Significance
Large floristic datasets that purportedly represent the diversity and composition of the Amazon tree flora are being widely used to draw conclusions about the patterns and evolution of Amazon plant diversity, but these datasets are fundamentally flawed in both their methodology and the resulting content. We have assembled a comprehensive dataset of Amazonian seed plant species from published sources that includes falsifiable data based on voucher specimens identified by taxonomic specialists. This growing list should serve as a basis for addressing the long-standing debate on the number of plant species in the Amazon, as well as for downstream ecological and evolutionary analyses aimed at understanding the origin and function of the exceptional biodiversity of the vast Amazonian forests.
Keywords: Amazonia, floristics, rain forests, seed plants, species diversity
Abstract
Recent debates on the number of plant species in the vast lowland rain forests of the Amazon have been based largely on model estimates, neglecting published checklists based on verified voucher data. Here we collate taxonomically verified checklists to present a list of seed plant species from lowland Amazon rain forests. Our list comprises 14,003 species, of which 6,727 are trees. These figures are similar to estimates derived from nonparametric ecological models, but they contrast strongly with predictions of much higher tree diversity derived from parametric models. Based on the known proportion of tree species in neotropical lowland rain forest communities as measured in complete plot censuses, and on overall estimates of seed plant diversity in Brazil and in the neotropics in general, it is more likely that tree diversity in the Amazon is closer to the lower estimates derived from nonparametric models. Much remains unknown about Amazonian plant diversity, but this taxonomically verified dataset provides a valid starting point for macroecological and evolutionary studies aimed at understanding the origin, evolution, and ecology of the exceptional biodiversity of Amazonian forests.
The Amazon is renowned for harboring the world’s largest expanse of rain forest, which spreads across the Amazon, Orinoco, and Atlantic North Coast river basins (including Essequibo and Cuarantyne), as well as the Tocantins and the Western Atlantic hydrological basins (including Mearim). The exceptional species diversity of these forests, here referred to collectively as the Amazon rain forest, has long captured the attention of scientists and explorers alike aiming to understand the origins, evolution, and ecology of this rich biota and the processes that created and now maintain its hyperdiverse communities (1–13). Long-standing debates about the number and identity of seed plant species found in the region remain unresolved. The Amazon basin has been estimated to host up to 50,000 plant species, depending on which model is used and how the region is defined (5). Of these, between 6,000 and 16,000 species are predicted to be trees reaching ≥10 cm stem diameter at breast height (DBH) (5, 14).
The uncertainty surrounding Amazon rain forest plant species richness and identity compromises downstream science focused on conservation (15) and the evolutionary and ecological patterns and processes that drive biodiversity (10–12, 16), leaving studies dependent on incomplete and/or extrapolated datasets (e.g., refs. 9, 14, 17), often resulting in incomplete and irreproducible conclusions. Floristic lists can now be generated quickly for any region through automated data harvesting (e.g., refs. 14, 17, 18), using the increasing amounts of digitally available occurrence data from specimens stored in the world’s herbaria; however, such approaches do not necessarily provide reliable or complete data for undercollected areas, such as the Amazon rain forests. They are also prone to a myriad of errors, due principally to the high percentage of incorrectly named specimens in herbaria worldwide (19) and the compilation of lists over time as accepted names change but old synonyms are not weeded out. A slower yet more scientifically accurate approach is to produce floristic checklists based on herbarium records verified by taxonomic specialists with updated synonymy following the most recent taxonomic revisions. Such floristic checklists have been accumulating for small regions of the Amazon (e.g., refs. 20–22), but because of the sheer area, megadiverse character, and the multinational nature of the Amazon, progress has been slow. Moreover, there are very few taxonomists working in the region (23, 24), and the current scientific funding structure in many nations discourages botanical exploration and long-term taxonomic or floristic projects.
Recent advances in the floristics of Amazonian countries resulting in published checklists and online floristic catalogs mean that revised estimates of species richness can now be derived. Checklists based on voucher specimens verified by taxonomists form the foundation of biodiversity knowledge and are essential baselines for studies aiming to fully understand the number and identity of plants (23, 25). Here we present a list of seed plant species growing in the Amazon rain forest at ≤1,000 m elevation based on recent national floristic efforts in Brazil, Bolivia, and Colombia, combined with previously published checklists for Ecuador, Peru, Venezuela, and the Guiana Shield (26–36). Together, these checklists cover all Amazonian countries and compile the work of hundreds of taxonomic specialists using taxonomically validated voucher specimens. Our list includes all currently known seed plant species found in the lowland rain forest biome growing across a broad range of Amazonian vegetation types (periodically flooded, terra-firme, and white-sand forests), but it excludes species known exclusively from other major biomes (savannas, seasonally dry tropical forests, and montane biomes).
Results
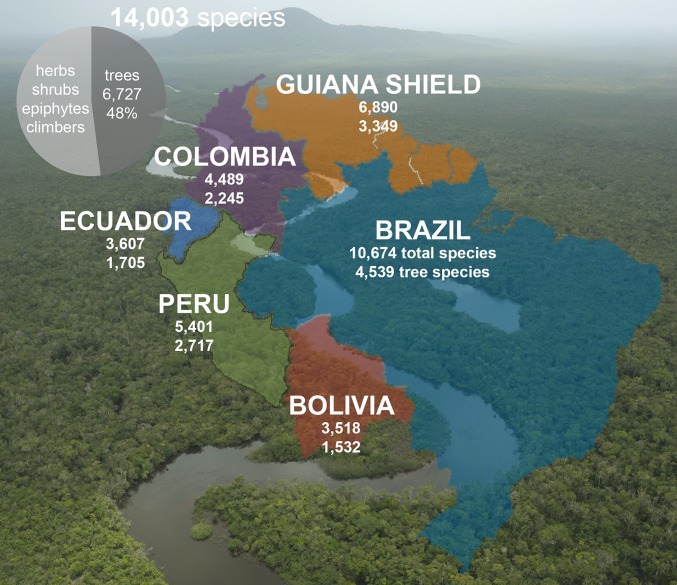
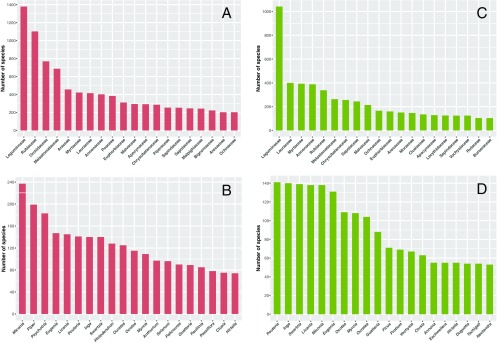
Our taxonomic dataset records 14,003 species, 1,788 genera, and 188 families of seed plants in the Amazonian lowland rain forest, with one-half of these trees that can reach ≥10 cm DBH (6,727 species, 48% of the total flora; 803 genera, 45% of the total genera; Fig. 1 and Dataset S1). More than one-half of seed plant species diversity in the Amazonian rain forests comprises shrubs, small trees, lianas, vines, and herbs (7,276 species, 52% of total flora). Leguminosae is the most species-rich family, with 1,379 species recorded, followed by Rubiaceae (1,102), Orchidaceae (769), Melastomataceae (687), Araceae (456), Myrtaceae (422), Lauraceae (415), Annonaceae (402), Poaceae (384), and Euphorbiaceae (311) (Fig. S1). Three of these top 10 families are exclusively herbaceous (Araceae, Orchidaceae, and Poaceae, except for bamboos such as Guadua species, which can attain diameters >10 cm DBH but are not defined as trees in most plot-based studies). The majority of species of two additional species-rich families are largely shrubby, herbaceous, or climbing (Melastomataceae and Rubiaceae; Dataset S1). The largest Amazonian seed plant genera are Miconia (237 species; Melastomataceae), Piper (199; Piperaceae), Psychotria (183; Rubiaceae), Eugenia (147; Myrtaceae), Licania (145; Chrysobalanaceae), Pouteria (141; Sapotaceae), Inga (140; Leguminosae), Swartzia (140; Leguminosae), Philodendron (128; Araceae), and Ouratea (125; Ochnaceae) (Fig. S1). Of these top 10 most species-rich genera, three are mainly herbs, shrubs, climbers, epiphytes, and/or small trees that do not normally reach ≥10 cm DBH (Piper, Philodendron, and Psychotria; Dataset S1).
