Significance
We propose a mechanistic framework that explains the activity and selectivity of an important class of compounds known as linear cationic antimicrobial peptides. These molecules have the potential to be developed into highly potent and selective pharmaceuticals, as they are able to discriminate between mammalian and bacterial membranes, and so destroy pathogens. By comparing both selective and nonselective peptides, we show that their activity is governed by topological and electrostatic interactions between the membrane-bound peptide and the surrounding lipids. This framework could underpin strategies for the rational design of therapeutic agents that are potentially able to bypass the mechanisms of acquired bacterial drug resistance.
Keywords: antimicrobial peptides, microfluidic, giant unilamellar vesicle, magainin, melittin
Abstract
Linear cationic antimicrobial peptides are a diverse class of molecules that interact with a wide range of cell membranes. Many of these peptides disrupt cell integrity by forming membrane-spanning pores that ultimately lead to their death. Despite these peptides high potency and ability to evade acquired bacterial drug resistance, there is a lack of knowledge on their selectivity and activity mechanisms. Such an understanding would provide an informative framework for rational design and could lead to potential antimicrobial therapeutic targets. In this paper, we use a high-throughput microfluidic platform as a quantitative screen to assess peptide activity and selectivity by precisely controlling exposure to vesicles with lipid compositions that mimic both bacterial and mammalian cell membranes. We explore the complexity of the lipid–peptide interactions governing membrane-disruptive behaviors and establish a link between peptide pore formation and both lipid–peptide charge and topological interactions. We propose a topological model for linear antimicrobial peptide activity based on the increase in membrane strain caused by the continuous adsorption of peptides to the target vesicle coupled with the effects of both lipid–peptide charge and topographical interactions. We also show the validity of the proposed model by investigating the activity of two prototypical linear cationic peptides: magainin 2 amide (which is selective for bacterial cells) and melittin (which targets both mammalian and bacterial cells indiscriminately). Finally, we propose the existence of a negative feedback mechanism that governs the pore formation process and controls the membrane’s apparent permeability.
The emergence of drug-resistant bacteria presents a pressing challenge to medicine (1, 2). New therapeutic compounds are needed to break the cycle of resistance that occurs after the introduction of new antibiotics (3, 4). Linear cationic antimicrobial peptides (LCAMPs) are potential antibiotic candidates, with many of them showing high potency against Gram-negative and Gram-positive bacteria at low micromolar concentrations (5). They are particularly interesting, as they are immune from bacterial drug resistance mechanisms (5, 6). The classes of LCAMPs are diverse, with over 1,000 members expressed in widely separated taxonomic groups and characterized by their amphipathic and cationic membrane-bound helices (7–9). Unlike most peptide families, it is the physiochemical properties of the LCAMPs’ assembled helices, which render them homologous, rather than their amino acid sequence (10, 11). Some LCAMPs are selective for bacterial cells [e.g., magainin 2 amide (m2a) from the African clawed frog Xenopus laevis (8)]. Others target bacterial and mammalian cells indiscriminately [e.g., melittin from the venom of the bee Apis mellifera (9)].
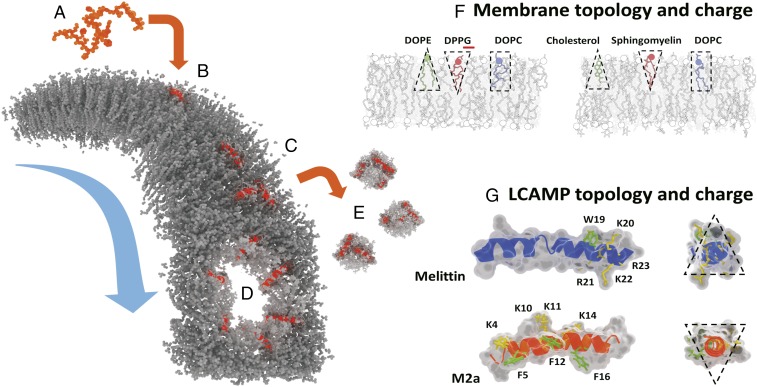
Although many complex lipid–peptide interactions involving, for example, electrostatics and peptide hydrophobic moments have been proposed to influence their behavior (12, 13), a full understanding of the relationship between LCAMPs and the cell’s membrane is not complete (6, 7). One mechanism widely used to describe LCAMP behavior is the Shai–Matsuzaki–Huang (SMH) model shown in Fig. 1 A–E (6, 14). Briefly, LCAMPs display random coil conformations in aqueous environments before spontaneously binding to lipid bilayers, where they adopt an amphipathic helical configuration. The peptides initially insert parallel to the membrane plane, leading to membrane thinning, as LCAMP helices increase their outer leaflet volume (7, 8, 14). After a threshold concentration of membrane-bound peptide is achieved, membrane integrity becomes compromised through a variety of peptide-induced effects (PIEs), including bursting, a detergent-like carpet mechanism, and formation of membrane-spanning toroidal pores (7, 9, 15, 16). The latter is marked by a shift in helical orientation (from parallel to perpendicular to the membrane plane) and by translocation of lipid and peptide material from the outer to the inner membrane leaflet. Although the SMH model provides a basic mechanistic framework for LCAMP activity, it cannot predict peptide activity within specific membrane systems (e.g., it is not able to explain LCAMP selectivity between bacterial and mammalian membranes, which are characteristically different in both charge and topology) (Fig. 1F).
Fig. 1.
The SMH model of LCAMP activity in lipid bilayers (gray). (A) LCAMPs (orange) bind to the outer membrane leaflet as amphipathic helices (B). Membrane-bound peptides (C) produce a variety of effects, including toroidal pore formation (D) and the release of lipid–peptide micelles in a detergent-like carpet mechanism (E). (F) Bacterial and mammalian membranes are characteristically different in charge and lipid topology. Bacterial outer leaflets contain lipids with large anionic head groups (e.g., DPPG) and conical nonbilayer lipids (e.g., DOPE), giving them a negative net charge and curvature profile. Mammalian outer leaflets contain bilayer lipids, like sphingomyelin, along with the conical geometry cholesterol, a lipid completely absent from bacterial membranes. They are net neutral in charge and curvature profile. (G) Similarly, melittin (blue) and m2a (orange) vary in charge and topological character. The four cationic lysines (K; yellow) and three phenylalanines (F; green) of m2a are distributed along its length, while the cationic arginines (R; yellow) and lysines (K; yellow) of melittin are clustered at the C terminus along with its single tryptophan residue (W; green). Melittin acts as a negative curvature membrane wedge (conical geometry), while m2a acts as a positive curvature wedge (inverse conical geometry).
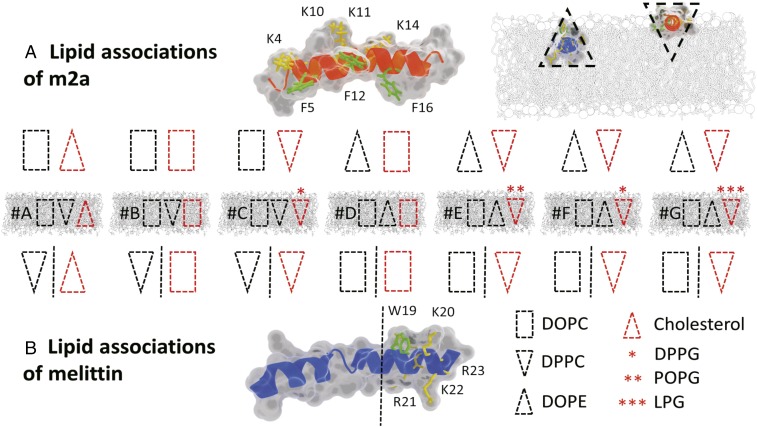
Bacterial cells contain high proportions of anionic and conical geometry nonbilayer lipids, whereas the outer leaflets of mammalian membranes are zwitterionic with a neutral curvature profile (17, 18). These marked differences in membrane charge and topology are reflected in the physiochemical properties of two LCAMPs, the selective m2a and the nonselective melittin (Figs. 1G and 2). The cationic residues of m2a are distributed along its length in close proximity to three sterically active phenylalanine residues and form a large angle on the helical face (19). The wide polar angle renders m2a unable to fully insert into the hydrophobic core of lipid bilayers, acting as a membrane “wedge” that induces positive curvature (9, 10). In contrast, melittin has a single sterically active tryptophan near its C terminus, where it clusters with its cationic residues, with a narrow polar angle formed on its helical face (7, 19). Melittin acts as a negative curvature-inducing membrane wedge owing to the deep penetration of its helix into the lipid bilayer (20). We now show that these inherent differences in lipid and peptide charges and topologies underpin LCAMPs activity and their selectivity between mammalian and bacterial cells.
Fig. 2.
The lipid associations of melittin and m2a in the membrane systems A–G, with lipid topology indicated by the block shapes. A red outline indicates lipids with a clustering mechanism either via electrostatics for the anionic lipids or via aromatic π-stacking with phenylalanine (F) and tryptophan (W) for cholesterol. (A) The block shapes indicate the topology of the lipids expected to associate with m2a. (B) The lipid topologies expected to associate with melittin. Shapes to the left of the dashed line associate with the peptides’ helical face, while those to the right cluster with the peptides’ tryptophan and cationic residues at the C terminus. (A, Top Right, Inset) The deeper penetration of melittin into the outer leaflet allows it to act as a negative curvature membrane wedge, while the shallower penetration of m2a results in a positive curvature wedge effect indicated by the appropriate block shapes.
