Significance
Cutaneous leishmaniasis and schistosomiasis are neglected tropical diseases for which there are no effective vaccines and limited treatment strategies. To develop vaccine and therapeutic alternatives, a detailed understanding of host immunity is essential. We show a role for IL-4Rα–responsive B cells in host susceptibility to Leishmania major and protection against Schistosoma mansoni infection through the production of early IL-4, which in turn regulates Th2 cell polarization and disease outcome in mice. These important findings highlight the significant impacts that B cell-specific IL-4Rα and IL-4 responsiveness have in the context of type 1 (L. major) and type 2 (S. mansoni) pathogens. Thus, vaccine and therapeutic development should aim to target both B and T cell immunity for optimal efficacy.
Keywords: IL-4R alpha, B cells, leishmaniasis, schistosomiasis, mouse
Abstract
Interleukin-4 (IL-4)–induced T helper (Th) 2 cells promote susceptibility to the protozoan parasite Leishmania major, while conferring immunity to the intestinal trematode Schistosoma mansoni. Here, we report that abrogation of IL-4 receptor alpha (IL-4Rα) signaling on B cells in BALB/c mice (mb1creIL-4Rα–/lox) transformed nonhealer BALB/c to a healer phenotype with an early type 1 and dramatically reduced type 2 immune response and an absence of ulceration and necrosis during cutaneous leishmaniasis. From adoptive reconstitution and mixed bone-marrow chimera studies in B cell-deficient (µMT) mice, we reveal a central role for B cell-derived IL-4 and IL-4Rα in the optimal induction of the susceptible type 2 phenotype to L. major infection. We further demonstrate that the absence of IL-4Rα signaling on B cells exacerbated S. mansoni-induced mortality and pathology in BALB/c mice, due to a diminished type 2 immune response. In both disease models, IL-4Rα–responsive B cells displayed increased IL-4 production as early as day 1 after infection. Together, these results demonstrate that IL-4–producing and IL-4Rα–responsive B cells are critical in regulating and assisting early T helper dichotomy toward Th2 responses, which are detrimental in cutaneous leishmaniasis but beneficial in acute schistosomiasis.
B cells are well known for their ability to differentiate into antibody-secreting plasma cells in response to foreign antigens or pathogens (1). However, recent studies suggest that B cells appear to regulate both protective and pathologic immune responses by antibody-independent mechanisms. These mechanisms range from the organization of secondary lymphoid tissues and lymphangiogenesis to recognition of antigenic ligands by Toll-like receptors (1, 2). Activated B cells are also excellent antigen-presenting cells (APCs) capable of internalizing antigen through the B cell receptor (BCR), processing, and presenting peptides to CD4+ T cells in an MHCII-independent manner (1–5). In fact, growing evidence suggests that antigen-presenting B cells assist in regulating the quality and quantity of both primary and memory CD4+ T helper (Th) cell responses (2, 5–7).
Besides antibody production and antigen presentation, B cells also influence host immunity by producing cytokines (1, 2, 8). However, the importance of cytokine-producing B cells in health and disease remained an unexplored aspect until the last decade. Resurgence in this field of B cell biology has led to the subdivision of B cells into distinct cytokine-producing “regulatory” or “effector” subsets, detected both in vivo and in vitro. Regulatory B cells (Bregs) produce IL-10 and TGF-β and are critical in protection against allergic airway inflammation (9), colitis (10), and collagen-induced arthritis (11). On the other hand, defined effector B cell subsets produce cytokines such as IFN-γ, IL-12p40, TNF-α or IL-2, IL-6, IL-4, and IL-13 (8, 12, 13). These B cell-derived cytokines have been described to influence the outcome of infectious diseases. IFN-γ– and IL-12p40–producing B cells have been detected in vivo during infection with a type 1 polarizing parasite Toxoplasma gondii (8, 12), while infection with the type 2 polarizing parasite Heligmosomoides polygyrus has been shown to induce differentiation of IL-4– and IL-2–producing B cells in vivo (2, 8, 13). Altogether, these observations demonstrate that B cells produce cytokines in response to foreign antigens or pathogens that might function to initiate and/or maintain the magnitude and quality of CD4+ Th cell-dependent immune responses toward Th1 or Th2 phenotypes. The exact role of cytokine-producing B cells in vivo during cutaneous leishmaniasis, a type 1-controlled disease caused by Leishmania major, and acute schistosomiasis, a type 2-controlled disease caused by Schistosoma mansoni, is not yet established.
