Significance
Prolactin-responsive neurons in the medial preoptic area project widely throughout the brain. After targeted deletion of prolactin receptors in the preoptic area of adult female mice, mice were able to get pregnant and give birth normally. However, mothers lacking prolactin receptors in the medial preoptic area abandoned their litters soon after birth, establishing a critical role for prolactin/placental lactogen action in this area for establishment and maintenance of normal parental care.
Keywords: maternal behavior, prolactin, prolactin receptor, medial preoptic area
Abstract
Pregnancy hormones, such as prolactin, sensitize neural circuits controlling parental interactions to induce timely activation of maternal behaviors immediately after parturition. While the medial preoptic area (MPOA) is known to be critical for maternal behavior, the specific role of prolactin in this brain region has remained elusive. Here, we evaluated the role of prolactin action in the MPOA using complementary genetic strategies in mice. We characterized prolactin-responsive neurons within the MPOA at different hormonal stages and delineated their projections in the brain. We found that MPOA neurons expressing prolactin receptors (Prlr) form the nexus of a complex prolactin-responsive neural circuit, indicating that changing prolactin levels can act at multiple sites and thus, impinge on the overall activity of a distributed network of neurons. Conditional KO of Prlr from neuronal subpopulations expressing the neurotransmitters GABA or glutamate within this circuit markedly reduced the capacity for prolactin action both in the MPOA and throughout the network. Each of these manipulations, however, produced only subtle impacts on maternal care, suggesting that this distributed circuit is robust with respect to alterations in prolactin signaling. In contrast, acute deletion of Prlr in all MPOA neurons of adult female mice resulted in profound deficits in maternal care soon after birth. All mothers abandoned their pups, showing that prolactin action on MPOA neurons is necessary for the normal expression of postpartum maternal behavior in mice. Our data establish a critical role for prolactin-induced behavioral responses in the maternal brain, ensuring survival of mammalian offspring.
Maternal care is critical to survival of dependent offspring in mammals. Seminal work from Rosenblatt (1), published 50 y ago, showed that maternal behavior can be exhibited in ovariectomized, hypophysectomized rats, suggesting an underlying neural basis that was not dependent on hormonal inputs. Subsequent studies have characterized a complex neural circuitry controlling parental interactions (2, 3), with distributed sites mediating different components of the behavior (4). While the neural circuit controlling maternal behavior is not thought to be dependent on hormonal inputs, it is clear that pregnancy hormones, particularly rising levels of estradiol, oxytocin, prolactin, and placental lactogen, coupled with an abrupt decrease in progesterone can sensitize the underlying circuitry to induce timely activation of maternal behaviors immediately after parturition (5). The medial preoptic area (MPOA) forms a critical nexus (2, 3), integrating a range of hormonal and sensory inputs into the maternal circuit.
Within the complex hormonal milieu of pregnancy, the specific role of prolactin in maternal behavior has remained elusive. This is largely because prolactin and the related placental lactogen have an obligate role in sustaining ovarian progesterone production during pregnancy in rodents (6), meaning that traditional approaches, such as pharmacological inhibition of prolactin secretion and antagonism or KO of prolactin receptors (Prlr), will terminate the pregnancy, making study of postpartum maternal behavior impossible. Circumvention of this issue has usually necessitated investigation of the effects of prolactin on maternal behaviors in artificial, nonpregnant models. For example, virgin Prlr KO mice (Prlr−/−) show a deficit in pup-induced maternal care (7), but these mice are infertile because of the absence of luteotrophic support of the ovary (8), preventing investigation of normal postpartum maternal behavior. Similarly, the key evidence showing that prolactin is involved in the initiation of maternal care has come from studies administering hormones to nonpregnant rats (9, 10). Importantly, these studies have identified the MPOA as a major region where prolactin action can facilitate the onset of maternal behavior in nonpregnant females (10).
To specifically evaluate the role of prolactin action in the MPOA in maternal behavior in the context of a normal pregnancy, we used complementary genetic strategies in mice. We characterized prolactin-responsive neurons within the MPOA at different hormonal stages and delineated their projections in the brain. Most (75%) of the MPOA neurons expressing c-Fos during maternal behavior are GABAergic (3), and we have previously observed extensive Prlr expression on GABAergic neurons in this region (11). In addition, this region is also rich in glutamatergic neurons (although only a small proportion of these express c-Fos during maternal behavior) (3). Hence, we next generated conditional KO animals and deleted Prlr in GABA and/or glutamate neurons in the brain, while retaining Prlr expression in peripheral reproductive tissues. This allowed us to evaluate maternal behavior in the context of a normal pregnancy but with markedly reduced capacity of the maternal circuits to sense prolactin. Finally, we specifically evaluated the role of Prlr in the MPOA using a viral approach to acutely ablate Prlr specifically within this region in the adult brain, while leaving Prlr expression in other parts of the maternal circuit intact.
Results
The MPOA Forms the Nexus of a Prolactin-Sensitive Neural Network.
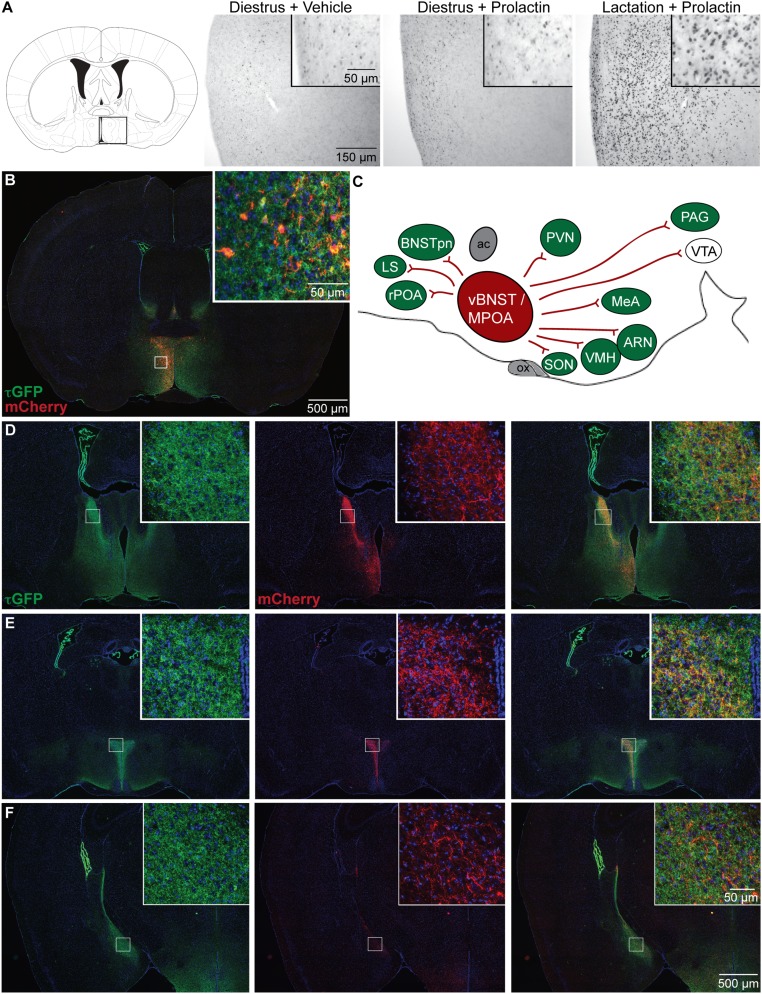
The MPOA contains a large number of neurons expressing Prlr mRNA (12). Prolactin typically activates transcriptional responses through the JAK/STAT signal transduction pathway mediated by phosphorylation of STAT5 (pSTAT5) (13). We found that the number of MPOA neurons showing prolactin-induced pSTAT5 was relatively low in nonpregnant mice but markedly increased during lactation (Fig. 1A). These data suggest considerable plasticity in the MPOA during lactation, with enhanced transcriptional responses to prolactin, although there is no change in Prlr mRNA expression in this region at this time (14).
Fig. 1.