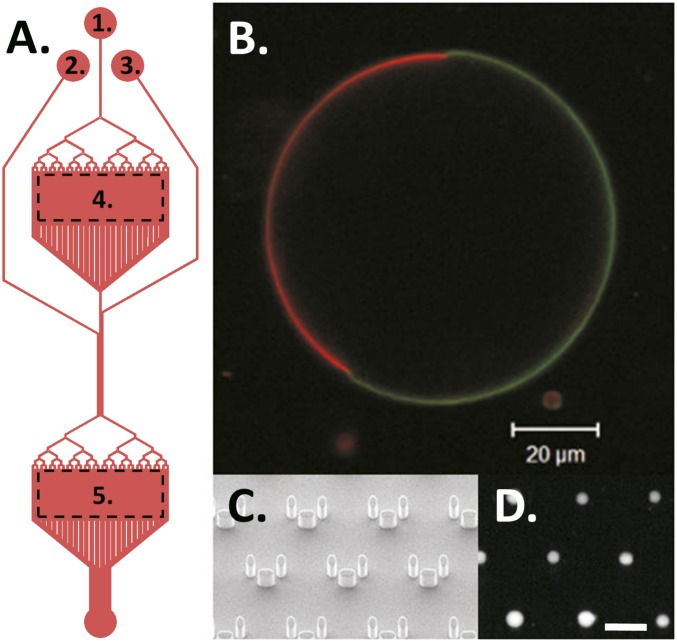
We have used giant unilamellar vesicles (GUVs) as model systems of cell membranes. GUVs with different lipid topology and charge but with comparable size and curvature to mammalian cells are exposed to LCAMP within a high-throughput microfluidic device (Fig. S1A). Dye leakage experiments have been used for the investigation of lipid–peptide interactions (21, 22). We have previously used such a technique within a microfluidic platform to precisely control the exposure of GUVs to LCAMPs (23). An advantage of this approach is the greatly increased experimental throughput over conventional techniques, which typically study only one GUV at a time.
Fig. S1.
(A) Plan view of the device with the following parts labeled. (A1) Flow channel. (A2) Peptide channel. (A3) Wash channel. (A4) Electroformation chamber. (A5) Microtrap array chamber. (B) A mammalian biomimetic GUV (system A) with the liquid-disordered DOPC domain visualized with 0.1 mol% 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE)-rhodamine (red) and the liquid-ordered domain visualized using 0.5 mol% cholesterol-TopFluor (green). (C) SEM image of the microarray traps. (D) Vesicles trapped within the microarray, encapsulating 3 kDa dextran-AlexaFluor488. (Scale bar: 100 µm.)
By generating extensive dye leakage datasets (e.g., Fig. S2) from over 1,500 GUVs with different lipid compositions, we are able to gain insights into lipid–peptide topology and electrostatic interactions that are consistent with the existing SMH model. In particular, we provide not only a mechanistic explanation of LCAMP activity and selectivity but also, a predictive framework for peptide action within specific membranes. We show that the diverse membrane-disruptive activity of LCAMPs can be explained through both the accumulation of membrane strain and the relationship between lipid and peptide shape and charge. This extended model describes the selectivity mechanism of m2a for bacterial over mammalian cells as well as the nonselective nature of melittin (8, 9). Finally, we propose a negative feedback mechanism within the pore formation process that controls the membranes’ apparent permeability.
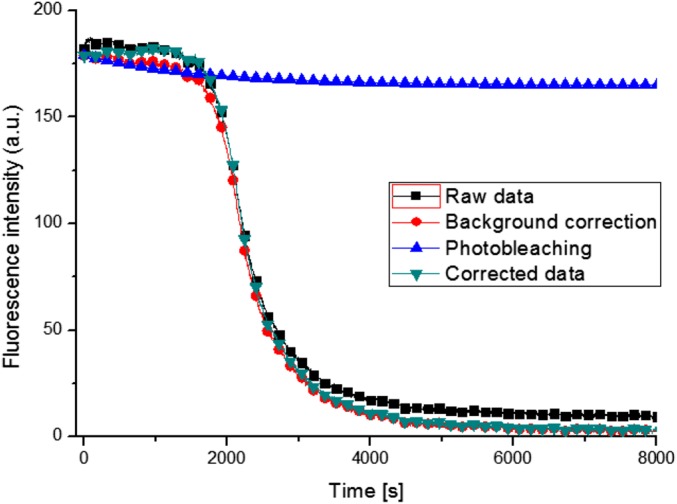
Fig. S2.
Graph displaying the data processing to correct a 3-kDa dextran dye leakage trace for fluorescence background and photobleaching. Data collected from a DOPC vesicle after exposure to 1 µM LCAMP melittin.
Results
We describe the effects of lipid charge and topological character on overall LCAMP lipid clustering and activity as well as the classification of PIEs and the dynamics of pore-mediated dye leakage.
Activity.
Overall LCAMP activity was determined by the relationship between peptide and lipid topology with m2a and melittin activity arising in systems with opposing lipid geometries (Tables 1 and 2). Membrane-bound proteins form dynamic lipid rafts shown to be capable of preferentially associating with specific lipids, including cholesterol and sphingolipids (24). Fig. 2 shows the expected lipid associations of both m2a and melittin within all seven membrane compositions investigated (A–G), with consideration given to four factors.
-
i)
Membrane curvature. This is a modulator of lipid and protein localization as membranes seek to minimize free energy through reduction of their internal packing strain (25–27). By considering the established ability of melittin and m2a to induce opposing curvature in similar membranes, the peptides minimize packing frustration around them by clustering with lipids opposing the peptide-induced curvature (9, 15, 20). For example, membrane systems B and C contain positive curvature lipids [dipalmitoyl-phosphatidylcholine (DPPC)] that oppose melittin’s negative curvature induction (i.e., they lower raft energy by clustering around the peptide helix).
-
ii)
Aromatic interactions. It is known that cholesterol forms π-stacking interactions with tryptophan and phenylalanine residues (28, 29). Here, cholesterol can cluster with phenylalanine residues of m2a and the sole tryptophan of melittin. The aromatic interactions bring a negative curvature lipid into contact with LCAMPs in the mammalian biomimetic membrane (system A).
-
iii)
Electrostatic interactions. These are powerful mediators of lipid–LCAMP associations (12). For example, system G contains the anionic lipid lysophosphoglycerol (LPG), which will form clusters around the cationic residues of both peptides.
-
iv)
Residue distribution. The positioning of cationic and sterically bulky residues affects lipid distribution around the helix (12, 28, 29); m2a distributes these residues along its helical length, and the lipids that associate with them (i.e., anionic lipids and cholesterol) will also locate along its helical length (Fig. 2). Melittin isolates these residues at the C terminus, and anionic lipids and cholesterol will similarly locate there, leaving the helical face to associate with other lipid components.
Table 1.
PIEs of the LCAMP melittin
| System | Composition | No effect (%) ± SD | Pore (%) ± SD | Burst (%) ± SD | Carpet (%) ± SD | Other (%) ± SD |
| A | DOPC:DPPC:chol | 0 | 37 ± 7 | 22 ± 4 | 40 ± 10 | 1 ± 2 |
| B | DOPC:DPPC:DOPG | 3 ± 4 | 58 ± 13 | 3 ± 3 | 30 ± 12 | 6 ± 7 |
| C | DOPC:DPPC:DPPG | 22 ± 13 | 54 ± 6 | 10 ± 6 | 10 ± 11 | 4 ± 4 |
| D | DOPC:DOPE:DOPG | 0 | 17 ± 12 | 33 ± 17 | 47 ± 8 | 2 ± 3 |
| E | DOPC:DOPE:POPG | 32 ± 12 | 22 ± 12 | 43 ± 14 | 2 ± 3 | 2 ± 3 |
| F | DOPC:DOPE:DPPG | 31 ± 12 | 14 ± 7 | 39 ± 10 | 13 ± 11 | 3 ± 6 |
| G | DOPC:DOPE:LPG | 27 ± 17 | 19 ± 2 | 27 ± 9 | 18 ± 7 | 9 ± 6 |
PIEs of LCAMP melittin in membrane systems A–G. Listed are the membrane compositions and the PIEs recorded for each lipids system after exposure to 1 µM peptide, including no effect, pore-mediated leakage, carpet mechanism, and other. Each dataset is composed of at least 34 vesicles from at least three independent experiments, and the SD is included. Bold values are the results discussed within the text and are a guide to the eye only. chol, cholesterol.
Table 2.
PIEs of the LCAMP m2a
| System | Composition | No effect (%) ± SD | Pore (%) ± SD | Burst (%) ± SD | Carpet (%) ± SD | Other (%) ± SD |
| A | DOPC:DPPC:chol | 69 ± 12 | 3 ± 5 | 18 ± 9 | 10 ± 6 | 1 ± 1 |
| B | DOPC:DPPC:DOPG | 82 ± 6 | 7 ± 2 | 6 ± 5 | 0 | 6 ± 8 |
| C | DOPC:DPPC:DPPG | 51 ± 13 | 38 ± 11 | 0 | 7 ± 9 | 4 ± 3 |
| D | DOPC:DOPE:DOPG | 94 ± 6 | 0 | 3 ± 3 | 3 ± 3 | 0 |
| E | DOPC:DOPE:POPG | 84 ± 5 | 0 | 2 ± 5 | 9 ± 7 | 5 ± 10 |
| F | DOPC:DOPE:DPPG | 28 ± 12 | 42 ± 26 | 12 ± 12 | 15 ± 4 | 3 ± 4 |
| G | DOPC:DOPE:LPG | 49 ± 5 | 14 ± 3 | 28 ± 9 | 8 ± 14 | 0 |
PIEs of LCAMP m2a in membrane systems A–G. Listed are the membrane compositions and the PIEs recorded for each lipids system after exposure to 1 µM peptide, including no effect, pore-mediated leakage, carpet mechanism, and other. Each dataset is composed of at least 34 vesicles from at least three independent experiments, and the SD is included. Bold values are the results discussed within the text and are a guide to the eye only. chol, cholesterol.