Most studies on the contribution of B cells to host defense or susceptibility to L. major or S. mansoni infection have been conducted in BALB/c mice that lack mature B cells due to disruption of the IgM transmembrane domain (μMT). B cell-deficient μMT mice were found to be intermediately resistant to L. major infection (14) but developed exacerbated egg pathology and increased mortality in response to S. mansoni infection (15, 16). However, deletion of the complete B cell population provides very little information on the specific contribution of B cell subsets and derived cytokines to disease outcome. We therefore used a newly generated BALB/c mouse lacking IL-4Rα expression specifically on B cells, mb1creIL-4Rα–/lox BALB/c mice (17), to investigate the role of IL-4Rα–responsive B cells during parasitic infection. The IL-4Rα chain is a common receptor of both the type I (IL-4) and type II (IL-4/IL-13) receptor complexes. Both these cytokines have pleiotropic immune functions, being shown to be responsible for intracellular parasitism caused by L. major (18–21) and mediating protection to S. mansoni infection (22–24).
Using this model, we show that while IL-4Rα–unresponsive B cells are beneficial in cutaneous leishmaniasis, leading to host protective immunity in mb1creIL-4Rα–/lox BALB/c mice, these cells are detrimental in acute schistosomiasis, leading to increased host pathology. We further demonstrate that IL-4Rα–deficient B cells differentially regulate mRNA cytokine expression, consistent with Th1 cell dichotomy, as early as day 1 after infection. Importantly, we show that IL-4–producing B cells are critical in driving the susceptible type 2 immune response to cutaneous leishmaniasis in BALB/c mice. Together, these data demonstrate that early IL-4Rα–responsive B cells producing IL-4 influence early Th polarization toward detrimental Th2 responses that drive L. major-induced cutaneous leishmaniasis and protect the host against S. mansoni-induced pathology.
Results
B Cell-Specific IL-4Rα–Deficient BALB/c Mice Are Protected from Cutaneous Leishmaniasis.
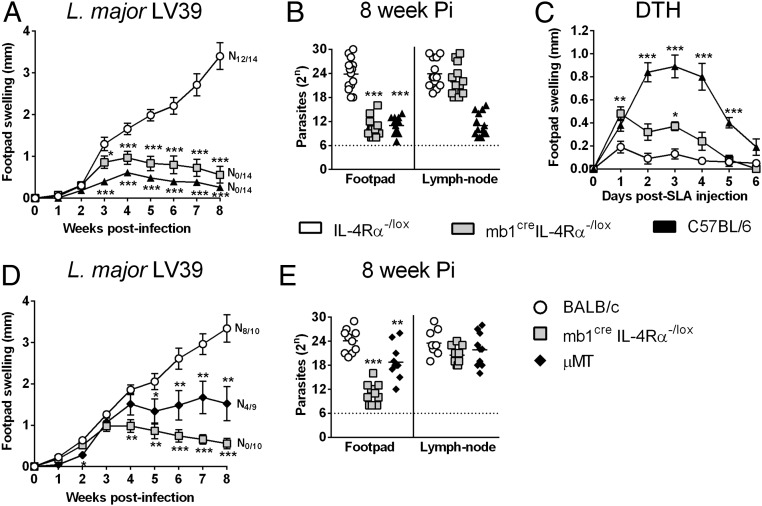
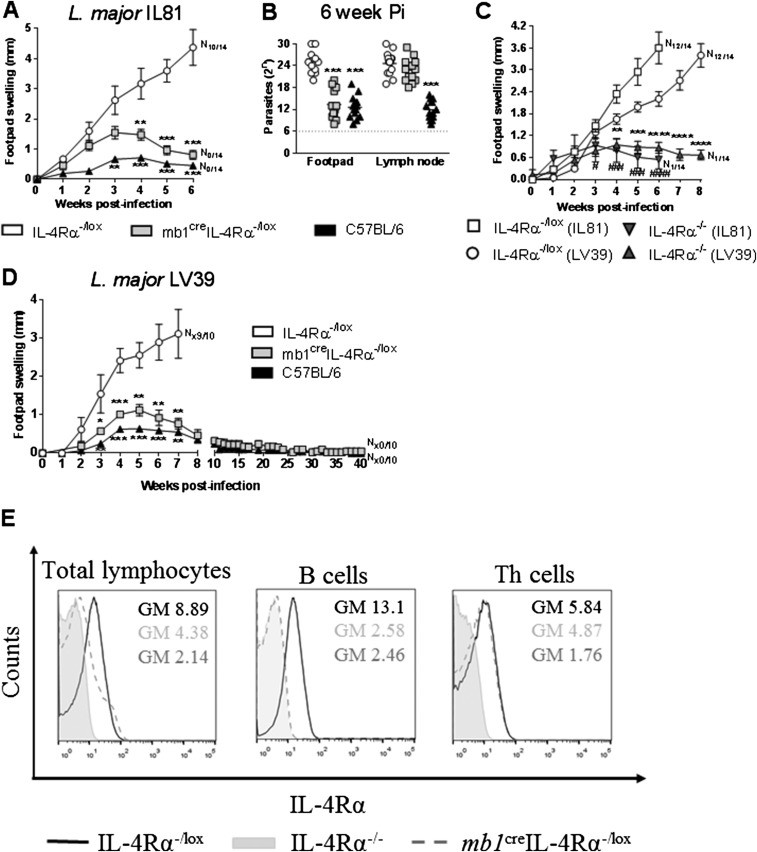
In a loss-of-function approach, we recently generated B cell-specific IL-4Rα–deficient BALB/c mice (mb1creIL-4Rα–/lox) (17) to investigate a possible role for IL-4–responsive B cells in type 1-controlled cutaneous leishmaniasis. mb1creIL-4Rα–/lox, hemizygous littermate control IL-4Rα–/lox BALB/c (expressing functional IL-4Rα), and C57BL/6 mice were infected s.c. with 2 × 106 stationary phase metacyclic