions in the cytoplasm, serving as a reversible tag to direct mRNAs to processing bodies (P bodies) (12). Moreover, several studies have demonstrated that P bodies dynamically associate with siRNA bodies, and the latter are implicated in posttranscriptional gene silencing (PTGS) through the synthesis of dsRNAs to generate siRNAs (32, 33). We reasoned that atALKBH9B-forming granules might be related to siRNA and/or P bodies. To test this hypothesis, we performed colocalization experiments in mock and AMV-infected N. benthamiana leaves by coexpressing atALKBH9B with DCP1 (a decapping enzyme located in P bodies) and SGS3 (a component of siRNA bodies) (32, 34) fused to fluorescent proteins. CLSM images showed that, in both healthy and infected tissues, atALKBH9B granules perfectly colocalized with siRNA bodies, whereas ∼40% of the atALKBH9B granules were spatially associated with P bodies (Fig. 3A and Fig. S5). These results suggest that atALKBH9B might be a new component of siRNA bodies and P bodies.
Fig. S3.
Confocal laser microscope scanning images of N. benthamiana cells agroinfiltrated with the GFP fused to the N- and C-terminal ends of atALKBH9B.
Fig. S4.
Rootless phylogenetic tree derived from the Clustal Omega analysis between proteins of the AlkB family belonging to different species. Basic local alignment search tool (BLAST) web tool (National Center for Biotechnology Information) was used to compare the amino acid sequence of the protein atALKBH9B against a large number of sequences available in the databases. A total of 34 protein sequences were selected with which a multiple alignment was then performed by Clustal Omega (51), available from the European Molecular Biology Laboratory–European Bioinformatics Institute website. Finally, using this alignment a rootless phylogenetic tree was constructed in the MEGA v.6.0 program (52). The minimum evolution method was selected as the statistical method, and the bootstrap (53) analysis, with 10,000 replicates, was tested as a phylogeny test. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were calculated using the Poisson correction method and they are found in units of number of amino acid substitutions per site. The minimal evolution tree was recorded using the close neighbor interchange (CNI) algorithm at a search level of 1. The neighbor-joining algorithm was used to generate the initial tree. All ambiguous positions were removed for each pair of sequences. A pairwise deletion method was selected for the treatment of gaps.atALKBH9B protein (at2g17970), circled in red, enclosed in a small cluster that encompasses its homologous proteins from other vegetables and this, in turn, in a larger group including the ALKBH5 protein, already described and studied in mammals. The bootstrap values are indicated on each node. At, Arabidopsis thaliana; br, Brassica rapa; fc, Felis catus; hs, Homo sapiens; md, Malus domestica; nt, Nicotiana tomentosiformis; oz, Oryza sativa; and rn, Rattus norvegicus.
Fig. 3.
The atALKBH9B protein colocalizes with siRNA-body/P-body components in noninfected tissues. (A and B) Confocal laser scanning microscope (CLSM) images of N. benthamiana leaf epidermal cells coinfiltrated with the DNA constructs indicated Above the images. Overlay panels are the superposition of images from the green and red channels. Arrowheads indicate granules with both proteins. (C) vRNAs accumulation in upf1.5 mutant with respect to WT. SD values are shown. Asterisks indicate significant differences from the WT (**P < 0.01) using the t test (n = 20).
Fig. S5.
The atALKBH9B protein colocalizes with siRNA-body/P-body components in infected tissues. N. benthamiana leaves were inoculated with AMV viral particles and 24 h postinfection were agroinfiltrated with the indicated coinfiltrated DNA constructs indicated Above the images. Confocal laser scanning microscope images were taken 48 h postinfiltration. Overlay panels are the superposition of images from the green and red channels. Arrowheads indicate granules with both proteins.
Recently, the nonsense-mediated mRNA decay system (NMD) has been proposed to work as viral restriction mechanism in plants (35). The regulator of nonsense transcripts 1 (UPF1) is a critical component of this RNA quality control system and is found to be linked to P bodies (36). To shed light on which viral functions might be modulated by m6A modification, we have analyzed the impact of the NMD in AMV infection. Subcellular localization of UPF1 by CLMS showed that the construct atUPF1:GFP also formed cytoplasmic discrete granules colocalizing with GFP:atALKBH9B (Fig. 3B). Further, the analysis of vRNAs accumulation revealed a significant enhancement in systemic leaves of Arabidopsis upf1.5 mutant plants (at5g47010; SALK_112922) (Fig. 3C). Altogether, our results suggest that atALKBH9B might be a new component of siRNA bodies and P bodies and, as found in other plant viruses (35), NMD would restrict AMV infection.
atALKBH9B Catalyzes m6A Demethylation of RNA in Vitro.