These four factors underpin LCAMP activity, which we describe in more details in the following sections. Anionic lipids with low hydrophobic volumes and inverse conical geometry will activate m2a. For example, membrane systems C [dipalmitoyl-phosphoglycerol (DPPG)], F (DPPG), and G (LPG) contain inverse conical anionic lipids with saturated 16:0 palmitoyl fatty acids and return activities of 49, 72, and 51%, respectively. Substituting DPPG with 1-palmitoyl-2-oleoyl-phosphoglycerol (POPG; i.e., replacing a palmitoyl chain with an unsaturated 18:1; 9Z oleic chain) results in a fourfold decrease in activity between membranes F (DPPG: 72%) and E (POPG: 16%). Conversely, lipids with large hydrophobic volumes [e.g., dioleoyl-phosphatidylcholine (DOPC), dioleoylphosphatidyl-ethanolamine (DOPE), and 1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phospho-(1′-rac-glycerol) (DOPG)] from their unsaturated 18:1 oleic fatty acid chains or cholesterol [a lipid with large negative curvature (18)] inactivate m2a. Indeed, membranes A (cholesterol), B (DOPG), and D (DOPG) return activities of 31, 18, and 6%, respectively. Similarly, within the context of topological constraints, melittin shows an opposing trend compared with m2a. Its activity is enhanced by interaction with large-hydrophobic volume lipids and reduced by the presence of inverse conical low-hydrophobic volume lipids. For instance, although melittin is generally more active than m2a, systems C (DPPC and DPPG), F (DPPG), and G (LPG) show the highest activities for m2a, whereas they yield low melittin activities of 78, 69, and 73%, respectively.
PIE Classification.
m2a and melittin behavior can be further classified into three groups: pore-mediated leakage, where dye efflux occurs with no loss of membrane volume (7, 15, 16); a detergent-like carpet mechanism, where dye efflux is coupled to a loss of membrane volume (7, 9); and bursting, where dye loss is complete and instantaneous (Figs. S3–S7 and Movies S1–S3). Tables 1 and 2 report the percentage values of the PIEs recorded for the membrane systems A–G. It is clear that the two LCAMPs investigated vary widely in their membrane-disruptive behavior, even when tested using identical membranes. Below, we describe each of these leakage processes and build a correlation between them and their associated lipid–peptide interactions.
Fig. S3.
Description of the PIEs recorded after vesicle exposure to the LCAMPs melittin and m2a categorized as shown in Figs. S2, S4, and S8. Vesicles can be left unchanged (no effect), burst, or exhibit dye leakage via three mechanisms: gradient or all or none pore-mediated leakage or a detergent-like carpet mechanism.
Fig. S7.
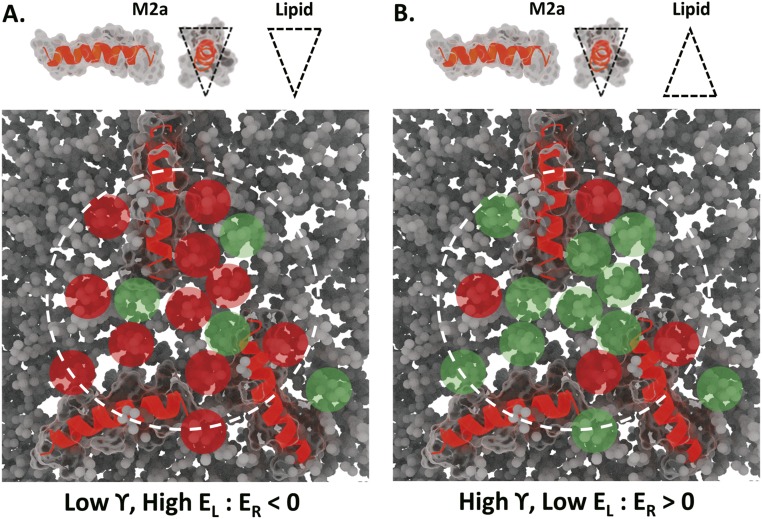
The aggregation of LCAMP helices within a small prepore region, indicated by the dashed white line, can potentially accumulate significant membrane packing frustration (EL) within a small region. (A) m2a helices surrounded by inverse conical lipids form high-energy lipid–peptide interactions (red shading). Together with the efficient pore leaflet fold packing of inverse conical lipids, this satisfies the conditions for pore formation (i.e., renders ER < 0). (B) m2a surrounded by conical lipids generates low EL (green shading). Conical lipids also increase γ, leaving the raft assembly unable to satisfy the conditions for pore formation.
Pore Events.
The three highest levels of melittin pore activity occur in systems A (37%), B (58%), and C (54%). Notably, they all contain the zwitterionic inverse conical lipid DPPC, which is expected to cluster along the peptides’ helical face (Fig. 2). As LCAMP pore formation is coupled to a shift in peptide orientation from parallel to the membrane plane to perpendicular to it (14, 16) by inserting the center of the helix deep into the membrane core, it is likely that the pore rim is primarily composed of those lipids along the helical face. The opposite situation, where melittin clusters with high-hydrophobic volume lipids along its helical face (i.e., DOPC in systems D–G), produces low levels of pore activity: 17, 22, 14, and 19%, respectively. Pore formation is, therefore, favored by the presence of positive curvature lipids coupled to a clustering mechanism that will enrich these lipids along the helical face. m2a displays high levels of pore formation in systems C (38%) and F (42%), where the peptide will cluster with the positive curvature anionic lipid DPPG through electrostatic interactions with the cationic residues along the helical face. Membrane C presents the highest relative pore formation of any lipid–peptide system tested, with 78% of active GUVs displaying pore-mediated leakage events.
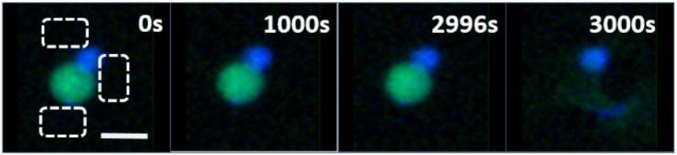
Carpet Events.
Melittin displays a significant number of carpet events in systems A (40%), B (30%), and D (47%), where it associates with high-hydrophobic volume lipids at its C terminus either via electrostatic clustering with anionic conical geometry lipids or via π-stacking interactions between tryptophan and cholesterol (12, 28, 29) (Fig. 2). Conversely, our results (Tables 1 and 2) show that carpet events are suppressed when melittin clusters with low-hydrophobic volume lipids at its C terminus [i.e., POPG in system E (2%) and DPPG in systems C and F (10 and 13%, respectively)]. m2a displays its highest levels of carpet events when clustered with lipids matching its own positive curvature induction, returning 15% in system F (DPPG).
Bursting Events.
Melittin displays its highest levels of vesicle bursting in lipid compositions D (33%), E (43%), and F (39%), where the peptide tends to form lipid rafts enriched with the high-hydrophobic volume lipid DOPC. Conversely, bursting is suppressed when melittin associates with lipids having low hydrophobic volumes [i.e., DPPC in systems A (22%), B (3%), and C (10%)]. m2a displays high levels of bursting in membranes where it forms rafts enriched with lipids having high hydrophobic volume [i.e., cholesterol and DOPC in membrane A (18%) and DOPE in membranes F and G (12 and 28%, respectively)]. Bursting events are also strongly disfavored in system with composition C (no bursting activity; of 59 GUVs observed), where m2a associates with the low-hydrophobic volume anionic lipid DPPG.
Pore-Mediated Efflux Dynamics.
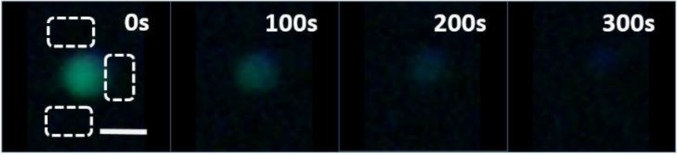
A subpopulation of dye efflux traces displays complex multimodal dynamics, with distinct changes in dye efflux over the timescale of the leakage process. Fig. 3A shows an example of a multimodal dye leakage trace for a system C vesicle (DOPC:DPPC:DPPG) after exposure to 1 µM of m2a.
Fig. 3.
(A) The normalized fluorescence intensity (NFI) function as a measure of the dye efflux of a system C GUV exposed to 1 µM LCAMP m2a. The trace displays multiple changes in leakage dynamics. (B) The −Ln(NFI) plot of the same trace shown in A. (C) This plot shows an example of the continuous change in membrane apparent permeability over the course of the entire leakage process from the data presented in A (as described in the SI Materials and Methods). D–G are the apparent membrane permeability histograms of (D) system A (mammalian biomimetic membrane) exposed to 1 µM m2a, which show the presence of distinct characteristic membrane flux rates or equivalently leakage characteristic times (τ; top axis). (E) System C (bacterial biomimetic) vesicles exposed to 1 µM m2a. (F) System A exposed to 1 µM melittin featuring the presence of flux groupings, although less evident than the case with m2a. (G) System C GUVs exposed to 1 µM melittin.
The data extracted from these dye efflux traces (Fig. 3 B and C) show the existence of well-defined characteristic efflux rates when membranes are exposed to either melittin or m2a. The membranes’ apparent permeability centers around a few common values for all of the explored systems as shown in Fig. 3 D–F (Fig. S8 for details on processing). A Shapiro–Wilks test was performed to show that the data were not a random sampling of a normal distribution to a high degree of confidence for all groupings (P < 0.05). This discretization phenomenon is not supported by the SMH model.
Fig. S8.
Calculations for the conversion of NFI as a function of time into the leakage characteristic time (τ) and the membrane’s apparent permeability. The calculations were performed via a dedicated LabView (National Instruments) virtual instrument, which is available at DOI:10.5525/gla.researchdata.434.
Discussion
As stated, Tables 1 and 2 support the hypothesis that lipid topology is an important modulator of LCAMP activity, with positive curvature lipids activating m2a and negative curvature lipids activating melittin. Here, we discuss in greater detail the impact of topological drivers on peptide activity and propose a model for LCAMP pore formation and selectivity.
Pore Formation.
After their formation, lipid–LCAMP rafts diffuse laterally in the outer leaflet and create transient raft assemblies, which may form a membrane “hotspot”—a prepore region with increased packing frustration (30). To form a pore, the lipid–peptide system must reorganize from a bilayer into a higher energy, tightly curved structure. The pore formation process in peptide-free membranes under mechanical stress provides a useful framework to describe the energy difference (ER) between pore-containing and pore-free membranes (31, 32). Based on the same principles, here we describe the interaction between LCAMPs and the membranes by means of Eq. 1:
| [1] |
Eq. 1 shows the energy difference between pore-free and pore-containing membranes (ER); γ is the pore rim line tension, R is the pore radius, is the bulk (global) membrane tension after LCAMP binding from solution, and is the sum of the energy produced by lipid–peptide interactions within the prepore region.