promastigotes of L. major LV39 strain (MRHO/SV/59/P) into the hind footpad. As expected following L. major infection (Fig. 1 A and B), littermate IL-4Rα–/lox BALB/c mice developed rapidly growing necrotic lesions (N12/14), leading to fulminant cutaneous leishmaniasis within 8 wk of infection, similar to WT BALB/c (IL-4Rα+/+ mice) (21, 25, 26), whereas the healer C57BL/6 strain controlled tissue pathology without development of necrosis (N0/14) and ulceration (19, 21, 25, 26). Deficiency of the IL-4Rα chain on B cells resulted in control of the disease, as presented by reduced footpad swelling, absence of necrotic lesions (Fig. 1A), and ∼1,000-fold fewer parasites in the footpad but not in the draining lymph nodes (dLNs) of mb1creIL-4Rα–/lox BALB/c mice compared with susceptible IL-4Rα–/lox littermates (Fig. 1B). Infection with a more virulent L. major IL81 strain (MHOM/IL/81/FEBNI), which is faster developing and IL-4–dependent similar to our LV39 strain (25), confirmed the resistant phenotype for mb1creIL-4Rα–/lox BALB/c mice with strikingly reduced swelling and reduced parasite burden in the footpad (Fig. S1 A and B), analyzed 6 wk postinfection. As expected, global IL-4Rα−/− knockout mice controlled both LV39 and IL81 infection in the footpad in the acute phase (Fig. S1C) in line with previous reports (21, 25). Challenging with soluble L. major antigen (SLA) in 6 wk-infected mb1creIL-4Rα–/lox BALB/c mice resulted in increased delayed-type hypersensitivity (DTH) (Fig. 1C), implying heightened memory responses to L. major infection, as known for the healer C57BL/6 strain. Indeed, acute resistance translated to chronic disease control, as demonstrated by the absence of footpad swelling, similar to the C57BL/6 healer strain (Fig. S1D). Cre-mediated IL-4Rα deletion on B cells was also efficient during infection with impaired IL-4Rα expression only on B cells (Fig. S1E). In summary, these data suggest a direct role for IL-4Rα–responsive B cells in host susceptibility to cutaneous leishmaniasis. In the absence of B cells during acute LV39 infection, B cell-deficient mice (µMT BALB/c) were moderately susceptible, showing slightly ameliorated footpad swelling and pathogen burden (Fig. 1 D and E). However, fulminant cutaneous leishmaniasis arose in µMT mice, whereas mb1creIL-4Rα–/lox BALB/c mice mounted a protective immune response altogether, supporting a beneficial role for IL-4Rα–unresponsive B cells during cutaneous leishmaniasis.
Fig. 1.