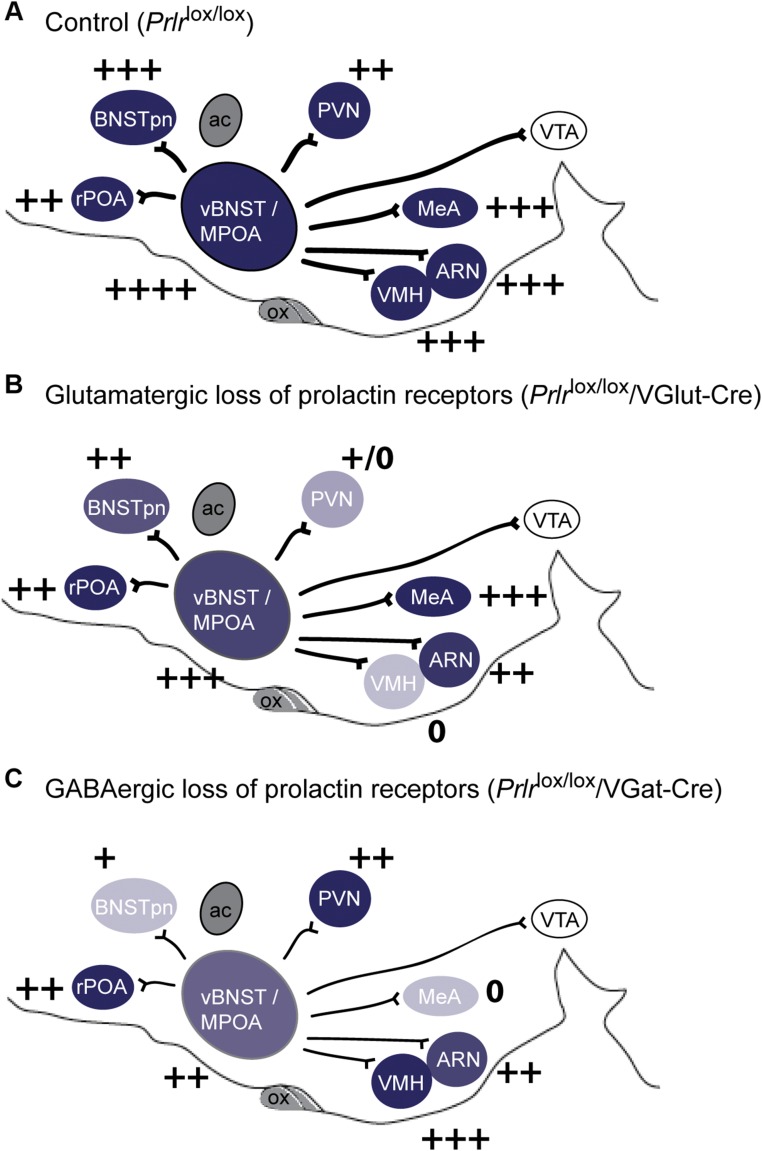
A prolactin-sensitive neural network centered on the medial preoptic area (MPOA). (A) Changes in prolactin-induced signal transduction in Prlr-expressing MPOA neurons. Photomicrographs show immunohistochemical labeling of pSTAT5 (black nuclear staining) after prolactin administration in diestrus nonlactating mice and day 7 lactating mice compared with vehicle-treated controls. Prolactin induces widespread and intensive pSTAT5 labeling in lactating mice but not in diestrus mice. (B–F) Distribution of projections from Prlr-expressing neurons in the MPOA. Prlr-iCre/eR26-τGFP mice received a unilateral injection of AAV5-EF1a-DIO-hChR2(H134R)-mCherry-WPRE-pA into the MPOA to drive Cre-dependent expression of mCherry specifically in the MPOA. Images were captured using a 10× objective using a Zeiss Axio Imager2 microscope with a motorized stage, with multiple images combined to form a composite image using the MozaiX module in the Axiovision software. (B) Site of the injection into the MPOA, with Prlr-expressing cells being labeled with τGFP (green) and a subset of Prlr-expressing neurons in the MPOA on one side expressing mCherry (red). (C) Schematic representation of mCherry-labeled projections from the MPOA in the sagittal plane, with regions containing Prlr-expressing cells colored in green and regions lacking Prlr-expressing cell bodies in white. ac, anterior commissure; ARN, arcuate nucleus; BNSTpn, bed nucleus of the stria terminalis principle nucleus; LS, lateral septum; MeA, medial amygdala; ox, optic chiasm; PAG, periaqueductal gray; PVN, paraventricular nucleus; rPOA, rostral preoptic area; SON, supraoptic nucleus; vBNST, ventral bed nucleus of the stria terminalis; VTA, ventral tegmental area. (D–F) Representative sections illustrating the distribution of τGFP labeling showing Prlr expression (green), mCherry-positive projections of MPOA prolactin receptor-expressing cells (red), and the composite images through the forebrain (Figs. S1 and S2 and Table S1).
To analyze the role of prolactin-responsive neurons specifically in the MPOA, we next developed a mouse line in which Cre recombinase is expressed under the control of the Prlr promoter using an internal ribosome entry site (IRES) (15). We inserted the IRES-Cre construct immediately downstream of exon 10 of the Prlr gene, resulting in Cre expression in cells that express the long form of the Prlr (Prlr-iCre). We then crossed the Prlr-iCre animals with mice expressing a Cre-dependent τGFP reporter (ROSA26-CAGS-τGFP; eR26-τGFP) (16) to fluorescently label prolactin-responsive neurons. Because τGFP associates with microtubules, it effectively labels neuronal processes of Prlr neurons as well as their cell bodies. We found that τGFP expression was restricted to areas known to express Prlr in the adult brain (12). In particular, we detected high levels of τGFP-positive cell bodies in the MPOA, bed nucleus of the stria terminalis (BNST), paraventricular nucleus (PVN), ventromedial hypothalamic nucleus (VMH), arcuate nucleus (ARN), and medial amygdala (MeA) (Fig. 1 and Fig. S1), all regions that have previously been implicated in the neural circuitry governing parental behavior (4, 5). We also detected τGFP-positive fibers throughout the brain, including a number of areas, such as the supramammillary region and ventral tegmental area (VTA), that were devoid of Prlr-expressing cell bodies (12).
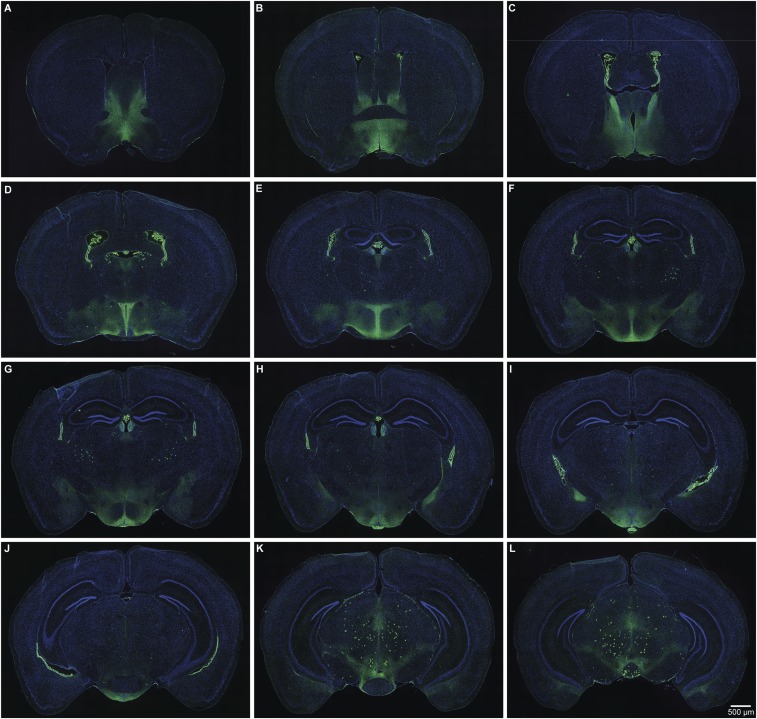
Fig. S1.
Serial sections through a single animal showing the distribution of τGFP-labeled Prlr-expressing cell bodies and fibers in Prlr-iCre/eR26-τGFP mice. Images were captured using a 10× objective using a Zeiss Axio Imager2 microscope with a motorized stage, with multiple images combined to form a composite image using the MozaiX module in the Axiovision software. Note high levels of labeling in the anteroventral periventricular region (A); MPOA (B); BNST (B and C); choroid plexus (B–J); PVN (D and E); ARN, VMH, and median eminence (G–I); medial amgydala (H and I); and VTA (J–L).