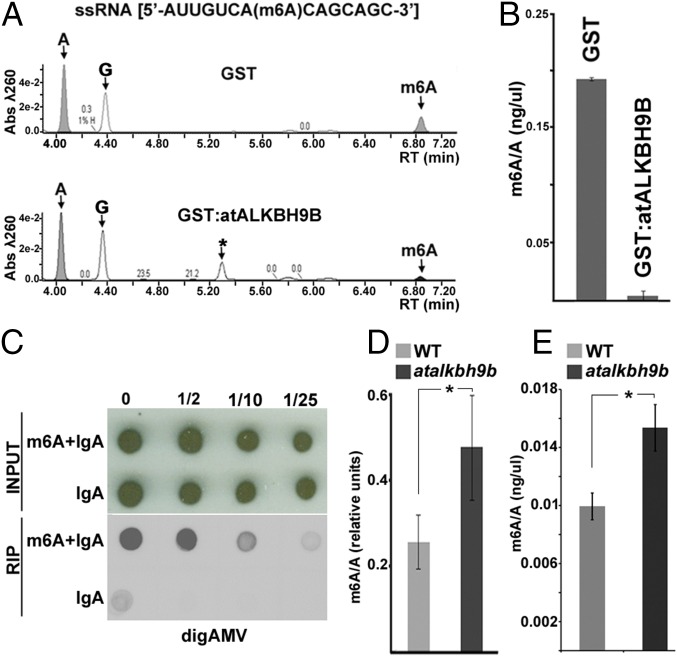
To assay the m6A demethylation activity of atALKBH9B, the protein was fused to the glutathione S-transferase (GST) protein (GST:atALKBH9B) and purified from E. coli using the GST purification system. GST:atALKBH9B or GST alone was incubated with a synthetic single-stranded RNA oligonucleotide (ssRNA) with a single specifically incorporated m6A, followed by digestion to nucleosides and ultra-performance liquid chromatography–photodiode detector–quadrupole/time-of-flight–mass spectrometry (UPLC-PDA-TOF-MS) analysis. We found that GST:atALKBH9B almost completely demethylates m6A in the ssRNA substrate (Fig. 4 A and B). Therefore, atALKBH9B is a protein described in plants with ssRNA m6A demethylase activity.
Fig. 4.
atALKBH9B catalyzes demethylation of m6A in ssRNA in vitro and modulates methylation of vRNAs. (A) Representative UPLC-PDA-Q/TOF-MS chromatogram showing the retention times of the nucleosides adenosine (A) and N6-methyladenosine (m6A) after incubation of the m6A-containing ssRNA substrate with GST:atALKBH9B and GST as the negative control. The peak (G) corresponds to the nucleoside guanosine. The peak denoted as (*) could not be unequivocally identified although it was determined to present a molecular weight of 343 with a maximum absorption at 261 nm. (B) Graph representing the demethylation activity in three independent experiments. (C–E) Genomic AMV RNAs are m6A hypermethylated in atalkbh9b plants. (C) RIP of vRNAs with a specific anti-m6A antibody. Total RNA extracted from WT plants infected with AMV was incubated with anti-m6A plus IgA or IgA alone. Dilutions of the immunoprecipitated RNAs (indicated on Top) were blotted on nylon membranes and the vRNAs were detected with DigAMV. (D) Average ratios of m6A in vRNAs obtained by quantification of m6A on three different Northwestern blots from AMV-infected WT and atalkbh9b plants. (E) Graphic showing the average m6A/A ratios obtained by UPLC-Q-Tof-MS after digestion of vRNAs extracted from virions purified from AMV-infected WT and atalkbh9b plants. In B, D, and E error bars represent the SEM and asterisks indicate significant differences from the WT (*P < 0.05) using the t test (n = 3).
m6A Abundance in AMV vRNAs Correlates with Viral Fitness.
Given that atALKBH9B has been shown to possess m6A demethylase activity and that its depletion influences AMV infection, we investigated the presence of the m6A modification in AMV vRNAs. Total RNA was purified from Arabidopsis WT AMV-infected plants, and an RNA immunoprecipitation assay (RIP) using the anti-m6A antibody and immunoglobulin-A was performed to immunoprecipitate the m6A-modified RNAs. Subsequent hybridization of RIP products with the DigAMV probe clearly detected the presence of the AMV vRNAs (Fig. 4 C and D). These results demonstrate that in Arabidopsis, adenosine residues in the AMV genome become modified to m6A during the infection process. We next determined whether atALKBH9B depletion in atalkbh9b plants modulates m6A levels in the AMV genome. In this case, vRNAs extracted from AMV particles isolated from WT or atalkbh9b plants were electrophoresed in agarose gels, transferred to nylon membranes, and immunoblotted using the m6A antibody (Fig. S6). In parallel, vRNAs were digested to single nucleosides and the m6A abundance was quantified by UPLC-PDA-Tof-MS. The m6A/adenosine ratio (m6A/A) was reduced ∼35% in WT compared with atalkbh9b plants (Fig. 4E), indicating that depletion of atALKBH9B correlates with hypermethylation of the AMV vRNAs.
Fig. S6.
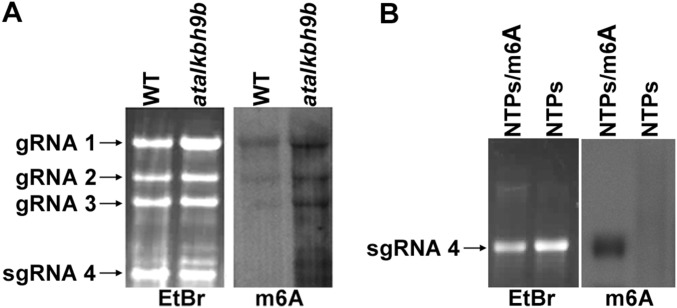
m6A antibody recognizes AMV vRNAs by Northwestern blot. (A) Representative nylon membrane stained with ethidium bromide (EtBr) and Northwestern blot with m6A antibody (m6A) of vRNAs extracted of virions purified from AMV-infected WT and alkbh9b plants. Positions of the genomic AMV RNAs are indicated on the Right of EtBr. (B) In vitro transcripts of vRNA 4 obtained with NTPs (non-methylated) or NTPs+m6A (methylated) were used as negative and positive Northwestern blot controls, respectively.
