Significance
Engineered cardiac muscle can be used to promote the structural and functional maturation of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs). However, previous studies have not yet produced cardiac tissues with metabolic and proliferative maturation. Here, we develop a 96-well screening platform and screen for cardiac maturation conditions in engineered cardiac muscle. We found that simulating the postnatal switch in metabolic substrates from carbohydrates to fatty acids promoted a switch in metabolism, DNA damage response, and cell cycle arrest in hPSC-CM. Our study shows that this mechanism can be harnessed to enhance the maturation of human hPSC-CM and cardiac tissues, which has major implications for stem cell sciences, drug discovery, and regenerative medicine.
Keywords: heart development, regeneration, tissue engineering, pluripotent stem cells, metabolism
Abstract
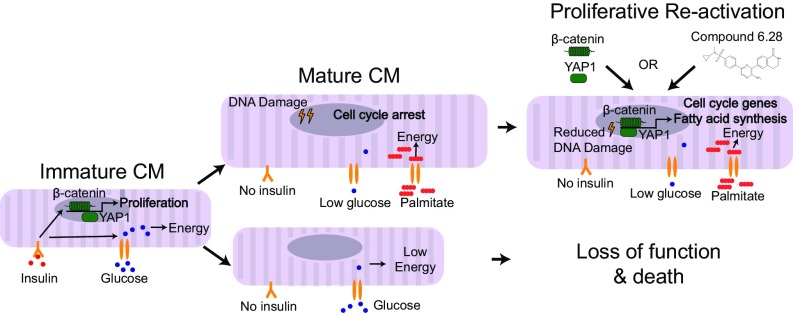
The mammalian heart undergoes maturation during postnatal life to meet the increased functional requirements of an adult. However, the key drivers of this process remain poorly defined. We are currently unable to recapitulate postnatal maturation in human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs), limiting their potential as a model system to discover regenerative therapeutics. Here, we provide a summary of our studies, where we developed a 96-well device for functional screening in human pluripotent stem cell-derived cardiac organoids (hCOs). Through interrogation of >10,000 organoids, we systematically optimize parameters, including extracellular matrix (ECM), metabolic substrate, and growth factor conditions, that enhance cardiac tissue viability, function, and maturation. Under optimized maturation conditions, functional and molecular characterization revealed that a switch to fatty acid metabolism was a central driver of cardiac maturation. Under these conditions, hPSC-CMs were refractory to mitogenic stimuli, and we found that key proliferation pathways including β-catenin and Yes-associated protein 1 (YAP1) were repressed. This proliferative barrier imposed by fatty acid metabolism in hCOs could be rescued by simultaneous activation of both β-catenin and YAP1 using genetic approaches or a small molecule activating both pathways. These studies highlight that human organoids coupled with higher-throughput screening platforms have the potential to rapidly expand our knowledge of human biology and potentially unlock therapeutic strategies.
Maturation of cardiomyocytes occurs during early postnatal life and imposes numerous adaptations, including electrophysiological, structural, and metabolic changes (1), which occur coincident with loss of proliferative capacity and regenerative potential (2, 3). The discovery of key upstream drivers of cardiomyocyte maturation and cell cycle arrest remains one of the most important unanswered questions in cardiac biology. Discovery of these drivers would facilitate current attempts to promote cardiomyocyte maturation in vitro for drug discovery and to dedifferentiate adult cardiomyocytes in vivo for regenerative medicine.
There are considerable changes in metabolic substrate provision during early postnatal life. The mammalian heart relies on high concentrations of carbohydrates and the presence of insulin in utero but later switches to fatty acid-dominated substrates present in milk and low insulin levels postbirth (4). To adapt to these changes in substrates, cardiomyocytes up-regulate the genes required for fatty acid oxidation after birth (5). The importance of these metabolic adaptations for cardiomyocyte maturation has been difficult to study, because genetic disruption of fatty acid oxidation components in vivo can have a broad range of negative health impacts (6). Therefore, there is a need to develop alternative approaches for studying the impact of cardiomyocyte metabolism on the maturation process.
Human pluripotent stem cells (hPSCs) are now widely used for the generation of defined human somatic cell types, including cardiomyocytes. Human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) have been used extensively for developmental studies, drug screening, disease modeling, and heart repair. However, lack of maturity and inappropriate responses to pharmacological agents have been identified as limitations in 2D or embryoid body-based differentiation strategies (7). In an effort to better simulate heart muscle structure and function, cardiac tissue engineering to form 3D engineered heart muscle has been used (8–14). These recent advances in human cardiac tissue engineering have greatly enhanced structural and functional maturation of hPSC-CMs. However, metabolic, transcriptional, and proliferative maturation have not yet been achieved.
We developed a 96-well device, the heart dynamometer (Heart-Dyno), for high-throughput functional screening of human pluripotent stem cell-derived cardiac organoids (hCOs) to facilitate screening for maturation conditions. The Heart-Dyno is designed to facilitate automated formation of dense muscle bundles from minimal cells and reagents, while also facilitating culture and automated force of contraction analysis without any tissue handling. Using the Heart-Dyno, we define serum-free 3D culture conditions that promote metabolic and proliferative maturation of hCOs. Furthermore, we uncover a metabolic mechanism governing hPSC-CM cell cycle arrest through repression of β-catenin- and Yes-associated protein 1 (YAP1)-dependent signaling.
Results
Heart-Dyno: A Miniaturized 96-Well hCO Screening Platform.
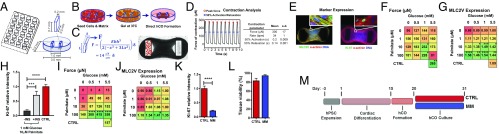
To facilitate the automated formation and analysis of cardiac organoids comprising dense muscle bundles, we used SU-8 photolithography and polydimethylsiloxane (PDMS) casting to fabricate a 96-well plate containing culture inserts (Fig. 1A). We designed the elliptical geometry, such that a 3.5-µL volume containing 50,000 cardiac cells would automatically condense around two elastic posts over 2 d, forming hCOs 1 mm in length (Fig. 1B and Fig. S1A). hPSC-derived cardiac cells are composed of ∼70% α-actinin+/CTNT+ hPSC-CMs, with the rest being predominantly CD90+ stromal cells (13). This ratio of hPSC-CMs to stromal cells is essential and optimal to form a functional hCO (13, 14). The elastic posts provide mechanical resistance to the contraction of the hCOs (Movie S1), which is required to enhance function (15). This design also allows contractile force to be approximated by tracking the movement of the poles (Fig. 1C and Movie S2), which we validated using force transducers (SI Methods). In addition, a custom-designed high-content imaging system was developed to capture 10-s videos of each hCO from each well at high speed (50 frames per 1 s). Video files were subsequently batch analyzed using a custom-written Matlab program to produce force traces and contraction data for each hCO (Fig. 1D). We further validated our 3D tissue culture and contraction analysis pipeline by assessing hCO responses to stimuli that alter the force of contraction (Fig. S1B) and prolong relaxation time (Fig. S1C). Importantly, the Heart-Dyno was able to predict physiological responses to known pharmacological agents, including compounds with human ether-a-go-go-related gene potassium channel (hERG) toxicity that entered the clinic and were subsequently withdrawn because of arrhythmogenic side effects (cisapride) (Fig. S1C).
Fig. 1.
The Heart-Dyno microtissue platform facilitates automated formation, mechanical loading, and analysis of hCOs. (A) Schematic representation of the 96-well plate Heart-Dyno. Each well has a culture insert containing an elliptical seeding well that contains two elastomeric posts. (B) Automatic tissue formation within the Heart-Dyno. hPSC-CMs and fibroblasts are seeded within ECM and allowed to gel for 30 min at 37 °C. Cells subsequently condense around the two elastomeric poles, resulting in the automated formation of an hCO. (C) Pole tracking can be used to approximate the force of contraction. (Left) Pole deflection schematic with force approximation formulas (SI Methods). Based on the parameters of our system, this results in a force equal to 14 µN/µm of postdeflection. (Right) Phase contrast image of a hCO; crosses on the left pole indicate tracking points selected by automated contraction analysis software. (D) Batch video analysis can be performed on each hCO to obtain contractile properties. (Left) Representative force trace curve. (Right) Overall contraction parameters ± SD from n = 31 hCO cultured for 7 d in CTRL medium. (E) Whole-mount immunostaining can be used to assess hCO marker expression for screening. Representative screening images with confocal close-up of MLC2v, Ki-67, and α-actinin immunostaining. (F) Screening identifies optimal glucose and palmitate concentrations for hCO force of contraction. Force heat map in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen. Force was assessed after 5 d of serum-free culture after 2 d of hCO formation in CTRL medium; n = 7–13 from three experiments; HES3-derived hCOs (SI Methods). (G) Palmitate induces MLC2v expression. MLC2v heat map in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen. MLC2v expression was assessed after 5 d of serum-free culture after 2 d of hCO formation in CTRL medium; n = 7–13 from three experiments; HES3-derived hCOs. MLC2v expression is relative to control serum-free conditions (5.5 mM glucose, no palmitate). (H) Ki-67 expression is induced by insulin. hCO were assessed after 11 d of serum-free culture after 5 d of hCO formation in CTRL medium; n = 12–13 from three experiments; HES3-derived hCOs. Ki-67 expression is relative to CTRL medium. (I) Screening identifies optimal glucose and palmitate levels in the absence of insulin. Force heat map in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen without the presence of insulin. Force was assessed after 11 d of serum-free culture after 5 d of hCO formation in CTRL medium; n = 6–15 from three experiments; HES3-derived hCOs. (J) Palmitate induces MLC2v expression. MLC2v heat map in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen in the absence of insulin. MLC2v expression was assessed after 11 d of serum-free culture after 5 d of hCO formation in CTRL medium; n = 6–9 from three experiments; HES3-derived hCOs. MLC2v expression is relative to control serum-free conditions (5.5 mM glucose, no palmitate). (K) Ki-67 expression is reduced in serum-free medium containing 1 mM glucose and 100 μM palmitate without insulin (now termed MM). hCOs were assessed after 11 d of serum-free culture after 5 d of hCO formation in CTRL medium. Ki-67 expression is relative to CTRL medium; n = 6–8 from two experiments; HES3-derived hCOs. (L) Culture of hCO maintains high tissue viability in CTRL medium or MM after 11 d of culture after 5 d of hCO formation in CTRL medium. Viability is defined as a live intact tissue with no deformities; n = 16 experiments with 733 and 717 tissues, respectively; HES3-derived hCOs. (M) Culture schematic of finalized cardiac maturation protocol indicating timing and duration of hPSC expansion, cardiac differentiation, and hCO formation and culture in CTRL medium or MM. Data are mean ± SEM; SI Methods has data variability for the heat maps (F, G, I, and J). #P = 0.07; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001 using one-way ANOVA with Tukey’s posttest (H), Dunnet’s posttest relative to 5.5 mM glucose and no palmitate (F, G, I, and J), or t test (K).
Fig. S1.
Development, optimization, and characterization of Heart-Dyno (supporting data for Fig. 1). (A) Photomicrographs of Heart-Dyno culture inserts with hCOs in a 96-well plate. (B) Force of contraction responses to known repressors/activators of force of contraction; n = 5. (C) Proarrhythmogenic compounds cause prolonged relaxation time in hCO as expected; n = 5. (D) Validation of Ki-67 marker activation using whole-mount immunostaining; n = 5–7 from two experiments. hCOs treated with 0.05% DMSO and 5 µm CHIR 99021. (E) Lactate dehydrogenase (LDH) levels in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen in the presence of insulin. LDH levels were assessed after 5 d of serum-free culture after 2 d of hCO formation in CTRL medium; n = 8–9 from three experiments (HES3-derived hCOs). (F) Cardiac troponin (CTNI) levels for serum-free conditions. CTNI levels were assessed after 5 d of serum-free culture after 2 d of hCO formation in CTRL medium; n = 2–3 from one experiment (HES3-derived hCOs). −VE, negative control; +VE, positive control (mechanically crushed hCO). (G) Whole-mount images of hCOs stained with α-actinin that mechanically failed from broken arms (Upper) and necking (Lower) issues after 5 d of serum-free culture after 2 d of hCO formation in CTRL medium. (Scale bars: 200 μm.) (H) Force heat map in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen. Force was assessed after 5 d of serum-free culture after 2 d of hCO formation in CTRL medium; n = 8–12 tissues from three experiments (H9-derived hCOs). (I) Whole-mount MLC2v expression in response to a full factorial glucose (0, 0.5, 1, and 5.5 mM) and palmitate (0, 1, 10, and 100 μM) screen. MLC2v expression was assessed after 5 d of serum free culture after 2 d of hCO formation in CTRL medium; n = 8–12 from three experiments (H9-derived hCOs). MLC2v expression is relative to control serum-free conditions (5.5 mM glucose, no palmitate). (J) TGFβ-1 is detrimental to hCO function. Force heat map in response to TGFβ-1 concentration (0, 1, 3, and 10 ng/mL) and duration (days 2–5, 2–7, and 2–9). Force was assessed after 10 d of serum-free culture after 2 d of hCO formation in CTRL medium; n = 13–16 from three experiments (H9-derived hCOs). (K) Increased collagen I and Matrigel improve tissue viability. Tissue viability was assessed after 10 d of serum-free culture following 2 d of hCO formation in CTRL medium. n = 18 from three experiments (H9-derived hCOs). Low Col = 1.6 mg/mL collagen matrix, High Col = 3.2 mg/mL collagen matrix, High Col + MG = 2.6 mg/mL collagen plus 9% (vol/vol) Matrigel matrix. SF, Serum -free medium (1 mM Glucose, 10 μM Palmitate with insulin). (L) Influence of the amount and composition of ECM on the force of hCO. Tissue viability was assessed after 10 d of serum free culture after 2 d of hCO formation in CTRL medium; n = 6–15 from three experiments (H9-derived hCOs). Data are mean ± SEM. High Col, 3.2 mg/mL collagen matrix; High Col + MG, 2.6 mg/mL collagen plus 9% (vol/vol) Matrigel matrix; Low Col, 1.6 mg/mL collagen matrix; SF, serum-free medium (1 mM glucose, 10 μM palmitate with insulin). *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001, one-way ANOVA plus Dunnet’s posttest relative to baseline or 0.2 mM Ca2+ (B and C); t test (D) with Dunnet’s posttest relative to no TGFβ-1 (J); or using ANOVA comparing either CTRL medium or SF groups only with Tukey’s posttest (L).
In addition to semiautomated analyses of force of contraction, we also developed a protocol for postanalysis of hCOs for the expression of different markers using whole-mount immunostaining combined with high-content image analysis (Fig. 1E). We validated this approach using α-actinin and Ki-67 staining to detect the proproliferative effects of glycogen synthase kinase (GSK3) inhibition using CHIR99021 (16) (Fig. S1D). These initial studies validated the Heart-Dyno as a high-throughput, high-content screening platform that facilitates chronic stimulation as well as analysis of contractile properties and marker expression.
Screening for Optimal Metabolic Substrates for hCO Maturation.
We next determined whether switching metabolism from glycolysis to fatty acid oxidation could induce hCO maturation. We screened a full factorial interplay between glucose and palmitate on cardiac maturation in serum-free medium. We chose to use palmitate as a fatty acid substrate, as it is one of the most abundant fatty acids circulating during the neonatal period, representing 36% of all long-chain free fatty acids (17). Cardiac maturation was assessed via three primary readouts: cardiac function (assessed by force of contraction), hPSC-CM proliferation as a marker of immaturity (assessed by Ki-67 expression), and expression of ventricular myosin light-chain 2 (MLC2v) as a maturation marker (18).
The hCO force of contraction showed a trend to increase with the addition of 10 and 100 μM palmitate under serum-free conditions (pooling all glucose concentrations for each palmitate concentration: P = 0.007 and P = 0.07 compared with 0 μM palmitate, respectively). The highest forces were produced within medium containing 1 mM glucose and 10 or 100 μM palmitate (Fig. 1F). Concurrently, MLC2v expression also increased with the addition of palmitate (Fig. 1G). All hCOs cultured in 100 μM palmitate in addition to hCOs cultured in 1 mM glucose with 10 μM palmitate had increased MLC2v expression relative to serum-free controls (5.5 mM glucose without palmitate). To assess if these serum-free conditions had any detrimental effects on cell viability, we performed ELISAs for lactate dehydrogenase and cardiac troponin I and found that their levels were unaffected by the addition of palmitate, indicating that our serum-free culture conditions in hCOs did not overtly cause cell death (Fig. S1 E and F). SI Results has more information regarding our iterative screening process, including optimization of serum-free medium and matrix composition.