Fig. 1.
Species richness of seed plants in the lowland Amazon rain forests (<1,000 m). Numbers of species are shown by country (and the Guiana Shield area) for all seed plants and for trees. The background shows the Amazon forest in Serra da Mocidade National Park, Brazil. Image courtesy of Ricardo Azoury (photographer).
Fig. S1.
Species-rich seed plant families and genera recorded in lowland Amazon rain forests. The 20 most species-rich families (A) and genera (B) of the entire flora, and the 20 most-species rich families (C) and genera (D) for trees only.
Of the 6,727 species of trees ≥10 cm DBH in our verified checklist of the Amazon rain forest, the 10 most species-rich families contain 55% of the total tree species: Leguminosae (1,042 species), Lauraceae (400), Myrtaceae (393), Annonaceae (388), Rubiaceae (338), Melastomataceae (263), Chrysobalanaceae (256), Sapotaceae (244), Malvaceae (214), and Ochnaceae (166) (Fig. S1). This pattern agrees strongly with all previous studies of the Amazon flora (9, 14, 37). The largest Amazonian genera in terms of tree species are Pouteria (141 species), Inga (140), Swartzia (139), Licania (138), Miconia (138), Eugenia (131), Ocotea (109; Lauraceae), Myrcia (108; Myrtaceae), Ouratea (104), and Guatteria (88; Annonaceae) (Fig. S1). These data also corroborate previous floristic and plot-based studies (9, 22, 38, 39).
Discussion
Source of Diverging Species Number Estimates in the Amazon.
Recent counts of global seed plant species diversity, calculated mainly from the World Checklist of Selected Plant Families (40), vary from 370,492 (41) to 296,462 (42). Thus, our estimate of 14,003 species from the lowland Amazon rain forests suggests that these forests shelter between 3.8% and 4.7% of all seed plant species. Taking into account the area of Amazonia (∼5,500,000 km2, corresponding to ∼3.6% of the global land surface), our estimate roughly matches the overall global species/area average for seed plants. Our taxonomically verified list suggests that the tree species diversity in the Amazon rain forests is lower than that found in other aggregation studies (5, 9, 14). The 6,727 tree species cataloged here, representing ∼11% of the 60,065 tree species estimated to occur worldwide (43), support estimates derived from nonparametric ecological models that predict ∼6,000–7,000 tree species for the Amazon basin and Guiana Shield (in ref. 14), ∼5,000–5,600 tree species for the forests of tropical America (44), and the recently published country-level checklist of trees for the world that lists 23,000 species of trees for all biomes and countries of the Neotropics, where a more relaxed definition of trees was used (height of ≥2 m or ≥5 cm DBH; ref. 43). Parametric ecological models used to estimate the numbers of trees (≥10 cm DBH only) have produced considerably higher estimates: ∼11,210 tree species for the Brazilian part of the Amazon basin alone (5), and up to ∼16,000 tree species for the entire Amazon basin (9). More recently, such high estimates were supported by ter Steege et al. (14), who listed 11,676 tree species compiled from forest inventory plots and specimen occurrence records retrieved from automated data harvesting of online platforms, but with limited taxonomic verification by specialists.
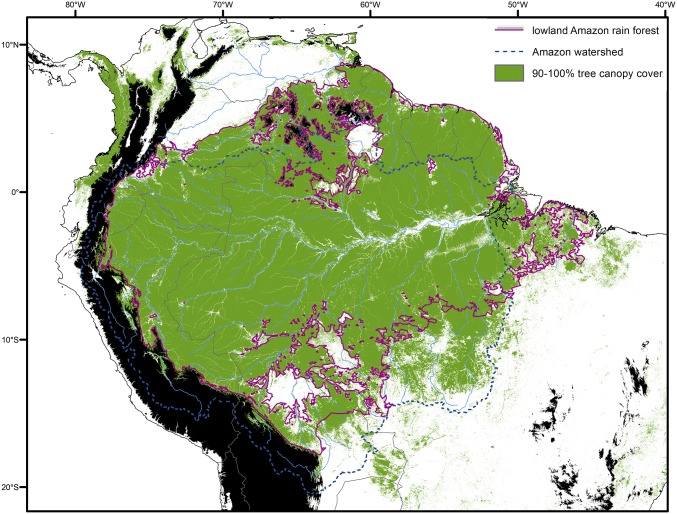
Incongruent species numbers can be the result of the different definitions of the Amazon. Previous studies (e.g., ref. 14) defined the Amazon as the entire watershed of the Amazon River, which includes several biomes and an elevation range of sea level to >4,000 m and encompasses huge variations in annual rainfall, temperature, and seasonality. We use a more biologically focused concept of the Amazon as a lowland rain forest biome occurring across the Amazon, Orinoco, and Atlantic North Coast river basins (including Essequibo, Cuarantyne, etc), as well as the Tocantins and Atlantic Western hydrological basins (including Mearim), with high above-ground biomass, relatively low seasonality, and high annual rainfall (Fig. 2). This definition is similar to that adopted in many ecological studies of the Amazon (e.g., refs. 6, 9–11, 13). Our definition of Amazonia excludes savannas and dry forests, as well as habitats at elevations >1,000 m, to focus on a single system within which organisms are thought to evolve and interact as a metacommunity over evolutionary time scales (48, 49).