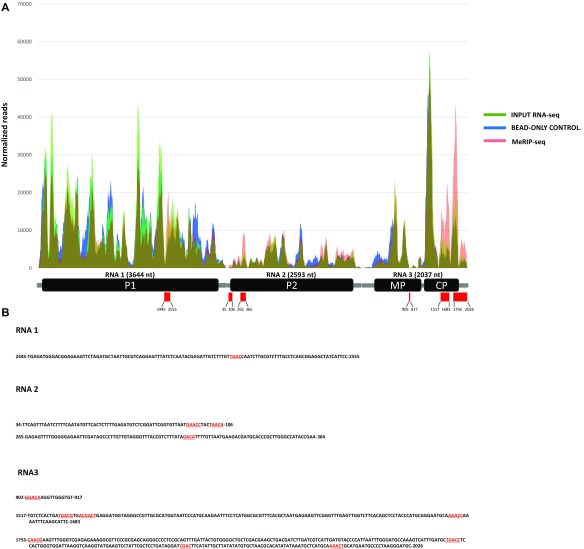
Finally, we performed a methylated RNA immunoprecipitation sequencing (MeRIP-seq) experiment to map m6A sites within the AMV genome. For this, vRNAs extracted from viral particles isolated from atalkbh9b AMV-infected plants were immunoprecipitated with an m6A-specific antibody and RNAs from input, bead-only control and MeRIP-seq samples were used to generate RNA sequence libraries. We identified six discrete peaks distributed along the AMV genome, which contained three of the common m6A consensus motifs [(G,A,U)/(G,A)/A/C/(A,C,U)], [(A,C)/G/A/C/(G,U)] and [UGAC] (Fig. S7).
Fig. S7.
Map of m6A sites within AMV genomic RNAs by MeRIP-seq. (A) Read coverage of the three RNAs on input RNA-seq (green), bead-only control (blue), and MeRIP-seq (pink). Reads were normalized to the total number of reads mapping to the viral genome. Below, a schematic diagram of the AMV genome is presented. Red rectangles indicate the six m6A peaks identified and numbers show their nucleotide positions in each vRNA. (B) Nucleotide sequence of the six m6A peaks identified, showing in red the [(G,A,U)/(G,A)/A/C/(A,C,U)], [(A,C)/G/A/C/(G,U)], and [UGAC] consensus motifs.
m6A Is Present in the Genomic RNAs of Other Members of the Bromoviridae Family.
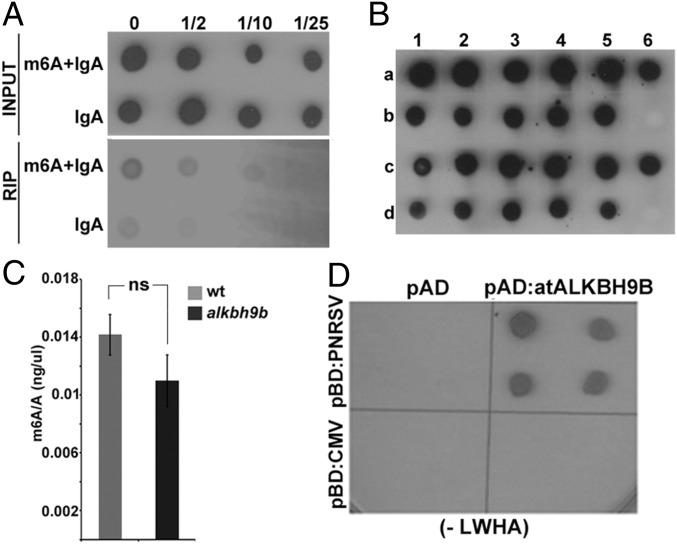
Since m6A appears to regulate AMV infection, we asked whether other viruses in the Bromiviridae family might also be influenced by this modification. We chose cucumber mosaic virus (CMV), the type member of the genus Cucumovirus, which infects Arabidopsis and is not closely related to AMV. First, the presence of m6A was examined in CMV vRNAs by RIP from total RNA of WT CMV-infected plants using the m6A antibody. Hybridization of RIP products with the DigCMV probe showed that the CMV genome contains m6A as well (Fig. 5A). We then evaluated whether, similar to AMV, atALKBH9B activity might influence viral infectivity and/or m6A modification in CMV vRNAs. To address this, total RNA was isolated from upper floral stems to look for systemic viral movement, and blot hybridization with the DigCMV probe showed that 100% of atALKBH9B plants were systemically infected by the virus (Fig. 5B). We also extracted vRNAs from viral particles isolated from WT and atalkbh9b plants infected with CMV. After digestion to single nucleosides, UPLC-PDA-Tof-MS showed no significant differences in the m6A/A ratios in purified vRNAs from WT compared with atALKBH9B plants (Fig. 5C). Therefore, the CMV genome contains m6A, but the methylation levels of vRNAs and viral infection are not regulated by atALKBH9B. We hypothesized that our findings might correlate with a lack of interaction between atALKBH9B and the CMV CP; hence, this interaction was investigated by Y2H analysis. Growth on interaction selective medium of yeast cells coexpressing both proteins showed that the viral protein fails to interact with atALKBH9B in vivo (Fig. 5D). Interestingly, we found that atALKBH9B interacts with the CP of Prunus necrotic ringspot virus (PNRSV) (Fig. 5D), a virus that is functionally and phylogenetically closely related to AMV (37, 38). Unfortunately, since Arabidopsis is not a host for PNRSV, we could not assay the virus infectivity in atalkbh9b plants. This finding suggests that atALKBH9B may regulate the viral life cycle of other Bromoviridae family members.
Fig. 5.