To specifically trace the projections of MPOA Prlr neurons, we stereotaxically injected a recombinant adeno-associated virus (AAV) encoding Cre-dependent mCherry into this brain area. mCherry is anterogradely transported within neuronal processes labeling axons of the Cre-expressing cells. After unilateral AAV injection into the MPOA of Prlr-iCre/eR26-τGFP mice, we found that a subset of the Prlr neurons (green in Fig. 1B) expressed mCherry (red in Fig. 1B). While mCherry-positive cell bodies were restricted to the side of the injection, we identified extensive mCherry-positive projections from the MPOA Prlr neurons (Fig. 1), predominantly but not exclusively on the ipsilateral side (quantified in Table S1). Many of the major projections connected to regions that also showed high levels of τGFP in cell bodies, indicating prolactin-responsive neurons, such as the BNST, PVN, ARN, and VMH (Fig. 1, Figs. S1 and S2, and Table S1). Importantly, few of these areas showed mCherry-positive staining in the cell bodies, which would have been indicative of retrograde transport of the marker, suggesting that the observed fiber staining was localized almost exclusively in projections from the MPOA. Taken together, these data suggest that MPOA Prlr-expressing neurons form the nexus of a complex prolactin-responsive neural circuit, indicating that changing prolactin levels may significantly impinge on the overall activity of a distributed network of neurons.
Table S1.
Projections of prolactin-sensitive neurons from the MPOA
| Brain regions | Abbreviations | Projections/infected cells |
| Hypothalamus | ||
| MPOA | MPOA | ++/cc |
| Medial preoptic nucleus, lateral part | MPOL | ++/c |
| Medial preoptic nucleus, medial part | MPOM | ++/c |
| Anteroventral periventricular nucleus | AVPV | ++/c |
| Periventricular hypothalamic nucleus | Pe | ++/c |
| Ventromedial preoptic nucleus | VMPO | ++ |
| Median preoptic nucleus | MnPO | ++ |
| Subfornical organ | SFO | ++ |
| Paraventricular thalamic nucleus | PVN | ++ |
| Tuber cinereum area | TC | ++ |
| Arcuate hypothalamic nucleus | Arc | ++ |
| Median eminence | ME | ++ |
| Medial tuberal nucleus | Mtu | ++ |
| Supraoptic nucleus | SO | ++ |
| Dorsomedial hypothalamic nucleus | DMH | ++ |
| Ventromedial hypothalamic nucleus | VMH | ++ |
| Premammillary nucleus, ventral part | PMV | ++ |
| Supramammillary nucleus | SuM | ++ |
| Vascular organ of the lamina terminalis | VOLT | + |
| Anterior hypothalamic area, anterior part | AHA | + |
| Lateral hypothalamic area | LH | + |
| Lateroanterior hypothalamic nucleus | LA | + |
| Paratenial thalamic nucleus | PT | + |
| Zona incerta | ZI | + |
| Paraventricular thalamic nucleus, anterior part | PVA | + |
| Anterior hypothalamic area, central part | AHC | + |
| Subthalamic nucleus | STh | + |
| Paraventricular thalamic nucleus | PV | + |
| Posterior hypothalamic area | PH | + |
| Parasubthalamic nucleus | PSTh | + |
| Supramammillary nucleus, lateral part | SuML | + |
| Supramammillary nucleus, medial part | SuMM | + |
| Ventral tuberomammillary nucleus | VTM | + |
| Thalamus | ||
| Intermediodorsal thalamic nucleus | IMD | + |
| Posteromedian thalamic nucleus | PoMn | + |
| Pallidum | ||
| BNST, medial division, posteromedial part | BNSTMPM | ++/c |
| BNST, medial division, anterior part | BNSTMA | ++ |
| BNST, medial division, ventral part | BNSTMV | + |
| Striatum | ||
| Lateral septal nucleus, ventral part | LSV | + |
| Central amygdaloid nucleus, medial division, anterodorsal part | CeMAD | + |
| Central amygdaloid nucleus, medial division | CeM | + |
| Central amygdaloid nucleus, capsular part | CeC | + |
| Medial amygdaloid nucleus, posterodorsal part | MePD | + |
| Midbrain | ||
| Periaqueductal gray | PAG | + |
| VTA | VTA | + |
| Lateral periaqueductal gray | LPAG | + |
| Interfascicular nucleus | IF | + |
| Cortical subplate | ||
| Amygdalohippocampal area, posteromedial part | AHiPM | + |
| Fiber tracts | ||
| Stria terminalis | st | + |
| Periventricular fiber system | pv | + |
Brain regions where no projections were found are not included in the table. Areas in which projections occupy 2–20% or <2% of the region of interest were defined as dense or sparse projection areas, respectively. To quantify the number of infected cells that are mCherry-positive, the area of each brain region was measured in ImageJ, and the number of cells within that region was counted. Brain regions were labeled according to the cell density; regions with 1–20 cells per 1 mm2 were classified as containing few cells (c) and those with 20–110 cells per 1 mm2 were classified as containing many cells (cc). c, Few mCherry+ cells; cc, many mCherry+ cells; +, sparse mCherry+ projections; ++, dense mCherry+ projections.
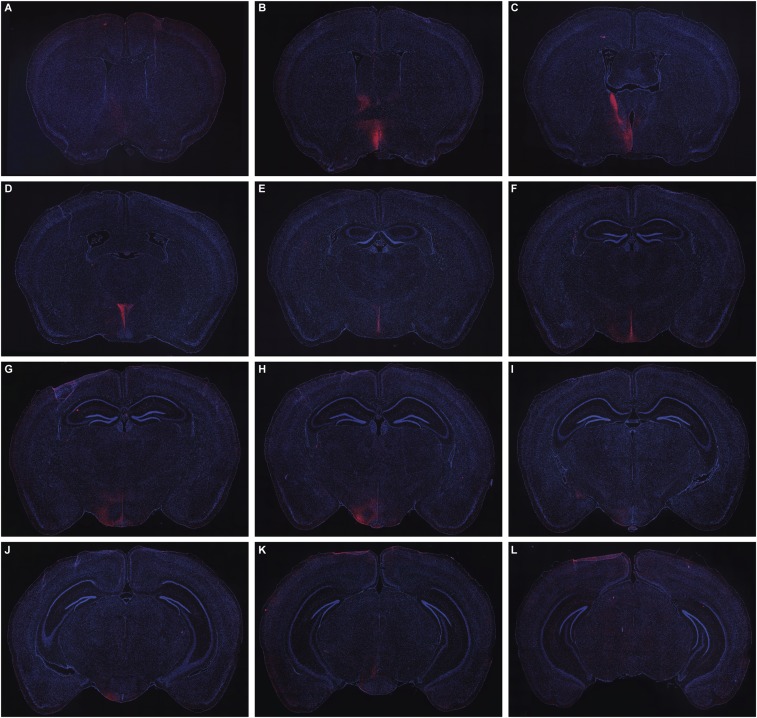
Fig. S2.
Serial sections through a single animal showing the distribution of mCherry-labeled fibers (red) derived from prolactin-responsive neurons in the MPOA after administration of AAV5-EF1a-DIO-hChR2(H134R)-mCherry-WPRE-pA into the MPOA of Prlr-iCre/eR26-\x{03c4}GFP mice. Note: These are the same sections as Fig. S1, using the same magnification. Images were captured using a 10× objective using a Zeiss Axio Imager2 microscope with a motorized stage, with multiple images combined to form a composite image using the MozaiX module in the Axiovision software. Note the predominantly ipsilateral distribution extending from the rostral preoptic area (A), BNST (B and C), PVN (D), periventricular nucleus (D–F), ARN and VMH (G–I), and VTA (J–L).
Conditional KO Mice Lacking Prolactin Receptors in Glutamate and/or GABA Neurons Display Mild Perturbations of Maternal Behaviors.