Fig. 2.
Biologically meaningful delineation of the lowland rain forest biome across Amazonia (light-green outline). Areas >1,000 m elevation are shown in black (https://www2.jpl.nasa.gov/srtm/), major rivers are shown by light-blue lines, and the Amazon watershed itself is outlined with a dark-blue dotted line. Areas with >90% tree canopy cover are shown in green based on satellite data from 2000 (45). Our delineation (purple line) was derived by visualizing areas within the multiple watersheds ≤1,000 m elevation that have >1,300 mm annual mean rainfall [slightly below the threshold of Malhi et al. (46)], 18 °C minimum and 24 °C maximum annual mean temperature (lower limit follows the Koppen classification for tropical forests), and climatic water balance (precipitation minus potential evapotranspiration) >0 throughout the year. The northern limit shows complexity, with multiple excluded areas around the tepuis due to high elevation and/or low annual mean rainfall. Large areas highlighted in white, notably in northern Bolivia (Beni savanna/llanos de Moxos) and in the border area of Venezuela, Guyana, and Brazil (Guianan savannas), are excluded due to higher annual mean temperatures (>24 °C). Climatic data were obtained from ref. 47.
The analysis presented here represents our attempt to catalog Amazonian rain forest species of seed plants based entirely on taxonomically verified herbarium specimens or falsifiable reports. We argue that the comparatively low number of species presented here, supported by nonparametric ecological models (44), is closer to the actual number of seed plant species found in the Amazon than the higher estimates derived from parametric ecological models and unverified aggregated lists (9, 14). The basis for our argument is threefold: (i) the known proportion of tree species in neotropical lowland rain forest communities and current estimates of overall seed plant diversity both within Brazil and across the Neotropics; (ii) inflation of species number values in previous unverified aggregated lists; and (iii) issues related to the use of parametric ecological models that use regional-level plot data to estimate near-continental scale species diversity across the entire Amazon basin. We discuss these factors in detail below.
Known proportions of tree species.
Complete plot surveys have shown that ∼15–34% of species in neotropical lowland rain forests are trees ≥10 cm DBH (37, 50–54). This suggests that our checklist, listing 48% of all seed plants as trees, is potentially an overestimate of the number of tree species in the Amazon rain forest, or an underestimation of nontree species (e.g., due to a larger proportion of nontree species remaining to be described). Assuming that trees constitute ∼15–34% of plant species in a lowland neotropical forest community, estimates of ∼16,000 tree species from parametric ecological models imply a total Amazonian flora of 47,000–107,000 seed plant species. Such high numbers are not credible, given that only 34,215 seed plant species are currently listed for all of Brazil, including extensive diversity from dry habitats and the exceptional species diversity of the Atlantic forest (54–56). Projected estimates of seed plant species diversity range from 40,000 to 60,000 species for all of Brazil (54) and ∼90,000 species for the Neotropics as a whole, including montane, savanna, and dry habitats. Thus, it is likely that extremely high species diversity estimates derived from parametric ecological models are too high, even when considering that modeled numbers refer to all species (described and as-yet undescribed), while empirical lists such as ours refer to only currently recognized species (described only). While acknowledging that the taxonomic dataset presented here does not account for the vast areas of the Amazon that are still alarmingly undercollected (57, 58), the taxonomically verified, collection-based data do not support the argument that the Amazon rain forest holds >10,000 tree species.
Inflated values in previous aggregated checklists.
We argue that some previous numbers cited as evidence of higher species diversity are significantly inflated due to large numbers of taxonomic, geographic, and ecological errors. To demonstrate this, we evaluated three published lists (14, 17, 18). A total of 144 genera (23% of the total) included in the lists of Feeley and Silman (17) and Dexter and Chave (18) are either exclusively non-Amazonian, cultivated, herbs, epiphytes, climbers, shrubs, or treelets <10 cm DBH (Dataset S2). An even higher level of inaccuracy was found in the checklist of trees published by ter Steege et al. (14) at the species level, for which we exhaustively reviewed all names (9,346) listed for 55 plant families (80% of total names, 38% of total families) (Dataset S2). Our review shows that 40% (3,794 of 9,527) of the names listed in these families are mistakes. If the same trend applies to the entire list, this implies that ∼4,670 of the 11,676 total names listed by ter Steege et al. (14) are not correct, for various reasons (see below). If these errors are corrected, then a revised list comprises 7,006 tree species for the Amazon, greatly reduced from the original estimate of 11,676. The sources of error detected in the list of ter Steege et al. (14) include (i) 2,757 demonstrably non-Amazonian species (25% of the names in our exhaustively reviewed families); (ii) individual species listed more than once, as synonyms and spelling variants (786 names; 7% of the total); (iii) listing of nontree species, including herbs, shrubs, vines, and epiphytes (1,138 names; 11% of the total); (iv) the inclusion of Old World species not native to the Neotropics, probably due to database errors or perhaps to the listing of species from cultivation (96 names; 0.8% of the total); and (v) the inclusion of non-Amazonian cultivated species (53 names; 0.4% of the total). Examples of these erroneous citations include the listing of Nothofagus (Nothofagaceae, a family endemic to southern temperate regions); several endemic Australian species of Acacia, the Southern Magnolia or Bull Bay (Magnolia grandiflora), a widely cultivated species native to the southeastern United States; species endemic to the high-elevation Andes; species characteristic of South American seasonally dry tropical forests and the frost-prone Chaco; emblematic endemics from the Brazilian coastal Atlantic forest (e.g., the Brazilwood Paubrasilia echinata, the palmito-juçara Euterpe edulis, the jequitibá Cariniana legalis, and the genera Arapatiella, Curitiba, Harleyodendron, and Neomitranthes); the confusing duplicate listing of species under generic names treated as synonyms for at least 100 y (e.g., Acinodendron, Aulomyrcia, Uragoga), with Myrcia guianensis and Myrcia splendens collectively listed 24 separate times under different synonyms; as well as the inclusion of widely cultivated crop and ornamental species native to the Old World (e.g., Cassia javanica, Corymbia torelliana, Moringa oleifera, Solanum macrocarpon). Perhaps the most striking error is the listing of many herbaceous taxa that cannot be considered trees, such as the herbs Diodella (Rubiaceae), Schwenckia (Solanaceae), Spigelia (Loganiaceae), and Zornia (Leguminosae); the straggly subshrubs Pluchea (Asteraceae) and epiphytes Hillia (Rubiaceae); and Psychotria and Solanum, both of which comprise species that are predominantly herbaceous or shrubby, as among the 10 most species-diverse tree genera in the Amazon. Most of the mistaken records in the list of Steege et al. (14) derive from aggregated databases compiled from herbarium data available online (3,698 species names; 85% of the 4,367 total errors detected). This is not surprising given the recent estimate that >50% of specimens from tropical regions in global herbaria are likely misnamed or have not had a name update to reflect the most current taxonomic treatment (19), and a taxonomic review of plot voucher specimens highlighting up to 50% misidentifications in some genera (16). Taxonomic vetting and updating of specimen records will undoubtedly lead to a significant reduction in species numbers and revision of names in the previously published lists of Amazonian tree species (14, 18). In addition, many databases contain numerous typographical errors; thus, basic editing should also reduce the number of species found by automated aggregation and counting.