CMV genomic RNAs contain the m6A modification. (A) Detection of the m6A modification in CMV by RIP of viral RNAs with a specific anti-m6A antibody plus IgA or IgA alone. Dilutions of the immunoprecipitated RNAs (indicated on Top) were blotted on nylon membranes and vRNAs were detected with DigCMV. (B) Graphic showing the average m6A/A ratios obtained by UPLC-Q-Tof-MS after digestion of vRNAs extracted from virions purified from CMV-infected WT and atalkbh9b plants. Error bars represent SEM; ns, no significant differences (P > 0.05) from the WT using the t test (n = 3). (C) CMV systemic infection is not affected in atalkbh9b plants. Dot-blot hybridization of floral stems to analyze systemic viral movement. Samples b6 and d6 are the negative controls. Dots in rows a and b correspond to WT plants; dots in c and d correspond to atalkbh9b plants. (D) Yeast two-hybrid analysis of the interaction between CPs of CMV and PNRSV with atALKBH9B. Interacting colonies were identified by growth after 5 d on medium lacking leucine, tryptophan, histidine, and adenine (−LWHA). ns, not significant.
Discussion
In the last few years, m6A modification has emerged as an important mechanism to regulate mRNA biology (1, 2, 4–7). Components of this regulatory system (methyltransferases, demethylases, and protein effectors) have been identified in mammals and yeast (12). Several recent studies have shown that m6A modification is also a conserved feature of mRNA in plants (20, 39), and that it plays a critical regulatory role in plant development (21–23). However, as far as we know, only two components of the methylase complex, MTA and FIP37, have been identified at present (21, 23). In this work, using a biochemical test, we demonstrate that atALKBH9B possesses m6A demethylase activity toward single-stranded RNA in vitro, and similar to the results of a previous study, we found that this protein localizes exclusively to the cytoplasm (24). Several studies have previously reported the presence of the cellular machinery controlling m6A modification in the cytoplasm of mammalian cells (17, 19,). Thus, atALKBH9B may be a new component of the cellular machinery controlling N6 methylation of adenosine in plant mRNAs, and similar to the situation in mammals, m6A modification could take place in the cytoplasm after the export of mRNAs from the nucleus.
A detailed examination showed that atALKBH9B forms discrete granules either in healthy or infected tissues that colocalize with SGS3 and UPF1, and some of these granules presented a spatial association with DCP1. SGS3 and DPC1 are components of siRNA bodies and P bodies, respectively (33, 34), whereas that UPF1 is transported from the cytoplasm to P bodies by SMG7 (36). In the cytoplasm, binding of different proteins to m6A mediates host mRNA destination. For instance, binding of YTHDF1 or eIF3 has been found to favor mRNA translation (3, 4), whereas the binding of HNRNPC regulates mRNA degradation (7), and YTHDF2 directs m6A-marked mRNAs to P bodies for RNA decay (4). RNA turnover in P bodies and PTGS in siRNA bodies are conserved eukaryotic mechanisms to regulate mRNA integrity that have been found to be functionally and spatially associated (33, 40). Our findings suggest that atALKBH9B m6A activity might be linked to mRNA silencing and the mRNA decay processes. In this sense, we found that NMD, a surveillance system linked to P bodies proposed to work as viral restriction mechanism in plants, could act on AMV infection, since UPF1 depletion increased viral accumulation (35).
Recent studies have shown that the m6A machinery modifies the viral RNA genomes of several animal viruses belonging to the Flaviviridae family, indicating that m6A modification is a conserved mark that regulates viral infection (17, 19). In this paper, we report that adenosines in the genomes of AMV and CMV are also modified to m6A, suggesting that this feature is a conserved phenomenon in viruses that replicate in the cytoplasm of both mammalian and plant cells. In the case of AMV, we showed that AMV accumulation was reduced in inoculated leaves of atalkbh9b plants, and systemic infection of the plant was also severely impaired. Moreover, AMV genomic RNAs presented higher m6A levels in these mutant plants, providing evidence that m6A modification negatively affects viral infection. These findings are in agreement with those recently reported for HCV and ZIKV, in which viral infections are regulated by m6A modification of their genomic RNAs. Specifically, knockdown of ALKBH5 and FTO increased m6A abundance in the ZIKV genome, negatively affecting the viral titer (17), while depletion of FTO but not ALKBH5 decreased the production of infectious virus (19). Thus, the host RNA methyltransferase machinery may represent an additional host regulatory mechanism to counter infection by some plant viruses.
Gokhale et al. (19) proposed that regulation of m6A abundance in genomic RNAs of HCV would allow the virus to replicate at low rates, evading the host immune system and enabling the establishment of persistent infections. In plants, a conserved AlkB domain in the genomes of several single-stranded RNA plant viruses belonging to the Flexiviridae family has been identified (25, 41). Furthermore, a functional characterization analysis showed that these viral AlkB domains repaired deleterious RNA genome methylation damage, suggesting that this domain may be biologically relevant in preserving the viability of the viral genome (25). Interestingly, most of the viruses containing this AlkB domain infect woody or perennial plants, where they have to establish infections that persist for years (25, 42). In the case of AMV, a similar scenario could occur, since alfalfa (Medicago sativa) plants, its natural host, are generally maintained for a minimum of 5 y before the crop is replanted (42). However, unlike viruses in the Flexiviridae family, the AMV genome lacks the Alkb domain, so the virus may have the ability to usurp this host function for its long-term accumulation.
We found that the CMV genome also contains m6A, but differs from AMV in that neither m6A vRNA abundance nor virus infection was modified in atalkbh9b plants compared with WT plants. Remarkably, the CMV CP did not interact with atALKBH9B in vivo. An important difference between AMV and CMV is that, whereas the latter can replicate in the absence of its CP (43), the CP of AMV is a multifunctional protein that interacts with a variety of host factors and is indispensable for replication and translation (26–29). Thus, it may be possible that the interaction between atALKBH9B and the CP is essential to usurp atALKBH9B activity.