Initial glucose-palmitate screening was performed in the presence of insulin, which is commonly used in most serum-free medium supplements to improve survival and function. However, as insulin induces glycolysis and could potentially promote proliferation through its actions on PI3K/GSK3 signaling (19), it could also be preventing cardiomyocyte maturation. We found that insulin was inducing hPSC-CM proliferation under serum-free conditions (1 mM glucose and 10 μM palmitate) (Fig. 1H). Although the removal of insulin reduced cell cycle activity, this condition could potentially have effects on cellular metabolism under serum-free conditions with 1 mM glucose and 10 μM palmitate. Therefore, we next screened the full factorial interplay between glucose and palmitate in the absence of insulin. We again saw an increase in force in the presence of palmitate, with significantly higher forces produced within tissues cultured in 100 μM palmitate, even in the presence of various concentrations of glucose (0.5, 1, and 5.5 mM glucose) (Fig. 1I). Palmitate once again promoted a trend to increased MLC2v expression (Fig. 1J). Culture of hCOs in serum-free medium (1 mM glucose with 100 µM palmitate and no insulin) also reduced cell cycle activity fourfold relative to the results obtained in control (CTRL) medium (Fig. 1K). These conditions allowed robust generation and culture of hCOs, derived from multiple hPSC lines, with viable, intact, and functional tissues produced greater than 90% of the time (Fig. 1L). We subsequently termed this medium “maturation medium” (MM) and performed extensive phenotypic analyses of hCOs using this maturation protocol (Fig. 1M).
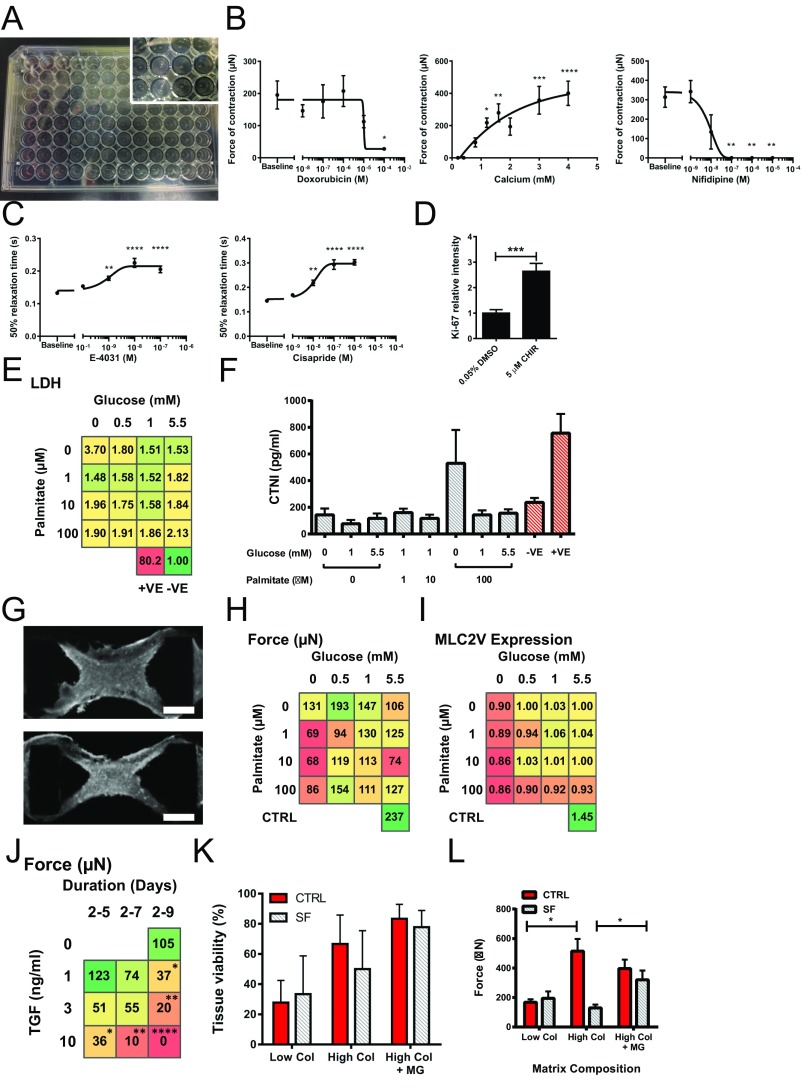
MM Does Not Alter Cellular Composition in hCO.
Consistent with the drop in Ki-67, we found that hCOs cultured in MM had a reduced number of cells compared with CTRL medium based on DNA intensity analysis (Fig. S2A). After dissociating hCOs, we determined that there are similar percentages of hPSC-CMs (α-actinin) present under both CTRL medium and MM conditions (Fig. S2 B and C). We also found a small but significant decrease in CD90+ cells cultured in MM (13% CTRL to 10% MM, P < 0.05) (Fig. S2D). Additionally, we used whole-mount immunostaining to examine multiple cardiac cell populations within the hCOs and found that hPSC-CMs (α-actinin or MLC2v), stromal cells (CD90), endothelial cells assembled into tubes (CD31), and epicardial cells (WT-1) were all present in tissues cultured in CTRL medium or MM (Fig. S2 E and F). In contrast to larger tissue formats, such as our recent publication (13), this suggests that endothelial structures are better supported in our dense miniaturized hCO format.
Fig. S2.
Culture of hCO in MM does not alter cellular composition. (A) DNA intensity in hCO indicates fewer cells in MM; n = 70–74 from 14 experiments. (B) Single hPSC-CMs dissociated from hCO. (Scale bars: 10 µm.) (C) Flow cytometry profiling of hPSC-CMs (α-actinin) dissociated from hCOs reveals a similar purity in both CTRL medium and MM (not significant). (D) Flow cytometry profiling of stromal cells (CD90+) dissociated from hCO reveals a very modest decrease in CD90+ cells in hCOs cultured in MM vs. CTRL medium (P < 0.05); n = 5. (E) CTRL medium hCOs have endothelial tubular structures (CD31) and stromal cells (CD90) present through the hPSC-CMs (MLC2v). There are also epicardial cells (WT-1) present on the outer surface of the hCO. Low magnification images of the entire hCO are shown on the left for each stain, and high magnification confocal images are shown on the right. (F) MM hCOs have endothelial tubular structures (CD31) and stromal cells (CD90) present through the hPSC-CMs (MLC2v). There are also epicardial cells (WT-1) present on the outer surface of the hCO. Low magnification of the entire hCO is shown on the left for each stain, and high-magnification confocal images are shown on the right. Data are presented as mean ± SEM. (Scale bars: low magnification, 200 µm; high magnification, 20 µm.) ***P < 0.001 using t test (A); statistics analyzed using t test (C and D).
MM Does Not Further Enhance hPSC-CM Function in hCO.
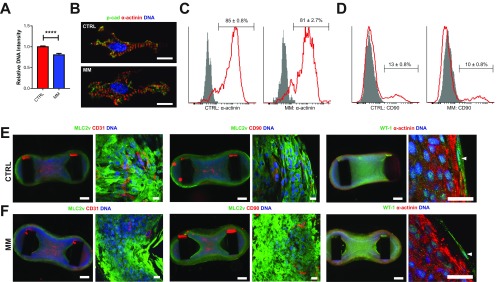
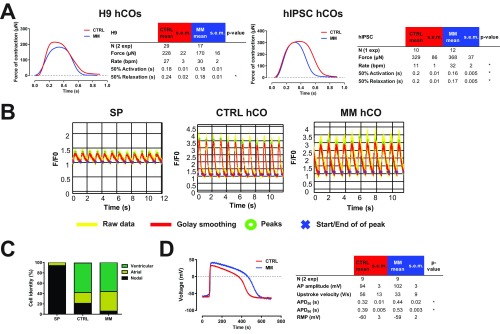
We analyzed the contractile properties of hCOs cultured in both CTRL medium and MM in detail. We found that hCOs cultured in MM had similar forces of contraction but reduced activation time [time from 50% activation to peak (Ta)] and reduced relaxation time [time from peak to 50% relaxation (Tr)] relative to hCO cultured in CTRL medium (Fig. 2A), reflecting the changes which occur during functional maturation during human cardiomyocyte development (20). To confirm these findings, we also profiled the contraction kinetics of hCOs derived from two additional cell lines: the human embryonic stem cell (hESC) line H9 and a commercially available human induced pluripotent stem cell (hiPSC) line. Both HES3-derived and hiPSC-derived hCOs displayed increased rates of contraction and reduced Ta in MM vs. CTRL medium. However, H9-derived hCO did not have increased rates of contraction or Ta in MM vs. CTRL medium (Fig. S3A). This indicates that changes in Ta are rate-dependent. In contrast, all lines tested had a reduced Tr in MM compared with those cultured in CTRL medium, regardless of rate (Fig. 2A and Fig. S3A). This may be caused by the reduction in endogenous ECM synthesis (Fig. 3 F and G), as ECM production also correlates with increased relaxation time in patients with heart disease (21).
Fig. 2.
Maturation of electrophysiological and contractile properties are determined by the tissue-engineered environment. (A) Representative individual contraction curves and contraction parameters of hCOs in CTRL medium or MM. (B) Isoprenaline stimulation induces an increased rate of contraction in hCOs; n = 5–7. (C) Isoprenaline increases the force of contraction in hCOs cultured in MM; n = 5–7 from two experiments. (D) hCOs cultured in MM have decreased calcium sensitivity. As isoprenaline stimulation experiments were performed in culture medium (Ca2+ = 1.8 mM), the effects of isoprenaline may be more pronounced at lower calcium concentrations; n = 4–7 from two experiments. (E) Isoprenaline increases force of contraction and decreases contraction duration under paced conditions (1 Hz) at the calcium EC50 (CTRL = 0.3 mM, MM = 1.0 mM); n = 7. (F) Representative individual calcium indicator (Fluo-4) recordings and parameters from individual hPSC-CMs dissociated from hCOs cultured in CTRL medium or MM paced at 1 Hz at 37 °C. (G) Representative individual action potential recordings and parameters from individual hPSC-CMs dissociated from hCOs cultured in CTRL medium or MM paced at 1 Hz at 37 °C. Data are mean ± SEM. The response curves to calcium of hCO in CTRL medium and MM are statistically significant using two-way ANOVA (P < 0.05) (D). APD, action potential duration; CMP, clamped membrane potential; RMP, resting membrane potential. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001 using t test (A and E), two-way ANOVA (indicates difference relative to baseline; B and C), or ANOVA with Tukey’s posttest (F and G).
Fig. S3.
Supporting data for functional analysis presented in Fig. 2. (A) H9- and human induced pluripotent stem cell (hiPSC)-derived hCOs have similar contraction properties as HES3 hCOs cultured in MM. Representative contraction curves and parameters of H9- or IPSC-derived hCOs in CTRL medium or MM. (B) Raw and processed data of representative calcium traces recorded from SP and hCOs cultured in CTRL medium and MM with Fluo-4 AM at 1-Hz pacing at 37 °C. (C) Characterization of hPSC-CM subtype using patch clamp (more detail is in SI Methods). (D) Electrophysiological recordings using impaling electrodes. Data are mean ± SEM. RMP, resting membrane potential. *P < 0.05 using t test (A, B, and D).
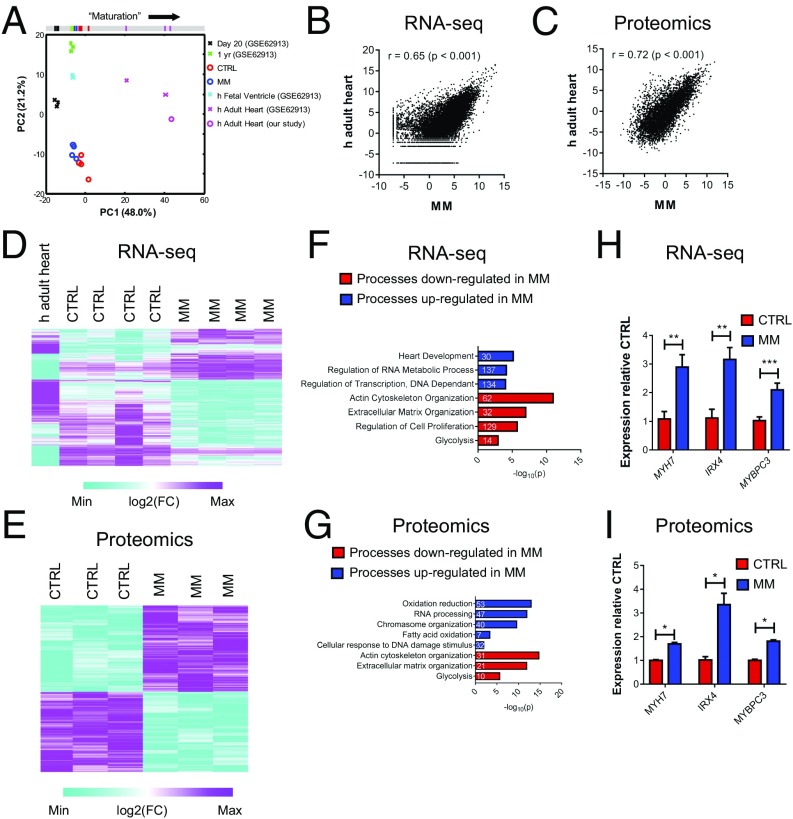
Fig. 3.
Culture in MM induces transcriptional and proteomic maturation in hCOs. (A) PCA of RNA-seq data using a subset of genes identified by Delaughter et al. (27) to profile maturity (genes from table S6 in ref. 27). RNA-seq data derived from hCOs cultured in CTRL medium or MM (n = 4 experiments, each 20 pooled hCOs) and our human adult heart sample (n = 1, three pooled hearts) combined with RNA-seq data from Gene Expression Omnibus accession number GSE62913 (25) containing 2D hPSC-CMs at 20 d (n = 3) and 1 y (n = 3), human fetal ventricles (n = 2), and human adult hearts (n = 2). All genes were >10 counts per million for at least one sample. Bar at the top is a projection of PC1, the component that correlates with maturation. (B) Pearson correlation coefficient indicates that hCOs cultured in MM are significantly correlated to human adult heart tissue. Graph is of log2(cpm) of human adult heart (n = 1, three pooled hearts) or average gene expression of hCOs cultured in MM (n = 4). (C) Pearson correlation coefficient indicates that hCOs cultured in MM are significantly correlated to human adult heart tissue. Graph is of log2(iBAQ) of human adult heart (n = 1) or pooled hCOs cultured in MM (n = 3, each nine pooled hCO). iBAQ, intensity-based absolute quantification. (D) Clustered heat map of genes regulated (FDR < 0.05) between hCOs cultured in CTRL medium vs. MM. (E) Clustered heat map of proteins regulated (q value < 0.05) between hCOs cultured in CTRL vs. MM. (F) GO term analysis of RNA-seq reveals that multiple processes are decreased or increased in hCOs cultured in MM vs. CTRL medium. These are consistent with processes that occur during postnatal heart maturation (5). Numbers in the bars indicate numbers of genes identified in that particular process. (G) GO term analysis of proteomics reveals that multiple processes are decreased and increased in hCOs cultured in MM vs. CTRL medium. These are consistent with processes that occur during postnatal heart maturation (5). Numbers in the bars indicate numbers of genes identified in that particular process. (H) RNA-seq markers of maturation: genes in the cardiac development GO term, which are also up-regulated in proteomics data. (I) Proteomics markers of maturation: genes in the cardiac development GO term. Data are mean ± SEM. *P < 0.05; **P < 0.01; and ***P < 0.001, FDR (H) or q value (I) for statistical analysis (SI Methods).
We also assessed the chronotropic and inotropic responses to isoprenaline in hCOs cultured in both CTRL medium and MM. Isoprenaline increased the rate of contraction in both media (Fig. 2B). However, isoprenaline only induced an increase in the force of contraction in hCOs cultured in MM (Fig. 2C), which is indicative of a great contractile reserve under the culture conditions tested. We found that hCOs cultured in MM had a higher calcium EC50 for force of contraction (1.0 mM Ca2+) than those cultured in CTRL medium (0.3 mM Ca2+) (Fig. 2D). A low calcium EC50 may result in a blunted isoprenaline-induced inotropic response under our hCO culture conditions containing 1.8 mM Ca2+, as the isoprenaline response depends on the contractile reserve (22). To investigate this, we assessed the effects of isoprenaline under highly controlled paced conditions (1 Hz) at the calcium EC50 for CTRL medium (0.3 mM) and MM (1.0 mM) hCO. Under these conditions, both CTRL medium and MM had similar increases in force of contraction and decreased 50% contraction time (Fig. 2E). The increased calcium EC50 in hCOs cultured in MM is indicative of maturation toward adult cardiac muscle [EC50 = 2.6–6.0 mM in adult (23)].
Calcium kinetics during contraction were also assessed using Fluo-4 AM calcium imaging on single cells dissociated from hCOs at 1-Hz pacing (37 °C). Single-cell calcium recordings were obtained from the starting population (SP) of hPSC-CMs as a reference, and hPSC-CMs dissociated from hCOs in CTRL medium and MM (Fig. S3B). These experiments revealed that there was increased peak amplitude, rising slope, and decay in hCO relative to the SP, indicative of a more mature calcium handling system, but no differences were observed between hCOs cultured in the different media (Fig. 2F). The lack of difference in calcium handling kinetics between CTRL and MM hCOs (Fig. 2F) also supports the notion that the reduced contractile Tr in hCOs cultured in MM is caused by reduced ECM production rather than changes in hPSC-CM calcium handling properties.