Problems with the use of parametric ecological models.
Here we highlight some issues that have been previously raised concerning the use of Fisher’s alpha (59) from regional plot data to derive species diversity estimates on continental scales such as the Amazon. Data from ecological models using Fisher’s alpha have been used to explain the discrepancy between the currently recorded number of tree species and the expected ∼16,000 trees for the Amazon. Estimates from fitted mean rank-abundance data from established and exhaustively censused tree plots using Fisher’s log-series distribution predict that up to 6,000 tree species in the Amazon have populations of fewer than 1,000 individuals (9). Species with such low population sizes have been argued to be “undetectable” given the current low collection densities across the Amazon basin (57). Such parametric models have been criticized, however, because estimating species richness on a continental scale such as the entire Amazon from regional-level plot data through rank-log abundance distributions assumes that plant communities are homogeneous in species abundance and composition at the scale of sampling. It is clear that this assumption is violated for lowland rain forest communities due to biogeographic, environmental, and spatial structuring of these communities, as shown by the incomplete leveling off of the Fisher’s alpha curve for tropical America (44).
A recent test of the parametric approach based on Fisher’s alpha and rank-abundance curve with North American tree data clearly demonstrated that estimating species richness on a continental scale from rank-abundance plots constructed from limited sampling is prone to errors of up to ±40% (60). Furthermore, current ecological models do not incorporate spatially explicit range size estimates, an issue that has been discussed elsewhere (17). Both of these factors have the potential for greatly inflating or deflating estimates of species diversity using Fisher’s alpha. Estimating species richness based on the metabolic scaling theory provides an alternative not yet explored for the Amazon; however, a study in Central Africa has shown that while the species richness of the largest trees often predicts a nonnegligible share of total species richness, this relationship varies strongly across sites (61).
Challenging Checklists.
Our study highlights the importance of taxonomically verified checklists as baseline infrastructures for engaging in accurate science. Checklists and floras, when vouchered and verified taxonomically, provide a falsifiable list of names on which further knowledge can be built (16, 24, 25, 62). Until now, no taxonomically verified checklist of seed plants or trees has been available for the Amazon rain forest. As discussed above, previously published lists of Amazonian tree species have been based largely on unverified species lists harvested from large datasets that have not been adequately vetted to exclude non-Amazonian species, duplicated names due to synonymy and spelling variants, herbaceous and shrubby species that do not reach ≥10 cm DBH, Old World endemics, and exotic cultivated species.
We argue that the uncritical and naïve use of checklists that are rapidly and routinely assembled through aggregation of unverified data has serious implications for comparative biology. Although in theory this data mining approach enables rapid construction of preliminary regional and continental lists, it cannot be a substitute for expertly vetted checklists (62). To emphasize the perils of scientifically unsound and taxonomically unverified lists as the basis of ecological analyses, previous aggregated checklists including many non-Amazonian species, such as species from the high-elevation Andes, temperate Chile, and the Old World, would lead to extremely misleading conclusions on the phylogenetic community structure of lowland Amazonian forests. This would be the equivalent of listing elephants, kangaroos, and the Andean spectacled bear as present in the lowland Amazon rain forests, and drawing conclusions based on these erroneous data.
What has led to the use of the uncritically assembled aggregated lists even though country-level taxonomically verified checklists have already been published for all Amazonian countries? Currently, a significant gap—the taxonomic impediment—separates the users and producers of floristic inventories, that is, the wide research community focused on broader ecological and evolutionary research questions versus taxonomy-centered inventories (63). The ecological-evolutionary community requires biome-specific, ecology-driven catalogs, while floristic inventory projects focus on delivering smaller-scale checklists of verified, falsifiable taxon occurrence records. Most biological processes are thought to take place within the bounds of ecological metacommunities (e.g., biomes, vegetation types), leading to the need for biome-specific information, while floristic work is most effectively done within political boundaries to maintain manageable project sizes in relation to available funding, to coordinate with specialists, and to secure collecting permits (54, 64). This gap further reflects funding differentials, with significantly more resources currently invested in conducting ecological and evolutionary analyses of the Amazon flora than in building the underlying taxonomic and floristic datasets that fuel those analyses (65, 66).
This imbalance is now leading to an increase in the production and publication of large, and in our analysis, flawed floristic datasets (14, 17, 18) containing numerous inaccuracies that are then used to draw fundamental conclusions about the patterns, evolution, and function of Amazonian plant diversity. As discussed above, previously published lists of Amazon tree flora contain major errors (14, 17, 18), yet have been used in large-scale analyses to infer patterns and processes of historical community assembly (e.g., ref. 18). It is difficult to draw conclusions regarding the ecological and evolutionary processes that have led to the assembly and maintenance of the Amazon tree flora based on a list of 631 genera, which includes 140 (22%) that do not occur in the Amazon or are not trees and omits 332 tree genera known to occur in the Amazon. Better data are available, yet compiling these data is complicated and challenging, requiring ongoing revision and verification. With the list that we have assembled here, we hope to begin closing the gap between large-scale ecological analyses and floristic work.
Implications for Conservation and Future Work.
In this paper, we have collated the best available knowledge on the floristic composition of the lowland Amazon rain forest, including the most relevant data sources assembled by hundreds of plant taxonomists working to document and describe the flora of these extraordinary forests. By no means do we claim that the work of documenting and describing Amazonian plant diversity has been completed, but we have provided a much-needed, biome-focused floristic baseline for use by the scientific community at large. Representing the accumulated knowledge of the past decades is of fundamental importance to science, to enable robust analyses aimed at understanding the complex ecology and evolution of Amazon plant diversity. We believe that the numbers presented here, with 14,003 seed plant species including 6,727 trees, provide the current best species counts for seed plants recorded in the Amazon rain forest.
Ongoing taxonomic and floristic efforts across Latin America, such as the web-based platforms of the Catálogo de Colombia and Flora do Brasil (26, 28), are the best way to track progress and updates in near-real time, which is why we used them here. Given the collaborative and interactive format of these databases and digital resources, they will continue to be actively revised as knowledge continues to increase with the accumulation of studies based on increasing numbers of herbarium specimens, field work, and molecular data.