But on the other hand, the Arabidopsis genome encodes five putative demethylase orthologs, so a protein different from atALKBH9B could very well participate in the CMV m6A regulation process. In fact, it has been reported that depletion of FTO negatively affects HCV infection, while depletion of ALKBH5 has no effect on the HCV cycle (19). Finally, we cannot rule out the possibility that m6A modification does not influence CMV viral infection per se.
Materials and Methods
Analysis of GST:atALKBH9B-Viral RNA Interaction by Northwestern Assay.
Dilutions of GST or GST:ALKBH9B purified proteins were electrophoresed in 12% SDS/PAGE and transferred to PDVF membranes. Membranes were incubated overnight at 4 °C (10 mM Tris⋅HCl pH 7.5, 1 mM EDTA, 0.1 M NaCl, 0.0005% Triton X). After two washes of 5 min each with the same buffer, membranes were incubated with 20 mL of buffer B (10 mM Tris⋅HCl pH 7.5, 1 mM EDTA, 0.1 M NaCl) containing 50 ng/μL of the AMV RNA 4 labeled with digoxigenin for 2 h at 25 °C. Then digoxigenin detection procedures were carried out as detailed in SI Materials and Methods.
BiFC and Subcellular Localization Study.
atALKBH9B and atUPF1 ORFs were amplified with specific primers designed for cloning using the Gateway System (Invitrogen) and recombined into binary destination vectors expressing the fluorescent proteins mCherry or GFP for subcellular localization studies and the N-terminal part of the YFP for BiFC analysis, following manufacturer recommendations (GFP:atALKBH9B, atALKBH9B:GFP; NYFP:atALKBH9B, atUPF1:GFP). For details, see SI Materials and Methods.
Virus Isolation and Viral Genomic RNA Purification and Rip Using m6A Antibody.
Virus purification was performed following PEG protocol with some modifications (SI Materials and Methods). For vRNAs purification, pellets were directly resuspended in 1 mL of RiboZol reagent (Amresco) and RNA extraction was carried out following manufacturer recommendations.
RNAs with m6A modification were immunoprecipitated as previously reported with some modifications as detailed in SI Materials and Methods.
Purification of atALKBH9B Protein.
atALKBH9B was subcloned into pGEX-KG (GE Healthcare Life Sciences) to generate a construct with atALKBH9B fused to the C-terminal part of the GST. GST and GST:atALKBH9B proteins were expressed in BL21 (DE3) E. coli cells and purified with glutathione sepharose 4B beads (GE Healthcare Life Sciences) following manufacturer recommendations. All of the protein purification procedures were performed at 4 °C.
In Vitro m6A Demethylation Assays.
The m6A demethylase activity assay was performed by incubating 2.5 µg of GST or GST:ALKBH9B proteins and 1 µg of m6A monomethylated ssRNA (Dharmacon, Inc.) oligonucleotide for 3 h at 25 °C in a reaction mixture containing 50 mM of Hepes buffer (pH 7.0), 10 μM α-ketoglutarate, 100 μM l-ascorbic acid ascorbate, 20 μM (NH4)2Fe(SO4)2·6H2O. Finally, reactions were quenched by heating at 95 °C for 10 min. UPLC-PDA-Tof-MS analysis of vRNAs and ssRNA oligonucleotide demethylation were performed as described in SI Materials and Methods.
MeRip-Seq.
Immunoprecipitation of m6A RNA fragments was performed using 200 µg of purified vRNAs as previously described (44). For details, see SI Materials and Methods.
SI Materials and Methods
Y2H Screening.
Yeast two-hybrid (Y2H) screening performance of Arabidopsis proteins interacting with AMV CP has been previously described (45).
BiFC and Subcellular Localization Study.
GFP:atALKBH9B, atALKBH9B:GFP; NYFP:atALKBH9B, atUPF1:GFP fusion proteins were cloned using the Gateway System (Invitrogen) following the manufacturer’s recommendations. Plasmid expressing the AMV CP fused to the C-terminal part of the YFP (CYFP:CP) has been previously described (46). All binary vectors were transformed into Agrobacterium tumefaciens C58 cells. Cultures were diluted at 0.2 OD600 in infiltration solution (10 mM Mes pH 5.5, 10 mM MgCl2) and infiltrated into 3-wk-old N. benthamiana plants. Confocal images were taken at 48 h after agroinfiltration with a Zeiss LSM 780 AxiObserver microscope. All images correspond to single slices of 1.8-μm thickness of epidermal cells. Excitation and emission wavelengths were 488 and 508 nm for GFP, 514 and 527 nm for YFP, and 545 and 572 nm for mCherry, respectively.
Analysis of atALKBH9B-AMV Viral Particle Interaction.
Full-length atALKBH9B ORF and the N-terminal part of the yellow fluorescent protein (amino acids 1–160) were cloned in plasmid pET28a (Novagen) fused in frame with the histidine tag to generate pET28a/His-atALKBH9B and pET28a/His-NYFP. BL21(DE3) E. coli cells transformed with the plasmids were grown at 37 °C and induced with 1 mM IPTG for 3 h. Cells were resuspended in buffer A (10 mM Tris pH 7.5; 150 mM NaCl; 0.5% Triton X-100; 20 mM imidazol). Soluble fractions were incubated with Ni-NTA column (Qiagen) for 1 h at 4 °C. After four washes with buffer A, Ni-NTA columns containing His-atALKBH9B or His-NYFP proteins were incubated with purified AMV virions for 1 h at 4 °C. After three washes with buffer A, protein complexes bound to Ni-NTA columns were eluted with buffer B (10 mM Tris pH 7.5; 150 mM NaCl; 0.5% Triton X-100; 250 mM imidazol). Purified complexes were separated in SDS/PAGE gels and transferred to PDVF membranes in duplicate (Amersham). Western blot analysis using anti-AMV CP (Loewe) or anti-His (Roche) antibodies were conducted following the manufacturer’s recommendations.