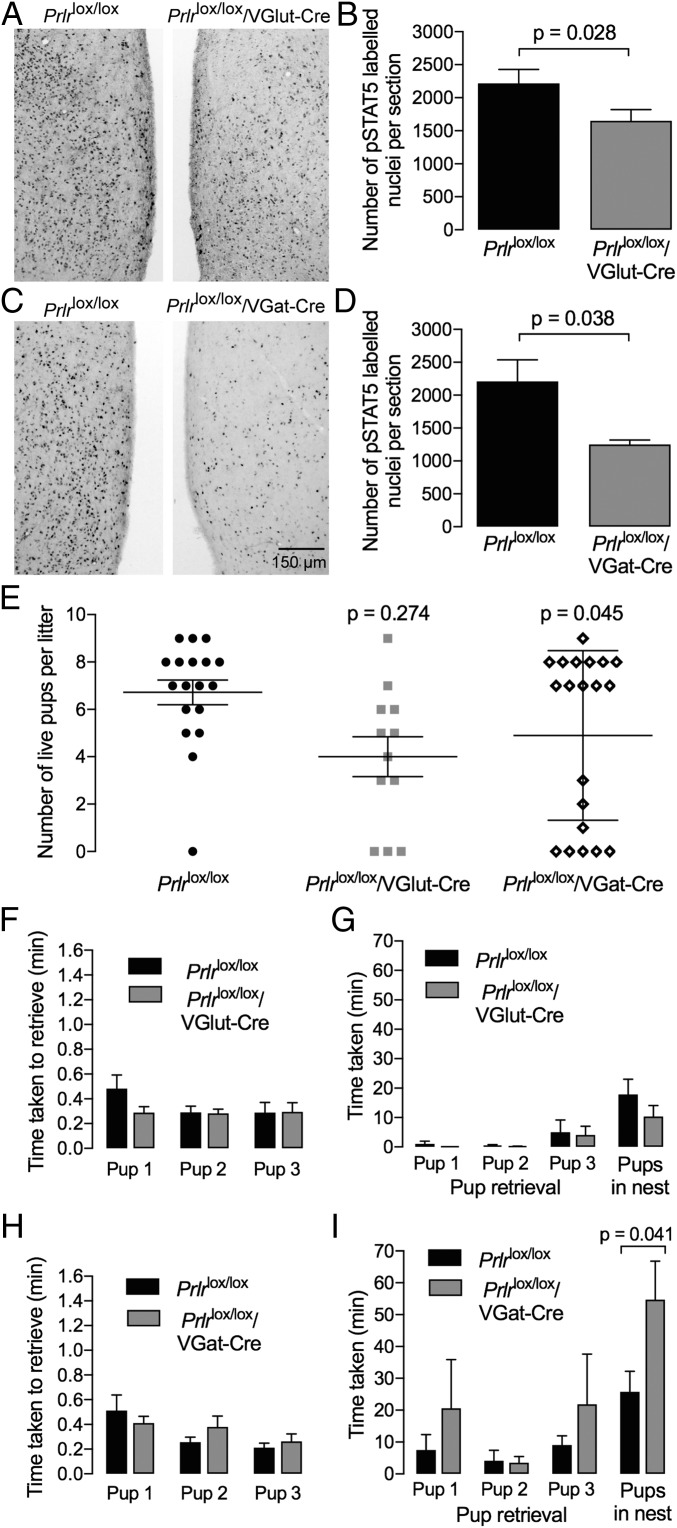
To investigate the functional role of prolactin in GABAergic and/or glutamatergic neuronal populations, we deleted the Prlr gene from GABA and/or glutamate neurons using VGat-Cre and VGlut2-Cre mouse strains (17) in combination with Prlrlox/lox animals (11). In Prlrlox/lox/VGlut2-Cre mice, there was a 25% loss of prolactin-induced pSTAT5 in the MPOA (P = 0.028), confirming successful deletion of Prlr in glutamate neurons in this area (Fig. 2 A and B). In addition, these animals showed an almost complete loss of prolactin-induced pSTAT5 in the PVN and VMH, suggesting that all prolactin-responsive neurons in these regions are glutamatergic. Conditional deletion of Prlr in GABA neurons (Prlrlox/lox/VGat-Cre) resulted in a 50% decrease in prolactin-induced pSTAT5 in the MPOA (P = 0.038) (Fig. 2 C and D). These mice also had an almost total loss of pSTAT5 in the MeA as well as reduced STAT5 activation in the ARN and BNST. The overall change in numbers of neurons responding to prolactin in each of the prolactin-responsive nuclei in the maternal circuit is illustrated schematically in Fig. S3. Both conditional KO strains were fertile and able to carry a pregnancy to term, although an increased number of the animals abandoned their litters (two or fewer pups alive on day 3 of lactation) compared with controls. While this difference for Prlrlox/lox/VGlut2-Cre mice (3 of 12 abandoned litters) was not significantly different from controls (1 of 18; P = 0.274), Prlrlox/lox/VGat-Cre mice showed higher levels of litter loss (7 of 20; P = 0.045) (Fig. 2E). Nevertheless, the majority of animals in each genotype was able to maintain their litters. In mice with surviving litters, we completed a pup retrieval experiment both in the home cage on day 3 postpartum and in a novel cage [a slightly more rigorous test of maternal behavior (18)] on day 5 postpartum. There was no significant effect of loss of Prlr in either GABA or glutamate neurons on pup retrieval in the home cage of postpartum female mice (Fig. 2 F and H). In the novel cage compared with the home cage, all mice took significantly longer to retrieve their pups and crouch over them in a newly constructed nest. Prlrlox/lox/VGlut2-Cre mice were not significantly different from control animals in performing this task in the novel cage. In contrast, while Prlrlox/lox/VGat-Cre exhibited normal retrieval of the pups, they took significantly longer to crouch over the pups and exhibit full maternal behavior (P = 0.041) (Fig. 2 G and I).
Fig. 2.
Conditional deletion of the Prlr from glutamate or GABA neurons. Prolactin-induced pSTAT5 (black nuclear staining) in the MPOA showing functional loss of Prlr signaling in the glutamate-specific Prlr KO mice (Prlrlox/lox/VGlut-Cre; A) and GABA-specific Prlr KO mice (Prlrlox/lox/VGat-Cre; C) compared with the Cre-negative controls (Prlrlox/lox). The numbers of pSTAT5-positive cells in this region are quantified in B (n = 8) and D (n = 4). Note that each genotype exhibits a partial loss of prolactin-responsive cells (P < 0.05). Fig. S3 shows quantification of numbers of pSTAT5 neurons in other brain regions in each genotype. E shows the effect of conditional KO of the Prlr on pup survival. While the majority of animals were able to successfully raise their litters, a significantly larger number of animals exhibited a loss of their litters in the Prlrlox/lox/VGat-Cre mice (P < 0.05). F–I show effect of conditional deletion of Prlr in GABA (Prlrlox/lox/VGat-Cre) or glutamate (Prlrlox/lox/VGlut-Cre) neurons on pup retrieval in the home cage (F and H; n = 8–12) or in a novel cage (G and I; n = 7–8). Mothers were separated from their pups; then, three pups were placed into the cage, and the time taken to retrieve pups was recorded. Note the difference in scale on the y axis, with retrieval taking much longer in the novel cage. As there was no preformed nest in the novel cage, the final measure was a grouping of the three pups into a new “nest” formed during the performance of this task. There was no significant difference in pup retrieval time in the home cage for either group. In the novel cage, however, GABA-specific Prlr KO animals took significantly longer to complete the retrieval task (P < 0.05).
Fig. S3.
Conditional deletion of the Prlr from glutamate or GABA neurons. Schematic representation of levels of Prlr expression in the maternal circuit in the sagittal plane, with intensity of color representing levels of Prlr expression and + symbols representing numbers of prolactin-induced pSTAT5 neurons detected in each region (A). Note the reduced expression of Prlr and prolactin-induced pSTAT5 in specific regions after Cre-mediated deletion of Prlr in glutamate (VGlut-Cre; B) or GABA (VGat-Cre; C). ac, anterior commissure; ARN, arcuate nucleus; BNSTpn, bed nucleus of the stria terminalis principle nucleus; MeA, medial amygdala; MPOA, medial preoptic nucleus; ox, optic chiasm; PVN, paraventricular nucleus; rPOA, rostral preoptic area; vBNST, ventral bed nucleus of the stria terminalis; VTA, ventral tegmental area.
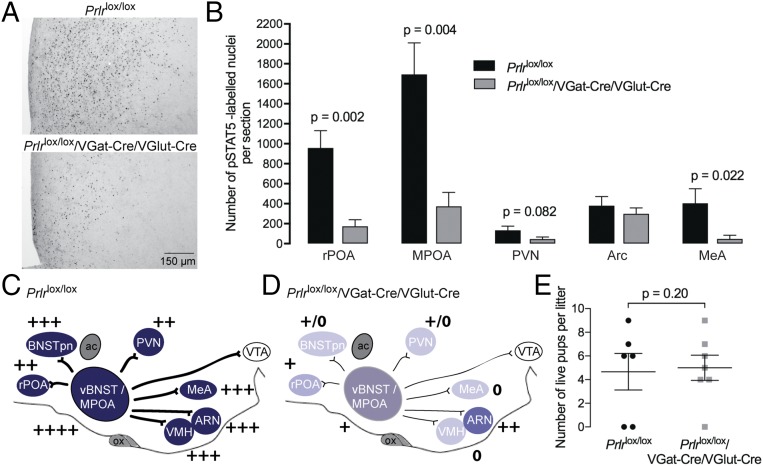
Combining both Cre alleles to generate mice lacking Prlr in both GABA and glutamate neurons resulted in approximately additive effects in terms of loss of prolactin responses. These animals had markedly reduced prolactin-induced pSTAT5 in the MPOA (∼75%; P = 0.004) as well as almost total loss of responses in the PVN, VMH, and MeA (Fig. 3). Despite this, the animals were no worse off in terms of maternal behavior, and most of them were able to care for their litters. Importantly, however, none of the manipulations above generated a complete loss of Prlr throughout the circuit, raising the possibility that the few remaining prolactin-responsive neurons in the MPOA were sufficient to sustain maternal behavior that was only mildly impaired compared with controls.