An important message derived from our study is that the flora of the lowland Amazon rain forest remains incomplete in terms of both collecting effort and taxonomic revision. Synergistic work by plant taxonomists, tropical ecologists, and parataxonomists (16, 67–70) is needed to complete the task of exploring, describing, revising, and mapping plant species across the vast expanses of the Amazon forests as anthropogenic habitat alteration proceeds in the region (64). It should be kept in mind that for many tropical plant groups, modern revisions are lacking and no specialists are available. To save time, parataxonomists are frequently asked to identify plants for inventory projects. They may be highly competent in the areas in which they were trained by specialists but are prone to errors in others, especially when working in regions outside their expertise or facing poorly known, rare taxa.
Vastly increased collecting in largely underrepresented sites across the Amazon basin is still required to increase our understanding of the flora (14, 57, 71, 72). New collections and examination of specimens already held in global herbaria lead to the recognition of new species and genera through detailed taxonomic monographic work (19, 25, 73, 74, 75). Approximately 582 new angiosperm species were published for the Brazilian Amazon during the 17 y between 1990–2006, while four times that many were described from non-Amazonian biomes in Brazil (73). Such low rates of species discovery in the Amazon compared with other biomes likely reflect low collection efforts, and demonstrate the need for increased investment to complete the task of cataloging the Amazon flora (54, 56, 64, 66). Intensified collecting efforts will undoubtedly continue to reveal additional diversity; for example, the number of species in the Reserva Ducke near Manaus in Brazil (76) nearly doubled over the course of 6 y of systematic collecting (22). The addition of Amazonian species in local areas within the basin itself is to be expected, but the gains in species numbers are unlikely to be on the same scale at the large, near-continental scale of the entire Amazon basin.
Detailed taxonomic monographic work is also needed to update the synonymy of species across countries and regions, leading to congruence in cases where different names are currently being used for a single species in each Amazonian country (19, 25, 74). To speed up taxonomic monographic work, more specimens should be exchanged among the Amazonian countries to allow verification of voucher specimens and better collaborative working. As taxonomic data accumulate, data on the geographic distribution and abundance of species are still needed to fully understand species distribution patterns within the lowland rain forests in Amazonia (72, 77, 78).
The species list presented here reflects our current state of knowledge. Many species remain to be cataloged, described, and recorded for the Amazon (56, 66). We hope that future projects can use expertly delivered, taxonomically verified checklists as a foundation for exploring the origin, evolution, ecology, and conservation of the Amazon’s spectacular species diversity. Our lower species number reported here (14,003 plant species) does not diminish the value of Amazon rain forest biodiversity; rather, it highlights the need for further exploration of the vast expanses of these still poorly collected forests. Much of the Amazonian flora remains undiscovered, and we need investment in taxonomy, herbarium collections, virtual herbarium platforms, and new collections through field work to provide the elements necessary for description and cataloging of the Amazon flora in its entirety, and to answer large scientific questions on the origin, evolution, and ecological processes that maintain these majestic forests.
Materials and Methods
We collated all published national-level floristic checklists for Amazonian countries, including Brazil, Colombia, Ecuador, Peru, Venezuela, and Bolivia (26–33), and the checklist for the Guiana Shield (Venezuela, Surinam, Guyana, and French Guiana; refs. 34–36). The data are derived from voucher specimens annotated by taxonomic specialists, taking into account the most current synonymy available. The largest part of the Amazonian flora, Brazil’s online checklist and ongoing flora project (www.floradobrasil.jbrj.gov.br), was followed for synonymy. New species of seed plants published since the publication of the reviewed checklists were added (73, 75). Subspecies, varieties, hybrids, and nonnative species were not counted. For 66 families (Dataset S1), the list was revised and updated based on the ongoing work by taxonomic specialists (47 families) and by nonspecialist taxonomists based on a monograph/taxonomic treatment written by a family specialist (19 families). Previously published lists of Amazonian tree species and genera (14, 18) were reviewed by comparing them against published data sources (refs. 26–36 and Dataset S2). Fifty-five families, accounting for 9,346 species (80% of the total listed; Dataset S2), were reviewed by taxonomic specialists to calculate data-quality estimates that were then extrapolated to the entire dataset. Details are provided in SI Materials and Methods.
SI Materials and Methods
We collated all published national-level floristic checklists for Amazonian countries, including Brazil, Colombia, Ecuador, Peru, Venezuela, and Bolivia (26–33), and the checklist for the Guiana Shield (Venezuela, Surinam, Guyana, and French Guiana; refs. 34–36). These checklists represent the ongoing work of hundreds of taxonomists worldwide, aiming to accurately describe and characterize the seed plant flora. The data are derived from voucher specimens annotated by taxonomic specialists, taking into account the most current synonymy available. The largest part of the Amazonian flora, Brazil’s online checklist and ongoing flora project (www.floradobrasil.jbrj.gov.br), was followed for synonymy in cases of disagreement between floristic works. New species of seed plants published since the publication of the reviewed checklists were added (73, 75). Subspecies, varieties, hybrids, and nonnative species were not counted. For 66 families—Achariaceae, Anacardiaceae, Annonaceae, Apocynaceae, Araceae, Araliaceae, Asteraceae, Begoniaceae, Bignoniaceae, Boraginaceae, Bromeliaceae, Burseraceae, Calophyllaceae, Cannabaceae, Caricaceae, Caryocaraceae, Chloranthaceae, Chrysobalanaceae, Clusiaceae, Combretaceae, Connaraceae, Costaceae, Dichapetalaceae, Droseraceae, Elaeocarpaceae, Eriocaulaceae, Euphorbiaceae, Humiriaceae, Hypericaceae, Lauraceae, Lecythidaceae, Leguminosae, Malpighiaceae, Melastomataceae, Meliaceae, Moraceae, Myristicaceae, Myrtaceae, Ochnaceae, Olacaceae, Oleaceae, Opiliaceae, Orchidaceae, Passifloraceae (excluding former Turneraceae), Peraceae, Peridiscaceae, Phyllantaceae, Picramniaceae, Picrodendraceae, Poaceae, Proteaceae, Putranjivaceae, Rhizophoraceae, Rubiaceae, Rutaceae, Sapotaceae, Simaroubaceae, Siparunaceae, Solanaceae, Ulmaceae, Urticaceae, Violaceae, Vitaceae, Vochysiaceae, and Zingiberaceae—the list was revised and updated based on the ongoing work by taxonomic specialists (47 families) and by nonspecialist taxonomists based on a monograph/taxonomic treatment written by a family specialist (19 families). This work included adding to existing checklists more recently described species, newly documented range extensions, and revised synonymies based on newer taxonomies, and covered 10,555 species (75% of the total). Angiosperm family names follow those of Angiosperm Phylogeny Group IV (79). Growth form data were taken from original checklists and herbarium labels.