mb1creIL-4Rα–/lox BALB/c mice efficiently control cutaneous L. major infection. (A) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with stationary phase 2 × 106 metacyclic promastigote L. major LV39 (MRHO/SV/59/P) parasites into the hind footpad, and footpad swelling was measured at weekly intervals. (B) At week 8 postinfection, parasite burden was determined by limiting dilution of homogenized footpads and draining LNs. (C) At 6 wk after L. major LV39 infection, mb1creIL-4Rα–/lox mice and controls were injected s.c. with 10 µg SLA in the contralateral hind footpad. A DTH response was monitored by measuring footpad swelling daily for 6 d. (D and E) B cell-deficient (μMT) BALB/c mice were infected with L. major LV39 parasites to determine footpad swelling (D) and parasite burdens in footpads and draining LNs (E) at week 8 after infection. A pool of three individual experiments is shown with mean ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice as significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). N#/14, # represents number of mice in a group of 14 showing necrosis/ulceration.
Fig. S1.
mb1creIL-4Rα–/lox and IL-4Rα−/− BALB/c mice control L. major LV39 and IL81 infection with efficient chronic disease control and Cre-mediated IL-4Rα deletion on B cells in mb1creIL-4Rα–/lox mice. (A and B) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with the more virulent 2 × 105 L. major IL81 (MHOM/IL/81/FEBNI) parasites into the hind footpad to determine weekly footpad swelling (A) and parasite burden in homogenized footpads and draining LNs (B) at week 6 after infection. (C) IL-4Rα–/lox and IL-4Rα−/− BALB/c mice were infected s.c. with 2 × 105 L. major IL81 and 2 × 106 L. major LV39 promastigotes into the hind footpad and footpad swelling monitored at weekly intervals until 8 and 6 wk postinfection, respectively. (D) Footpad swelling was monitored weekly until the chronic phase in L. major-infected mb1creIL-4Rα–/lox BALB/c and control mice. A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c (LV39) control mice as significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001) or to littermate IL-4Rα–/lox BALB/c (IL81) mice as significant (#P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001, ####P ≤ 0.0001). (E) Cell-surface IL-4Rα expression was analyzed in total lymphocytes, B cells (CD19+B220+CD3−), and Th cells (CD3+CD4+CD19−) in the draining LNs of L. major-infected-IL-4Rα−/− (gray tinted), IL-4Rα–/lox (black line), and mb1creIL-4Rα–/lox (dark gray dashed line) mice by flow cytometry. Data represent individual histogram plot of one of two independent experiments with n = 5 mice per group.