Plant Growth Conditions, Virus Inoculation, and Northern Blots.
N. benthamiana and Arabidopsis ecotype Col-0 WT, upf1.5 (N9902, SALK_112922), and atalkbh9b (N671317, SALK_015591) plants were grown in 6-cm diameter pots in a growth chamber at 24 °C with a photoperiod of 24 °C-16 h light/20 °C-8 h dark. Plants were mechanically inoculated with purified virions (1 mg/mL) of AMV PV0196 isolate (Plant Virus Collection, DSMZ) in 30 mM sodium phosphate buffer pH 7. For CMV infections, N. benthamiana leaves infected with CMV-Fny were grounded in 30 mM sodium phosphate buffer pH 7 (wt/vol 1:1) and sap extracts were used for mechanical inoculations. Detection of viral RNAs was carried out by Northern or direct-dot blot analysis. Inoculated leaves and upper systemic floral stems were harvested at 3, 6, and 15 dpi, respectively. Tissues were grounded in liquid nitrogen with mortar and pestle and total RNA was extracted from 0.1 g leaf material using RiboZol reagent protocol (Amresco). A total of 1 µg of total RNA was denatured by formaldehyde treatment and analyzed by Northern blot hybridization. Otherwise, for direct-dot blot analysis, 1 µg of total RNA was directly blotted onto nylon membranes. Viral RNAs were visualized on blots using digoxigenin-labeled riboprobes to detect the four vRNAs of AMV or the vRNA 3 and sgRNA 4 of CMV. Synthesis of the digoxigenin-labeled riboprobes, hybridization, and digoxigenin-detection procedures were carried out as previously described (47).
Viral Particle Purification.
Viral particles were purified from inoculated leaves collected at 9 dpi. Leaves were macerated with mortar and pestle in two volumes (wt/vol) of isolation buffer (100 mM K2HPO4; 100 mM ascorbic acid; 20 mM EDTA; pH 7.1). The macerate was filtered through miracloth and mixed with 1/2 volume of butanol-chloroform (1:1). The emulsion was centrifuged at 8,000 × g for 15 min and 1/5 volume of polyethylenglycol (MW 20000) was added to the aqueous phase. After a 15-min incubation in ice, the solution was centrifuged at 8,000 × g for 15 min and supernatants were discarded. Pellets were resuspended with phosphate buffer (10 mM sodium phosphate pH 7, 1 mM EDTA) and Triton X-100 was added at a final concentration of 0.5%. Tubes were incubated for 30 min in ice and centrifuged at 8,000 × g for 5 min. Supernatants were transferred to fresh tubes and centrifuged 76,000 × g for 3 h. For vRNAs purification, pellets were directly resuspended in 1 mL of RiboZol reagent (Amresco) and RNA extraction was carried out following manufacturer recommendations.
m6A RIP of viral genomic RNAs.
Immunoprecipitation of m6A viral RNA was performed as previously described (44). Purified total RNAs (250 µg) from WT or atalkbh9b plants were incubated in a final volume of 500 µL with 1× IP buffer [50 mM Tris⋅HCl, 150 mM NaCl, 0.5% (vol/vol) Nonidet P-40], 200 U RNasin and 12.5 µg of specific m6A antibody (Synaptic Systems) in rotation for 2 h at 4 °C. Then, 200 μL of recombinant protein A beads in 1× IP buffer were added and additionally incubated in rotation for 2 h at 4 °C. After this, beads were washed four times with 1× IP buffer supplement with RNasin and incubated for 1 hr with vigorous shaking in elution competition buffer [50 mM Tris⋅HCl, 150 mM NaCl, 0.5% (vol/vol) Nonidet P-40, 200 U RNasin and 6.7 mM m6A] to elute m6A containing RNAs. As negative controls the same reactions without specific m6A antibody were performed. Eluted RNAs and serial dilutions were directly blotted onto nylon membranes and hybridized with DigAMV or DigCMV probes as described above.
UPLC-PDA-Tof-MS Analysis.