The biologically meaningful concept of lowland Amazon rain forest used here refers to the tropical rain forest biome with high above-ground biomass, high annual rainfall, and low seasonality without evolutionary adaptation to fire, long dry season, low annual rainfall, or low temperatures that occur across the Amazon, Orinoco, and Atlantic North Coast river basins (including Essequibo, Cuarantyne, etc.), as well as the Tocantins and Atlantic Western hydrological basins (including Mearim). Thus, our definition excludes savannas, seasonally dry forests, and montane areas at elevations >1,000 m, to focus on a single system within which organisms are thought to evolve and interact as a metacommunity over evolutionary time scales (48, 49). Our circumscription of the Amazon rain forest differs from the Amazon phytogeographic domain used in the Flora do Brasil. All species that reach ≥10 cm DBH during their life were considered trees, following previous studies of Amazon tree species diversity (5, 14).
Delivering a biome-specific checklist poses many challenges, a major one being that most published checklists follow political boundaries and usually include many different biomes. Such checklists do not always record in which biome(s) particular species occur, and in cases where they do, circumscriptions of biomes based on explicit criteria can differ. To deliver a taxonomically verified checklist that considers synonymy, growth form, and ecological conditions, we downloaded data from published checklists, filtering for species occurring <1,000 m elevation. The following search criteria for the country-level checklists were used: Bolivia: status endemic, native, or naturalized; distribution Beni, Cochabamba, La Paz, Pando, Santa Cruz; region zonas bajas; vegetation zone Bosque humedo, bosque semideciduo chiquitano, campos amazonicos; elevation 0–1,000 m; Colombia: status native or naturalized; biogeographic region Amazonia; elevation 0–1,000 m; Ecuador: status endemic, introduced, or native; provinces Morona-Santiago, Napo, Pastaza, Sucumbios, Zamora-Chinchipe; regions “Amazonian,” “Andean and Amazonian,” “Coastal, Andean and Amazonian,” Galapagos and Amazonian,” Galapagos, Andean and Amazonian,” “Galapagos, Coastal, and Amazonian,” and “Galapagos, Coastal, Andean, and Amazonian”; elevation 0–1,000 m; Peru: department Amazonas, Cuzco, Huanuco, Junin, Loreto, Madre de Dios, Pasco, Puno, San Martin, Ucayali; region Amazonian; elevation 0–1,000 m.
Results of each country-specific download were passed through the flora R package (80) to link with the most updated synonymy according to Flora do Brasil. We then ran occurrence record search for all resulting accepted species names from GBIF using the rgbif R package (81). Searches were limited to georeferenced records without coordinate issues. Climatic variables were then extracted for each occurrence point for mean annual temperature, minimum temperature of the coldest month, and mean annual precipitation derived from the interpolated temperature (WORLDCLIM v.1.3 Rel. 3; ref. 82) and the radar detected precipitation variables (TRMM; www.ambiotek.com/1kmrainfall/). Median values for each species were used to identify outliers (i.e., non-Amazonian species) within our dataset; these outliers were deleted after verification by taxonomic specialists confirmed their absence from Amazonian lowland rain forests.
In addition, previously published lists of Amazonian tree species and genera (14, 18) were reviewed by comparing them against published data sources (26–36). Fifty-five families, accounting for 9,346 species (80% of the total listed; see the list of families above, except for Araceae, Begoniaceae, Bromeliaceae, Costaceae, Droseraceae, Eriocaulaceae, Orchidaceae, Poaceae, Vitaceae, and Zingiberaceae), were reviewed by taxonomic specialists to calculate data quality estimates that were then extrapolated to the entire dataset. The families reviewed in detail include some of the most species-rich and dominant groups in the Amazonian flora, represent all growth forms and occur across a broad range of vegetation types within the Amazon rain forest (periodically flooded, terra-firme, and white-sand forests). In cases when a single taxon was referred to by multiple names due to the listing of synonyms and/or spelling variants, these names were considered duplicated synonyms. In cases when a single name had to be synonymized with a name not already listed, these names were not counted as errors.
Supplementary Material
Acknowledgments
We thank all botanical collectors and taxonomists over the years for their efforts; Iván Jiménez, Toby Pennington, and two anonymous reviewers for providing insightful comments on the manuscript; and Tom Croat and Xavier Delannay (Araceae), Julio Schneider (Quiinoideae, Ochnaceae), and Thiago André (Costaceae, Zingiberaceae) for revising their specialist families. Financial support was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Research Productivity scholarships, to A.M.A., A.R., C.V.d.B., D.C., J.R.P., J.R.S., L.P.d.Q., M.T., N.R., P.F., R.C.F., R.G., and R.M.-S.); Sistema de Informação sobre a Biodiversidade Brasileira, supporting the Flora do Brasil 2020 project; Programa de Apoio a Pesquisadores Emergentes da Universidade Federal da Bahia and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (process 23038.009148/2013-19, to D.C.); the Alexander von Humboldt Foundation and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/203.269/2016–JCNE and E-26/010.000990/2016–APq1, to M.T.); the National Science Foundation (Grant DEB-0316614, to S.K. and L.L.G. and Grant DEB-0818399, to F.A.M. and R.G.); the British Council [Researcher Link 127407379 and the Royal Society of London (International Exchange IE140292, to T.S.)]; Fundação de Amparo à Pesquisa do Estado de São Paulo (2015/50488-5, to D.C. and L.P.d.Q); and the Natural Environment Research Council (NE/N012488/1; to T.S. and P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706756114/-/DCSupplemental.