The term γ2πR represents the pore line tension, which will vary depending on the lipid composition of the membrane (26), while σGπR2 is the work required to open the pore against membrane tension. The final term () represents the energy contained within the lipid–LCAMP rafts within the prepore region. When conditions reach ER < 0, pore structures become thermodynamically favored. Lipid–LCAMP interactions affect all three terms of Eq. 1 and provide specific predictions from the topological model concerning LCAMP pore formation behavior as described below.
Pore Rim Line Tension ().
Toroidal pores possess a tightly positively curved leaflet fold structure, where the inner and outer membrane leaflets bend into one another. Lipid curvature has been shown to stabilize and/or induce nanoscale membrane curvature, reducing lipid packing frustration (26).
The sterically bulky amino acid residues, phenylalanine and tryptophan, are key mediators of LCAMP activity, although their precise role during pore formation remains unknown (7). Within the topological model, we propose that phenylalanine and tryptophan act as positionally flexible membrane topology wedges that are able to shift position within the membrane and manipulate the topography of the surrounding lipids. The positioning of one of these sterically bulky amino acids within the interfacial area of the outer leaflet increases the positive curvature of the adjacent lipids and favors pore formation through the stabilization of the leaflet fold structure (i.e., low value). Both phenylalanine and tryptophan residues are known to perform similar gating functions (shifting position within the membrane from the hydrophobic core to the interfacial region) for several transmembrane receptor proteins (33).
This process can be evaluated using the packing parameter (S), a metric which links lipid geometrical properties with their preferred supramolecular packing organization in aqueous environments (34). This measure is defined as S = V/(a × l), where V is the hydrocarbon volume, a is the head group area, and l is the hydrocarbon chain length. Fig. 4 shows this concept for m2a using three lipids in our dye leakage experiments (i.e., DOPE, DPPC, and DOPC). The new packing parameter, S*, is calculated by adding the area of the main steric component of a phenylalanine residue [a benzene ring with a cross-sectional area of 40 Å2 (28)] to the head group area, a.
Fig. 4.
The effect of phenylalanine (F) and tryptophan (W) residues on the native packing parameters (S) and supramolecular packing arrangements of the lipids DOPE, DOPC, and DPPC. The new packing parameter (S*) and packing arrangement (Packing*) after increasing the lipid head group area by the phenylalanine sidechains of m2a are shown. DOPE changes from reverse hexagonal phase (HII) to bilayer packing, and DOPC and DPPC change from bilayer to hexagonal phase (HI) packing. Values for calculations are taken from refs. 17, 18, 20, 25, 35, and 38.
The conical nonbilayer lipid DOPE has a native S value of 1.41, forming inverse hexagonal phases in aqueous solution (34). When the head group area is occupied by the phenylalanine residues of m2a, the S value drops to 0.8, predictive of a bilayer system [1.20 > S > 0.74 is the requirement for stable bilayer formation (35]). The cylindrical DOPC and the inverse conical DPPC possess native S values indicative of bilayer structures. In these cases, m2a forces S* values below the bilayer threshold into geometries preferring hexagonal phase structures (0.30 < S < 0.74) (35). This packing change is of particular interest because of the similarities between the lipid packing of hexagonal phases and idealized toroidal pore structures (Fig. S9).
Fig. S9.
Comparison between the leaflet fold moiety of an idealized toroidal pore (A) and the hexagonal phase packing arrangement of inverse conical lipids in aqueous environments (B). Both lipid packing arrangements involve tight positive curvature, bending the lipids from head group to tail group, which is shown by the white arrows.
Given that pore formation is linked to peptide insertion parallel to the membrane normal, it is likely that the lipids along the helical face form the curved leaflet fold structure (7, 8). LCAMPs that associate with positive curvature lipids along their helical face produce low γ values, favoring pore formation, whereas in systems where the peptide associates with either neutral or negatively curved lipids, higher γ values are produced; consequently, pore formation is decreased.
Work to Open a Pore ().
This term is modified from previous work (31, 32) by making the membrane tension sensitive to LCAMP binding. During the continuous exposure of a vesicle to LCAMPs within the microfluidic device, peptides constantly bind to the outer leaflet, with the consequence of thinning and weakening the membrane (9) (i.e., σG continuously decreases with time) in proportion to the amount of membrane-bound LCAMP. Melittin inserts deep into the bilayer and results in a greater leaflet area asymmetry per peptide monomer than m2a (8, 20), providing a higher contribution toward σG and hence, an increased activity. Moreover, the topological model predicts that pore formation should be dependent on vesicle size caused by the inverse scaling of membrane tension with vesicle radius (36), which will alter σG. Consequently, a prediction of the topological model is that pores in small GUVs are harder to open than those in large GUVs, as they require greater work to open against their increased membrane tension. This is a marked departure from the established SMH model, which does not comment on vesicle size.
Lipid–Peptide Interaction Energy (EL).
LCAMPs surrounded by lipids with matching curvature properties will increase raft packing frustration (37) and generate high EL. Within anionic (i.e., bacterial) membranes, these high-energy interactions can be forced via electrostatics (12). The topological model proposes that, for m2a, inverse conical anionic lipids (e.g., DPPG) can be brought into close proximity to the peptides’ three phenylalanine (F) residues because of their close spatial relationship with the peptides' cationic lysine (K) residues (Fig. 2), specifically K4 and F5, K10/11 and F12, and K14 and F16. These large head group lipids will compete for interfacial area with the F residues of m2a, generating increased lipid–peptide packing frustration and increasing EL. Conversely, melittin will generate large EL values when surrounded by conical lipids owing to its induction of negative curvature.
Extended LCAMP Model.
Here, based on our experimental results, we summarize our proposed topological model (Fig. 5 A–E), which adds both explanatory and predictive power to the SMH model (Fig. 6). LCAMP behavior can be understood by means of simple concepts, namely lipid–peptide charge and topological interactions. The process is driven by the accumulation of strain within the target membrane through the occupation of outer leaflet volume by the LCAMP binding from solution. The precise nature of PIEs seen within a membrane is controlled by the charge and topological interactions occurring within the lipid–peptide rafts.
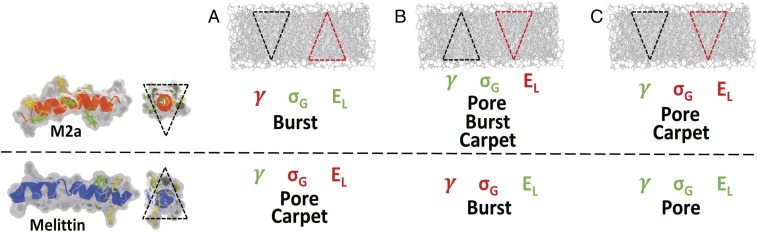
Fig. 5.
(A) The topological model proposes that lipid–peptide shape and charge interactions govern the behavior of LCAMPs. B–E show the specific lipid clustering and geometries responsible for individual PIEs, with lipid geometry shown using block shapes and clustering lipids given as a red outline. The terms of Eq. 1 associated with each PIE are also shown. (A) Peptide binds to the outer leaflet, generating internal membrane strain (σG), which controls overall activity (B). Both peptides are more active when clustered with lipids matching their own curvature strain: melittin with conical lipids and m2a with inverse conical lipids. (C) Clustering with lipids matching peptide curvature also governs the carpet mechanism by generating high EL values and stabilizing the high-curvature lipid–peptide micelles produced. (D) Bursting events for melittin occur when the membrane contains nonclustering conical lipids, which will generate high EL raft assemblies that cannot react via pore formation (high γ); instead, they cause complete vesicle failure. For m2a, both clustering and nonclustering conical lipids cause bursting. (E) Melittin generates pore activity (low γ) when inverse conical lipids associate with its helical face, while m2a generates pores when clustered with inverse conical lipids. Open pores allow interleaflet material transfer, lowering σG and generating a negative feedback system between the rate of LCAMP binding from solution (RS) and the rate of material flow through open pores (RP), with three possible cases. (i) RS > RP shown in F; there is a continuous increase in σG, causing pore opening (blue arrows) until the membrane failure point is reached and the vesicle bursts (red arrows). Examples are shown for system A exposed to m2a (F, Left) and for system C for melittin (F, Right), which display complex leakage dynamics, as several pores open (blue arrows) before a stable leak is established. (ii) RS = RP, resulting in a stable leak (G), shown for system C for m2a (G, Left) and melittin (G, Right). (iii) RP > RS, decreasing σG and favoring pore closure. This renders RS > RP, increasing σG and reinitiating pore formation. This results in pore opening and closing cycles. H, Left shows a pore-cycling leakage trace (system F; m2a), with its smoothed average (light blue) and fitted single-exponential decay curve (red). (H, Right) The residual between the two curves shown in H, Left (red) compared with the residual for a stable case ii leak (black). The residual shows dynamic cycling between at least two different leakage rates, with intervals between the minima of 1,517, 1,512, and 1,611 s. Consistent cycle spacing is caused by the constant RS within the microfluidic device.
Fig. 6.
Predictions for LCAMP behavior within membranes based on the lipid–peptide topological interactions and their effects on the terms of Eq. 1 (γ, pore rim line tension; σG, membrane tension; and EL, lipid–peptide interaction energy). The lipid topology of the membrane systems is indicated by block shapes, with lipids having a clustering mechanism given as a red outline. Within A, m2a generates high γ values (red) but low σG and EL (green) through clustering with conical lipids along its helical face. Pore mechanism is suppressed via inefficient leaflet fold packing, while carpet mechanism is suppressed by nullification of the peptides’ positive curvature. m2a will, therefore, react via bursting. Melittin clusters the conical lipids at its C terminus and associates with the inverse conical lipids along its helical face. This generates low γ combined with high EL at the C terminus and will respond via both pore (low γ) and carpet mechanisms (high EL). (B) In this membrane, m2a clusters inverse conical lipids at its helical face, generating low γ and high EL, which together with the presence of conical nonbilayer lipids allows the peptide to produce all three PIEs. Melittin generates higher γ and lower EL and primarily reacts via bursting. (C) m2a produces low γ and high EL through clustering with inverse conical lipids along its helical face. This will generate pore and carpet mechanisms. Melittin generates low γ and EL and will be forced to react mainly via bursting.