We next determined the electrophysiological properties of hPSC-CMs using whole-cell patch-clamp recordings from single cells dissociated from hCOs cultured in CTRL medium or MM and SP hPSC-CMs as a reference. We found that the action potential profile in hPSC-CMs from hCOs in both CTRL medium and MM resembles that of adult ventricular cardiomyocytes (Fig. 2G and ventricular hPSC-CMs quantified in Fig. S3C). However, we found no differences between hPSC-CMs derived from CTRL medium or MM hCO (Fig. 3G). As the resting membrane potentials of the hPSC-CMs dissociated from hCOs were relatively depolarized (Fig. 2G), we also recorded electrophysiological parameters in situ in the hCOs using impaling electrodes (Fig. S3D). Using this approach, we found that the hPSC-CMs in situ had resting membrane potentials of approximately −60 mV (Fig. S3D). The depolarized membrane potentials in the initial patch-clamp experiments performed on enzymatically dissociated hPSC-CMs (Fig. 2G) were likely caused by the dissociation process. Importantly, the action potential recordings using impaling electrodes in situ also confirmed that hPSC-CMs in the hCOs in both CTRL medium and MM had adult-like ventricular action potentials (Fig. S2D). Together, these results suggest that the tissue-engineered culture environment is supportive of the development of in vivo-like functional maturation, as has been previously reported (10, 14).
MM Does Not Further Enhance Structural Organization Supported by hCO Culture.
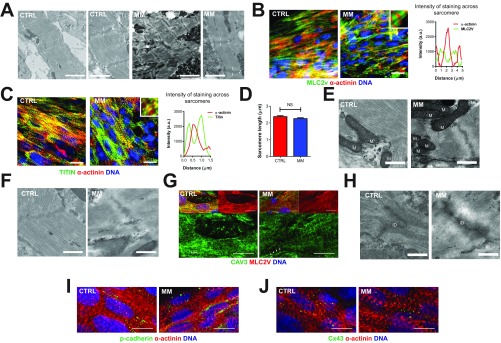
To determine whether there were other sarcomere-related changes in MM, we profiled the structural organization of the hPSC-CMs in the hCOs. Transmission electron microscopy (TEM) was used to confirm the presence of clear Z bands and I bands in the hCO in both CTRL medium and MM (Fig. S4A). This was confirmed using MLC2v and α-actinin staining revealing a highly organized expression pattern, with α-actinin localized to Z bands and MLC2v localized to I and A bands in hCOs cultured in either CTRL medium or MM (Fig. S4B). Titin and α-actinin staining also revealed clear delineation of α-actinin expression in the Z bands and titin expression in the I and A bands (Fig. S4C). The sarcomere length was ∼2.3 µm in both media (Fig. S4D), which is consistent with adult rather than immature cardiomyocytes (24). We also observed well-developed mitochondria (Fig. S4E) and t tubules (Fig. S4F) adjacent to the sarcomeres using TEM. We confirmed the presence of t tubules using caveolin-3 immunostaining (Fig. S4G).
Fig. S4.
hCOs cultured in both CTRL medium and MM exhibit in vivo-like structure. (A) Transmission electron micrographs of CTRL medium and MM hCOs for an overview and higher focus on sarcomeres. Z, I–Z, and I bands, respectively. (Scale bars: overview, 2 µm; higher focus, 1 µm.) (B) Whole-mount immunostaining of MLC2v and α-actinin reveals the presence of well-developed sarcomeres in hCOs in both CTRL medium and MM. Note the 5× Inset and intensity profiles, where the MLC2v/α-actinin costaining reveals α-actinin in the Z bands and MLC2v only in the rest of the sarcomeres. (Scale bars: 20 µm.) (C) Whole-mount immunostaining of titin and α-actinin reveals the clear delineation of α-actinin in the Z bands and titin in the I bands. Note the 5× Inset and intensity profiles, where the titin/α-actinin costaining reveals α-actinin in the Z bands and titin only in the I bands. (Scale bars: 20 µm.) (D) Quantification of sarcomere length using α-actinin staining; n = 20 cells from two experiments. Data are mean ± SEM. NS, not significant using t test (P < 0.05). (E) Transmission electron micrographs of mitochondria in CTRL medium and MM hCOs. M, mitochondria. (Scale bars: 0.5 µm.) (F) Transmission electron micrographs of t tubules in CTRL medium and MM hCOs. T, t tubules. (Scale bars: 0.5 µm.) (G) Whole-mount immunostaining of CAV3 confirms t-tubule structures in hCOs in CTRL medium and MM. (Scale bars: 10 µm.) (H) Transmission electron micrographs of intercalated discs in CTRL medium and MM hCOs. ID, intercalated disc. (Scale bars: 0.5 µm.) (I) Whole-mount immunostaining of pancadherin confirms presence of intercalated discs in hCOs in CTRL medium and MM. (Scale bars: 10 µm.) (J) Whole-mount immunostaining of connexin 43 confirms presence of intercalated discs in hCOs in CTRL medium and MM. (Scale bars: 10 µm.)
Using TEM, we found that there were also highly organized intercalated discs (Fig. S4H), and we confirmed the formation of cell–cell junctions using pancadherin (Fig. S4I) and connexin 43 staining (Fig. S4J). Together, these results further suggest that the tissue-engineered culture environment is supportive of the development of in vivo-like structure, as has been previously reported (10, 14).
MM Induces Enhanced Maturation of Cardiac Developmental Factors, Metabolism, and Cell Cycle.
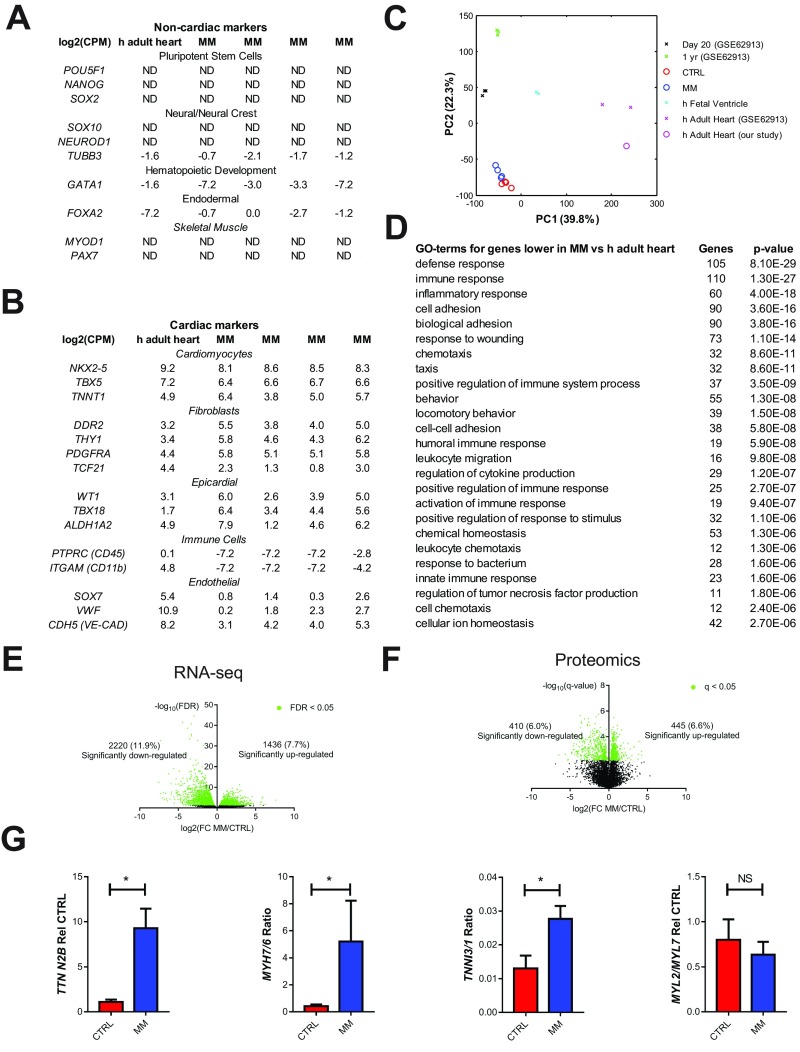
To get a broader view of the effects of MM on hCOs, we next performed RNA sequencing (RNA-seq) on hCOs cultured in CTRL medium or MM and a commercially available adult heart sample (three pooled male hearts, 30–39 y old). Very few contaminating cell types from other lineages were present in the hCOs, with most markers for potentially contaminating lineages expressed at similar levels in hCOs and human adult heart tissue (Fig. S5A). When we examined markers of the most prominent cell types in the heart, we found that our protocol generated hPSC-CMs, epicardial cells, and fibroblastic cells, with a similar abundance of transcripts for these lineages present in hCO and human adult heart tissue (Fig. S5B). Endothelial transcripts were also present but at lower abundance, and leukocyte transcripts were low or absent in the hCOs relative to human adult heart tissue (Fig. S5B). These results are consistent with our flow cytometry and immunostaining (Fig. S2).
Fig. S5.
Supporting data for the RNA-seq and proteomic expression analyses of hCOs presented in Fig. 3. (A) RNA-seq expression of markers for potentially contaminating cell types; n = 4 experiments. (B) RNA-seq expression of markers expressed in different cardiac cell populations; n = 4 experiments. (C) PCA of our RNA-seq data combined with RNA-seq data from Gene Expression Omnibus accession number GSE62913 (25) containing hPSC-derived 2D hPSC-CMs at 20 d (n = 3) and 1 y (n = 3), human fetal ventricles (n = 2), and human adult hearts (n = 2). All genes were >10 counts per million for at least one sample. (D) Top 25 GO terms for the 1,000 genes with highest differential expression between hCOs in MM and human adult heart that are more abundant in the human adult heart. (E) Volcano plot illustrating the differential analysis of the RNA-seq data; n = 4 experiments. (F) Volcano plot illustrating the differential analysis of the proteomic data; n = 3 samples. (G) qPCR of sarcomeric genes known to switch/increase with maturation (TTN N2B, MYH7/6, TNNI3/1, and MYL2/7); n = 4 experiments. Data are mean (A and B) or mean ± SEM (F). *P < 0.05 using Mann–Whitney (G). ND, not determined due to low abundance.
Principal component analysis (PCA) was performed to determine differences between our samples. For these analyses, we also included additional publically available reference data (GSE62913) including day 20 and 1-y-old hPSC-CMs, human fetal ventricles, and human adult hearts (25). When we used all transcripts (>10 counts per million), we found that hCOs clustered distinctly from other samples, indicating good reproducibility between experiments (Fig. S5C). However, no two conditions clustered together, which was most likely because of the influence of different cell populations being present in each different sample [i.e., hPSC-CM samples from Kuppusamy et al. (25) are pure, while our hCO samples and the heart tissues contain multiple cell types]. Indeed, hCOs have fewer endothelial cells and lack leukocytes (Fig. S5B), which are abundant in native heart tissue (26). In support of this notion, the top 25 Gene Ontology (GO) terms for the top 1,000 genes that were more highly expressed in human adult heart tissue relative to hCOs cultured in MM were mostly related to immune processes (Fig. S5D). Nevertheless, we used a similar approach as Delaughter et al. (27) to profile the developmental stage of our hCO by performing PCA on transcripts expressed in cardiomyocytes (table S6 in ref. 27) (795 transcripts >10 counts per million). We found that the hCOs from both CTRL medium and MM conditions in 1-y-old hPSC-CMs and human fetal ventricles clustered on principal component 1 (PC1) using this approach and were more mature than 20-d-old hPSC-CMs (Fig. 3A), consistent with the maturation profiling presented in the work by Delaughter et al. (27). Despite these differences, which are accentuated by PCA, we found that hCOs cultured in MM highly correlated to adult human heart tissue based on RNA-seq data (Fig. 3B). We also performed proteomics on hCOs cultured in CTRL medium or MM and adult human heart tissue and found that hCOs cultured in MM also closely resembled human adult heart tissue based on proteomic data (Fig. 3C).
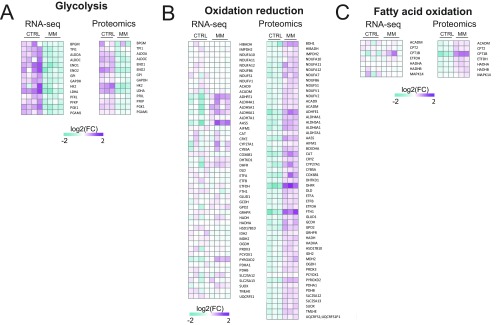
We identified 3,856 transcripts and 855 proteins that were differentially regulated between hCOs in CTRL medium or MM [false discovery rate (FDR) < 0.05 and q value < 0.05, respectively] (Fig. S5 E and F). Using the differentially expressed genes (Fig. 3D) or proteins (Fig. 3E), we performed GO term analysis independently on the RNA-seq (Fig. 3F) and proteomic datasets (Fig. 3G). In both cases, reductions in glycolysis, ECM organization, and actin cytoskeleton organization were identified in hCO cultured in MM (Fig. 3 F and G). Processes that were consistently enhanced between the two datasets included RNA processing/regulation of RNA metabolic process and regulation of transcription/chromosome organization (Fig. 3 F and G). Interestingly, oxidation reduction, fatty acid oxidation, and cellular response to DNA damage stimulus were only up-regulated in the proteomic dataset (Fig. 3 F and G). These biological processes are highly indicative of mammalian postnatal maturation of cardiomyocytes in vivo (5, 28, 29), which additionally highlights the importance of performing proteomic analyses in addition to transcriptomics. Consistent with postnatal maturation, the GO term cell cycle regulation was reduced by MM specifically in the RNA-seq dataset, likely because of the low abundance of proteins involved in the cell cycle, even in CTRL medium (Fig. 3 F and G). The GO term heart development was also up-regulated in hCOs cultured in MM in the RNA-seq dataset (Fig. 3F), and key factors involved in cardiac maturation in this GO term (MYH7, IRX4, and MYBPC3) were also up-regulated in the proteomics analysis (Fig. 3 H and I). MYH7 is known to increase during human heart maturation (30), IRX4 nuclear translocation increases during postnatal cardiac maturation (31) and is critical for maintaining cardiac function (32), and cardiomyocytes undergo an additional round of division before postnatal cell cycle arrest in MYBPC3 KO mice (33). We also validated that sarcomeric isoforms known to switch/increase during maturation (24), such as TTN N2B, MYH7/6, and TNNI3/1, increased in hCO cultured in MM using quantitative PCR (qPCR) (Fig. S5G). Collectively, these results show that hCOs cultured in MM undergo multiple postnatal maturation processes, including induction of cardiac developmental factors, metabolic switching, DNA damage, and a reduction in cell cycle activity. However, our findings also highlight that hCO culture in MM specifically matures these processes, while other structural and functional properties associated with maturation are not further enhanced by culture in MM (as found in Fig. 2 and Figs. S3 and S4).
MM Induces a Metabolic Switch from Glycolysis to Fatty Acid Oxidation.
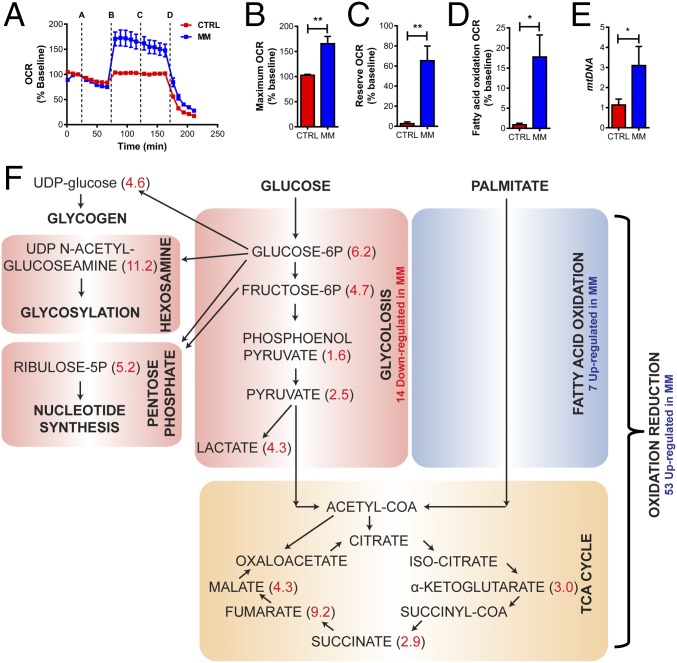
Our RNA-seq and proteomics analyses (Fig. 3) revealed that hCO culture in MM represses many glycolysis components and activates many fatty acid oxidation components in hCO (Fig. S6). We next profiled metabolism of hCOs using the Seahorse XF Bioanalyzer mitochondrial stress test with an additional step to measure endogenous fatty acid oxidation using etomoxir (Fig. 4A). In support of a switch to fatty acid oxidation, hCOs from MM had a higher maximal oxygen consumption rate (OCR) (Fig. 4B), OCR reserve (Fig. 4C), and increased fatty acid oxidation (Fig. 4D) under the Seahorse test conditions. These changes in oxidative capacity were associated with an increase in mitochondrial content (mtDNA) (Fig. 4E). As ATP uncoupling using carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) can lead to large increases in extracellular acidification rate in mature cardiomyocytes, even in the absence of glucose (34), we chose to use metabolomics to further profile glycolytic and branching pathway metabolic flux in hCO under CTRL medium and MM conditions. Metabolites in the glycolysis pathway as well as pathways branching from it, including hexosamine, pentose phosphate, and glycogen pathways, were all reduced in MM compared with CTRL medium (Fig. 4F). Together, our RNA-seq, proteomics, Seahorse data, and metabolomics confirm a switch in metabolism from glycolysis to fatty acid oxidation.