B Cell-Specific IL-4Rα–Deficient BALB/c Mice Show Strikingly Impaired Type 2 Responses.
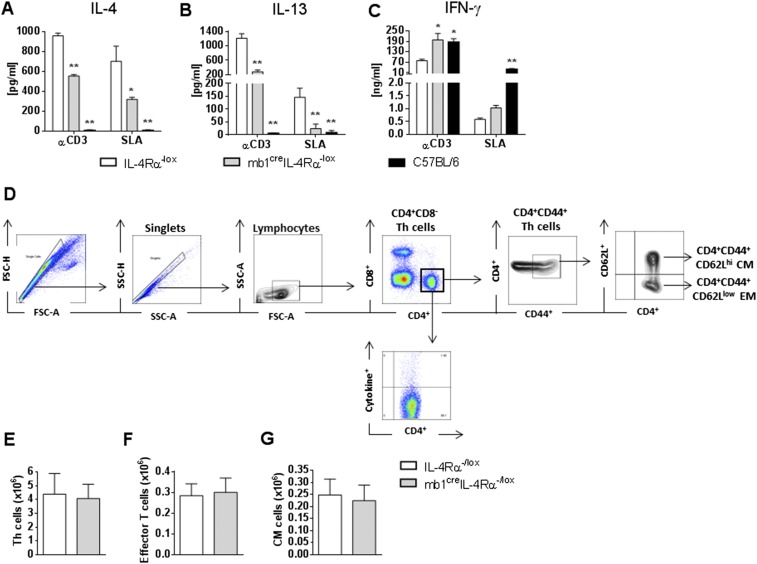
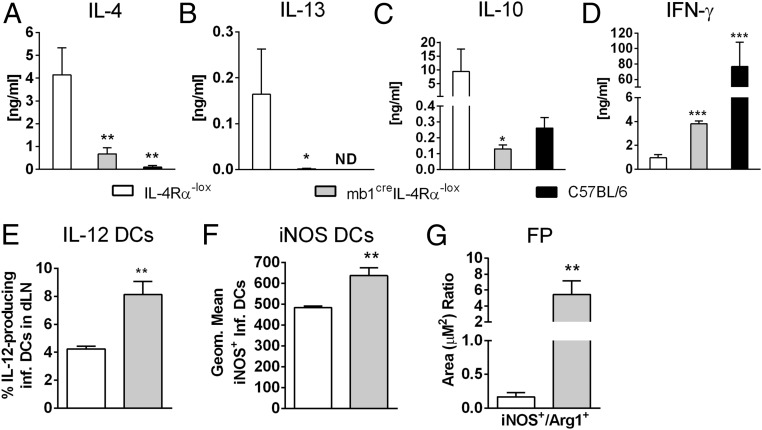
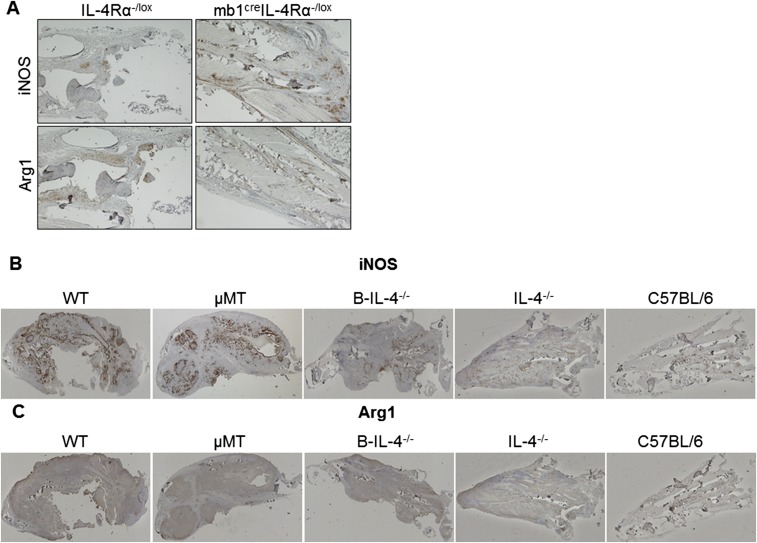
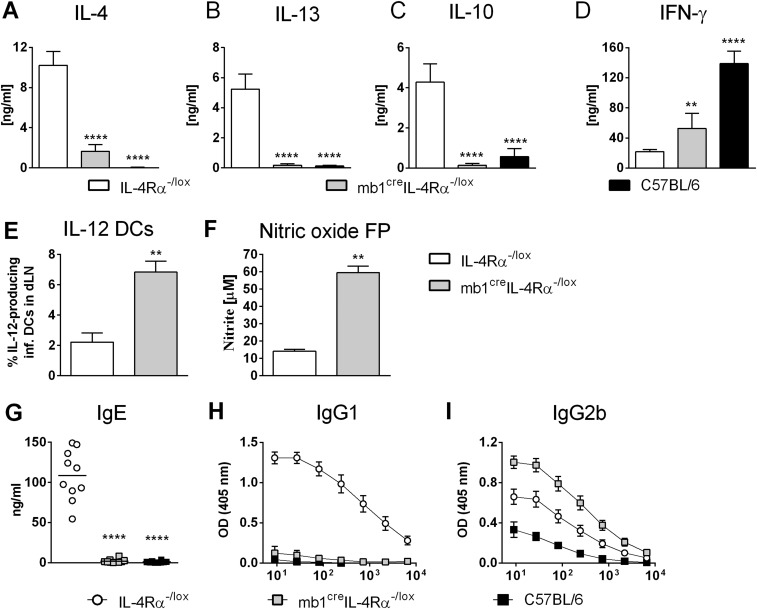
Protection from L. major-induced cutaneous leishmaniasis depends on IL-12–driven Th1 responses (20). Draining LN cells from 8 wk-infected mb1creIL-4Rα–/lox BALB/c mice (LV39 strain) were restimulated with mitogenic α-CD3 or with antigen-specific SLA. Total LN lymphocytes from mb1creIL-4Rα–/lox BALB/c mice responded with a significantly drastic reduction in the IL-4 and IL-13 cytokines in comparison with littermate control mice (Fig. S2 A and B) and a concomitant increase in IFN-γ secretion (Fig. S2C). This was relatively due to a striking 7–100-fold decrease in Th2 cells secreting IL-4, IL-13, and IL-10, as demonstrated by FACS-sorted LN CD4+ Th cells from infected mb1creIL-4Rα–/lox BALB/c mice, restimulated with L. major antigen in the presence of fixed APCs (Fig. 2 A–C). CD4+ Th1 cells from infected mb1creIL-4Rα–/lox BALB/c mice secreted twofold more IFN-γ compared with littermate control mice (Fig. 2D). Collectively, the composition of CD4+ Th cells, central memory and effector memory Th cells were similar between mb1creIL-4Rα–/lox BALB/c mice and littermate control IL-4Rα–/lox mice (Fig. S2 D–G). CD11chiMHCIIhiCD11b+Ly6chi inflammatory dendritic cells (DCs) from the LNs of infected mb1creIL-4Rα–/lox BALB/c mice