Overall activity is controlled by clustering with lipids that match the peptides’ own curvature—negative (conical) and positive (inverse conical) curvatures for melittin and m2a, respectively (Fig. 5B), that generate high σG. The carpet mechanism is promoted by clustering with similar lipids at the C terminus of melittin and along the helical face of m2a (Fig. 5C), causing high EL. The combination of peptide and lipid curvature can stabilize the tightly curved lipid–peptide micelles produced by the carpet mechanism. Bursting is favored by conical lipids for both peptides, although melittin associating with high-hydrophobic volume clustering lipids tends to react via the carpet mechanism, while m2a shows sensitivity to both clustering and nonclustering conical lipids (Fig. 5D). The data suggest that bursting occurs when the LCAMPs associate with lipids that disfavor other PIEs, such as clustering with high-hydrophobic volume lipids, which disfavor efficient leaflet fold packing, or with lipids opposing the peptides’ own curvature induction, supressing the carpet mechanism. Pore formation is favored via the association of inverse conical positive curvature lipids for both melittin and m2a (Fig. 5E). A negative feedback system between LCAMPs binding from solution and lipid–peptide transfer between leaflets through open pores governs peptide pore formation (Fig. 5 F–H).
Model Predictions.
Using the patterns in lipid–peptide topological interactions and their effects on the terms of Eq. 1, it is possible to make general predictions concerning the PIE activity of LCAMPs within membrane systems of defined topology and charge (Fig. 6).
Negative Feedback and Toroidal Pores.
Pore leakage dynamics can be explained by the inclusion of a negative feedback system within the topological model (Fig. 5 F–H). Toroidal pores are dynamic structures facilitating lipid and peptide transfer between the membrane leaflets (7, 8, 22), which amounts to a membrane relaxation process, reducing leaflet area asymmetry. Each pore opening renders subsequent pore openings less likely, creating negative feedback between the rate of LCAMP binding from solution (RS), the flow of the lipid–LCAMP material through open pores (RP), and the energy needed to open a pore structure (σG). This framework leads to three possible outcomes, all of which are consistent with the leakage dynamics recorded for m2a and melittin (Fig. 5 F–H).
-
i)
RS < RP. Leaflet asymmetry continually increases, allowing raft assemblies of lower total EL to create pores (Fig. 5F). A cascade of pore formation occurs until the membrane failure is reached and the GUV bursts.
-
ii)
RS = RP. When the rates are in equilibrium during the leakage process (Fig. 5G). Initially, RS is higher than RP, and as membrane tension increases, this causes pore formation and increases RP. When RS and RP are equal, a stable leakage state can occur.
-
iii)
RP > RS. Loss of outer leaflet volume decreases the area asymmetry between the outer and inner leaflets, decreasing membrane tension and causing pore closure until RS > RP and pore activity resumes. The negative feedback loop leads to cycles of high and low pore activity, as membrane tension repeatedly crosses the energy threshold required for lipid–LCAMP rafts to form pores (Fig. 5H).
We propose that this negative feedback explains the discretizing of LCAMP pore leakage behavior observed in Fig. 3, where leakage traces arrange in groups with similar dynamics, which are controlled by the pore size and number within the membrane (i.e., by their apparent permeability). We next apply the model to the selectivity mechanism of LCAMPs between mammalian and bacterial cells.
Implications for Selectivity.
Deciphering and controlling selectivity is a key consideration in the development of antimicrobial agents, and our topological model provides a powerful tool to understand this process. To succeed as antimicrobial agents, LCAMPs must form pores in bacterial membranes (i.e., render ER < 0), while leaving mammalian membranes intact (ER > 0). Using our lipid systems as examples, we explore how the characteristic electrostatic and topological differences between bacterial and mammalian membranes affect the terms in Eq. 1, yielding important considerations for peptide selectivity.
The opposing charge and topological characters of the nonselective melittin and the selective m2a mean that, within identical membranes, they can form rafts enriched with different lipids (Fig. 2). The clustering of melittin’s charged and sterically bulky residues at one end of the helix leaves the helical face free to interact with zwitterionic membrane components, making melittin more sensitive to zwitterionic lipid topology than m2a, which has cationic residues that are distributed along its helical length.
Bacterial outer membrane leaflets contain anionic lipids, many of which have large head groups and inverse conical geometries (12, 18). Electrostatic interactions can force these lipids into close contact with the helical face of m2a, favoring pore formation through efficient pore leaflet fold packing (i.e., low γ) and forcing unfavorable high EL topological interactions. For example, in system C (DOPC:DPPC:DPPG), m2a is an efficient pore former, returning the highest relative ratio of pore activity (78%). Bacterial membranes also contain a large proportion of conical nonbilayer lipids, like DOPE (19), similar to Fig. 6B. These lipids induce membrane packing frustration into the bilayer. This lowers the barrier to pore formation (i.e., lower contribution from to render ER < 0). Notably, pore formation by m2a in system F occurred faster than in any other membrane system tested (Tables S1 and S2), although the reduced positive curvature lipid content compared with system C produced lower relative pore formation (54%). SI Materials and Methods contains a more detailed discussion of the implications of the model for the timings of PIE initiation for membrane systems A–G.
Table S1.
Melittin PIE initiation timings
| Membrane system | Lipid composition | Vesicle size (µm ± SD) | Pore initiation (s ± SD) | Bursting initiation (s ± SD) | Carpet initiation (s ± SD) |
| A | DOPC:DPPC:chol | 25 ± 9 | 1,615 ± 744 | 1,125 ± 723 | 1,203 ± 728 |
| B | DOPC:DPPC:DOPG | 19 ± 6 | 451 ± 175 | 616 ± 227 | 527 ± 328 |
| C | DOPC:DPPC:DPPG | 17 ± 6 | 2,541 ± 1,375 | 1,418 ± 879 | 2,554 ± 2,921 |
| D | DOPC:DOPE:DOPG | 19 ± 8 | 1,557 ± 1,217 | 1,187 ± 596 | 1,650 ± 1,258 |
| E | DOPC:DOPE:POPG | 12 ± 7 | 4,351 ± 1,672 | 4,367 ± 2,244 | N/A |
| F | DOPC:DOPE:DPPG | 12 ± 5 | 4,042 ± 2,575 | 3,736 ± 2,596 | 258 ± 232 |
| G | DOPC:DOPE:LPG | 17 ± 7 | 2,223 ± 1,366 | 1,795 ± 2,170 | 1,812 ± 1,852 |
Average PIE initiation times for the biomimetic lipid systems A–G after exposure to 1 µM of the LCAMP melittin. The average initiation times for bursting, pore-mediated leakage, and carpet mechanism are listed together with their SDs from at least three independent experiments. chol, cholesterol; N/A, not available.
Table S2.
m2a PIE initiation timings
| Membrane system | Lipid composition | Vesicle size (µm ± SD) | Pore initiation (s ± SD) | Bursting initiation (s ± SD) | Carpet initiation (s ± SD) |
| A | DOPC:DPPC:chol | 20 ± 7 | 3,036 ± 2,427 | 2,215 ± 2,149 | 2,906 ± 2,204 |
| B | DOPC:DPPC:DOPG | 19 ± 8 | 1,815 ± 1,087 | 1,594 ± 1,939 | N/A |
| C | DOPC:DPPC:DPPG | 25 ± 8 | 3,255 ± 1,620 | N/A | 1,770 ± 1,657 |
| D | DOPC:DOPE:DOPG | 16 ± 6 | N/A | N/A | N/A |
| E | DOPC:DOPE:POPG | 19 ± 8 | N/A | 525 ± 531 | 1,242 ± 1,683 |
| F | DOPC:DOPE:DPPG | 14 ± 6 | 290 ± 302 | 4,407 ± 2,038 | 1,808 ± 2,183 |
| G | DOPC:DOPE:LPG | 20 ± 8 | 3,192 ± 3,033 | 1,678 ± 2,438 | 188 ± 60 |
Average PIE initiation times for the biomimetic lipid systems A–G after exposure to 1 µM of the LCAMP m2a. The average initiation times for bursting, pore-mediated leakage, and carpet mechanism are listed together with their SDs from at least three independent experiments. chol, cholesterol; N/A, not available.
Mammalian cells maintain zwitterionic outer membrane leaflets composed of bilayer lipids with large head groups, like phosphatidylcholine, and contain cholesterol (18), a membrane component completely absent from bacteria (similar to Fig. 6A). Within mammalian membranes, m2a is free from forced electrostatic interactions and can associate with lipids that minimize its raft energy (Fig. 2). m2a pore activity is notably suppressed in the mammalian biomimetic membrane (system A), where the peptide is expected to form cholesterol-enriched rafts through π-stacking interactions with its phenylalanine residues (28, 29). This puts a negative curvature, high-hydrophobic volume lipid into association with the peptide, which will not efficiently pack the pores leaflet fold (i.e., high γ), both lowering raft energy (i.e., low EL) and disfavoring pore formation. m2a was generally less active than melittin; however, in membranes with specific charge and topologies, it is capable of fast and efficient pore formation.
SI Materials and Methods
Chemicals and Reagents.
All lipids were purchased from Avanti Polar Lipids and used without further purification; 3-kDa dextran-AlexaFluor488 fluorescent dye was purchased from Molecular Probes (Life Technologies Ltd.). Melittin and m2a were obtained from Sigma-Aldrich Company Ltd. Sylgard 184 polydimethylsiloxane (PDMS) monomer and curing agent were purchased from Dow Corning.
Microfabrication.