Fig. S6.
Heat maps of RNA-seq and proteomic data for metabolism genes supporting the data presented in Fig. 4. (A) Heat map data from significantly regulated targets (CTRL medium vs. MM) in RNA-seq or proteomics data for the GO term glycolysis. (B) Heat map data from significantly regulated targets (CTRL medium vs. MM) in RNA-seq or proteomics data for the GO term oxidation reduction. (C) Heat map data from significantly regulated targets (CTRL medium vs. MM) in RNA-seq or proteomics data for the GO term fatty acid oxidation. Data are presented as log2 expression relative to mean for all conditions; n = 4 experiments for RNA-seq and n = 3 experiments for proteomics.
Fig. 4.
Metabolism switches from glycolysis to fatty acid oxidation in hCOs cultured in MM. (A) Seahorse mitochondrial stress test. A, oligomycin; B, FCCP; C, etomoxir; D, antimycin A/rotenone. The Seahorse stress tests were run with n = 6 independent recordings from n = 8 pooled hCOs per recording from hCOs cultured in CTRL medium or MM for 9 d (B–D). (B) Maximum OCR is higher in hCOs cultured in MM. (C) Reserve metabolic capacity is higher in hCOs cultured in MM. (D) Fatty acid oxidation is higher in hCOs cultured in MM. (E) mtDNA is higher in hCOs cultured in MM (qPCR using ND1 and RNA18S5 as the nuclear DNA controls); n = 4 experiments. (F) Measurements of carbon metabolites (n = 2, each 12–14 pooled hCOs) confirm that the metabolism in hCOs cultured in MM switches from glycolysis to fatty acid oxidation. Values in red indicate fold higher in CTRL medium vs. MM. Metabolomics values are normalized to the DNA intensity in hCOs cultured in MM vs. CTRL medium. Data are mean ± SEM. *P < 0.05; **P < 0.01, t test (B–D) or ratio paired t test (E).
MM Inhibits Cell Cycle and Is Associated with Repression of β-Catenin and Induction of the DNA Damage Response.
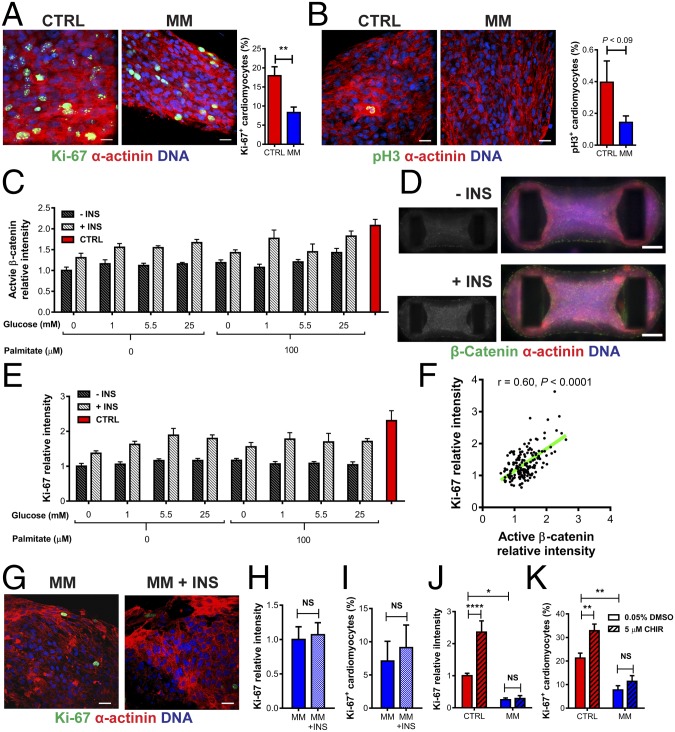
We firstly confirmed that MM induces a decrease in Ki-67 intensity in multiple additional hPSC cell lines (H9 and hiPSC) (Fig. S7 A and B). Additionally, we confirmed our initial whole-mount fluorescence intensity screening data (Fig. 1K) using high-resolution confocal microscopy staining and quantification of hPSC-CM cell cycle activity. We found that hCO culture in MM caused a reduction in percentage of hPSC-CMs in the cell cycle using the general cell cycle marker, Ki-67 (Fig. 5A), and the mitosis-specific marker, phosphohistone H3 (Ser10) (pH3) (Fig. 5B). hPSC-CM proliferation was markedly reduced in hCOs cultured in MM, with very low overall rates of hPSC-CM mitosis (∼0.2% pH3+ hPSC-CMs), which is similar to the postnatal human heart (35).
Fig. S7.
Ki-67 intensity in hCO derived from different lines and force of contraction under different metabolic conditions in support of the data presented in Fig. 5. (A) Ki-67 intensity in H9-derived hCOs; n = 10. (B) Ki-67 intensity in hiPSC-derived hCOs; n = 4. (C) Force of contraction in HES3-derived hCOs cultured in different metabolic conditions after 48 h; n = 11–15 from three experiments. Data are mean ± SEM. **P < 0.01 using t test (A and B). INS, insulin.
Fig. 5.
hPSC-CM proliferation and WNT–β-catenin signaling are repressed in hCOs cultured in MM. (A) Culture of hCOs in MM reduces hPSC-CM (α-actinin) proliferation (Ki-67); n = 11–14 hCOs from three to four experiments. The 4,396 hPSC-CMs were manually counted. (Scale bars: 20 μm.) (B) Culture of hCOs in MM reduces hPSC-CM (α-actinin) mitosis (pH3); n = 10 from five experiments. The 7,838 hPSC-CMs were manually counted. (C) Activated β-catenin intensity in hCOs cultured in different metabolic conditions after 48 h reveals that lack of insulin is responsible for a decrease in activated β-catenin. Data are normalized to the 0 mM glucose, 0 µM palmitate condition; n = 6–13 from three experiments. (D) Representative immunostaining of activated β-catenin in hCO in MM with and without insulin. (Scale bars: 200 μm.) Insets are activated β-catenin alone. (E) Ki-67 intensity in hCOs cultured in different metabolic conditions after 48 h reveals that lack of insulin is responsible for the initial drop in cell cycle activity. Data are normalized to the 0 mM glucose, 0 µM palmitate condition; n = 6–13 from three experiments. (F) Cell cycle activity (Ki-67) and activated β-catenin were highly correlated in hCOs cultured under different metabolic conditions; n = 146 from three experiments. (G) Reintroduction of insulin for 48 h after longer term culture (9 d in MM) could not restore proliferation (Ki-67) of hPSC-CMs (α-actinin; representative immunostaining). (Scale bars: 20 μm.) (H) Ki-67 intensity in hCOs after reintroduction of insulin for 48 h after longer term culture (9 d in MM) could not restore proliferation; n = 10–11 hCOs from two experiments. (I) Quantification of proliferating (Ki-67) hPSC-CMs (α-actinin) reveals that reintroduction of insulin for 48 h after longer term culture (9 d in MM) could not restore proliferation; n = 3–4 hCOs from two experiments. The 2,441 hPSC-CMs were manually counted. (J) hCOs cultured in MM have a blunted proliferative response (Ki-67 intensity) to CHIR99021 (24-h treatment); n = 11–14 hCOs from three to four experiments. (K) Quantification of proliferating (Ki-67) hPSC-CMs (α-actinin) confirms that hCOs cultured in MM have a blunted proliferative response to CHIR99021 (24-h treatment); n = 7–8 from two experiments. The 10,609 hPSC-CMs were manually counted. Data are mean ± SEM. P values were calculated using t test (B, H, and I) or Pearson correlation (r) and P value (F). INS− is statistically different from INS+ (P < 0.0001) using two-way ANOVA (C and E). *P < 0.05; **P < 0.01; and ****P < 0.0001 using t test (A) or ANOVA with Tukey’s posttest (J and K). INS, insulin.
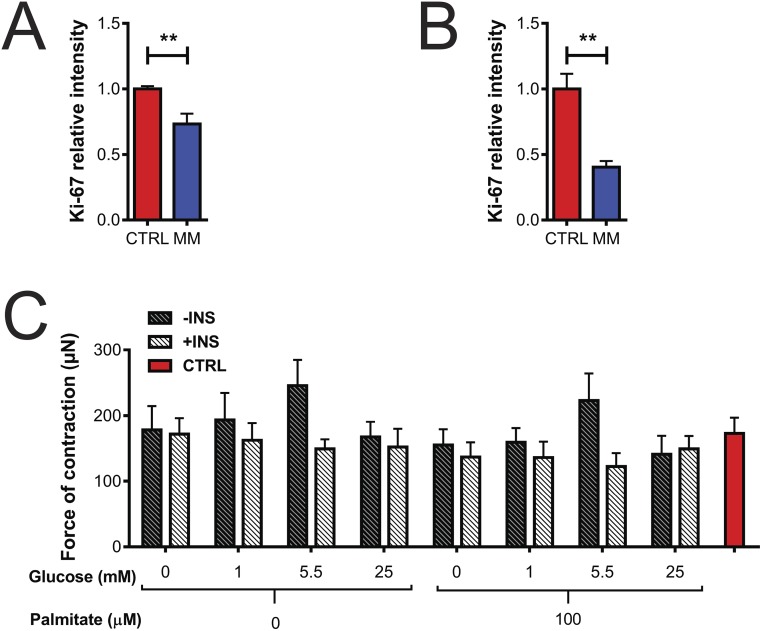
We next wanted to assess how metabolic substrates influenced the cardiac cell cycle. We performed a factorial experiment with glucose, insulin, and palmitate after the hCO formation phase. Importantly, all conditions had a similar force of contraction during the first 48 h of culture (Fig. S7C), indicating that the hCOs have similar functional properties and viability at this early stage, even in the absence of glucose and palmitate (in contrast to longer term cultures) (Fig. 1I). In these studies, we specifically examined the Wnt/β-catenin signaling pathway, as we have previously identified CHIR99021 as one of the most potent activators of human cardiomyocyte proliferation (16) and have also found this pathway to be transcriptionally repressed during postnatal maturation (5). As a readout of β-catenin activity in our screen, we performed quantification of activated β-catenin using an antibody that only binds to activated, nuclear-localized β-catenin (36). Activated β-catenin was highly dependent on insulin, regardless of the presence of either glucose or palmitate (Fig. 5 C and D), as was proliferation (Fig. 5E), and there was a highly significant correlation between activated β-catenin and Ki-67 intensity (Fig. 5F) (over all hCOs in all conditions). Despite this dependence on insulin signaling for proliferation 2 d after culture in MM, the addition of insulin after 11 d in MM was not sufficient to reactivate hPSC-CM proliferation (Fig. 5 G–I). This indicates that longer term culture in MM induces desensitization of hPSC-CMs to the proliferative actions of insulin.
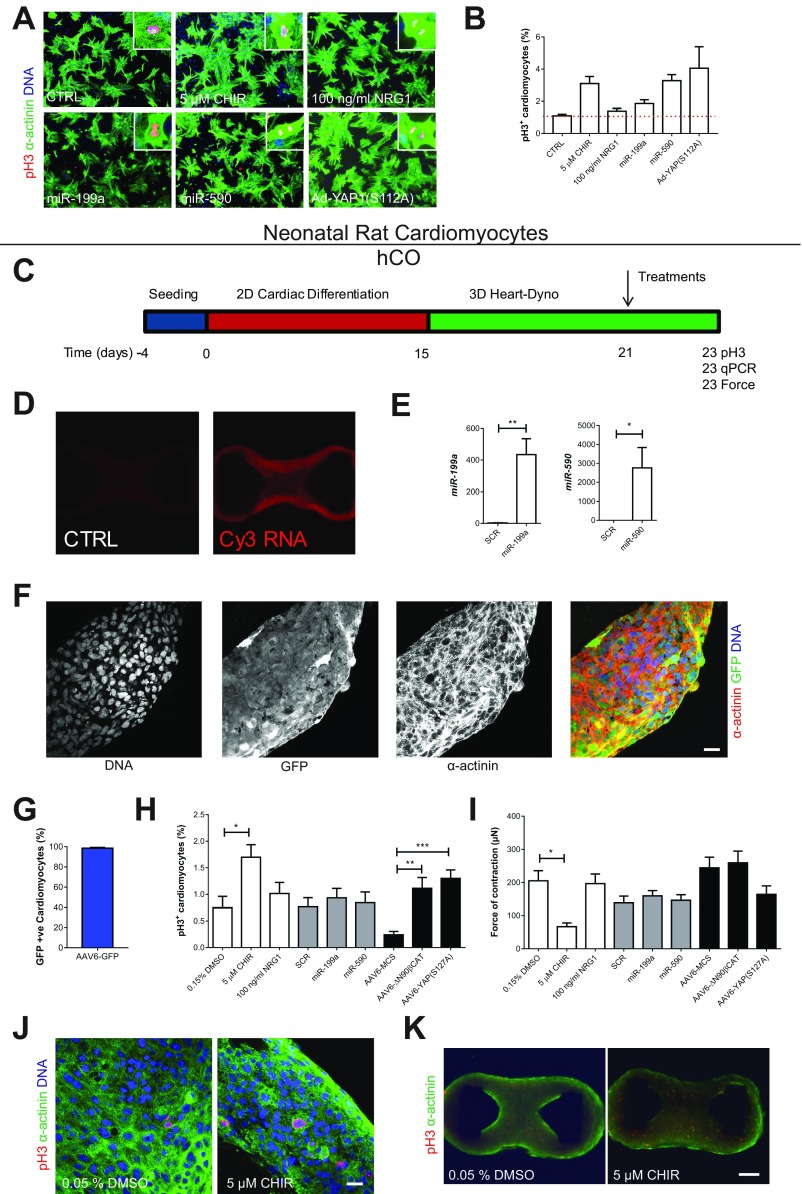
As mature cardiomyocytes are largely refractory to proliferative stimuli, we next wanted to test whether potent cardiomyocyte mitogens could stimulate proliferation in hCO cultured in MM. We initially tested previously reported mitogens including growth factors, small molecules, microRNAs (miRs), and transcription factors, in commonly used neonatal rat cardiomyocyte cultures. We found that CHIR99021 (16), miR-199a (37), miR-590 (37), and overexpression of constitutively active murine YAP1(S112A) (38) were all capable of inducing mitosis in neonatal rodent cardiomyocytes marked by pH3+ cardiomyocytes with disassembled sarcomeres (Fig. S8 A and B). Neuregulin (35) was unable to induce proliferation over baseline in neonatal rat cardiomyocyte cultures, although this could be caused by the presence of serum in our cultures, which induces a similar proliferative rate as neuregulin (39). We then tested whether these mitogens could induce proliferation in immature hCO (after only 6 d of culture in CTRL medium following seeding) (Fig. S8C). We firstly confirmed robust overexpression of miRs 48 h after transfection (Fig. S8 D and E) and very high infection efficiency (>98%) with AAV6-GFP (Fig. S8 F and G). We then screened the mitogens and found that only CHIR99021, constitutively active β-catenin (ΔN90), and constitutively active human YAP1(S127A) induced proliferation above baseline in these immature hCO (Fig. S8H). Interestingly, only CHIR99021 treatment resulted in a reduction of force of contraction at 48 h, indicating that it is the inhibition of GSK3 but not the activation of β-catenin or proliferation per se that results in a reduction in contractile function (Fig. S8I). These results confirm that Wnt/β-catenin is a highly conserved and potent regulator of cardiomyocyte proliferation in rodent and human cardiomyocytes.
Fig. S8.
Screening for the most potent mitogens in immature hCO. (A) Validation of proliferation induction in neonatal rat cardiomyocytes. Representative immunostaining of mitotic (pH3+) cardiomyocytes (α-actinin+) after all treatments after 48 h of culture. (B) Quantification of mitotic (pH3+) cardiomyocytes (α-actinin+) revealed that CHIR99021, miR-199a, miR-590, and Ad-YAP(S112A) were capable of inducing proliferation in neonatal rat cardiomyocytes; n = 4 replicates per group. The 14,339 cardiomyocytes were manually counted. (C) Schematic outlining protocol for directed differentiation of hESCs into cardiac cells (15 d) and formation and exercise of hCO in the Heart-Dyno (6 d). The tissues were then stimulated with mitogens for 2 d before analysis. (D) Whole-tissue imaging after transfection of a Cy3-labeled siRNA shows efficient transfection of small RNAs throughout the hCO in the Heart-Dyno. (E) qPCR shows increased expression of miR-199a or miR-590 after transfection vs. a scramble control; n = 5–6 from two experiments. (F) Staining of GFP-infected hCOs after 48 h of AAV6-GFP treatment. (Scale bar, 20 µm.) (G) Quantification of GFP+ hPSC-CMs (α-actinin+) after 48 h of AAV6-GFP treatment; n = 4. (H) Quantification of mitotic (pH3+) hPSC-CMs (α-actinin+) after all treatments after 48 h of treatment; n = 8–21 from two to three experiments. The 35,690 hPSC-CMs were manually counted for this analysis. (I) Analysis of force of contraction reveals that CHIR99021 decreases force; however, constitutively active β-catenin does not; n = 11–29 from two to three experiments. (J) Representative immunostaining of mitotic (pH3+) hPSC-CMs (α-actinin+) after 48-h treatment with CHIR99021. (Scale bar, 20 μm.) (K) Representative immunostaining of mitotic (pH3+) hPSC-CMs (α-actinin+) after 48-h treatment with CHIR99021, revealing that proliferating hPSC-CMs are located throughout the hCO. Data are mean ± SEM. (Scale bar, 200 μm.) *P < 0.05; **P < 0.01; and ***P < 0.001, using t test (E) or ANOVA with Tukey’s posttest (H and I). CHIR, CHIR99021.