References
- 1.Humboldt AL, Bonpland A. Essai sur la Géografie des Plantes. Chez Levrault, Schoell; Paris: 1807. [Google Scholar]
- 2.Wallace AR. My Life: A Record of Events and Opinions. Chapman & Hall; London: 1905. [Google Scholar]
- 3.Gentry AH. Neotropical floristic diversity: Phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny? Ann Mo Bot Gard. 1982;69:557–593. [Google Scholar]
- 4.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton Univ Press; Princeton, NJ: 2001. [DOI] [PubMed] [Google Scholar]
- 5.Hubbell SP, et al. Colloquium paper: How many tree species are there in the Amazon and how many of them will go extinct? Proc Natl Acad Sci USA. 2008;105:11498–11504. doi: 10.1073/pnas.0801915105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kursar TA, et al. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc Natl Acad Sci USA. 2009;106:18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoorn C, et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli A, Sanmartín I. Why are there so many plant species in the Neotropics? Taxon. 2011;60:403–414. [Google Scholar]
- 9.ter Steege H, et al. Hyperdominance in the Amazonian tree flora. Science. 2013;342:1243092. doi: 10.1126/science.1243092. [DOI] [PubMed] [Google Scholar]
- 10.Baker TR, et al. Fast demographic traits promote high diversification rates of Amazonian trees. Ecol Lett. 2014;17:527–536. doi: 10.1111/ele.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauset S, et al. Hyperdominance in Amazonian forest carbon cycling. Nat Commun. 2015;6:6857. doi: 10.1038/ncomms7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho de Souza F, et al. Evolutionary heritage influences Amazon tree ecology. Proc Biol Sci. 2016;283:20161587. doi: 10.1098/rspb.2016.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dexter KG, et al. Dispersal assembly of rain forest tree communities across the Amazon basin. Proc Natl Acad Sci USA. 2017;114:2645–2650. doi: 10.1073/pnas.1613655114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ter Steege H, et al. The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Sci Rep. 2016;6:29549. doi: 10.1038/srep29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ter Steege H, et al. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci Adv. 2015;1:e1500936. doi: 10.1126/sciadv.1500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker TR, et al. Maximising synergy among tropical plant systematists, ecologists, and evolutionary biologists. Trends Ecol Evol. 2017;32:258–267. doi: 10.1016/j.tree.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Feeley KJ, Silman MR. Extinction risks of Amazonian plant species. Proc Natl Acad Sci USA. 2009;106:12382–12387. doi: 10.1073/pnas.0900698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dexter K, Chave J. Evolutionary patterns of range size, abundance and species richness in Amazonian angiosperm trees. PeerJ. 2016;4:e2402. doi: 10.7717/peerj.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin ZA, Harris DJ, Filer D, Wood JR, Scotland RW. Widespread mistaken identity in tropical plant collections. Curr Biol. 2015;25:R1066–R1067. doi: 10.1016/j.cub.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Mori S, et al. Guide to the Vascular Plants of Central French Guiana. New York Botanical Garden; New York: 1997. [Google Scholar]
- 21.Vásquez R. Flórula de las Reservas Biológicas de Iquitos, Perú. Missouri Botanical Garden; St. Louis: 1997. [Google Scholar]
- 22.Hopkins MJG. Flora da Reserva Ducke, Amazonas, Brasil. Rodriguésia. 2005;56:9–25. [Google Scholar]
- 23.Crane PR. Documenting plant diversity: Unfinished business. Philos Trans R Soc Lond B Biol Sci. 2004;359:735–737. doi: 10.1098/rstb.2003.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funk V. Floras: A model for biodiversity studies or a thing of the past? Taxon. 2006;55:581–588. [Google Scholar]
- 25.Thomas WW, Forzza RC, Leitman P, Michelangeli F, Giulietti AM. Large-scale monographs and floras? The sum of local floristic research. Plant Ecol Divers. 2012;5:217–223. [Google Scholar]
- 26. Jardim Botânico do Rio de Janeiro. Flora do Brasil 2020. Available at floradobrasil.jbrj.gov.br/. Accessed September 11, 2016.
- 27.Brako L, Zarucchi JL. 1993. Catalogue of the Flowering Plants and Gymnosperms of Peru. Monographs in Systematic Botany (Missouri Botanical Garden, St. Louis), Vol 45, pp 1–1286.
- 28.Bernal R, Gradstein SR, Celis M, editors. 2015 Catálogo de Plantas y Líquenes de Colombia (Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia). Available at catalogoplantasdecolombia.unal.edu.co.
- 29.Jørgensen PM, León-Yánez S, editors. 1999. Catalogue of the Vascular Plants of Ecuador. Monographs in Systematic Botany (Missouri Botanical Garden, St. Louis), Vol 75, pp 1–1182.
- 30.Jørgensen PM, Nee MH, Beck SG. 2014. Catalogo de las Plantas Vasculares de Bolivia. Monographs in Systematic Botany (Missouri Botanical Garden, St. Louis), Vol 127, pp 1–1741.
- 31.Ulloa C, Zarucchi JL, León B. Diez Años de Adiciones a la Flora del Peru: 1993-2003. Universidad Privada Antenor Orrego; Trujillo, Peru: 2004. [Google Scholar]
- 32.Ulloa C, Neill DA. Cinco Años de Adiciones a la Flora del Ecuador: 1999-2004. Missouri Botanical Garden; St. Louis: 2005. [Google Scholar]
- 33.Ulloa C, Neill DA. Adiciones a la Flora del Ecuador: Segundo Suplemento, 2005-2010. Fundación Jatun Sacha; Quito, Ecuador: 2011. [Google Scholar]
- 34.Funk V, Hollowell T, Berry P, Kelloff C, Alexander SN. 2007. Checklist of the Plants of the Guiana Shield (Venezuela: Amazonas, Bolívar, Delta Amacuro; Guyana, Surinam, French Guiana). Contributions from the United States National Herbarium (Department of Botany, National Museum of Natural History, Washington, DC), Vol 55, pp 1–584.
- 35.Hokche O, Berry P, Huber O. Nuevo Catálogo de la Flora Vascular de Venezuela. Fundación Instituto Botánico de Venezuela; Caracas, Venezuela: 2008. [Google Scholar]
- 36.Feuillet C. Checklist of the Plants of the Guiana Shield, 1: An update to the angiosperms. J Bot Res Inst Tex. 2009;3:799–814. [Google Scholar]
- 37.Gentry AH. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard. 1988;75:1–34. [Google Scholar]
- 38.Valencia R, Balslev H, Paz Y Miño C G. High tree alpha-diversity in Amazonian Ecuador. Biodivers Conserv. 1994;3:21–28. [Google Scholar]
- 39.ter Steege H, et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature. 2006;443:444–447. doi: 10.1038/nature05134. [DOI] [PubMed] [Google Scholar]
- 40.Kew Royal Botanical Gardens 2016 World Checklist of Selected Plant Families. Available at apps.kew.org/wcsp/prepareChecklist.do;jsessionid=6D94027836575767F7F8A626E1593CB9?checklist=selected_families%40%40244010920171649499. Accessed April 1, 2017.