Fig. 3.
Effect of conditional deletion of Prlr in both GABA and glutamate neurons. (A) Prolactin-induced pSTAT5 (black nuclear staining) in the MPOA showing functional loss of Prlr signaling in combined GABA and glutamate Prlr KO mice (Prlrlox/lox/VGat-Cre/VGlut-Cre) together with quantification of the numbers of pSTAT5-positive cells in selected brain regions (n = 4–5). (B) Note the significant loss of prolactin-responsive cells in most brain regions. This is represented schematically in the diagrams of the brain in the sagittal plane (C and D), with intensity of color representing levels of Prlr expression and + symbols representing numbers of prolactin-induced pSTAT5 neurons detected in each region. Note the marked loss of Prlr expression throughout that neural network. E shows pup survival measured on day 3 of lactation. Although some mothers abandoned their litters in each group, there was no significant effect of Prlr deletion on postpartum maternal behavior compared with Cre-negative controls. ac, anterior commissure; ARN, arcuate nucleus; BNSTpn, bed nucleus of the stria terminalis principle nucleus; LS, lateral septum; MeA, medial amygdala; ox, optic chiasm; PAG, periaqueductal gray; PVN, paraventricular nucleus; rPOA, rostral preoptic area; SON, supraoptic nucleus; vBNST, ventral bed nucleus of the stria terminalis; VTA, ventral tegmental area.
Acute Deletion of Prolactin Receptors from the MPOA in Adults Abolishes Maternal Nursing Behavior.
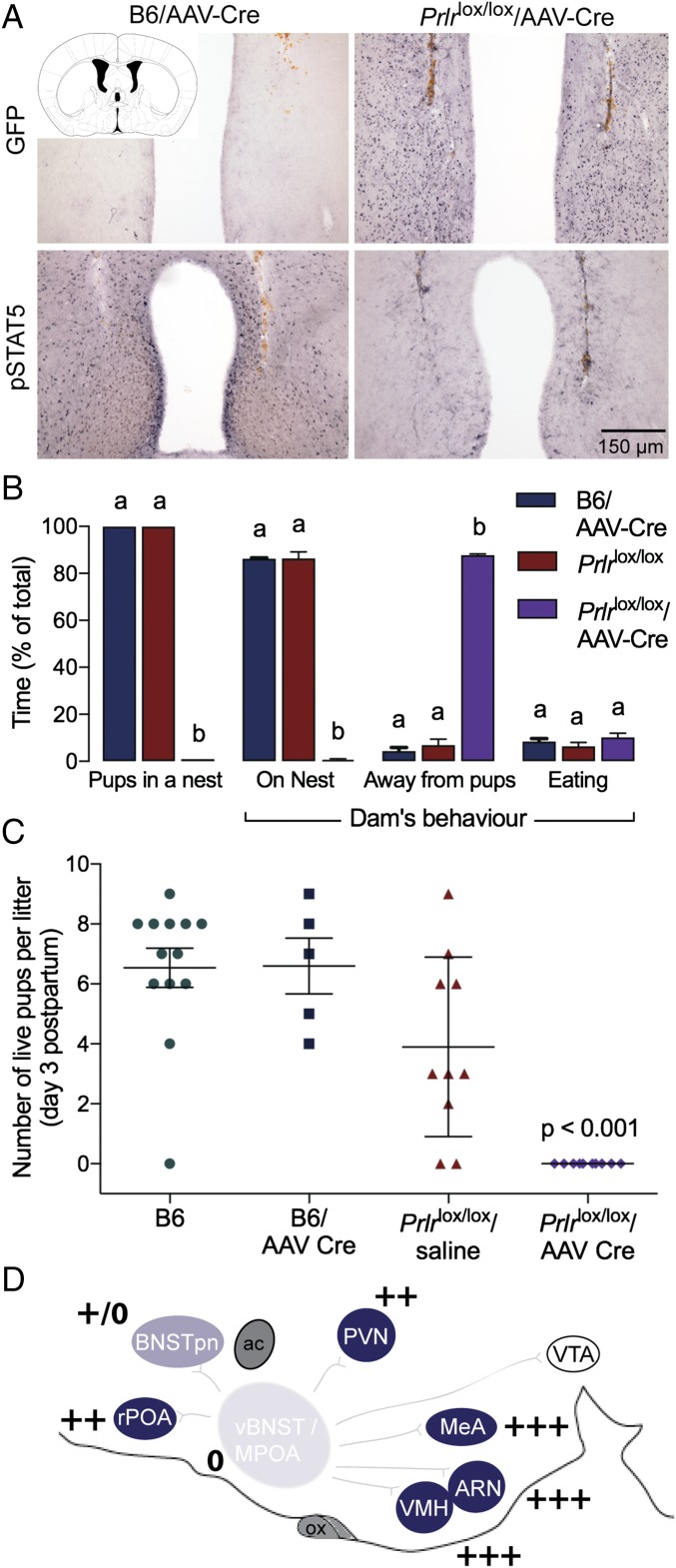
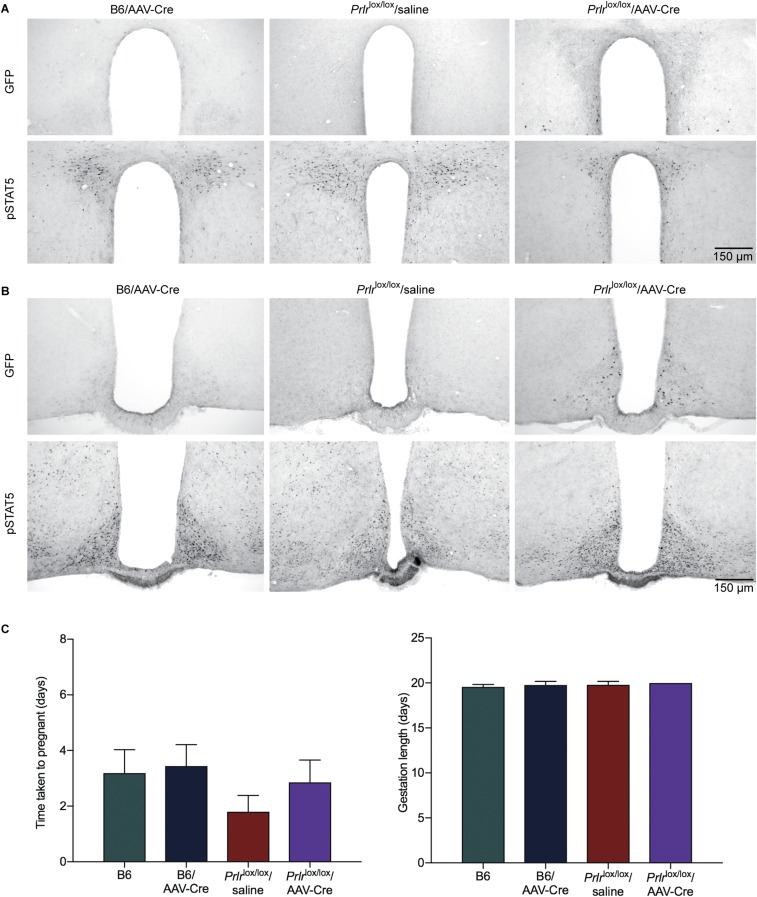
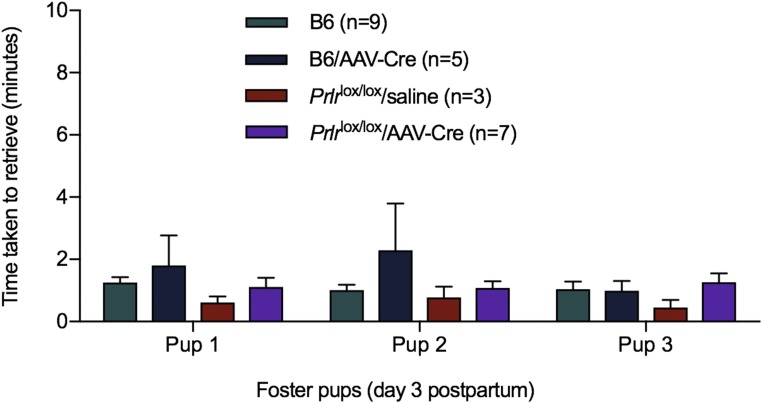
To test this hypothesis and specifically investigate the role of prolactin action in postpartum maternal behavior, we used an AAV to deliver Cre recombinase into the MPOA of adult female Prlrlox/lox mice (11). The Prlrlox/lox construct is designed such that Cre-mediated recombination will activate GFP expression and thus, report successful Prlr deletion (Fig. 4). AAV-Cre injection into the MPOA of Prlrlox/lox mice completely removed functional prolactin responses from this region (Fig. 4). The effect was highly localized, as there was little or no expression of GFP in the nearby PVN, and prolactin-induced pSTAT5 was unaffected in that and other nuclei (Fig. S4). Three days after injection, animals were mated and monitored throughout pregnancy. There was no effect of treatment on the time taken to get pregnant or the duration of pregnancy (Fig. S4). Animals gave birth to normal-sized litters and initiated the normal pup-directed activities (19), retrieving the pups to a nest, cleaning and grooming the pups, and eating the placenta. Having initiated these behaviors, however, the animals failed to establish full maternal care defined as crouching over the pups in a nest and assuming an arched back posture (kyphosis) to facilitate nursing. The animals failed to crouch over the pups, and over the course of the next 24 h, all of the mothers lacking Prlr in the MPOA abandoned their litters, resulting in death of all pups (Fig. 4). There was no evidence that the Prlrlox/lox/AAV-Cre animals ever suckled their young after birth. When examined in the first 6 h after birth (in the cross-fostering study; see below), the pups showed no signs of a milk band in their stomachs, and the mothers did not have elongated nipples or matted fur around the nipples, the normal signs of suckling seen in the controls. By contrast, control animals injected with AAV-Cre or saline-injected Prlrlox/lox animals showed normal maternal behavior and successfully raised their pups. Cross-fostering of pups confirmed a deficit in maternal care rather than in pup viability, with pups from two Prlrlox/lox/AAV-Cre litters surviving after cross-fostering to control Prlrlox/lox dams. Conversely, pups from control Prlrlox/lox dams did not survive after cross-fostering onto Prlrlox/lox/AAV-Cre dams, despite apparently normal retrieval (Fig. S5). Again, after initiation of normal pup-directed behaviors, the animals never established full maternal care. These data show that prolactin (or placental lactogen) action in the MPOA of the postpartum female, via the Prlr, is absolutely essential for normal expression of maternal care.
Fig. 4.
Effect of Prlr deletion from the MPOA on postpartum maternal behavior. (A) Bilateral administration (injection tracts are labeled brown) of AAV2-CMV-PI-Cre-rBG into the MPOA of adult Prlrlox/lox female mice resulted in extensive GFP and complete absence of pSTAT5 labeling, indicating successful deletion of the Prlr gene. GFP labeling was absent, and high levels of pSTAT5 labeling were observed in virus-injected WT lactating mice. (B) Video recordings 6–24 h postpartum revealed that maternal behavior was severely disrupted in mice with MPOA-specific Prlr deletion (Prlrlox/lox/AAV-Cre; n = 4) compared with control groups of AAV-Cre–injected WT C57BL/6J mice (B6/AAV-Cre; n = 2) and Prlrlox/lox mice (n = 4), with pups scattered and dams spending no time at the nest or with pups. Different letters represent statistically different groups (P < 0.01). (C) No pups from Prlrlox/lox/AAV-Cre dams survived beyond 2 d postpartum. (D) Schematic representation of levels of Prlr expression in the maternal circuit in the sagittal plane after AAV-Cre administration into the MPOA (using the same format as in Fig. 3). Note that Prlr deletion was highly localized to the site of injection apart from some loss of expression in regions immediately superior to the injection site surrounding the needle tract (Fig. S4). ac, anterior commissure; ARN, arcuate nucleus; BNSTpn, bed nucleus of the stria terminalis principle nucleus; LS, lateral septum; MeA, medial amygdala; ox, optic chiasm; PAG, periaqueductal gray; PVN, paraventricular nucleus; rPOA, rostral preoptic area; SON, supraoptic nucleus; vBNST, ventral bed nucleus of the stria terminalis; VTA, ventral tegmental area.