We next tested whether hCOs cultured in MM were refractory to activation of proliferation using CHIR99021, which potently activated proliferation throughout immature hCO (Fig. S8 J and K). While hCOs cultured (for standard 16 d in culture) in CTRL medium mounted a robust proliferative response to CHIR99021, mature hCOs cultured in MM had a blunted proliferative response (Fig. 5 J and K). This finding suggested that mature hPSC-CMs in MM were resistant to proliferative stimuli.
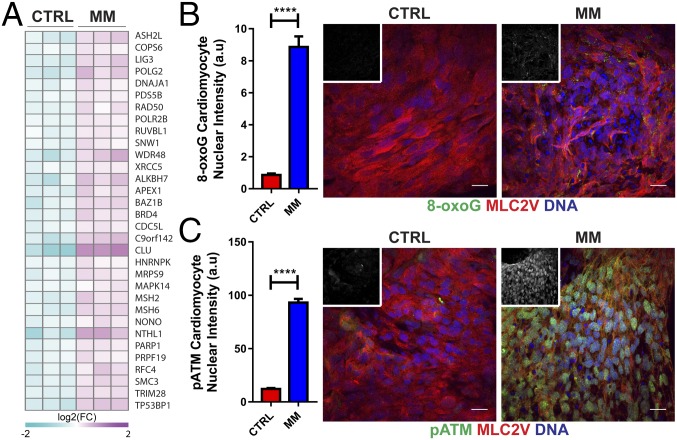
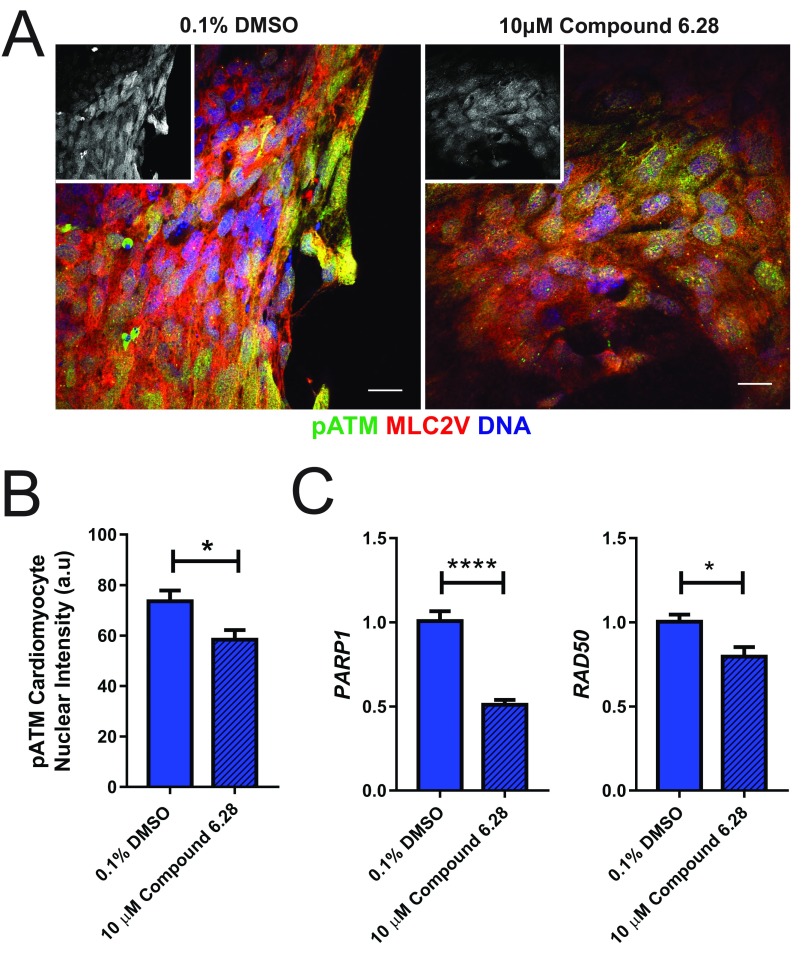
The highly oxidative postnatal environment in cardiomyocytes in vivo induces a DNA damage response (DDR), which has been proposed as a central mechanism driving cardiomyocyte terminal differentiation (29). Consistent with these findings in vivo, we also found that there was increased expression of DDR proteins in hCO cultured in MM (Figs. 3G and 6A). There was evidence for induction of the DDR in hCO cultured in MM, including increased expression of a marker of oxidative base modification in DNA 8-oxo-7,8-dihydroguanine (8-OxoG) (Fig. 6B) and increased Ser1987 phosphorylated ATM (pATM) (Fig. 6C). Therefore, consistent with in vivo postnatal maturation, the DDR is also associated with proliferative arrest in hCO cultured in MM.
Fig. 6.
hCO culture in MM induces a DDR. (A) Heat map showing significantly regulated proteins in CTRL medium vs. MM derived from proteomics data for the GO term cellular response to DNA damage stimulus. Data are presented as log2 expression relative to mean for all conditions; n = 3 experiments. (B) Nuclear intensity for the oxidative base modification in DNA, 8-OxoG, showing an increase in hPSC-CMs (MLC2v) cultured in MM; n = 120–180 hPSC-CM nuclei. Insets are 8-OxoG alone. (C) Nuclear intensity for pATM (Ser1987) showing an increase in hPSC-CM (MLC2v) cultured in MM; n = 120–240 hPSC-CM nuclei. Insets are pATM alone. Data are mean ± SEM. (Scale bars: 20 μm.) ****P < 0.0001 using t test (B and C).
Synergistic Activation of YAP1 and β-Catenin Results in Cell Cycle Reactivation and a Reduction in DDR.
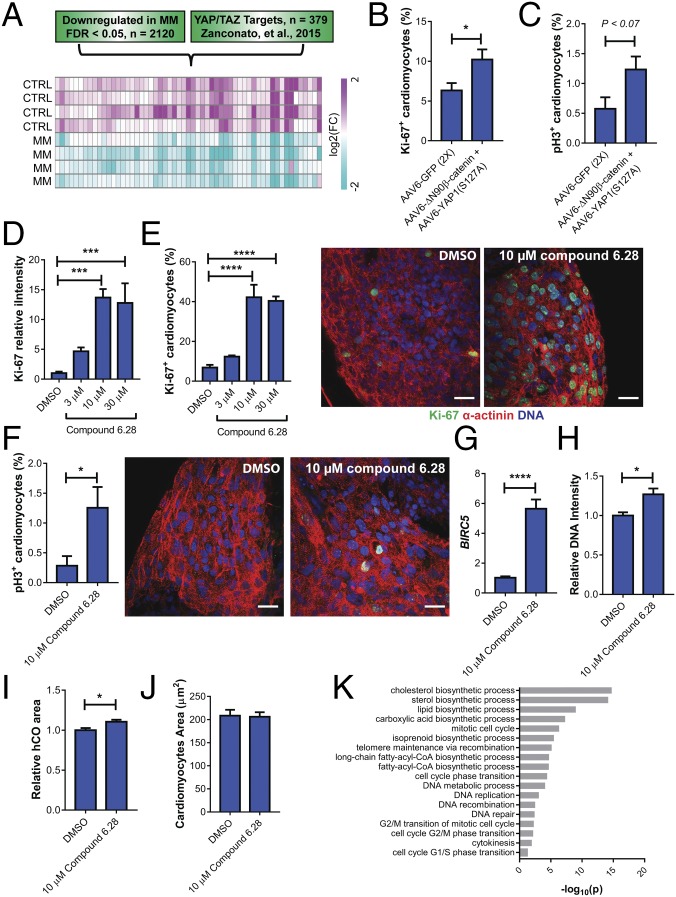
As CHIR99021 could not rescue the reduction in proliferation in MM, we next hypothesized that the β-catenin and YAP1 act in synergy to activate proliferation for a number of reasons: (i) both β-catenin and YAP1 activated proliferation in immature hCOs (Fig. S8H), (ii) both β-catenin and YAP1 have been shown to act cooperatively with the core cardiogenic transcription factor TBX5 to regulate the cell cycle (40), (iii) both β-catenin and YAP1 are required for cardiomyocyte proliferation during embryonic development (41, 42), and (iv) YAP1 can activate an antioxidant response in the heart (43). As YAP/TAZ mediates the transcription of the majority of its transcriptional targets through enhancers, we cross-referenced down-regulated genes in hCOs cultured in MM vs. CTRL medium (FDR < 0.05; 2,120 genes) to known YAP/TAZ targets that have been previously identified using chromatin immunoprecipitation sequencing and cross-referencing to the 3D chromatin interactome (44, 45). We found that 57 YAP/TAZ targets were significantly repressed by hCO culture in MM (Fig. 7A) (only 8 targets were up-regulated; a down-regulated list of gene names is in Fig. S9A). The YAP/TAZ targets repressed in hCOs cultured in MM included the well-characterized YAP/TAZ targets CTGF, CYR61, ANKRD1, and AXL (44). The top GO term for the repressed YAP/TAZ targets was regulation of cell cycle (10 genes, P = 9.7 × 10−4), thereby indicating that loss of YAP/TAZ transcriptional activity could by partially responsible for the cell cycle arrest observed in hCO cultured in MM.
Fig. 7.
β-Catenin and YAP1 coactivation restores proliferation in MM. (A) YAP/TAZ target genes are repressed in hCOs cultured in MM. Heat map data from significantly regulated YAP/TAZ targets in RNA-seq data; n = 4 experiments. (B) Delivery of constitutively active β-catenin and YAP1 cooperate to activate proliferation (Ki-67) of hPSC-CMs (α-actinin) in hCOs cultured in MM; n = 9 from three experiments. The 10,434 hPSC-CMs were manually counted. (C) Delivery of constitutively active β-catenin and YAP1 cooperate to induce mitosis (pH3) in hPSC-CMs (α-actinin) in hCOs cultured in MM; n = 3–4. The 3,770 hPSC-CMs were manually counted. (D) Compound 6.28 induces dose-dependent increases in proliferation (Ki-67 intensity) in hCOs cultured in MM for 2 d; n = 6–9 from two experiments. (E) Compound 6.28 induces dose-dependent increases in proliferation (Ki-67) of hPSC-CMs (α-actinin) in hCOs cultured in MM. Representative images of proliferating (Ki-67) hPSC-CMs (α-actinin) treated with DMSO or compound 6.28 for 2 d; n = 6–9 from two experiments. The 10,742 hPSC-CMs were manually counted. (F) Compound 6.28 induces mitosis (pH3) of hPSC-CMs (α-actinin) in hCOs cultured in MM. Representative images of mitotic (pH3) of hPSC-CMs (α-actinin) treated with DMSO or compound 6.28 for 2 d; n = 7 from two experiments. The 4,782 hPSC-CMs were manually counted. (G) Compound 6.28 induces expression of BIRC5 in hCOs cultured in MM; n = 5–6. (H) DNA intensity increases in hCO treated with compound 6.28 for 2 d; n = 7–9 hCOs from two experiments. (I) hCO area (using α-actinin area) increases after treatment with compound 6.28 for 2 d; n = 7–9 hCOs from two experiments. (J) Average hPSC-CM area per image does not change after treatment with compound 6.28 for 2 d; n = 13–15 hCOs from two experiments. (K) GO term analysis of proteomics reveals that multiple fatty acid biosynthesis and cell cycle processes are increased in hCOs treated with compound 6.28 for 2 d. Data are mean ± SEM. *P < 0.05; ***P < 0.001; and ****P < 0.0001, using ANOVA with Dunnet’s posttest (D and E) or t test (B, C, and F–I).
Fig. S9.
β-Catenin and YAP1 is synergistically required to induce hPSC-CM proliferation in hCO in support of the data presented in Fig. 7. (A) Heat map with gene names of YAP targets down-regulated hCOs in MM; n = 4 experiments from RNA-seq data. Log2 expression relative to mean for all conditions. (B) Delivery of constitutively active β-catenin or YAP1 individually does not activate proliferation (Ki-67 intensity) in hCOs cultured in MM; n = 11–13 from three experiments. (C) Delivery of constitutively active β-catenin or YAP1 individually does not activate proliferation (Ki-67) of hPSC-CMs (α-actinin) in hCOs cultured in MM; n = 10 from three experiments. The 17,214 hPSC-CMs were manually counted. (D) Delivery of constitutively active β-catenin or YAP1 individually does not activate mitosis (pH3) of hPSC-CMs (α-actinin) in hCOs cultured in MM; n = 6 from two experiments. The 10,243 hPSC-CMs were manually counted. (E) Delivery of constitutively active β-catenin or YAP1 individually does not activate BIRC5 in hCOs cultured in MM; n = 7–8 from two experiments. (F) Delivery of constitutively active β-catenin and YAP1 cooperates to activate proliferation (Ki-67 intensity) in hCOs cultured in MM; n = 11–14 from three experiments. (G) β-Catenin and YAP1 cooperate in hCOs cultured in MM to induce expression of BIRC5; n = 6–7 from two experiments. (H) Force of contraction is not affected by overexpression of constitutively active β-catenin and YAP1; n = 9–12. (I) Chemical structure of compound 6.28. (J) Dose–response curves for compound 6.28. (K) Volcano plot illustrating the differential analysis of the proteomics data for hCO treated with compound 6.28 for 2 d; n = 2 for DMSO control and n = 4 for compound 6.28. Data are mean ± SEM. **P < 0.01, using t test (F and G). FC, fold change.
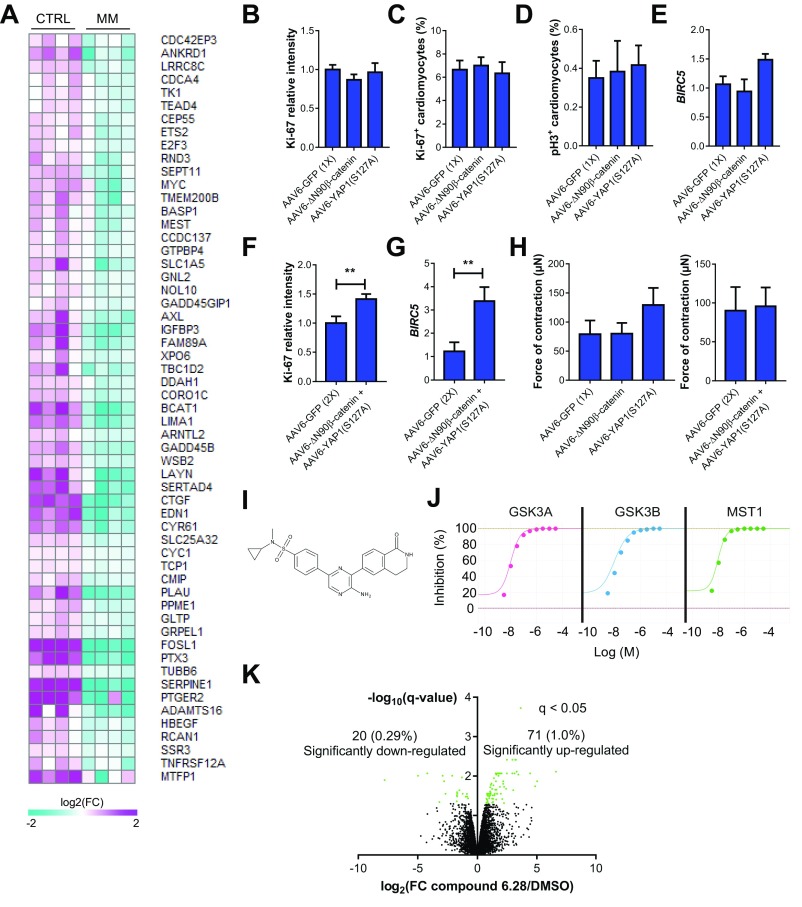
We next tested whether coactivation of β-catenin and YAP1 was sufficient to drive hPSC-CM cell cycle reentry in MM-cultured hCOs and rescue the proliferative block caused by the switch in metabolism. We found that overexpression of either constitutively active YAP1 (S127A) or constitutively active β-catenin (ΔN90) alone was insufficient to facilitate cell cycle reentry in hCO cultured in MM and failed to activate BIRC5 transcription (Fig. S9 B–E), which has been shown to be dependent on a β-catenin and YAP1 complex (40). However, overexpression of both β-catenin (ΔN90) and YAP1 (S127A) had a synergistic effect and together, augmented proliferation (Fig. 7B and Fig. S9F) and mitosis (Fig. 7C) of hPSC-CMs, as well as BIRC5 transcription (Fig. S9G) in the hCOs cultured in MM. This proliferative response did not alter the force of contraction (Fig. S9H).