- 41.Lughadha EN, et al. Counting counts: Revised estimates of numbers of accepted species of flowering plants, seed plants, vascular plants and land plants with a review of other recent estimates. Phytotaxa. 2016;272:82–88. [Google Scholar]
- 42.Christenjusz JM, Byng JW. The number of known plant species in the world and its annual increase. Phytotaxa. 2016;261:201–217. [Google Scholar]
- 43.Beech E, Rivers M, Oldfield S, Smith PP. GlobalTreeSearch: The first complete global database of tree species and country distributions. J Sustain For. 2017;36:454–489. [Google Scholar]
- 44.Slik JWF, et al. An estimate of the number of tropical tree species. Proc Natl Acad Sci USA. 2015;112:7472–7477, and correction (2015) 112:E4628–E4629. doi: 10.1073/pnas.1423147112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen MC, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. [DOI] [PubMed] [Google Scholar]
- 46.Malhi Y, et al. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc Natl Acad Sci USA. 2009;106:20610–20615. doi: 10.1073/pnas.0804619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deblauwe V, et al. Remotely sensed temperature and precipitation data improve species distribution modelling in the tropics. Glob Ecol Biogeogr. 2016;25:443–454. [Google Scholar]
- 48.Pennington RT, Lavin M, Oliveira-Filho A. Woody plant diversity, evolution and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu Rev Ecol Evol Syst. 2009;40:437–457. [Google Scholar]
- 49.Hughes C, Pennington RT, Antonelli A. Neotropical plant evolution: Assembling the big picture. Bot J Linn Soc. 2013;171:1–18. [Google Scholar]
- 50.Gentry AH. Patterns of neotropical plant species diversity. Evol Biol. 1982;15:1–84. [Google Scholar]
- 51.Foster RB. The floristic composition of the Río Manu Floodplain forest. In: Gentry AH, editor. Four Neotropical Rainforests. Yale Univ Press; New Haven, CT: 1990. pp. 99–111. [Google Scholar]
- 52.Foster RB, Hubbell SP. The floristic composition of the Barro Colorado Island Forest. In: Gentry AH, editor. Four Neotropical Rainforests. Yale Univ Press; New Haven, CT: 1990. pp. 85–98. [Google Scholar]
- 53.Hammel B. The distribution of diversity among families, genera, and habit types in the La Selva Flora. In: Gentry AH, editor. Four Neotropical Rainforests. Yale Univ Press; New Haven, CT: 1990. pp. 75–84. [Google Scholar]
- 54.Brazil Flora Group Growing knowledge: An overview of seed plant diversity in Brazil. Rodriguésia. 2015;66:1085–1113. [Google Scholar]
- 55.Forzza RC, et al. Catálogo de Plantas e Fungos do Brasil. Vol 1 Instituto de Pesquisas Jardim Botânico do Rio de Janeiro; Rio de Janeiro: 2010. [Google Scholar]
- 56.Forzza RC, et al. New Brazilian floristic list highlights conservation challenges. Bioscience. 2012;62:39–45. [Google Scholar]
- 57.Hopkins MJG. Modelling the known and unknown plant biodiversity of the Amazon Basin. J Biogeogr. 2007;34:1400–1411. [Google Scholar]
- 58.Schulman L, Toivonen T, Ruokolainen K. Analysing botanical collecting effort in Amazonia and correcting for it in species range estimation. J Biogeogr. 2007;34:1388–1399. [Google Scholar]
- 59.Fisher RA, Corbet AS, Williams CB. The relation between the number of species and the number of individuals in a random sample of an animal population. J Anim Ecol. 1943;12:42–58. [Google Scholar]
- 60.Ricklefs RE. How tree species fill geographic and ecological space in eastern North America. Ann Bot. 2015;115:949–959. doi: 10.1093/aob/mcv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bastin J-F, et al. Seeing Central African forests through their largest trees. Sci Rep. 2015;5:13156. doi: 10.1038/srep13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sosef MSM, et al. Exploring the floristic diversity of tropical Africa. BMC Biol. 2017;15:15. doi: 10.1186/s12915-017-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carvalho MR, et al. Taxonomic impediment or impediment to taxonomy? A commentary on systematics and the cybertaxonomic-automation paradigm. Evol Biol. 2007;34:140–143. [Google Scholar]
- 64.Medeiros H, et al. Botanical advances in southwestern Amazonia: The flora of Acre (Brazil) five years after the first catalogue. Phytotaxa. 2014;177:101–117. [Google Scholar]
- 65.Magallón S, et al. The influence of regional history and ecological affinity in the angiosperm composition of Mexican lowland tropical rainforests. In: Stevens WD, Montiel OM, Raven PH, editors. Paleobotany and Biogeography, A Festschrift for Alan Graham in His 80th Year. Missouri Botanical Garden; St. Louis: 2014. pp. 287–325. [Google Scholar]
- 66.Magnusson WE, et al. A linha de véu: A biodiversidade brasileira desconhecida. Parc Estrat. 2016;21:45–60. [Google Scholar]
- 67.Halme P, Kuusela S, Juslén A. Why taxonomists and ecologists are not, but should be, carpooling? Biodivers Conserv. 2015;24:1831–1836. [Google Scholar]
- 68.Basset Y, Novotny V, Miller SE, Pyle R. Quantifying biodiversity: Experience with parataxonomists and digital photography in Papua New Guinea and Guyana. Bioscience. 2000;50:899–908. [Google Scholar]
- 69.Sheil D, Lawrence A. Tropical biologists, local people and conservation: New opportunities for collaboration. Trends Ecol Evol. 2004;19:634–638. doi: 10.1016/j.tree.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Gotelli NJ. A taxonomic wish-list for community ecology. Philos Trans R Soc Lond B Biol Sci. 2004;359:585–597. doi: 10.1098/rstb.2003.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sousa-Baena MS, Garcia LC, Peterson AT. Completeness of digital accessible knowledge of the plants of Brazil and priorities for survey and inventory. Divers Distrib. 2014;20:369–381. [Google Scholar]
- 72.Oliveira U, et al. The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Divers Distrib. 2016;22:1232–1244. [Google Scholar]
- 73.Sobral M, Stehmann JR. An analysis of new angiosperm species discoveries in Brazil (1990-2006) Taxon. 2009;58:227–232. [Google Scholar]
- 74.Bebber DP, et al. Herbaria are a major frontier for species discovery. Proc Natl Acad Sci USA. 2010;107:22169–22171. doi: 10.1073/pnas.1011841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charity S, et al. 2016. Living Amazon Report 2016: A regional approach to conservation in the Amazon (WWF Living Amazon Initiative, Brasília, Quito)
- 76.Ribeiro JELS, et al. Flora da Reserva Ducke. Guia de Identificação das Plantas Vasculares de Uma Floresta de Terra Firme na Amazônia Central. Instituto Nacional de Pesquisas da Amazônia; Manaus, Brazil: 1999. [Google Scholar]
- 77.Feeley KJ, Silman MR. Keep collecting: Accurate species distribution modelling requires more collections than previously thought. Divers Distrib. 2011;17:1132–1140. [Google Scholar]
- 78.Hortal J, et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol Syst. 2015;46:523–549. [Google Scholar]
- 79.Angiosperm Phylogeny Group IV An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc. 2016;181:1–20. [Google Scholar]
- 80.Carvalho G. 2016 flora: Tools for interacting with the Brazilian Flora 2020. Available at www.github.com/gustavobio/flora. Accessed April 1, 2017.
- 81.Chamberlain S. 2016 rgbif: Interface to the global ‘biodiversity’ information facility API. R package version 0.9.5. Available at https://CRAN.R-project.org/package=rgbif. Accessed November 1, 2016.
- 82.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.