produced significantly more IL-12p40/70 and nitric oxide synthase 2 (NOS2), the latter responsible for nitric oxide (NO) production to kill intracellular Leishmania (27), compared with control IL-4Rα–/lox BALB/c mice, measured by flow cytometry (Fig. 2 E and F). At the site of infection, immunohistochemical staining revealed that footpads of mb1creIL-4Rα–/lox BALB/c had increased iNOS expression in relation to Arginase 1 (Arg1), indicating a shift to a classical activation profile and enhanced leishmanicidal effector functions in these animals compared with littermate control IL-4Rα–/lox mice (Fig. 2G and Fig. S3A). In response to IL81 infection, the cellular and humoral profile in mb1creIL-4Rα–/lox BALB/c mice mirrored the response during LV39 infection, shown by a significant down-regulation of Th2 cytokines (Fig. S4 A–C) and substantial up-regulation of IL-12–driven IFN-γ responses (Fig. S4 D and E), leading to increased NO production by total footpad cells stimulated with LPS in iNOS activity assays (Fig. S4F). Hence, impairment of IL-4Rα on B cells in L. major LV39 and IL81-infected BALB/c mice severely abrogated detrimental Th2 responses promoted by a beneficial IL-12–driven Th1 response. Thus, the extreme down-regulation of the type 2 response in mb1creIL-4Rα–/lox compared with WT littermate control IL-4Rα–/lox mice, rather than dramatic differences in the number of IFN-γ–secreting cells, is likely the reason behind the observed resistance to the parasite.