To confirm our findings with an independent method, we coactivated both signaling pathways using a small molecule, compound 6.28 (46) (Fig. S9I). Compound 6.28 inhibits both GSK3A/B (IC50 of 13 and 16 nM, respectively) and MST1 (IC50 of 12 nM) (Fig. S9J), which are critical upstream inhibitors of β-catenin (47) and YAP1 (48), respectively. Compound 6.28 induced concentration-dependent increases in hPSC-CM proliferation (Fig. 7 D and E) and also induced mitosis (Fig. 7F) and activation of BIRC5 (Fig. 7G) in hCOs cultured in MM. Treatment with compound 6.28 also resulted in increased DNA intensity (Fig. 7H) and an 11 ± 2.5% (Fig. 7I) increase in hCO size after 48 h without an increase in hPSC-CM size (Fig. 7J), indicative of bona fide proliferation. To determine the global effects of compound 6.28, we also performed proteomics and found 71 up-regulated and 20 down-regulated proteins (Fig. S9K). We performed GO term analysis, and consistent with our data on compound 6.28, we found that multiple aspects of cell cycle were activated, including (but not limited to) G1/S cell cycle phase transition, DNA replication, mitotic cell cycle, and cytokinesis (Fig. 7K). Fatty acid biosynthetic processes were also activated by compound 6.28 (Fig. 7K).
Taken together, our findings show that synergistic activation of β-catenin and YAP signaling induces bona fide hPSC-CM proliferation within our hCOs by rescuing the proliferative barrier imposed by culture in MM.
SI Methods
hPSCs.
HES3 (female) and H9 (female) hESCs (WiCell) or hiPSCs (female, Sendai virus-reprogrammed CD34+ cells ATCC-BXS0116; ATCC) were maintained as TypLE (Thermo Fisher Scientific) passaged cultures using mTeSR-1 (Stem Cell Technologies)/Matrigel (Millipore). Karyotyping and DNA fingerprinting were performed as a quality control.
Human RNA Sample.
The adult human heart sample was obtained from Clontech. The adult sample was pooled from three hearts from 30- to 39-y-old Caucasian males who died from trauma.
Human Proteomics Sample.
The human adult heart sample was obtained from a healthy 49-y-old female and snap frozen.
Neonatal Rat Ventricular Cardiomyocytes.
Cardiomyocytes were derived from P1 Sprague–Dawley neonatal rats as previously described (58); 1- to 2-d-old neonatal rats (Sprague–Dawley) were used for cardiomyocyte isolation. Briefly, neonatal rats were killed; hearts were excised and washed in ADS buffer (116 mM NaCl, 5.4 mM KCl, 1 mM NaH2PO4, 0.8 mM MgSO4, 5 mM glucose, 20 mM HEPES), and atria were removed. Myocytes were isolated using collagenase II and separated with Percoll gradients. Percoll gradients were constructed by layering a 1:1.2 Percoll:ADS layer on a 1:0.5 Percoll:ADS layer in a 15-mL Falcon tube. Isolated myocytes were plated in CTRL medium (see below) on gelatin-coated glass coverslips at 1 × 105 cells per 1 cm2 and allowed to recover overnight before experiments.
Heart-Dyno Fabrication.
Heart-Dyno culture inserts were fabricated using standard SU-8 photolithography and PDMS molding practices (16). Microfabricated cantilever array designs were drafted with DraftSight (Dassault Systems), and a number of different designs were initially tested for feasibility. Photomasks of the design were then plotted with an MIVA photoplotter onto 7-inch HY2 glass plates (Konica Minolta). SU-8 photolithography on 6-inch silicon wafer substrates formed the structures to a depth of ∼700 µm. Briefly, silicon wafers were cleaned with acetone, isopropanol, and N2 and then degassed at 150 °C for 30 min. SU-8 2150 photoresist (Microchem) was spin coated and soft baked four times to build the SU-8 to the required thickness. The wafer was then exposed to UV light under the photomask for a total dose of 1,082 mJ/cm2. The exposed wafer was then postexposure baked (5 min at 65 °C, 40 min at 95 °C, 4 min at 65 °C) and developed in propylene glycol monomethyl ether acetate for 45 min in a sonicator bath. Final feature height was measured with an optical surface profiler (Veeco). The Heart-Dyno was molded by soft lithography with PDMS (Sylgard 184; Dow Corning; mixed in 10:1 ratio of monomer:catalyst), with curing at 65 °C for 35 min. The molds were cut using a 6-mm hole punch and placed into 96-well plates, after which they were then sterilized with 70% ethanol and UV light, washed with PBS, and coated with 3% BSA (Sigma) to prevent cell attachment to the bottom of the wells.
Cardiac Differentiation.
Cardiac cells were produced using recently developed protocols (13, 55, 56). hPSCs were seeded at 2 × 104 cells per 1 cm2 in Matrigel-coated flasks and cultured for 4 d using mTeSR-1. They were then differentiated into cardiac mesoderm using RPMI B27− medium (RPMI 1640 GlutaMAX + 2% B27 supplement without insulin), 200 μM l-ascorbic acid 2 phosphate sesquimagnesium salt hydrate (Sigma), and 1% Penicillin/Streptomycin (all Thermo Fisher Scientific unless otherwise indicated) containing 5 ng/mL BMP-4 (RnD Systems), 9 ng/mL Activin A (RnD Systems), 5 ng/mL FGF-2 (RnD Systems), and 1 μM CHIR99021 (Stem Cell Technologies) with daily medium exchange for 3 d. Subsequently, they were specified into an hPSC-CM/stromal cell mixture using RPMI B27− containing 5 μM IWP-4 (Stem Cell Technologies) for 3 d, followed by another 7 d of RPMI B27+ (RPMI 1640 GlutaMAX + 2% B27 supplement with insulin, 200 μM l-ascorbic acid 2 phosphate sesquimagnesium salt hydrate, and 1% Penicillin/Streptomycin) with medium exchange every 2–3 d. The differentiated cells were then cultured in RPMI B27+ until digestion at 15 d using 0.2% collagenase type I (Sigma) in 20% FBS in PBS (with Ca2+ and Mg2+) for 60 min at 37 °C followed by 0.25% trypsin-EDTA for 10 min. The cells were filtered using a 100-μm mesh cell strainer (BD Biosciences), centrifuged at 300 × g for 3 min, and resuspended at the required density in CTRL medium: α-MEM GlutaMAX, 10% FBS, 200 μM l-ascorbic acid 2 phosphate sesquimagnesium salt hydrate, and 1% Penicillin/Streptomycin. Based on flow cytometry, the cells generated and used for tissue engineering were ∼70% α-actinin+/CTNT+ hPSC-CMs, with the rest being predominantly CD90+ stromal cells (13). The hPSC-CMs derived after 15 d of differentiation are defined as the starting population (SP).
hCO Fabrication.
CTRL medium: α-MEM GlutaMAX (ThermoFisher Scientific), 10% fetal bovine serum (FBS) (ThermoFisher Scientific), 200 μM l-ascorbic acid 2 phosphate sesquimagnesium salt hydrate (Sigma) and 1% Penicillin/Streptomycin (ThermoFisher Scientific). For each hCO, 5 × 104 cardiac cells in CTRL medium were mixed with collagen I to make a 3.5-μl final solution containing 2.6 mg/ml collagen I and 9% Matrigel. The bovine acid-solubilized collagen I (Devro) was first salt-balanced and pH-neutralized using 10X DMEM and 0.1 M NaOH, respectively, prior to mixing with Matrigel and cells. The mixture was prepared on ice and pipetted into the Heart-Dyno. The Heart-Dyno was then centrifuged at 100 × g for 10 s to ensure the hCO form halfway up the posts. The mixture was then gelled at 37°C for 30 min prior to the addition of CTRL medium to cover the tissues (150 μl/hCO). The Heart-Dyno design facilitates the self-formation of tissues around in-built PDMS exercise poles (designed to deform ∼0.07 μm/μN). The medium was changed every 2-3 days (150 μl/hCO).
hCOs were cultured in CTRL medium for formation and then changed to serum-free media as indicated for experiments. For all screening experiments, after hCO formation, hCOs were cultured in serum-free conditions comprising DMEM without glucose, glutamine, and phenol red (ThermoFisher Scientific) supplemented with 4% B27 (with or without insulin) (ThermoFisher Scientific), 1% GlutaMAX (ThermoFisher Scientific), 200 μM l-ascorbic acid 2 phosphate sesquimagnesium salt hydrate and 1% Penicillin/Streptomycin (ThermoFisher Scientific). Additions to the medium included glucose, palmitic acid (conjugated to bovine serum albumin within B27 by incubating for 2h at 37°C, Sigma), or TGFβ-1 (Peprotech). A timeline of the finalized hCO fabrication, culture and maturation protocol can be found in Fig. 1M.
Force Analysis of hCO in Heart-Dyno.
The pole deflection was used to approximate the force of contraction. A Leica DMi8 inverted high-content imager was used to capture a 10-s time lapse of each hCO contracting in real time at 37 °C. Custom batch processing files were written in Matlab R2013a (Mathworks) to convert the stacked .tiff image files to .avi movie files, track the pole movement (using vision.PointTracker), determine the contractile parameters, produce a force-time figure, and export the batch data to an Excel (Microsoft) spreadsheet (Matlab files are available on request).
The following formulas were used to determine the contractile force at each time point.
Maximum deflection at the end of a rectangular cantilever fixed at one end with force applied at a specified distance was
| [S1] |
| [S2] |
| [S3] |
where F = force, I = moment of inertia, E = Young’s modulus, b = length of the pole, h = width of the pole (direction of bending), L = height of the pole, x = position of tissue on the poles in the z direction, and δ = pole deflection.
Based on the parameters of our system, for each pole: E = 1,500 kPa, b = 0.5 mm, h = 0.2 mm, L = 0.7 mm, x = 0.35 mm (hCO halfway up the poles), k = 14 µN/µm, and
| [S4] |
We validated that these parameters using a sensitive isometric force transducer (ADInstruments) and measured k = 14.2 ± 2.4 µN/µm (n = 10).
Whole-Mount Immunostaining.
hCOs were fixed for 60 min with 1% paraformaldehyde (Sigma) at room temperature and washed three time with PBS, after which they were incubated with primary antibodies (Table S1) in Blocking Buffer, 5% FBS, and 0.2% Triton X-100 (Sigma) in PBS overnight at 4 °C. Cells were then washed in Blocking Buffer two times for 2 h and subsequently incubated with secondary antibodies (Table S1) and Hoescht (1:1,000) overnight at 4 °C. They were washed in Blocking Buffer two times for 2 h and imaged in situ or mounted on microscope slides using Fluoromount-G (Southern Biotech).
Table S1.
Antibodies used in this study
| Antibody | Species | Company | Catalog no. | Dilution |
| α-Actinin (clone EA-53) | Mouse IgG1 | Sigma | A7811 | 1:1,000 |
| Ki-67 (D3B5) | Rabbit IgG | Cell Signaling Technology | 9129 | 1:400 |
| Antiphospho-Histone H3 (Ser10; pH3) | Rabbit polyclonal | Millipore | 06–570 | 1:200 |
| Active β-catenin (PY489) | Mouse IgM | Developmental Studies Hybridoma Bank | PY489-B-catenin | 5 μg/mL |
| Titin | Mouse IgM | Developmental Studies Hybridoma Bank | 9 D10 | 5 μg/mL |
| MLC2v | Rabbit IgG | Protein Tech Group | 10906–1-AP | 1:200 |
| CD31 | Mouse IgG1 | Dako | M082329-2 | 1:200 |
| CD90 | Mouse IgG2A | RnD Systems | MAB2067 | 1:200 (After reconstitution using manufacturer’s guidelines) |
| WT-1 | Rabbit IgG | Abcam | AB89901 | 1:200 |
| Caveolin 3 | Mouse IgG1 | BD Transduction Laboratories | 610421 | 1:200 |
| pATM | Mouse IgG1 | Santa Cruz Biotechnology | sc-47739 | 1:100 |
| 8-OxoG | Mouse IgM | Abcam | Ab206461 | 1:100 |
| Pancadherin | Rabbit antiserum | Sigma | C3678 | 1:200 |
| Connexin 43 | Rabbit IgG | Abcam | ab11370 | 1:200 |
| GFP | Chicken polyclonal | Abcam | Ab13970 | 1:200 |
| Alexa Fluor 488 goat anti-rabbit IgG (H+L) | NA | Life Technologies | A-11034 | 1:400 |
| Alexa Fluor 488 goat anti-mouse IgG (H+L) | NA | Life Technologies | A-11029 | 1:400 |
| Alexa Fluor 488 goat anti-mouse IgM (µ chain) | NA | Life Technologies | A-21042 | 1:400 |
| Alexa Fluor 555 goat anti-rabbit IgG (H+L) | NA | Life Technologies | A-21428 | 1:400 |
| Alexa Fluor 555 goat anti-mouse IgG (H+L) | NA | Life Technologies | A-21422 | 1:400 |
| Alexa Fluor 633 goat anti-rabbit IgG (H+L) | NA | Life Technologies | A-21070 | 1:400 |
| Alexa Fluor 488 goat anti-chicken IgG (H+L) | NA | Life Technologies | A-11039 | 1:400 |
NA, not applicable.
Immunostaining Analysis.
For screening, hCOs were imaged using a Leica DMi8 high-content imaging microscope for in situ imaging. Custom batch processing files were written in Matlab R2013a (Mathworks) to remove the background, calculate the image intensity, and export the batch data to an Excel (Microsoft) spreadsheet.
For more detailed images, an Olympus IX81 confocal microscope or a Nikon Diskovery Spinning Disk confocal microscope for mounted imaging was used. For cell cycle analysis experiments, three random fields of view were imaged and manually quantified for proliferation. These were added together to calculate the percentage of hPSC-CM proliferation in each hCO.
Flow Cytometry.
Cells were dissociated to single cells for flow cytometry. hCOs were first washed twice in perfusion buffer at 37 °C (130 mM NaCl, 1 mM MgCl2, 5 mM KCl, 0.5 mM NaH2PO4, 10 mM Hepes, 10 mM Taurine, 10 mM glucose, 10 μM 2,3-butanedione monoxime, pH 7.4). hCOs were incubated in EDTA buffer at 37 °C for 5 min (130 mM NaCl, 5 mM KCl, 0.5 mM NaH2PO4, 10 mM Hepes, 10 mM Taurine, 10 mM glucose, 5 mM EDTA, 10 μM 2,3-butanedione monoxime, pH 7.4). hCOs were washed twice in perfusion buffer and then incubated in perfusion buffer plus 1 mg/mL collagenase B (Roche) for 30 min at 37 °C on a shaker at 750 rpm. hCOs were then centrifuged at 1,000 × g for 3 min, collagenase was removed, and they were incubated in 0.25% trypsin-EDTA for 15 min at 37 °C on a shaker at 750 rpm. Perfusion buffer with 5% FBS was then added, and the single cells were pelleted by centrifuging at 1,000 × g for 3 min. The cells were then stained for flow cytometry using published protocols (13), except that PBS was replaced by perfusion buffer to maintain cell viability of live cells. Flow cytometry was performed on a Becton Dickinson LSR Fortessa X-20 cytometer and analyzed using Cyflogic 1.2.1 (Cyflo Ltd).
hPSC-CM Dissociation for Single-Cell Electrophysiology and Calcium Imaging.
hPSC-CMs were dissociated for SP, using the same protocol as for hCO fabrication and seeded on gelatin-coated coverslips in CTRL medium. Cells were analyzed the following day.
hPSC-CMs were dissociated from hCOs 9 d after switching to MM by washing three times in calcium-free Tyrode’s buffer (120 mM NaCl, 1 mM MgCl2, 5.4 mM KCl, 22.6 mM NaHCO3, 0.42 mM NaH2PO4, 5.5 mM glucose, pH 7.4) with 10 μM 2,3-butanedione monoxime (dissociation buffer). Cells were dissociated using 1 mg/mL collagenase B in dissociation buffer for 30–60 min at 37 °C. The dissociated cells were washed in dissociation buffer and centrifuged at 100 × g for 3 min. They were resuspended in dissociation buffer, and the calcium concentration gradually increased to 10, 50, 250, and finally, 1,250 μM using CTRL medium or MM with 10 μM 2,3-butanedione monoxime. The cells were centrifuged at 100 × g for 3 min, resuspended in CTRL medium or MM with 10 μM 2,3-butanedione monoxime, and plated on growth factor-reduced Matrigel or laminin (Sigma)-coated coverslips. After 4 h of attachment, the medium was changed to CTRL medium or MM without 10 μM 2,3-butanedione monoxime, and the cells were analyzed the following day.
Electrophysiology.