Fig. S4.
Bilateral administration of AAV2-CMV-PI-Cre-rBG into the MPOA of adult Prlrlox/lox female mice did not induce GFP expression or restrict pSTAT5 expression in adjacent regions, including the PVN (A) or ARN (B), showing the localized deletion of the Prlr gene using this approach. Note that a few neurons in the ARN express GFP, suggesting that there may have been limited retrograde infection of neurons that project from the arcuate to the MPOA. (C) Reproductive data from the MPOA-specific Prlr KO animals (Prlrlox/lox/AAV-Cre). There was no effect on time to mating (n = 5–7) and duration of pregnancy in these animals (n = 5–7).
Fig. S5.
Effect of deletion of Prlr in the MPOA on pup retrieval. Prlrlox/lox/AAV-Cre–treated mice were examined for pup retrieval in the home cage. Mothers were separated from their pups; then, three foster pups were placed into the cage, and the time taken to retrieve pups was recorded. There was no significant difference in pup retrieval time.
Discussion
Our data establish a critical role for prolactin in mammalian reproduction that is more comprehensive than simply milk production. Prolactin-induced behavioral responses in the maternal brain are equally important in ensuring survival of mammalian offspring.
Maternal behavior can be broadly classified into two components that are mediated by separate but interacting neuronal circuits (4, 5). One circuit, involving structures such as the MeA and VMH, controls pup-directed avoidance and aggression. This must be inactivated to promote attraction and retrieval of pups. A second circuit, focused around the MPOA, is associated with the expression of parental care and must be activated to promote actions, such as nest formation, pup retrieval, grooming, and nursing of the pups. Our data suggest that prolactin can influence both of these circuits, with Prlr expression detected in multiple regions within these distributed neural networks. Given this widespread distribution of Prlr, it was surprising that conditional deletion of Prlr in GABAergic and/or glutamatergic neurons, which markedly reduced the number of neurons responding to prolactin, had only a subtle effect on maternal behavior. In particular, pup retrieval was relatively normal after all manipulations, raising the possibility that this function may be independent of prolactin action in mice. It was noteworthy that the most significant impact of the conditional deletion experiments came when Prlr were deleted in GABAergic neurons, which are the largest population of neurons normally activated during expression of maternal behavior (3). Collectively, however, the relatively subtle effects suggest that the maternal behavior circuit is very robust and that prolactin action within this circuit is somewhat redundant under normal conditions. This might be a consequence of the distributed nature of the network, with prolactin able to act on the circuit at multiple sites, or developmental compensation after constitutive loss of Prlr. In contrast to the subtle effects seen in the conditional KOs, complete deletion of Prlr from the MPOA in the adult profoundly impaired maternal nursing behavior. Whether similar adult-onset KO of Prlr in the other parts of the distributed network might have specific effects on different components of maternal behavior remains to be determined.
When pups from the mothers lacking Prlr in the MPOA were cross-fostered onto control mothers, they were able to suckle and appeared completely normal, showing that the deficit was a maternal one. One possibility is that specific deletion of Prlr in the MPOA caused a failure of lactation or milk ejection and that the mothers abandoned the litters because of an inability to feed the pups. The ability of these animals to maintain a pregnancy, however, suggests that prolactin (and placental lactogen) was adequate to sustain the corpus luteum (6) and therefore, likely sufficient for mammary gland development. Similarly, the ability of the animals to give birth normally suggests at least some level of function of the oxytocin system (20), meaning that milk ejection would likely have occurred had the animals suckled. In addition to maternal behavior, the MPOA has a number of important homeostatic roles, including thermoregulation, sleep, and social reward (21, 22). Potentially, maternal behavior could be impacted if losing the influence of prolactin on these functions also impaired pregnancy-related adaptations in the maternal brain. While these possibilities warrant additional investigation, the observation that the animals never properly initiated nursing of the pups suggests that the observed deficits were more likely to directly result from a lack of prolactin input into the circuitry controlling maternal behavior.