The device was manufactured as a PDMS cast from a silicon and SU8 master mold, as described in ref. 23, using standard photolithographic techniques. A plan view of the device is shown in Fig. S1A.
Microtrap geometry was characterized by SEM, shown in Fig. S1C. The device was plasma bonded to the conductive surface of an indium tin oxide (ITO)-coated microscope slide and then incubated overnight at 4 °C in a 0.1% BSA solution, coating the channels and microtrap arrays with BSA. The chip was then flushed with a solution of 100 mM glucose and 5 mM Hepes (pH adjusted to 7.4 using 25% KOH).
GUV Electroformation.
Vesicles were manufactured using modified versions of previously established protocols (39, 40), with lipid mixes of different charges and topographies as described in Table S3.
Table S3.
Membrane lipid compositions
| System | Lipid composition (60:40:40 mol %) | Melittin vesicles* | m2A vesicles* |
| A | DOPC:DPPC:chol | 121 | 354 |
| B | DOPC:DPPC:DOPG | 86 | 76 |
| C | DOPC:DPPC:DPPG | 71 | 59 |
| D | DOPC:DOPE:DOPG | 167 | 34 |
| E | DOPC:DOPE:POPG | 47 | 46 |
| F | DOPC:DOPE:DPPG | 44 | 55 |
| G | DOPC:DOPE:LPG | 138 | 35 |
The third and fourth columns indicate the numbers of GUVs exposed to melittin and m2a, respectively. chol, cholesterol.
Total number of vesicles from at least three experimental runs.
A 3.75 mg/mL solution of the desired lipids (60:40:40 mol %) in 95:5% chloroform:acetonitrile was spin-coated at 400 rpm onto the conductive surface of an ITO-coated glass slide, using a Headway Research Inc. PW32-PS-R790 spin coater. The slide was dried under vacuum for 90 min to remove all traces of organic solvent. The slide was then placed lipid film side down onto the PDMS device using a bespoke clamp, creating a central electroformation chamber. A solution of 100 mM sucrose, 5 mM Hepes (pH adjusted to 7.4 using 25% KOH), and 10 µM 3 kDa dextran-AlexaFluor488 was injected into the central chamber. An alternating current (AC)-field sequence was then applied across the two slides, as shown in Table S4.
Table S4.
Electoformation field parameters
| Frequency, Hz | Voltage (Vpp) | Waveform | Duration (m) |
| 10 | 0.1 | Sine | 10 |
| 10 | 0.5 | Sine | 20 |
| 10 | 1.0 | Sine | 30 |
| 10 | 1.6 | Sine | 120 |
| 3 | 2.0 | Square | 60 |
The AC electroformation pulse parameters for GUV production. The square wave field was applied to detach the GUVs from the slide. Electroformation for DPPC-containing GUVs (systems A, B, and D) were carried out at 60 °C, above the transition temperature of DPPC, to ensure mixing of the lipid components; 0.05 mol% 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE)-rhodamine was included within all lipid mixes to enable fluorescent visualization of the vesicles.
Care was taken to osmotically match the interior sucrose and external glucose solutions using a microosmometer (Advanced Instruments) to avoid osmotic stress on the GUVs. The vesicles produced were predominantly unilamellar (>90%) and typically 10–30 µm in diameter. The mammalian biomimetic GUVs (system A) displayed lateral phase separation between liquid-disordered DOPC and liquid-disordered DPPC domains (Fig. S1B). This phase separation replicates the in vivo lipid rafts that exist in mammalian cell membranes (18).
Experimental Protocol.
The device was loaded with vesicles as described in ref. 23 and was washed using isosmotic solution from the flow channel, which resulted in a high-density array of dye-loaded GUVs gently pinned against PDMS traps by microfluidic lamellar flow (shown in Fig. S1D). A 100 mM glucose and 5 mM Hepes (pH adjusted to 7.4 with KOH) solution doped with 1 µM either m2a or melittin was then flowed over the entrapped vesicles. The concentration of 1 µM is below the range of minimum inhibitory concentrations reported for both peptides in various bacterial strains (5, 10, 13). Because of the conditions of LCAMP binding within the microfluidic device, the use of a low concentration of peptide allowed helices to slowly accumulate within the vesicle outer leaflet, delaying the onset of LCAMP PIEs. The slow adsorption of peptides onto the GUVs membrane allowed details regarding their activity to be revealed, which may have been obscured by faster peptide addition. Data for the 3 kDa dextran-AlexaFluor488 were collected at a frequency of 0.25 Hz using a Zeiss LDM 5 Live confocal microscope (Carl Zeiss Ltd.). All data presented within the paper are from a minimum of three experimental runs.
Data Processing.
The fluorescence intensity data were corrected for background fluorescence and photobleaching as shown in Fig. S2. The data were then normalized for a better comparison between different measurements.
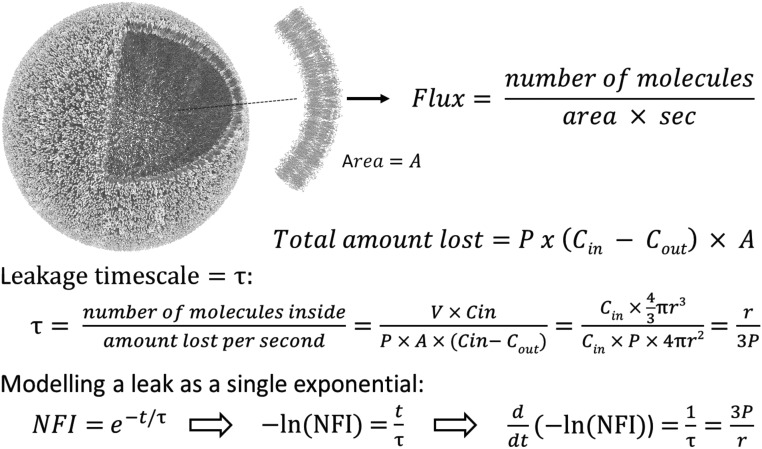
Analysis of Pore-Mediated Leakage Dynamics.
Fig. S8 outlines the analytical method for determining the apparent permeability of a GUVs membrane by monitoring the dye efflux process. In particular, there are two assumptions.
-
i)
The dye concentration inside (cin) is homogeneous, since the time for a molecule of 3 kDa dextran to diffuse a distance equal to an average GUV radius of r ∼ 10 µm is ∼2 s, while the monitoring rate is 0.25 s−1.
-
ii)
The dye concentration outside (cout) is equal to zero because of the continuous flow washing away any leaked dye.
To analyze the normalized fluorescence intensity (NFI), it is assumed that the differential loss of dye from a GUV of volume V equals the flux of dye through the membrane (of area A and apparent permeability P) occurring within the differential time dt. . It follows that the leakage dynamics can be described (instant by instant) by a single exponential: , where is the characteristic time of the process. In the case of multimode leakages, the above equation provides the opportunity to monitor the membranes’ apparent permeability during the changes in leakage dynamics as shown in Fig. 3 A–C and schematically described in Fig. S8. The NFI curves were analyzed, as described above, by means of a dedicated Labview (National Instruments) virtual instrument, which is available at DOI:10.5525/gla.researchdata.434.
The membranes’ apparent permeability showed grouping into allowed flux rates when compiled into histograms (Fig. 3). A Shapiro–Wilks test was performed, which showed that the data were not a random sampling of a normal distribution to a high degree of confidence for all groupings (P < 0.05).
PIE Classification.
LCAMP PIEs.
Fig. S3 presents the PIEs reported in the literature for LCAMP activity (6–9). For a vesicle to be categorized as belonging to a PIE, it had to meet the conditions given in the following sections, which describe the main forms of dye efflux processes (e.g., pore-mediated leakage, a detergent-like carpet mechanism, and vesicle bursting).
No effect.
GUVs can display no effect from peptide exposure over the experimental time course, defined as retaining 100% of the encapsulated dye, coupled to no loss in vesicle volume.
Pore-mediated leakage.
The literature describes two forms of pore-mediated leakage: (i) graded, where vesicles lose a portion of their encapsulated dye, and (ii) all or none, where vesicles lose 100% of their internal dye (41). To be classified as a pore-mediated leakage event, a GUV had to lose at least some of its contents, with no accompanying loss of vesicle volume. We found that all pore-mediated leakage events occurred via the all or none mechanism (shown in Fig. S4).
Fig. S4.
Time series of a pore-mediated leakage event occurring after exposure to 1 µM LCAMP m2a within a system C GUV. The event initiated after 200 s. Still images are taken from Movie S1. (Scale bar: 20 µm.)
Carpet mechanism.
Gradual dye efflux from a GUV accompanied by a loss of vesicle volume is characteristic of a carpet event, as shown in Fig. S5. Carpet mechanism is associated with the ejection of tightly curved lipid–peptide micelles from the membrane, which are responsible for the loss in vesicle volume.
Fig. S5.
Time series of a carpet mechanism leakage event within a system C exposed to 1 µM melittin. The event initiated after 2,800 s of exposure to peptide. Still images are taken from Movie S2. (Scale bar: 20 µm.)
Bursting.
Bursting events were classified as a total failure of a GUV’s membrane, resulting in instant total loss of vesicle contents and volume, as shown in Fig. S6.
Fig. S6.
Time series of a bursting event occurring after exposure to 1 µM m2a within a system F GUV. The event initiated after 168 s of exposure to peptide. Still images are taken from Movie S3. (Scale bar: 20 µm.)
Other.
A small proportion of vesicles (average ∼3%) displayed PIEs that did not classify according to the criteria described above. Some GUVs underwent a micellisation, which we defined as an instantaneous loss of both encapsulated dye and vesicle volume. Unlike bursting, where the vesicle membrane totally failed, after a micellization event, a new smaller vesicle remained. The authors have tentatively identified this event as the reformation of a vesicle after a bursting event.
Prepore regions.