Electrophysiological recordings were obtained at 37 °C using a TC-124A temperature controller (Warner Instruments) mounted onto the stage of an Olympus IX-51 inverted microscope. Data were acquired with pClamp 9 software (Axon Instruments) through a 16-bit AD/DA interface (Digidata 1322A; Axon Instruments) connected to an Axoclamp 200B amplifier (Axon Instruments). Recordings were sampled at 10 kHz, low pass Bessel-filtered at 5 kHz (−3-dB cutoff), and evaluated offline with Clampfit 10 and GraphPad Prism 6. Pipettes were prepared from standard wall borosilicate glass capillaries (BF 120–69-10; Sutter Instruments) on a P-87 horizontal puller (Sutter Instruments).
hCO single–hPSC-CM action potentials (APs) were recorded from dissociated cells bathed in 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 5 mM Hepes, and 5 mM glucose, pH 7.4. Pipette potential offset and capacitance neutralization preceded whole-cell patch-clamp measurements in the current-clamp mode. Patch pipettes had resistances of 1–3 MΩ when backfilled with an internal solution (10 mM NaCl, 140 mM KCl, 2 mM EGTA, 1 mM MgCl2, 0.1 mM Na-GTP, 5 mM Mg-ATP, 10 mM Hepes, pH 7.2). hPSC-CMs were “clamped” to a membrane potential of −80 mV by continuous current injection. APs were elicited at 1 Hz by applying 4-ms rectangular current pulses at 125% threshold level. hPSC-CMs were classified into ventricular-, atrial-, and nodal-like according to the following criteria. Ventricular-like APs had a clear plateau (a prolonged phase of at least 50 ms in duration with less than 20-mV drop in membrane potential), fast upstroke (>50 V/s), a large AP amplitude (>85 mV), and a small ratio of action potential duration at 90% (APD90) repolarization to action potential duration at 50% repolarization (APD50; APD90/APD50 < 2.3). Atrial-like APs exhibited no clear plateau but shared all other ventricular-like criteria. Finally, nodal-like APs lacked a plateau phase and were characterized by a slower phase of repolarization (APD90/APD50 > 2.3).
Membrane potentials and spontaneous electrical signals were recorded from intact hCOs bathed in CTRL medium or MM with 7 µM blebbistatin to inhibit contractions. Pipette potential offset and capacitance were neutralized before impaling the tissue. Sharp electrodes had series resistances of 30–50 MΩ when backfilled with 3 M KCl. Tip potentials and liquid junction potentials amounted to a few millivolts and were not subjected to correction.
Calcium Imaging.
Cells were loaded with 2.5 μM Fluo-4 AM (Thermo Fisher Scientific) added directly to the culture medium for 30 min at 37 °C. The medium was changed (CTRL medium or MM) and left for 30 min at 37 °C before recordings. For recordings, the cells were stimulated at 1 Hz (using a Panlab/Harvard Apparatus Digital Stimulator) at 37 °C on an Olympus IX81 confocal microscope using line scanning (∼1 ms per line for ∼10 s). Raw data were processed, peaks were identified, and parameters were calculated for each calcium transient in the recording and averaged for that particular cell. This was performed using a custom written program in Matlab R2013a (Mathworks) to improve accuracy and eliminate bias.
RNA Extraction.
RNA was extracted using TRIzol (Thermo Fisher Scientific), treated with DNase (Qiagen), and purified using RNeasy Minielute Cleanup Kit (Qiagen).
RNA-Seq.
For hCOs and the adult human heart sample, rRNA was depleted with Ribo Zero Gold, and cDNA was generated with SuperScript II Reverse Transcriptase (Thermo Fisher Scientific). RNA-seq libraries were created with TruSeq Stranded Total RNA kits (Illumina) and read with the HiSeq SR Cluster v4 kit (Illumina) on a HiSeq 2500 sequencer. Sample read quality was determined with FASTQC, and Trimmomatic (59) was used to trim poor-quality sequence (<25 phred score) and adapter sequence. Each sample was mapped to hg38 with STAR (60). Mapped reads were then counted with htseq-count on union mode, and differential expression analysis was performed with EdgeR(v3.2.4).
Proteomics Sample Preparation.
Nine hCOs from either CTRL medium or MM condition were pooled per replicate and washed two times in PBS. Tissues were lysed in by tip-probe sonication in 6 M guandinium chloride, 100 mM Tris, pH 8.0, 10 mM Tris(2-carboxyethyl)phosphine, and 40 mM 2-chloroacetamide and heated to 95 °C for 5 min. The samples were cooled to 4 °C and centrifuged at 20,000 × g for 10 min. The supernatant was diluted 1:1 with water followed by protein precipitation with 4 vol acetone. Protein pellets were washed with 80% acetone and resuspended in 10% trifluoroethanol and 100 mM Tris, pH 8.0. Protein was quantified by bicinchoninic acid assay, and 50 µg were digested with 1 µg LysC (Wako Chemicals) for 2 h at 37 °C followed by 1 µg trypsin (Sigma) for 16 h at 37 °C. Digests were diluted with 4 vol 0.5% trifluoroacetic acid and desalted with C18 microcolumns packed with POROS Oligo R2/R3 reversed phase particles (20 µm; Thermo Fisher Scientific). Peptides were eluted in 50% acetonitrile and 0.1% trifluoroacetic acid and dried by vacuum centrifugation. Peptides were quantified by Qubit fluorescence, and 1-µg aliquots were removed for direct analysis by liquid chromatography–tandem MS (LC-MS/MS). A second aliquot of peptides was removed from each of the hCO samples (totaling 30 µg) and pooled for fractionation. Peptides from pooled hCOs and myocardial tissue were fractionated on an 320 µm × 30 cm in-house packed C18 µHPLC column (3 µm ethylene bridged hybrid; Waters) using an Agilent 1200 HPLC. The gradient was 0–40% Buffer B over 60 min, with 2-min fractions collected followed by concatenation into 12 fractions for analysis by LC-MS/MS (Buffer A = 10 mM ammonium bicarbonate, pH 7.9; Buffer B = 90% acetonitrile).
LC-MS/MS and Data Analysis.
Peptides were analyzed on a Dionex 3500RS coupled to a Q-Exactive Plus with Tune v2.4.1824 in positive polarity mode. Peptides were separated using an in-house packed 75-μm × 50-cm pulled column (1.9-μm particle size, C18AQ; Maisch) with a gradient of 2–30% acetonitrile containing 0.1% formic acid over 120 min at 250 nL/min at 55 °C. An first-stage mass spectrometry scan was acquired from 300–1,500 m/z (70,000 resolution, 3e6 automated gain control, 100-ms injection time) followed by MS/MS data-dependent acquisition of the 20 most intense ions with higher energy collisional dissociation (17,500 resolution, 1e5 automated gain control, 60-ms injection time, 27 normalized collision energy, 1.2 m/z isolation width). Raw data were searched with Andromeda (61) in MaxQuant v1.5.3.30 (62) against the human UniProt database (January 2016) using all default settings with peptide spectral matches and protein FDR set to 1%. Label-free quantification (63) was enabled, including the “match between runs” option, where single-shot replicates of the hCOs were matched into the pooled fractionated sample. Statistical analysis was performed in Perseus (64) and included a two-sample t test corrected for multiple testing using Benjamini–Hochberg FDR. Only proteins quantified in all biological replicates were included in the final analysis, and significance was calculated based on q < 0.05.
Bioinformatics.
PCA was performed using Matlab R2013a (Mathworks) using normalized log2-transformed count per million data outputted from EdgeR(v3.2.4) (65). The PCA included RNA-seq of hPSC-CMs cultured for 1 y or 20 d and adult and fetal human heart tissue from a previous study for comparison (25). These 100-bp paired end read data were obtained from the Gene Expression Omnibus (accession no. GSE62913) and analyzed as above, except that Trimmomatic (59) and STAR (60) were run using paired end sequencing settings. For our RNA-seq and proteomics data, GO analysis was performed with DAVID (66), and heat maps and hierarchical clustering were performed using GENE-E (Broad Institute).
qPCR.
RNA was reverse transcribed using SuperScript III (random primers), and qPCR was performed using SYBR Mastermix (Thermo Fisher Scientific) on a Applied Biosystems Step One Plus to determine cycle number (Ct). The 2−ΔΔCt method was used to determine gene expression changes using HRPT1 as a housekeeping gene. Primer sequences are listed in Table S2 and were used at 200 nM. For miR quantification Applied Biosystems™ TaqMan™ MicroRNA Assays were used for miR-199a and miR-590 with RNU6B as a housekeeping control.
Table S2.
qPCR primers used in this study
| Gene | Forward | Reverse | Size (bp) | Accession no. |
| HPRT1 | AACCTCTCGGCTTTCCCG | TCACTAATCACGACGCCAGG | 150 | NM_000194.2 |
| RNA18S5 (genomic DNA) | GCTGAGAAGACGGTCGAACT | CGCAGGTTCACCTACGGAAA | 74 | NR_003286.2 |
| αMHC (MYH6) | CTCCTCCTACGCAACTGCCG | CGACACCGTCTGGAAGGATGA | 85 | NM_002471 |
| βMHC (MYH7) | GACCAGATGAATGAGCACCG | GGTGAGGTCGTTGACAGAACG | 63 | NM_000257 |
| MLC2a (MYL7) | CAGCGGCAAAGGGGTGGTGAAC | GGTCCATGGGTGTCAGGGCGAA | 113 | NM_021223.2 |
| MLC2v (MYL2) | GGCGCCAACTCCAACGTGTT | ACGTTCACTCGCCCAAGGGC | 149 | NM_000432.3 |
| TTN ALL (Ex49-50) | GTAAAAAGAGCTGCCCCAGTGA | GCTAGGTGGCCCAGTGCTACT | 68 | NM_001267550.1 |
| NM_001256850.1 | ||||
| NM_133437.3 | ||||
| NM_133432.3 | ||||
| NM_003319.4 | ||||
| TTN N2B (Ex50-219) | CCAATGAGTATGGCAGTGTCA | TACGTTCCGGAAGTAATTTGC | 93 | NM_133437.3 |
| NM_133432.3 | ||||
| NM_003319.4 | ||||
| cTNNI (TNNI3) | CCTCCAACTACCGCGCTTAT | CTGCAATTTTCTCGAGGCGG | 77 | NM_000363.4 |
| ssTNNI (TNNI1) | GCTCCACGAGGACTGAACAA | CTTCAGCAAGAGTTTGCGGG | 97 | NM_003281.3 |
| ND1 (mtDNA) | AACCTCAACCTAGGCCTCCT | GAGTTTGATGCTCACCCTGA | 86 | NC_012920.1 |
| BIRC5 | TTCTCAAGGACCACCGCATC | CCAAGTCTGGCTCGTTCTCA | 126 | NM_001012271.1 |
| NM_001168.2 | ||||
| NM_001012270.1 |
TEM.
Samples were processed for TEM as described previously (67). Sections were analyzed unstained in a Jeol1011 transmission electron microscope.
Metabolite Extraction.
To extract cellular metabolites, hCOs were pooled into n = 12 or 14 for hCOs cultured in CTRL medium or MM (for 9 d), respectively. They were washed three times in 3 mL ice cold 0.9% NaCl, and the metabolites were extracted using 1 mL ice cold 50% aqueous acetonitrile with multiple rounds of vortexing over a 10-min period (68). The samples were snap frozen at −80 °C until processing and analysis. The extraction solution contained 50 nM azidothymidine per sample as an internal standard to monitor extraction efficiency for HPLC-MS/MS analysis.
Central Carbon Metabolite Analysis.
Intermediates of central carbon metabolism (CCM) were analyzed following the method described in ref. 69 with the following modifications. Sample extracts were analyzed at two concentrations to enhance the likelihood of detection for low-abundance metabolites as well as to dilute highly abundant metabolites into range. Thus, 200 µL sample extract was dried down in a vacuum centrifuge (Eppendorf Concentrator Plus; Eppendorf) for ∼60 min with no heating (i.e., at room temperature) using the V-AQ program. The samples were resuspended in 50 µL 95:5 water:acetonitrile to provide a fourfold concentrated sample, 5 µL of which were removed to a fresh vial and diluted with 95 µL 95:5 water:acetonitrile to provide an effective fivefold dilution of the original extract. Samples were transferred to HPLC vials for CCM analysis by injection onto the HPLC-MS/MS system as described previously (69).
Seahorse Metabolic Profiling.
Cellular bioenergetics (including oxygen consumption rates and extracellular acidification rates) were determined on a Seahorse XF24 Extracellular Flux Bioanalyser (Seahorse Bioscience). Briefly, hCOs were washed in unbuffered assay medium (pH 7.4; Seahorse Bioscience) supplemented with glucose (5.5 mM; Sigma), sodium pyruvate (1.0 mM; Thermo Fisher Scientific), and GlutaMAX (2.0 mM; Thermo Fisher Scientific). After two washes, eight hCOs were seeded (in assay medium) into a 24-well XF24 cell culture microplate (Seahorse Bioscience). Eight wells, which contained unbuffered assay medium alone, were used as background controls. Specific aspects of mitochondrial and glycolytic bioenergetics were analyzed during a mitochondrial stress test using consecutive administration of oligomycin (2 µM), FCCP (1.5 µM), etomoxir (4 µM), and rotenone/antimycin A (2 µM) as described previously (70).
Mitogen Screening.
For neonatal rat cardiomyocytes, small molecules/growth factors were added to CTRL medium and given to the cells for 24 or 48 h: DMSO (Sigma), CHIR99021, and NRG-1 (RnD Systems). For transfection experiments, the cells were transfected for 8 h using Lipofectamine RNAiMax (3 μL per 24 wells) in 500 μL per 24-well OptiMEM followed by a medium change into CTRL medium. The cells were transfected at 50 nM with scramble miR control (All Stars Negative Control; Qiagen), miR mimic hsa-miR-199a-3p (Qiagen), or miR mimic hsa-miR-590–3p (Qiagen). For overexpression of constitutively active Yap1, cells were infected in CTRL medium with an adenovirus containing a mutated version of murine Yap1, CMV-YAP(S112A) or a GFP control (CMV-GFP), at an multiplicity of infection of 10.
hCOs were cultured for 6 d after seeding in the Heart-Dyno in CTRL medium before treatment. Small molecules were added and given to the cells for 48 h: DMSO, CHIR99021, and NRG-1. For transfection experiments, the cells were transfected for 4 h using Lipofectamine RNAiMax (3 μL per hCO) in 150 μL per hCO OptiMEM followed by a medium change into CTRL medium. The cells were transfected at 50 nM with scramble miR control, miR mimic hsa-miR-199a-3p, or miR mimic hsa-miR-590–3p. For overexpression of constitutively active YAP1, hCOs were infected with an AAV6 containing a mutated version of human YAP1, CMV-YAP(S127A) (Vector Biolabs), at 1.25–2.5 × 1010 viral genomes (vg) per hCO. For overexpression of constitutively active β-catenin, hCOs were infected with an AAV6 containing a mutated version of human CTNNB1 without the amino acids 2–90, AAV6-ΔN90βCAT (Vector Biolabs), at 1.25–2.5 × 1010 vg per hCO. Control AAV6-MCS or AAV6-GFP (Vector Biolabs) controls were used at the same titers in these experiments.
Quantification and Statistical Analyses.
All key hCO experiments were performed with multiple hCOs per condition in multiple experiments to ensure reproducibility. For screening or experiments where multiple groups were analyzed, all groups were present in each experiment, including controls, to ensure that results were not an artifact of comparing conditions over different experiments.
Data are presented as mean ± SEM unless otherwise noted. Statistics were analyzed using Microsoft Excel (Microsoft) or GraphPAD Prism 6 (Graphpad Software Inc.). Sample numbers, experimental repeats, statistical analyses, and P values are reported in the figures.
Dataset Availability.
RNA-seq data have been deposited in the Gene Expression Omnibus as accession number GSE93841, and CTRL medium vs. MM hCO proteomics data have been deposited in the PRIDE under accession number PXD005736. All Matlab m-files will be provided on request.
Discussion
hPSC-CMs have become widely used to study human cardiac biology, development, and physiology. However, they are typically immature, which limits their capacity to accurately model adult cardiac biology. While engineered heart muscle can improve hPSC-CM maturation in terms of structure and function (10, 14), the key upstream drivers of metabolic maturation and cell cycle arrest are largely unknown.
To identify central regulators of hPSC-CM maturation and cell cycle exit, we developed a miniaturized semiautomated cardiac organoid culture platform (Heart-Dyno) to facilitate screening. Through systematic and iterative screening, we were able to show that distinct physiological hallmarks of the maturation process were driven by different cues. For example, we found that maturation of many parameters, such as the structural, electrophysiological, and calcium handling as well as responses to adrenergic stimulation, were supported by the 3D engineered heart tissue environment. These parameters in our hCO were similar to those reported using other cardiac tissue engineering platforms (8–14). However, we found that switching metabolism to fatty acid oxidation was a key driver not only for shifting the expression of metabolism genes, mitochondrial biogenesis, and fatty acid oxidation but also, for increased expression of adult sarcomeric protein isoforms and cell cycle exit. Therefore, different aspects of the adult cardiomyocyte phenotype are governed by distinct cues, which need to be carefully controlled for the generation of mature hPSC-CMs.