The MPOA has a critical role in linking the multiple regions involved in maternal behavior and in integrating the various hormonal and sensory inputs that influence the behavior (4, 5). A number of studies have traced connections from the MPOA to other structures involved in the maternal circuit, and have demonstrated a major output of the system to midbrain effector regions, such as the VTA (and nucleus accumbens) (23, 24). Our data show that some of these projections are derived from prolactin-responsive cells. After deletion of Prlr in the MPOA in adult females, the mothers initially showed appropriate pup-directed behavior, grooming the pups after birth and consuming the placenta. However, these interactions were not sustained, and the mothers never exhibited full maternal care and abandoned their pups within 24 h of birth. Thus, it seems that prolactin action in the MPOA is necessary for the normal function of the maternal network, possibly altering the key output of the network to regions that reinforce the behavior (22). This might be mediated by prolactin itself but also by the closely related placental lactogens that act on the Prlr and are elevated in the blood throughout the second half of pregnancy (25). Importantly, unlike some recent reports that have used cell ablation approaches (26, 27) or blockade of neurotransmitter production (28, 29) to identify critical cell types mediating maternal behavior, in this model, the neuronal circuitry is completely intact. The profound deficit arises specifically from the lack of appropriate hormonal input into these circuits.
Methods
Animals.
The generation and genotyping of Prlr-iCre and Prlrlox/lox mice (11) are detailed elsewhere (SI Methods). Prlr-iCre mice were crossed with ROSA26-CAGS-τGFP reporter animals (16), generating mice that express τGFP specifically in Prlr-expressing neurons. VGat- and VGlut2-IRES-Cre mice, originally developed by Brad Lowell (17), were purchased from Jackson Labs [Slc32a1tm2(cre)Lowl/MwarJ (stock no. 028862) and Slc17a6tm2(cre)Lowl/MwarJ (stock no. 028863), respectively]. These mice were crossed with our Prlrlox/lox mice. As Cre-mediated inversion deletes the Prlr gene and knocks in EGFP in its place (11), EGFP expression in the brain was used as a marker for both successful recombination and for the normal pattern of receptor expression. Finally, double-Cre animals were generated initially by breeding male VGat-Cre +ve mice with female VGlut-Cre +ve mice. After a number of double-Cre males had been produced, the breeding strategy was changed to cross double-Cre male mice with homozygous Prlrlox/lox females (SI Methods). Groups of WT C57BL/6J (B6) mice were used as additional control groups in some experiments. All mice were used as adults (8–14 wk) and were group-housed under conditions of controlled temperature (22 °C ± 1 °C) and lighting (12-h light and 12-h dark cycles with lights on at 0600 hours), with ad libitum access to food and water. All animal experimental protocols were approved by the University of Otago or the University of Saarland animal ethics committee.
Stereotaxic Injections of AAV.
Adult mice (8–12 wk old) were anesthetized with isoflurane and placed in a stereotaxic apparatus. For tract tracing experiments, Prlr-iCre/eR26-τGFP females received unilateral 1-µL injections of AAV5-EF1a-DIO-hChR2(H134R)-mCherry-WPRE-pA (5 × 1012; UNC Vector Core) into the MPOA. For gene KO experiments, Prlrlox/lox animals received bilateral 1-μL injections of AAV2-CMV-PI-Cre-rBG (2.27 × 1013; Penn Vector Core) into the MPOA. All injections were given at a rate of 100 nL/min, and the syringes were left in situ for 3 min before and 10 min after injections. Coordinates for the MPOA were 0.07 mm anterior to Bregma and 0.3 mm lateral to midline, and depth was adjusted for age (8 wk: 4.7-mm depth; 10 wk: 4.9-mm depth; 12 wk: 5.0-mm depth). A control group of WT C57BL/6J mice received bilateral AAV-Cre injections, and a control group of Prlrlox/lox mice received saline injections.
Tract Tracing Experiments.
Two to four weeks after viral injection, mice were anesthetized with ketamine and xylazine and perfused transcardially with 4% paraformaldehyde, and brains were prepared for immunohistochemistry for mCherry and τGFP (SI Methods). Nuclei were visualized by Hoechst staining. Sections were imaged using the Axio Imager M2 microscope with AxioVision software (Zeiss) and then ImageJ. Images were split into single channels, regions of interest were assigned to individual brain regions, and percentages of each area occupied by mCherry-positive signal above threshold, as calculated by the Triangle method (30), were obtained (Table S1).
Monitoring of Maternal Behavior.
Seventy-two hours after AAV-Cre injection, females were individually housed with a WT C57BL/6J male mouse. Successful mating was confirmed by the presence of a vaginal plug, the male was removed, and day of parturition was recorded as day 1 of lactation. Pup survival was monitored daily, and total number of live pups was recorded on day 3 of lactation. Maternal behavior in the home cages (with their own pups) was recorded 6–24 h after parturition in groups of Prlrlox/lox (n = 4), B6/AAV-Cre (n = 2), and Prlrlox/lox/AAV-Cre (n = 4) mice. The percentages of time that the dam spent over the pups in the nest, elsewhere in the cage, and eating were calculated during both the light and dark periods. The percentage of time that pups were gathered in a nest was also calculated. To evaluate whether any deficit in maternal care was caused by pup-related factors, pups from two Prlrlox/lox/AAV-Cre mothers were cross-fostered onto Prlrlox/lox control dams, with control pups placed with the KO mothers. Pups were collected as soon as possible after birth (within 6 h) and were checked for general health and the presence of milk bands in their stomach. At the same time, the mothers were assessed for evidence of suckling (matted fur around the nipples and/or elongated nipples). Pups were then placed with the new mother and observed for the next 48 h. Pup retrieval behavior was tested in home cages on day 3 of lactation (SI Methods).
Immunohistochemistry.
To evaluate Prlr expression in the MPOA of Prlrlox/lox mice, brains were processed for the presence of EGFP immunoreactivity (to indicate Cre-dependent recombination in the Prlrlox/lox mice and therefore, represent cells that expressed Prlr before recombination) and prolactin-induced pSTAT5 [a sensitive and reliable marker of activated Prlr (12)]. Pups were removed from dams on days 7–10 of lactation at 0900 hours, and at 1215 hours, dams were administered with ovine prolactin (5 mg/kg injection i.p.). Mice were anesthetized with sodium pentobarbital and perfused transcardially 45 min after prolactin administration with 4% paraformaldehyde. Brains were removed, postfixed for 1 h in the same fixative, and cryoprotected in 30% sucrose overnight. Three sets of 30-μm-thick coronal sections through the forebrain from each animal were cut using a sliding microtome. One series of tissue each was used to examine pSTAT5 and EGFP expression by chromagen immunohistochemistry as previously described (11, 12) (SI Methods). Quantification of pSTAT5 labeling in the MPOA of lactating Prlrlox/lox (n = 4–8) mice was undertaken by counting the total number of pSTAT5-labeled nuclei in two sections per animal. Sections were anatomically matched between each different animal.
Images were collected with a Zeiss Plan-Apochromat 10× objective (or 20× for the inset figures in Fig. 1 D–F) using the Axio Imager M2 microscope with a motorised stage and filters for fluorescent dyes (Zeiss Fs 43, AHF Analysentechnik F36-526, and F36-500 for Cy3, Alexa488, and Hoechst, respectively). The MozaiX module in the AxioVision software (Zeiss) was used to acquire whole brain images. Briefly, the area containing a brain section was defined, focus was adjusted manually, the area was scanned with three filters, and individual images in the area were stitched together automatically.
SI Methods
Animals.
Prlr Cre mice were generated using homologous recombination in mouse ES cells (15). The targeting construct was designed to insert an IRES and the sequence for Cre recombinase immediately after exon 10 in the Prlr gene (specific for the long form of the Prlr), with a flippase recognition target (FRT)-flanked neomycin resistance cassette for selection of clones. Correctly targeted ES cells were injected into C57BL/6J blastocysts to generate chimeras that were backcrossed to C57BL/6J animals to give Prlr-iCreneo+ mice. To remove the neomycin resistance cassette, Prlr-iCreneo+ animals were bred to Flp recombinase deleter mice (31) to result in Prlr-iCreneo− (Prlr-iCre) mice. Flippase (flp)-mediated excision of the selection cassette was verified by Southern blot analysis. Prlr-specific expression of Cre was validated by crossing Prlr-iCre mice with ROSA26-CAGS-τGFP reporter animals (32), with expression of τGFP restricted to regions known to express the long form of Prlr (12).
Double-Vgat/VGlut Cre animals were generated initially by breeding male VGat-Cre +ve mice with female VGlut-Cre +ve mice. To genotype the double-Cre animals, it was necessary to use a strategy that specifically distinguished the presence of Cre within the VGat or VGlut gene (as opposed to a generic Cre genotype) using the following combinations of primers to generate bands of 300 bp in WT animals or 800 bp in the presence of the IRES-Cre cassette:
VGatP1 CTT CGT CAT CGG CGG CAT CTG,
VGatP2 CAG GGC GAT GTG GAA TAG AAA,
VGlutP1 CGG TAC CAC CAA ATC TTA CGG,
VGlutP2 CAT GGT CTG TTT TGA ATT CAG, and
CreP ATC GAC CGG TAA TGC AGG CAA.