Fig. S2.
Enhanced Th2 responses but normal recruitment and expansion of T cell populations in mb1creIL-4Rα–/lox mice infected with L. major LV39. (A–C) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with 2 × 106 L. major LV39 promastigotes into the hind footpad. At week 8 postinfection, total LN cells were restimulated with anti-CD3 or SLA for 72 h, and cell supernatants were analyzed for the production of IL-4 (A), IL-13 (B), and IFN-γ (C). (D–G) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with 2 × 106 L. major LV39 promastigotes into the hind footpad. Draining LN cells were FACS-stained and gated (D) for recruitment of CD3+CD4+ Th cells (E), expansion of effector memory CD4+ T cells (CD3+CD4+CD44hiCD62Llo) (F), and central memory CD4+ T cells (CD3+CD4+CD44hiCD62Lhi) (G). A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice as significant (*P ≤ 0.05, **P ≤ 0.01).
Fig. 2.
Impaired Th2 cytokine responses and killing effector functions in mb1creIL-4Rα–/lox mice infected with L. major LV39. (A–D) Total LN CD4+ T cells were restimulated for 72 h with fixed APCs and SLA. The production of IL-4 (A), IL-13 (B), IL-10 (C), and IFN-γ (D) in cell supernatants was determined by ELISA (ND, not detected). (E and F) CD11chiMHCIIhiCD11b+Ly6chi inflammatory DCs in the LN were analyzed for the production of IL-12 (E) and iNOS (F) by intracellular FACS staining. (G) Quantification of iNOS and arginase 1-positive areas in multiple sections of formalin-fixed footpads by immunohistochemical staining. Full sections of footpads were scanned at 2× objective lens using a Nikon 90i microscope and quantified using NIS-Elements advanced research software for quantification of positive staining expressed as a ratio of iNOS+/Arg-1+, serving as a proxy for classical and alternative activation, respectively. A representative of three individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice as significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Fig. S3.
iNOS and arginase staining in footpads of mice infected with L. major LV39. (A) iNOS and arginase 1-positive staining in formalin-fixed footpad sections of LV39-infected mb1creIL-4Rα–/lox BALB/c and control mice, and (B and C) respective chimeric mice (Fig. S7) by immunohistochemical staining. Representative images were acquired at 4× objective lens (A) and 2× objective lens (B and C) using a Nikon 90i microscope and scanned for quantification of positive staining expressed as a ratio of iNOS+/Arg-1+ (Figs. 2G and 5I), serving as a proxy for classical and alternative activation, respectively. (Scale bar: A, 500 µm; B and C, 1,000 µm.)
Fig. S4.