For UPLC-PDA-Tof-MS analysis, 10 μg of vRNAs or 1 μg of ssRNA oligonucleotide were digested by incubating for 2 h at 37 °C in 50 μL (final volume) reaction mixture containing 1× buffer C (25 mM Tris pH 8, 2 mM MgCl2, 1 mg/mL; BSA), 10 U Benzonase, 0.002 U phosphodiesterase I and 1.5 U alkaline phosphatase. Then, 5 μL of digested nucleosides was injected into a Micromass Q-TOF spectrometer coupled to an Acquity UPLC-PDA system (Waters) via an electrospray ionization (ESI) interface. Separation was performed on a Waters Acquity BEH C18 column (150 × 2.1 mm i.d., 1.7 µm). During sample running, the mobile phase consisted of 0.1% formic acid in water (phase A), and 0.1% formic acid in acetonitrile (phase B). The solvent gradient program is conditioned as follows: 100–90% solvent A over the first 20 min, 90–0% solvent A over 10 min, return to the initial 100% A in 5 min, and conditioning at 100% A. The flow rate was 0.4 mL/min and the column and sample temperatures were kept at 40 °C. UV spectra were acquired between 210 and 800 nm with a 1.2-nm resolution and 20 points s−1 sampling rate. The ESI source was operated in positive ionization mode with the capillary and cone voltages at 2.7 kV and 30 V, respectively. The temperature of the source and desolvation was set at 120 °C and 300 °C, respectively. The cone and desolvation gas (nitrogen) flows were 500 l h−1 and 50 l h−1, respectively. The collision energy was set at 5 eV. ESI data acquisition was collected in centroid mode in a full scan range from mass-to-charge ratio [m/z] 50–1,500 at 0.2 s per scan. The mass spectrometer was calibrated using a sodium formate mixture from 200 to 1,500 MW (resolution specification 5,000 FWHM, deviation <5 ppm RMS in the presence of a known lock mass). Leu-enkephalin was used as the lockmass using a LockSpray exact mass ionization source. All data were acquired using Masslynk NT4.1 software (Waters Corp.). The nucleosides were quantified using either the SIR monitoring of 282.1 (m6adenosine) and 268.1 (adenosine) m/z ratios or the λ 260-nm ratios. Quantification was performed by comparison with the standard curve obtained from authentic nucleoside standards (Sigma-Aldrich) running at the same batch of samples.
MeRip-Seq.
Immunoprecipitation of m6A RNA fragments was performed using 200 µg of purified vRNAs as previously described (5). Sequencing libraries were prepared from input, bead-only control and m6A-immunoprecipitated RNAs (MeRip-seq) with TruSeq stranded mRNA Library Preparation kit of Illumina. Libraries were sequenced 2 × 75 base pair reads on the NextSEq. 500 (Illumina) at the Genomic Service Unit (Servicio Central de Apoyo a la Investigación Experimental, Universitat de Valencia). After adapter removal, quality trimming and size filtering with cutadapt (6), complete paired-end reads longer than 20-bp were mapped to combined A. thaliana (TAIR10) and AMV genomic RNAs (RefSeq accessions: NC_001495.1, NC_002024.2, and NC_002025.1) using Bowtie2 (48). Only coherently virus-mapped paired reads (50–250 bp mapping distance) were used for subsequent analysis. Search for enriched peaks in the MeRIP-seq sample compared with the input or bead-only control were performed as described previously (49) with the difference that we calculated the sequencing coverage for every virus genome position using sequence fragments instead of sequence reads (sequence fragments included all of the bases contained between the leftmost and the rightmost base of each paired-end read obtained). For each AMV RNA, nucleotides with differential coverage between input and bead-only control or MeRIP-seq samples were identified using the trimmed mean of M-values (TMM) normalization and a negative binomial model with the edgeR package (50). A nucleotide was called positive if the false discovery rate (FDR) was <0.01 and the log2FC was ≥1, and peaks were considered as stretches of more than 10 consecutive positive nucleotides.
Acknowledgments
We thank L. Corachan for her excellent technical assistance, Dr. Emilio Martinez de Alba and Dr. Christophe Rizenthaler for kindly providing GFP:SGS3 and DCP2:mCherry plasmids, Prof. John W. S. Brown for providing a plasmid containing the ORF of atUPF1, and the Bioinformatics Core Service at the Instituto de Biología Molecular y Celular de Plantas (IBMCP) for the support provided in the data analysis. UPLC-PDA-Q/TOF-MS analyses were performed by the Metabolic Analysis Department of the IBMCP. F.A. and M.M.-P. were recipients of Contract RYC-2010-06169 from the Ramón y Cajal Program of the Ministerio de Educación y Ciencia, and Predoctoral Contract FPI-2015-072406 from the Subprograma FPI-MINECO (Formación de Personal Investigador–Ministerio de Economía y Competitividad), respectively. This work was supported by Grant BIO2014-54862-R from the Spanish granting agency Dirección General de Investigación Científica y Técnica and the Prometeo Program (GV2015/010) from the Generalitat Valenciana.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703139114/-/DCSupplemental.