During the first week of the postnatal period in vivo, a metabolic switch to fatty acid oxidation and cell cycle exit occurs in the heart in concert with a change in serum composition from glycolytic substrates to fatty acids (4). In this study, we found that mimicking these changes in metabolic substrate provision is a major driver of not only the metabolic maturation but also, the transcriptional and cell cycle maturation in human cardiomyocytes. Specifically, we found that a switch from commonly used high-insulin, carbohydrate-based medium to a low-carbohydrate, low-insulin, palmitate-based medium enhanced the maturation of hCOs. These maturation conditions are in contrast to the typical cell growth environments, which generally include glucose, insulin, and serum and are designed to increase cellular proliferation. Altering the metabolic environment was critical to promote maturation in hCO and may also represent a viable approach for promoting differentiation and functional maturation of other cell types.
Recent studies have suggested that oxygen tension is linked to cardiomyocyte proliferation (28, 29, 49) and revealed that a high oxygen environment is a key trigger for postnatal cardiomyocyte cell cycle exit (29). However, the in vitro culture of hPSC-CMs at atmospheric oxygen (∼21% O2) is not sufficient to drive cell cycle exit (16, 50). Our study shows that a switch to fatty acid oxidation via alteration of metabolic substrate utilization, in a high oxygen environment, is a driver of hPSC-CM cell cycle arrest. This suggests that the high oxygen environment works in concert with changes in metabolic substrate availability after birth to govern cardiomyocyte cell cycle arrest. This is also supported in vivo by a recent study showing a link between reduced oxygen concentrations and reactivation of cardiomyocyte proliferation and cardiac regeneration in the adult heart, which was associated with a metabolic switch to glycolysis (28). Together, these studies establish a causal link between postnatal metabolic adaptations, oxygen tension, and cardiomyocyte proliferation.
Our findings show that switching hPSC-CM metabolism to fatty acid oxidation induces long-lasting changes in β-catenin and YAP1 signaling as well as the DDR, which results in hPSC-CM cell cycle withdrawal. Importantly, simultaneous activation of β-catenin and YAP1 was required to overcome the metabolism-induced proliferative barrier in mature hCO. Cooperativity between β-catenin and YAP signaling has also been reported in the embryonic heart, where they interact to control cardiomyocyte proliferation during heart development (41). Moreover, Hippo/Yap signaling has emerged as a central regulator of cardiac regenerative capacity in the neonatal period (38, 51). Our study suggests that postnatal alterations in cardiomyocyte metabolism could operate as a key switch leading to cardiomyocyte cell cycle shut down via repression of β-catenin and YAP signaling after birth. Similarly, alterations in metabolism are known to influence β-catenin and YAP activity in other cell types (52, 53). Therefore, these findings support a model, whereby β-catenin, YAP1, metabolism, and the DDR are intimately linked and cooperate to regulate the cardiac cell cycle and maturation (Fig. 8).
Fig. 8.
Metabolism regulates cardiac maturation. Insulin can only be removed in long-term culture with maintenance of contractile function if fatty acids replace glucose substrates. Initially, the removal of insulin is responsible for a decrease in proliferation. However, in the longer term, cell cycle arrest cannot be rescued by addition of insulin, because there are alterations in metabolism, which repress β-catenin and YAP1 signaling and induce a DDR, thus resulting in cell cycle exit. CM, cardiomyocyte.
Although the mechanisms by which β-catenin and YAP1 interact to induce cell cycle reentry in metabolically mature cardiomyocytes require additional elucidation, we found that treatment with compound 6.28 activates fatty acid synthesis pathways and reduces the DDR in mature hCO. These findings are of particular interest, because fatty acid biosynthesis plays a key role in tumorigenesis (54). Key regulators of fatty acid biosynthesis that are up-regulated by compound 6.28 include fatty acid synthase and ATP citrate lyase, which are currently being pursued as targets for cancer therapeutics (54). While fatty acid synthesis can regulate many biological processes and signaling pathways, it can also protect cells from reactive oxygen species by altering the overall saturation levels of lipids in the cell membrane (54). Additionally, YAP1 has been implicated in cardiac regeneration by promoting an antioxidant response (43). In support of this notion, treatment with compound 6.28 also reduced pATM levels (Fig. S10 A and B) and decreased the expression levels of RAD50 and PARP1 (Fig. S10C), suggesting that β-catenin and YAP1 coactivation may be an approach to overcome the proliferative barrier imposed by the postnatal DDR in cardiomyocytes (29).
Fig. S10.
Treatment of hCOs cultured in MM with compound 6.28 results in a decrease in DDR. (A) hPSC-CMs (MLC2v) treated with compound 6.28 for 2 d have reduced DDR assessed using pATM. (Scale bars: 20 µm.) Inset is white-colored pATM alone. (B) hPSC-CM nuclear intensity of pATM decreased after treatment with compound 6.28, indicative of a reduced DDR; n = 120–241 hPSC-CM nuclei. (C) DDR genes PARP1 and RAD50 are reduced by treatment with compound 6.28 for 2 d using qPCR quantification; n = 5–6. Data are mean ± SEM. *P < 0.05; and ****P < 0.0001 using t test (B and C).
The production of human organoids has rapidly advanced over the past few years. Coupled with higher throughput screening platforms, such as the Heart-Dyno, organoid experiments have the potential to rapidly expand our knowledge of human biology and potentially unlock therapeutic strategies for many diseases.
Methods
Heart-Dyno Fabrication.
Heart-Dyno culture inserts were fabricated using standard SU-8 photolithography and PDMS molding practices (16).
Neonatal Rat Ventricular Cardiomyocytes.
Cardiomyocytes were derived from P1 Sprague–Dawley neonatal rats; 1- to 2-d-old neonatal rats (Sprague–Dawley) were used for cardiomyocyte isolation and handled in accordance with the Australian code of practice for care and use of animals for scientific purposes under ethics approval from the University of Queensland Ethics Committee.
Human Samples.
Ethical approval for the use of hESCs was obtained from The University of Queensland's Medical Research Ethics Committee (2014000801), and the human adult heart sample for proteomics was obtained under ethical approval from The University of Sydney (2012/2814). Ethical approval was carried out in accordance with the National Health and Medical Research Council of Australia regulations under informed consent. The adult human heart sample was obtained from Clontech.
Cardiac Differentiation and hCO Fabrication.
Cardiac cells were produced using recently developed protocols (13, 55, 56). For each hCO, 5 × 104 cardiac cells were mixed with collagen I to make a 3.5-μl final solution containing 2.6 mg/ml collagen I and 9% Matrigel. The bovine acid-solubilized collagen I (Devro) was first salt-balanced and pH-neutralized using 10X DMEM and 0.1 M NaOH, respectively, prior to mixing with Matrigel and cells. The mixture was prepared on ice and pipetted into the Heart-Dyno; more details are in SI Methods.
hCO Screening and Characterization.
Methods associated with hCO screening and characterization, including force analysis, staining and imaging, hPSC-CM dissociation, electrophysiology, calcium imaging, RNA extraction, qPCR, RNA-seq, proteomics, TEM, bioinformatics analysis, metabolite analysis, and metabolic profiling, can be found in SI Methods. Details of antibodies (Table S1) and primers (Table S2) can be found in SI Methods.
SI Results
Initial screens were performed using hCOs derived from the hESC line, HES3, which were seeded in 1.6 mg/mL collagen I and formed for 2 d in CTRL medium. Subsequently, hCOs were cultured under serum-free conditions in 4% B27 in DMEM with GlutaMAX and 10 ng/mL TGFβ-1. When the same conditions were assessed in H9-derived hCO, there were significant issues with cell viability and mechanical instability or tissue “necking” (57), resulting in substandard hCO integrity (Fig. S1G) and failure to recapitulate our initial screening results (Fig. S1 H and I). To ensure that culture conditions were robust and applicable to multiple hPSC lines, we optimized the serum-free medium and matrix composition. Removal of TGFβ-1 increased cell survival and increased function (Fig. S1J), while the addition of more collagen together with Matrigel supplementation prevented necking (Fig. S1K) and improved function (Fig. S1L). Additionally, we increased hCO formation time in CTRL medium from 2 to 5 d to allow for sufficient tissue condensation. These protocol modifications allowed improved tissue viability, prevented tissue necking, and allowed robust generation of hCOs derived from all hPSC lines tested (H9 hESC- and hiPSC-derived hCOs viability in MM of 93 and 90%, respectively).
Supplementary Material
Acknowledgments
We thank Shaun Walters for his assistance in setting up the customized Leica DMI8 microscope for our applications and Dr. Sean Lal (Sydney Heart Bank) for providing the human heart biopsy for proteomic analysis. We thank the Developmental Studies Hybridoma Bank for providing the β-catenin (PY489) and titin antibodies. We used the Australian National Fabrication Facility Queensland Node for the fabrication of the Heart-Dyno molds. We also thank QFAB bioinformatics for access to MetaCore as well as the GVL project and the Research Computing Centre for access to the Galaxy-qld server (https://galaxy-qld.genome.edu.au/galaxy). We acknowledge the use of the Australian Microscopy & Microanalysis Research Facility at the Center for Microscopy and Microanalysis at The University of Queensland. R.G.P. was supported by National Health and Medical Research Council of Australia Grants APP1037320, APP1058565, and APP569542 and the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology. D.A.E. and E.R.P. are supported by the Victorian Government’s Operational Infrastructure Support Program. E.R.P. and J.E.H. are supported by fellowships and project grants from the National Health and Medical Research Council, the National Heart Foundation, Stem Cells Australia, and The University of Queensland.
Footnotes
Conflict of interest statement: R.J.M., D.M.T., E.R.P., and J.E.H. are listed as coinventors on a pending patent held by The University of Queensland that relates to the Heart-Dyno device and maturation medium, which are described in this paper. R.J.M., G.A.Q.-R., E.R.P., and J.E.H. are listed as coinventors on a pending patent held by The University of Queensland that relates to the reactivation of cardiomyocyte cell cycle for cardiac regeneration. L.D., A.T.P., and Q.-D.W. are employees of AstraZeneca.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE93841), and the control medium vs. maturation medium human pluripotent stem cell-derived cardiac organoid proteomics data reported in this paper have been deposited in the PRIDE (accession no. PXD005736).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707316114/-/DCSupplemental.
References
- 1.Porrello ER, Olson EN. A neonatal blueprint for cardiac regeneration. Stem Cell Res (Amst) 2014;13:556–570. doi: 10.1016/j.scr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porrello ER, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girard J, Ferré P, Pégorier JP, Duée PH. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev. 1992;72:507–562. doi: 10.1152/physrev.1992.72.2.507. [DOI] [PubMed] [Google Scholar]
- 5.Sim CB, et al. Dynamic changes in the cardiac methylome during postnatal development. FASEB J. 2015;29:1329–1343. doi: 10.1096/fj.14-264093. [DOI] [PubMed] [Google Scholar]
- 6.Shekhawat PS, Matern D, Strauss AW. Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: Impact of expanded newborn screening on their diagnosis and management. Pediatr Res. 2005;57:78R–86R. doi: 10.1203/01.PDR.0000159631.63843.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiburcy M, Zimmermann WH. Modeling myocardial growth and hypertrophy in engineered heart muscle. Trends Cardiovasc Med. 2014;24:7–13. doi: 10.1016/j.tcm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Boudou T, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18:910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tulloch NL, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaaf S, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes SS, et al. Biowire: A platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voges HK, et al. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development. 2017;144:1118–1127. doi: 10.1242/dev.143966. [DOI] [PubMed] [Google Scholar]
- 14.Tiburcy M, et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation. 2017;135:1832–1847. doi: 10.1161/CIRCULATIONAHA.116.024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann WH, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 16.Titmarsh DM, et al. Induction of human iPSC-derived cardiomyocyte proliferation revealed by combinatorial screening in high density microbioreactor arrays. Sci Rep. 2016;6:24637. doi: 10.1038/srep24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bougnères PF, Karl IE, Hillman LS, Bier DM. Lipid transport in the human newborn. Palmitate and glycerol turnover and the contribution of glycerol to neonatal hepatic glucose output. J Clin Invest. 1982;70:262–270. doi: 10.1172/JCI110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiburcy M, et al. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res. 2011;109:1105–1114. doi: 10.1161/CIRCRESAHA.111.251843. [DOI] [PubMed] [Google Scholar]
- 19.Desbois-Mouthon C, et al. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- 20.Racca AW, et al. Contractile properties of developing human fetal cardiac muscle. J Physiol. 2016;594:437–452. doi: 10.1113/JP271290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreo A, et al. Influence of myocardial fibrosis on left ventricular diastolic function: Noninvasive assessment by cardiac magnetic resonance and echo. Circ Cardiovasc Imaging. 2009;2:437–443. doi: 10.1161/CIRCIMAGING.108.838367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann WH, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 23.Eder A, Vollert I, Hansen A, Eschenhagen T. Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev. 2016;96:214–224. doi: 10.1016/j.addr.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Pabon L, Murry CE. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res. 2014;114:511–523. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuppusamy KT, et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci USA. 2015;112:E2785–E2794. doi: 10.1073/pnas.1424042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto AR, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLaughter DM, et al. Single-cell resolution of temporal gene expression during heart development. Dev Cell. 2016;39:480–490. doi: 10.1016/j.devcel.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakada Y, et al. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 29.Puente BN, et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois NC, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson DO, Jin DX, Downs KM, Kamp TJ, Lyons GE. Irx4 identifies a chamber-specific cell population that contributes to ventricular myocardium development. Dev Dyn. 2014;243:381–392. doi: 10.1002/dvdy.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruneau BG, et al. Cardiomyopathy in Irx4-deficient mice is preceded by abnormal ventricular gene expression. Mol Cell Biol. 2001;21:1730–1736. doi: 10.1128/MCB.21.5.1730-1736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang J, et al. Cardiac myosin binding protein C regulates postnatal myocyte cytokinesis. Proc Natl Acad Sci USA. 2015;112:9046–9051. doi: 10.1073/pnas.1511004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Readnower RD, Brainard RE, Hill BG, Jones SP. Standardized bioenergetic profiling of adult mouse cardiomyocytes. Physiol Genomics. 2012;44:1208–1213. doi: 10.1152/physiolgenomics.00129.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polizzotti BD, et al. Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci Transl Med. 2015;7:281ra45. doi: 10.1126/scitranslmed.aaa5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturzu AC, et al. Fetal mammalian heart generates a robust compensatory response to cell loss. Circulation. 2015;132:109–121. doi: 10.1161/CIRCULATIONAHA.114.011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eulalio A, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 38.Xin M, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao YY, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbluh J, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao G, et al. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature. 2016;534:119–123. doi: 10.1038/nature17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin F, et al. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature. 2013;503:290–294. doi: 10.1038/nature12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Augeri DJ, et al. 2012. Patent WO/2012/121992, Germany.
- 47.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Xu CM, et al. Mst1 overexpression inhibited the growth of human non-small cell lung cancer in vitro and in vivo. Cancer Gene Ther. 2013;20:453–460. doi: 10.1038/cgt.2013.40. [DOI] [PubMed] [Google Scholar]
- 49.Kimura W, et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–230. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 50.Burridge PW, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morikawa Y, et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal. 2015;8:ra41. doi: 10.1126/scisignal.2005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chocarro-Calvo A, García-Martínez JM, Ardila-González S, De la Vieja A, García-Jiménez C. Glucose-induced β-catenin acetylation enhances Wnt signaling in cancer. Mol Cell. 2013;49:474–486. doi: 10.1016/j.molcel.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 55.Hudson J, Titmarsh D, Hidalgo A, Wolvetang E, Cooper-White J. Primitive cardiac cells from human embryonic stem cells. Stem Cells Dev. 2012;21:1513–1523. doi: 10.1089/scd.2011.0254. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann W-H, Hudson JE, Tiburcy M. 2015. Patent WO 2015/040142 Al, Germany.
- 57.Wang H, et al. Necking and failure of constrained 3D microtissues induced by cellular tension. Proc Natl Acad Sci USA. 2013;110:20923–20928. doi: 10.1073/pnas.1313662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas WG, et al. Adenoviral-directed expression of the type 1A angiotensin receptor promotes cardiomyocyte hypertrophy via transactivation of the epidermal growth factor receptor. Circ Res. 2002;90:135–142. doi: 10.1161/hh0202.104109. [DOI] [PubMed] [Google Scholar]
- 59.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cox J, et al. Andromeda: A peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 62.Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 63.Cox J, et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox J, Mann M. 1D and 2D annotation enrichment: A statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinformatics. 2012;13 Suppl 16:S12. doi: 10.1186/1471-2105-13-S16-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 67.Takasato M, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 68.Dietmair S, Timmins NE, Gray PP, Nielsen LK, Kromer JO. Towards quantitative metabolomics of mammalian cells: Development of a metabolite extraction protocol. Anal Biochem. 2010;404:155–164. doi: 10.1016/j.ab.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 69.Medina-Torres CE, van Eps AW, Nielsen LK, Hodson MP. A liquid chromatography-tandem mass spectrometry-based investigation of the lamellar interstitial metabolome in healthy horses and during experimental laminitis induction. Vet J. 2015;206:161–169. doi: 10.1016/j.tvjl.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 70.Nicholls DG, et al. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 2010;(46) doi: 10.3791/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.