Membrane-bound LCAMPs helices form dynamic lipid–peptide rafts within the outer membrane leaflet, continually exchanging lipids with the bulk membrane. Each lipid contributes to the raft energy through interactions with the peptides’ helical face, resulting in a heterogeneous population of rafts having different EL and γ. Lipids producing lower energy rafts (i.e., low σL and EL) will be retained longer than those producing higher energy rafts (27). Electrostatic interactions can force association between the cationic peptide and anionic lipids (12), increasing the frequency of high-energy rafts within the raft population. Lateral diffusion of the helices creates transient raft assemblies as shown in Fig. S7, a membrane region with the potential to form a pore. To satisfy the thermodynamic conditions for pore formation, the raft assembly must contain enough packing frustration that, when combined with the LCAMP-induced membrane thinning, it can overcome the integrity of the bilayer (i.e., render ER < 0) as shown in Fig. S7A. High-energy raft assemblies that do not satisfy the conditions for pore formation (e.g., rafts enriched with large-hydrophobic volume lipids) will react via other PIEs.
Hexagonal phase and leaflet fold packing.
The leaflet fold moiety of an idealized toroidal pore has a pronounced similarity with hexagonal phase packing structures adopted by positive curvature lipids in aqueous environments (35). Fig. S9 shows that both lipidic structures have tight positive curvature resulting from the bending of lipids from the head group toward the tail group. The pores’ leaflet fold moiety bends the outer and inner membrane leaflets together, while hexagonal phase packing is formed of tightly curved lipid cylinders.
PIE initiation timings.
The initiation times for the PIEs produced by m2a and melittin are presented in Tables S1 and S2 and discussed below in the context of the topological model for LCAMP activity.
Pore initiation.
Melittin induces fast pore formation in the membrane systems A (DOPC:DPPC:cholesterol), B (DOPC:DPPC:DOPG), and D (DOPC:DOPE:DOPG), displaying average initiation times of 1,614.8, 450.6, and 1,556.8 s, respectively. Large hydrophobic volume membrane components (i.e., DOPG and cholesterol) induce rapid but not necessarily numerous pore formation. In systems A (37%) and B (58%), pore formation is both rapid and numerous, but system D displays a lower event frequency of 17%. The discrepancy in pore frequency in these fast pore-forming membrane systems can be explained within the framework of the topological model through the consideration of the γ, σL, and EL values of the lipid–melittin rafts.
Lipid–melittin rafts enriched with DOPG and cholesterol possess high σG and EL values because of the synergy between lipid and peptide negative curvature topology, causing a high degree of packing frustration in the surrounding lipids. Raft assemblies of these high-energy rafts can quickly overcome the cohesiveness of the bilayer and induce quick PIEs. However, DOPG and cholesterol are inefficient packers of the pores rim because of their high hydrophobic volume, increasing γ. In system D, the helical face of melittin is enriched with DOPC, another lipid with high hydrophobic volume that will be inefficient at packing the pore. This creates a situation where a membrane-bound LCAMP helix forms high-energy rafts but cannot satisfy the conditions for pore formation because of a high γ. The lipid–peptide system will, therefore, react via another PIE rather than pore formation; in the case of system D, the carpet mechanism (47%) and bursting (33%) are preferred.
In systems A and B, the helical face of melittin will be enriched with the inverse conical lipid DPPC, a positively curved lipid that is an efficient packer of the leaflet fold structure, giving low γ and enhanced pore formation. Systems B and D differ only by the substitution of 20 mol% of the lipids DPPC and DOPE. System B (DPPC) shows the second fastest initiation of pore formation of any lipid–peptide system tested: over threefold faster than system D (DOPE). This shows the sensitivity of melittin to zwitterionic lipid topology caused by the charge distribution of melittin, leaving the helical face of the peptide free to interact with zwitterionic lipids.
Slowest pore formation for melittin occurred in systems C (2,541.0 s), E (4,351.4 s), and F (4,041.7 s). These membranes all form lipid–melittin rafts enriched with inverse conical anionic lipids: DPPG in system C, POPG in system E, and DPPG in system F. Although inverse conical lipids lower the pore energy (γ) through leaflet fold stabilization, they also act in opposition to a key factor controlling melittin activity—the large increase in hydrophobic volume caused by deep penetration into the outer membrane leaflet. The resulting decrease in σG and EL means more melittin monomers must bind to the membrane before sufficient membrane tension is achieved, leading to slower initiation of pore formation.
m2a shows significant pore activity in the membrane systems C (38%), F (42%), and G (14%), which return pore formation initiation times of 3,255.4, 289.5, and 3,191.7 s, respectively. System F (DOPC:DOPE:DPPG) shows the quickest initiation of pore-mediated leakage events for any of the lipid–LCAMP systems tested., Within system F membranes, m2a manipulates all three terms of the equation (γ, σG, and EL) and generates efficient pore formation. The electrostatically forced clustering of DPPG along the helical face of m2a lowers γ and increases both σG and EL. The positive curvature lipid DPPG stabilizes the pores leaflet fold structure, lowering the pore energy and favoring pore formation. The synergy with the positive curvature induction of m2a will result in a larger increase in leaflet area asymmetry per peptide monomer that binds to the membrane and generates a large amount of packing frustration around the membrane-bound peptide (i.e., high EL). σG will also be influenced by the presence of the nonbilayer lipid DOPE in the bulk membrane, which induces strain within bilayers (31) and reduces the negative work needed to open the pore structure. EL will be further increased by the steric crowding between the positive curvature anionic lipid DPPG and the sterically bulky F residues of m2a, generating high-energy lipid–m2a rafts. Together with the leaflet fold stabilization from the peptides’ three F residues, this combination of factors leads to rapid pore formation within system F vesicles, quickly rendering ER < 0 and favoring the formation of pores over other PIEs.
In a marked similarity with the melittin dataset, exchanging 20 mol % of the lipids DOPE and DPPC alters the initiation of pore formation for m2a. However, in contrast to melittin, it is the replacement of DPPC with DOPE that speeds pore formation rather than the converse. The selective m2a requires the nonbilayer lipid DOPE to weaken the membrane but can stabilize the pore leaflet fold structure caused by its three phenylalanine residues. The nonselective melittin destabilizes the membrane through its deeper penetration into the outer leaflet, but as the peptide contains only one tryptophan residue, it lacks the positive curvature stabilization of m2a and requires the presence of the inverse conical DPPC to generate low γ. The requirement for the presence of DOPE or DPPC to initiate the quickest pore formation is, therefore, reversed between the peptides (i.e., m2a requires DOPE to weaken the membrane, while melittin requires DPPC to form stable pore leaflet fold structures).
It is interesting to note that both melittin and m2a are capable of rapid pore formation in the membrane systems containing lipids characteristic of their intended targets; mammalian membranes are primarily composed of bilayer lipids, like DPPC (18), while bacterial membranes typical contain conical nonbilayer lipids, like DOPE (19). m2a is tuned by the relationship between its topological and charge properties and the lipid–peptide interactions with its membrane-bound helix to be selective for bacterial cells. It operates in the narrow gap between the lamellar to nonlamellar phase transition seen in bacterial membranes compared with mammalian membranes (27). m2a requires a more unstable membrane to initiate pore formation but under optimal conditions, forms rapid pores.
Carpet mechanism initiation.
System C (DOPC:DOPE:DPPG) shows the fastest initiation of the carpet mechanism PIE (258.3 s) when exposed to melittin, but the event is a low-frequency occurrence (10%), suggesting that it may be caused by relatively rare lipid–peptide interactions (e.g., the association of high-energy but low-frequency lipid–melittin rafts featuring DOPE). Melittin in conjunction with DOPE accumulates negative curvature within the membrane, stabilizing the formation of the tightly curved micellular structures, which are expelled from the membrane during carpet mechanism events. The membranes that generate the next two fastest initiation times for the carpet mechanism are mammalian biomimetic membrane system A (1,202.5 s) and bacterial biomimetic membrane system B (526.9 s), with both returning >30% event frequencies for the carpet mechanism. Both systems also feature a negative curvature membrane component that can be expected to become enriched within the lipid–melittin rafts. System A contains cholesterol, which will preferentially associate with the peptide through aromatic π-stacking interactions with melittin’s tryptophan residue (28, 29); system B contains the anionic lipid DOPG, which will associate through electrostatic interactions with the cluster of cationic residues at the peptides’ C terminus (12).
Conversely, m2a shows faster carpet mechanism events within membrane systems containing positively curved anionic lipids: systems E (DPPG) and G (LPG) return initiation times of 1,241.7 and 187.5 s, respectively. The rapid nature of carpet mechanism initiation in system G is particularly worthy of comment, being the fastest initiation of any PIE within any membrane system for either of the peptides. The association via electrostatics of the cationic positively curved m2a and the anionic highly positively curved LPG (S < 1/3) concentrates a large amount of positive curvature within the membrane: enough to stabilize the highly curved lipid–peptide micelles generated by the carpet mechanism. Membrane systems containing anionic lipids with larger hydrophobic volumes (i.e., DOPG in systems B and D) generate no carpet events, suggesting that clustering with unsaturated 18:1 (9Z) hydrocarbons is sufficient to suppress the carpet activity of m2a. The mammalian biomimetic GUV system A (DOPC:DPPC:cholesterol) generates the slowest carpet mechanism events through the enrichment of the lipid–m2a rafts with cholesterol. The presence of the negative curvature lipid cholesterol will counteract the positive curvature induction of m2a, preventing the accumulation of the curvature required to form micellular structures.
Image production.
The lipid and peptide rendered figures were assembled using two 3D modeling and lipid simulation tools: Lipidwrapper, an algorithm developed by Jacob Durrant at the University of California (42); and CHARMM, a program for the generation of molecular dynamics simulation models developed by the research group of Martin Karplus at Harvard University (43, 44). Pymol, an open source molecular visualization system, was used to generate the peptide models directly from Protein Data Bank files. Lipids and peptides were then assembled within Blender, an open source 3D creation suite, and rendered.