References
- 1.Wang X, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu C, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 3.Meyer KD, et al. 5′-UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu N, et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ping X-L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz S, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 13.Xiao W, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: Implications for RNA processing. Mol Cell Biol. 1985;5:2298–2306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirumuru N, et al. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife. 2016;5:e15528. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichinchi G, et al. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016;1:16011. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichinchi G, et al. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe. 2016;20:666–673. doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy EM, et al. Posttranscriptional m(6)A editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe. 2016;19:675–685. doi: 10.1016/j.chom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gokhale NS, et al. N6-methyladenosine in flaviviridae viral RNA genomes regulates infection. Cell Host Microbe. 2016;20:654–665. doi: 10.1016/j.chom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y, et al. Transcriptome-wide high-throughput deep m(6)A-seq reveals unique differential m(6)A methylation patterns between three organs in Arabidopsis thaliana. Genome Biol. 2015;16:272. doi: 10.1186/s13059-015-0839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong S, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodi Z, et al. Adenosine methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen L, et al. N(6)-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev Cell. 2016;38:186–200. doi: 10.1016/j.devcel.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielecki D, et al. Novel AlkB dioxygenases––alternative models for in silico and in vivo studies. PLoS One. 2012;7:e30588. doi: 10.1371/journal.pone.0030588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Born E, et al. Viral AlkB proteins repair RNA damage by oxidative demethylation. Nucleic Acids Res. 2008;36:5451–5461. doi: 10.1093/nar/gkn519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bol JF. Replication of alfamo- and ilarviruses: Role of the coat protein. Annu Rev Phytopathol. 2005;43:39–62. doi: 10.1146/annurev.phyto.43.101804.120505. [DOI] [PubMed] [Google Scholar]
- 27.Herranz MC, Pallás V, Aparicio F. Multifunctional roles for the N-terminal basic motif of Alfalfa mosaic virus coat protein: Nucleolar/cytoplasmic shuttling, modulation of RNA-binding activity, and virion formation. Mol Plant Microbe Interact. 2012;25:1093–1103. doi: 10.1094/MPMI-04-12-0079-R. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim A, Hutchens HM, Berg RH, Loesch-Fries LS. Alfalfa mosaic virus replicase proteins, P1 and P2, localize to the tonoplast in the presence of virus RNA. Virology. 2012;433:449–461. doi: 10.1016/j.virol.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Pallas V, Aparicio F, Herranz MC, Sanchez-Navarro JA, Scott SW. The molecular biology of ilarviruses. Adv Virus Res. 2013;87:139–181. doi: 10.1016/B978-0-12-407698-3.00005-3. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramaniam M, Kim BS, Hutchens-Williams HM, Loesch-Fries LS. The photosystem II oxygen-evolving complex protein PsbP interacts with the coat protein of Alfalfa mosaic virus and inhibits virus replication. Mol Plant Microbe Interact. 2014;27:1107–1118. doi: 10.1094/MPMI-02-14-0035-R. [DOI] [PubMed] [Google Scholar]
- 31.Aparicio F, Pallás V. The coat protein of Alfalfa mosaic virus interacts and interferes with the transcriptional activity of the bHLH transcription factor ILR3 promoting salicylic acid-dependent defence signalling response. Mol Plant Pathol. 2017;18:173–186. doi: 10.1111/mpp.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumakura N, et al. SGS3 and RDR6 interact and colocalize in cytoplasmic SGS3/RDR6-bodies. FEBS Lett. 2009;583:1261–1266. doi: 10.1016/j.febslet.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 33.Martínez de Alba AE, et al. In plants, decapping prevents RDR6-dependent production of small interfering RNAs from endogenous mRNAs. Nucleic Acids Res. 2015;43:2902–2913. doi: 10.1093/nar/gkv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingelfinger D, Arndt-Jovin DJ, Lührmann R, Achsel T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA. 2002;8:1489–1501. [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia D, Garcia S, Voinnet O. Nonsense-mediated decay serves as a general viral restriction mechanism in plants. Cell Host Microbe. 2014;16:391–402. doi: 10.1016/j.chom.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mérai Z, et al. The late steps of plant nonsense-mediated mRNA decay. Plant J. 2013;73:50–62. doi: 10.1111/tpj.12015. [DOI] [PubMed] [Google Scholar]
- 37.Aparicio F, Sánchez-Navarro JA, Olsthoorn RC, Pallás V, Bol JF. Recognition of cis-acting sequences in RNA 3 of Prunus necrotic ringspot virus by the replicase of Alfalfa mosaic virus. J Gen Virol. 2001;82:947–951. doi: 10.1099/0022-1317-82-4-947. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Navarro JA, Reusken CB, Bol JF, Pallás V. Replication of alfalfa mosaic virus RNA 3 with movement and coat protein genes replaced by corresponding genes of Prunus necrotic ringspot ilarvirus. J Gen Virol. 1997;78:3171–3176. doi: 10.1099/0022-1317-78-12-3171. [DOI] [PubMed] [Google Scholar]
- 39.Luo G-Z, et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun. 2014;5:5630. doi: 10.1038/ncomms6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory BD, et al. A link between RNA metabolism and silencing affecting Arabidopsis development. Dev Cell. 2008;14:854–866. doi: 10.1016/j.devcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Bratlie MS, Drabløs F. Bioinformatic mapping of AlkB homology domains in viruses. BMC Genomics. 2005;6:1. doi: 10.1186/1471-2164-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergua M. 2011. El virus del mosaico de la alfalfa (AMV) en España: incidencia y efectos en alfalfa y análisis de la diversidad biológica y genética de poblaciones procedentes de distintos huéspedes. PhD thesis (Universidad de Zaragoza/Centro de Investigación y Tecnología Agroalimentaria de Aragón, Zaragoza, Spain)
- 43.Ryabov EV, Roberts IM, Palukaitis P, Taliansky M. Host-specific cell-to-cell and long-distance movements of cucumber mosaic virus are facilitated by the movement protein of groundnut rosette virus. Virology. 1999;260:98–108. doi: 10.1006/viro.1999.9806. [DOI] [PubMed] [Google Scholar]
- 44.Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G. Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat Protoc. 2013;8:176–189. doi: 10.1038/nprot.2012.148. [DOI] [PubMed] [Google Scholar]
- 45.Németh K, et al. Pleiotropic control of glucose and hormone responses by PRL1, a nuclear WD protein, in Arabidopsis. Genes Dev. 1998;12:3059–3073. doi: 10.1101/gad.12.19.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aparicio F, Sánchez-Navarro JA, Pallás V. In vitro and in vivo mapping of the Prunus necrotic ringspot virus coat protein C-terminal dimerization domain by bimolecular fluorescence complementation. J Gen Virol. 2006;87:1745–1750. doi: 10.1099/vir.0.81696-0. [DOI] [PubMed] [Google Scholar]
- 47.Pallás V, Más P, Sánchez-Navarro JA. Detection of plant RNA viruses by nonisotopic dot-blot hybridization. Methods Mol Biol. 1998;81:461–468. doi: 10.1385/0-89603-385-6:461. [DOI] [PubMed] [Google Scholar]
- 48.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–12. [Google Scholar]
- 49.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]