Immunohistochemistry.
Immunohistochemistry for GFP and pSTAT5 was conducted as previously described (11, 12) using 30-µm sections cut on a sliding microtome. To label GFP, sections were incubated in polyclonal rabbit anti-GFP (1:10,000; A-6455; Life Technologies) for 48 h at 4 °C. To label pSTAT5, an antigen retrieval procedure was performed on all tissue before immunohistochemistry for pSTAT5. Sections were incubated in rabbit anti-pSTAT5 primary antibody (polyclonal rabbit anti-pSTAT5; Tyr-694, 1:1,000; Cell Signaling Technology, Inc.) for 72 h at 4 °C. For GFP and pSTAT5 labeling, sections were incubated for 90 min in biotinylated goat anti-rabbit IgG (1:400 for GFP, 1:200 for pSTAT5; Vector Laboratories) followed by a 90-min incubation in Vector Elite avidin–biotin–HRP complex (1:100). Peroxidase labeling was visualized with nickel-diaminobenzidine tetrahydrochloride using glucose oxidase to create a black nuclear precipitate. The GFP and prolactin-induced pSTAT5 labeling in the MPOA were assessed in all AAV-Cre administered mice. Only mice showing widespread GFP labeling and the complete absence of pSTAT5 labeling throughout the MPOA were included for analysis of maternal behavior. For tract tracing experiments, brains were removed and frozen in tissue freezing medium (Leica); 14-µm coronal sections were cut using a cryostat (Leica). To detect mCherry and τGFP expression, sections were incubated in rabbit anti-DsRed (1:1,000; catalog no. 632496; Clontech) and chicken anti-GFP (1:1,000; A10262; Invitrogen) antibodies overnight at 4 °C followed by goat anti-rabbit Cy3 and anti-chicken Alexa 488 (1:400; A10520 and A11039, respectively; Invitrogen) for 1 h at room temperature.
Maternal Behavior Testing.
Pup retrieval behavior was tested in home cages on day 3 of lactation in groups of Prlrlox/lox (n = 8), Prlrlox/lox/VGlut-Cre (n = 8), Prlrlox/lox/VGat-Cre (n = 12), B6 (n = 10), Prlrlox/lox (n = 8), B6/AAV-Cre (n = 4), Prlrlox/lox/saline (n = 3), and Prlrlox/lox/AAV-Cre (n = 7) mice. Between 0900 and 1100 hours on postpartum day 3, all pups were removed from the dam’s home cage, and 5 min later, three age-matched foster pups were placed into the cage at the opposite end from her nest. All pup-directed and other behaviors were recorded for 30 min. The time taken to retrieve each pup and to place it into her nest was assessed. After pup retrieval assessment, the dams own pups were returned to her. On postpartum day 5, pup retrieval in a novel cage was tested. The dams were moved into a clean, novel cage and allowed to settle for 10 min. Three of her own pups were then placed into three corners of the cage. Time taken to retrieve each pup and the time taken to form a nest with the three pups were recorded over a 3-h testing period. After testing, dams were returned to their home cage with their pups.
Statistical Analysis.
Data are reported as mean ± SEM; all statistical analyses were undertaken using GraphPad Prism 6 (GraphPad), and P < 0.05 was considered statistically significant. One-way ANOVAs and unpaired t tests were used to statistically compare the time taken from mating to presence of plug and gestation length between genotypes, and a Tukey post hoc test was used where appropriate. For analyzing numbers of neurons expressing pSTAT5 in specific regions, unpaired t tests were performed between control and Cre-expressing groups. Home cage maternal behavior 6–24 h postpartum was assessed by two-way ANOVA, and Sidak’s multiple comparisons test was used to identify statistically significant changes. One-way ANOVAs were used to compare pup retrieval behavior between MPOA AAV-Cre or saline-injected groups, and unpaired t tests were used to compare pup retrieval behavior in the home and novel cage tests between control and Cre-expressing groups. Pup survival was assessed by Fisher’s exact test to compare the number of litters with fewer than two live pups.
Acknowledgments
We thank Kendra Boyes and Hsin-Jui (Regina) Lien for completing the video analysis of behavior, and Dr. Hermans-Borgmeyer (Transgenic Animals Service Group, Center for Molecular Neurobiology) for ES cell work. This work was supported by Health Research Council of New Zealand Grant HRC 14/568, Marsden Grant 16-UOO-236 from the Royal Society of New Zealand, German Federal Ministry of Education and Research Grant BMBF NZL 12-011, and Deutsche Forschungsgemeinschaft Grant SFB 894.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708025114/-/DCSupplemental.
References
- 1.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 2.Numan M, Woodside B. Maternity: Neural mechanisms, motivational processes, and physiological adaptations. Behav Neurosci. 2010;124:715–741. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- 3.Tsuneoka Y, et al. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J Comp Neurol. 2013;521:1633–1663. doi: 10.1002/cne.23251. [DOI] [PubMed] [Google Scholar]
- 4.Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges RS. Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol. 2015;36:178–196. doi: 10.1016/j.yfrne.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 7.Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- 8.Binart N, et al. Rescue of preimplantatory egg development in prolactin receptor-deficient mice after progesterone administration. Endocrinology. 2000;141:2691–2697. doi: 10.1210/endo.141.7.7568. [DOI] [PubMed] [Google Scholar]
- 9.Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- 10.Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RS, et al. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2016;36:9173–9185. doi: 10.1523/JNEUROSCI.1471-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown RS, Kokay IC, Herbison AE, Grattan DR. Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol. 2010;518:92–102. doi: 10.1002/cne.22208. [DOI] [PubMed] [Google Scholar]
- 13.Grattan DR, LeTissier P. Hypothalamic control of prolactin secretion, and the multiple reproductive functions of prolactin. In: Plant TM, Zelesnik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th Ed. Vol 1. Elsevier; Amesterdam: 2015. pp. 469–526. [Google Scholar]
- 14.Brown RS, Herbison AE, Grattan DR. Differential changes in responses of hypothalamic and brainstem neuronal populations to prolactin during lactation in the mouse. Biol Reprod. 2011;84:826–836. doi: 10.1095/biolreprod.110.089185. [DOI] [PubMed] [Google Scholar]
- 15.Candlish M, De Angelis R, Götz V, Boehm U. Gene targeting in neuroendocrinology. Compr Physiol. 2015;5:1645–1676. doi: 10.1002/cphy.c140079. [DOI] [PubMed] [Google Scholar]
- 16.Wen S, et al. Genetic identification of GnRH receptor neurons: A new model for studying neural circuits underlying reproductive physiology in the mouse brain. Endocrinology. 2011;152:1515–1526. doi: 10.1210/en.2010-1208. [DOI] [PubMed] [Google Scholar]
- 17.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda KO, Tsuneoka Y. Assessing postpartum maternal care, alloparental behavior, and infanticide in mice: With notes on chemosensory influences. Methods Mol Biol. 2013;1068:331–347. doi: 10.1007/978-1-62703-619-1_25. [DOI] [PubMed] [Google Scholar]
- 20.Douglas AJ, Leng G, Russell JA. The importance of oxytocin mechanisms in the control of mouse parturition. Reproduction. 2002;123:543–552. doi: 10.1530/rep.0.1230543. [DOI] [PubMed] [Google Scholar]
- 21.McKinley MJ, et al. The median preoptic nucleus: Front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 2015;214:8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 22.McHenry JA, et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017;20:449–458. doi: 10.1038/nn.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- 24.Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev. 2011;35:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Soares MJ. The prolactin and growth hormone families: Pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott N, Prigge M, Yizhar O, Kimchi T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature. 2015;525:519–522. doi: 10.1038/nature15378. [DOI] [PubMed] [Google Scholar]
- 28.Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- 29.Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zack GW, Rogers WE, Latt SA. Automatic measurement of sister chromatid exchange frequency. J Histochem Cytochem. 1977;25:741–753. doi: 10.1177/25.7.70454. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez I, Feinstein P, Mombaerts P. Variable patterns of axonal projections of sensory neurons in the mouse vomeronasal system. Cell. 1999;97:199–208. doi: 10.1016/s0092-8674(00)80730-8. [DOI] [PubMed] [Google Scholar]