Conclusions
High-throughput analysis of dye leakage experiments of GUVs with different lipid compositions has enabled the in-depth study of the membrane-disrupting properties of LCAMPs. This has allowed us to propose the presence of a negative feedback system between membrane strain (σG) and pore opening that can be used to describe pore behavior. By using steric and electrostatic interactions as primary considerations, we have developed a model for LCAMP behavior that provides a predictive mechanism for activity and selectivity. The model has potential application in the rationale design of therapeutics, which are urgently required to combat the rapid spread of drug-resistant bacterial strains. By using the proposed models framework, LCAMPs can be designed to generate efficiently packed pores (low γ) together with high-leaflet area asymmetry (σG) and lipid–LCAMP interaction energy (EL) within a variety of membranes, providing the opportunity of tailoring peptide activity to specific bacterial membranes. We propose that lipids must be considered involved participants in the membrane disruption caused by LCAMPs instead of being merely passive in the process.
Materials and Methods
The materials and methods are described in detail within SI Materials and Methods. Briefly, GUVs were formed by in situ electroformation within the microfluidic device from seven different lipid compositions (A–G) of varying topology and charge (Table S3 for the composition of each system and Table S4 for the electroformation process parameters). System A is based on a typical mammalian membrane composition, and systems B–G are based on an Escherichia coli membrane, which is dominated by phosphatidylethanolamine (PE) and phosphoglycerol (PG) lipid species (38). Membrane topography was varied by changing the proportions of 16:0 and 18:1 fatty acid chains of the PG species and exchanging the PE head group for the larger phosphatidylcholine (PC) head group.
A fluorescent marker (AlexaFluor488-3k dextran) was incorporated within the GUVs, which were captured within a microfabricated trap array. Trapped GUVs were exposed to either a nonselective LCAMP (melittin) or a selective LCAMP (m2a) using microfluidic dispensation to precisely control both the duration of exposure and the final peptide concentration. GUVs were imaged during LCAMP exposure using confocal microscopy (Zeiss LSM 510 Live) at a data capture frequency of 0.25 frames per second (23). Activity was defined by the proportion of GUVs showing PIEs: pore-mediated leakage, bursting or carpet mechanism (defined as shown in Figs. S3–S7). Data were collated into characteristic profiles describing the frequency (percentage) of each of the three behaviors within the seven lipid compositions (A–G).
Supplementary Material
Acknowledgments
We thank Dr. Alasdair Clark (University of Glasgow) for SEM and the James Watt Nanofabrication Centre (University of Glasgow) for help with device fabrication. Research was funded by the Engineering and Physical Sciences Research Council Doctoral Training Centre (EPSRC DTC) Grant EP/F500424/1, Proxomics Grant EP/Io17887/1, and Frontiers Grant EP/K038885/1. M.T. acknowledges Royal Academy of Engineering/EPSRC Fellowship 10216/101. J.R. acknowledges the University of Glasgow Lord Kelvin and Adam Smith Research Fellowship. J.M.C. acknowledges support from EPSRC Personal Fellowship EP/K027611/1 and a European Research Council Advanced Grant Bio-Phononics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704489114/-/DCSupplemental.
References
- 1.Cohen ML. Changing patterns of infectious disease. Nature. 2000;406:762–767. doi: 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- 2. Center for Disease Control and Prevention (2013) Threat report: Antibiotic resistance threats in the United States (US Department of Health & Human Services, Atlanta), Technical Report CS239559-B.
- 3.Davies J. Bacteria on the rampage. Nature. 2006;383:219–220. doi: 10.1038/383219a0. [DOI] [PubMed] [Google Scholar]
- 4.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ani I, Zimmermann S, Reichling J, Wink M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine. 2015;22:245–255. doi: 10.1016/j.phymed.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;41:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 7.Raghuraman H, Chattopadhyay A. Melittin: A membrane-active peptide with diverse functions. Biosci Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki K, Sugishita K, Harada M, Fujii N, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of gram-negative bacteria. Biochim Biophys Acta. 1997;1327:119–130. doi: 10.1016/s0005-2736(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 9.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Haney EF, Hancock RE. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers. 2013;100:572–583. doi: 10.1002/bip.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oren Z, Shai Y. Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: Structure-function study. Biochemistry. 1997;36:1826–1835. doi: 10.1021/bi962507l. [DOI] [PubMed] [Google Scholar]
- 12.Epand EF, Maloy WL, Ramamoorthy A, Epand RM. Probing the “charge cluster mechanism” in amphipathic cationic antimicrobial peptides. Biochemistry. 2010;49:4076–4084. doi: 10.1021/bi100378m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dathe M, Wieprecht T, Nikolenko H, Bienert M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997;403:208–212. doi: 10.1016/s0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 14.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by K-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 15.Brogden KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Meer G, de Kroon AIPM. Lipid map of the mammalian cell. J Cell Sci. 2011;124:5–8. doi: 10.1242/jcs.071233. [DOI] [PubMed] [Google Scholar]
- 18.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta. 1999;1462:71–87. doi: 10.1016/s0005-2736(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 20.Batenburg AM, van Esch JH, de Kruijff B. Melittin-induced changes of macroscopic structure of phosphatidylethanolamines. Biochemistry. 1988;27:2324–2331. doi: 10.1021/bi00407a013. [DOI] [PubMed] [Google Scholar]
- 21.Ladokhin AS, Selsted ME, White SH. Sizing membrane pores in lipid vesicles by leakage of coencapsulated markers. Biophys J. 1997;72:1762–1766. doi: 10.1016/S0006-3495(97)78822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaki K, Yoneyama S, Miyajima K. Pore formation and translocation of melittin. Biophys J. 1997;73:831–838. doi: 10.1016/S0006-3495(97)78115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paterson DJ, Reboud J, Wilson R, Tassieri M, Cooper JM. Integrating microfluidic generation, handling and analysis of biomimetic giant unilamellar vesicles. Lab Chip. 2014;14:1806–1810. doi: 10.1039/c4lc00199k. [DOI] [PubMed] [Google Scholar]
- 24.Hebbar S, et al. A fluorescent sphingolipid binding-domain peptide probe interacts with sphingolipids and cholesterol-dependent raft domains. J Lipid Res. 2008;49:1077–1089. doi: 10.1194/jlr.M700543-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Callan-Jones A, Sorre B, Bassereau P. Curvature-driven lipid sorting in biomembranes. Cold Spring Harb Perspect Biol. 2011;3:a004648. doi: 10.1101/cshperspect.a004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerberg J, Koslov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 27.Almeida PF, Pokorny A, Hinderliter A. Thermodynamics of membrane domains. Biochim Biophys Acta. 2005;1720:1–13. doi: 10.1016/j.bbamem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front Physiol. 2013;4:1–9. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Xu J. Free cholesterol induces higher β-sheet content in Aβ peptide oligomers by aromatic interaction with Phe19. PLoS One. 2012;7:e46245. doi: 10.1371/journal.pone.0046245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahalka AK, Kinnunen PKJ. Binding of amphipathic α-helical antimicrobial peptides to lipid membranes: Lessions from temporins B and L. Biochim Biophys Acta. 2009;1788:1600–1609. doi: 10.1016/j.bbamem.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Lee M-T, Chen F-Y, Huang HW. Energetics of pore formation induced by membrane active peptides. Biochemistry. 2004;43:3590–3599. doi: 10.1021/bi036153r. [DOI] [PubMed] [Google Scholar]
- 32.Leontiadou H, Mark AE, Marrink SJ. Molecular dynamics simulations of hydrophilic pores in lipid bilayers. Biophys J. 2004;86:2156–2164. doi: 10.1016/S0006-3495(04)74275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelkar DA, Chattopadhyay A. Membrane interfacial localization of aromatic amino acids and membrane protein function. J Biosci. 2006;31:297–302. doi: 10.1007/BF02704101. [DOI] [PubMed] [Google Scholar]
- 34.Isrealachvili J, Mitchel J, Ninham B. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Faraday Trans 2. 1976;72:1525–1568. [Google Scholar]
- 35.Kumar VV. Complementary molecular shapes and the addivity of the packing parameter of lipids. Proc Natl Acad Sci USA. 1991;88:444–448. doi: 10.1073/pnas.88.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karatekhin E, et al. Cascades of transient pores in giant vesicles: Line tension and transport. Biophys J. 2003;84:1734–1749. doi: 10.1016/S0006-3495(03)74981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneggenburger PE, Beerlink A, Weinhausen B, Salditt T, Diederichsen U. Peptide model helices in lipid membranes: Insertion, positioning, and lipid response on aggregation studied by X-ray scattering. Eur Biophys J. 2011;40:417–436. doi: 10.1007/s00249-010-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oursel D, et al. Lipid composition of membranes of Escherichia coli by liquid chromatography/tandem mass spectrometry using negative electrospray ionization. Rapid Commun Mass Spectrom. 2007;21:1721–1728. doi: 10.1002/rcm.3013. [DOI] [PubMed] [Google Scholar]
- 39.Angelova M, Soleau S, Meleard P, Faucon JF, Bothorel P. Preparation of giant vesicles by external A.C. electric fields. Kinetics and applications. Prog Colloid Polym Sci. 1992;89:127–131. [Google Scholar]
- 40.Estes DJ, Mayer M. Giant liposomes in physiological buffer using electroformation in a flow chamber. Biochim Biophys Acta. 2005;17:152–158. doi: 10.1016/j.bbamem.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Wheaten SA, Lakshmanan A, Almeida PF. Statistical analysis of peptide-induced graded and all-or-none fluxes in giant vesicles. Biophys J. 2013;105:432–443. doi: 10.1016/j.bpj.2013.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durrant JD, Amaro RE. Lipidwrapper: An algorithm for generating large-scale membrane models of arbitrary geometry. PLOS Comput Biol. 2014;10:e1003720. doi: 10.1371/journal.pcbi.1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng Y, Shukla D, Pande VS, Roux B. Transition path theory analysis of c-Src kinase activation. Proc Natl Acad Sci USA. 2016;113:9193–9198. doi: 10.1073/pnas.1602790113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Versace RE, Lazaridis T. Modelling protein-micelle systems in implicit water. J Phys Chem B. 2015;119:8037–8047. doi: 10.1021/acs.jpcb.5b00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.