Impaired Th2 cytokine responses and killing effector functions in mb1creIL-4Rα–/lox mice infected with L. major IL81. (A–C) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with 2 × 105 L. major IL81 promastigotes into the hind footpad. At week 6 postinfection, total LN CD4+ T cells were restimulated for 72 h with fixed APCs and SLA. The production of IL-4 (A), IL-13 (B), IL-10 (C), and IFN-γ (D) in cell supernatants was determined by ELISA. (E) CD11chiMHCIIhiCD11b+Ly6chi inflammatory DCs in the LN were analyzed for the production of IL-12 by intracellular FACS staining at week 6 after IL81 infection. (F) Production of NO in total footpad cells. Total cells were isolated from footpads at week 6 after IL81 infection and stimulated with 10 ng/mL LPS for 72 h. Production of NO was determined in cell supernatants. (G–I) At week 6 post-IL81 infection, total IgE (G), antigen-specific IgG1 (H), and IgG2b (I) antibody isotypes were quantified from infected sera by ELISA. A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice as significant (**P ≤ 0.01, ****P ≤ 0.0001).
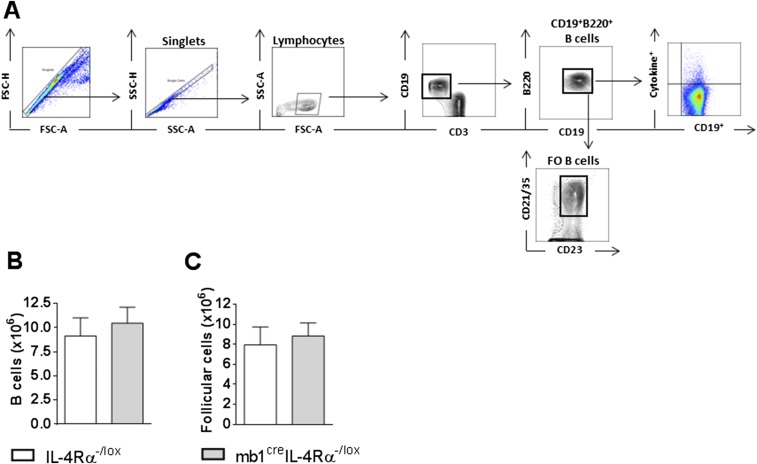
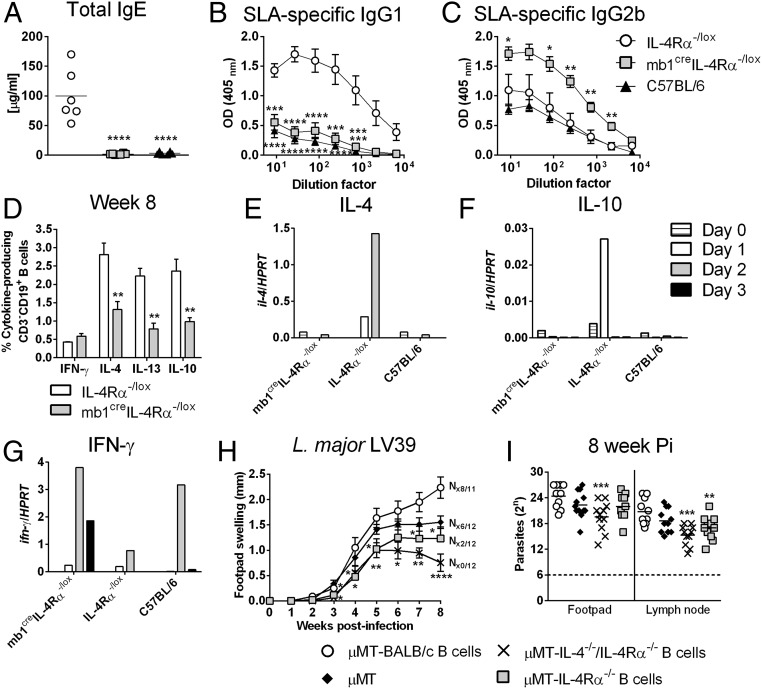
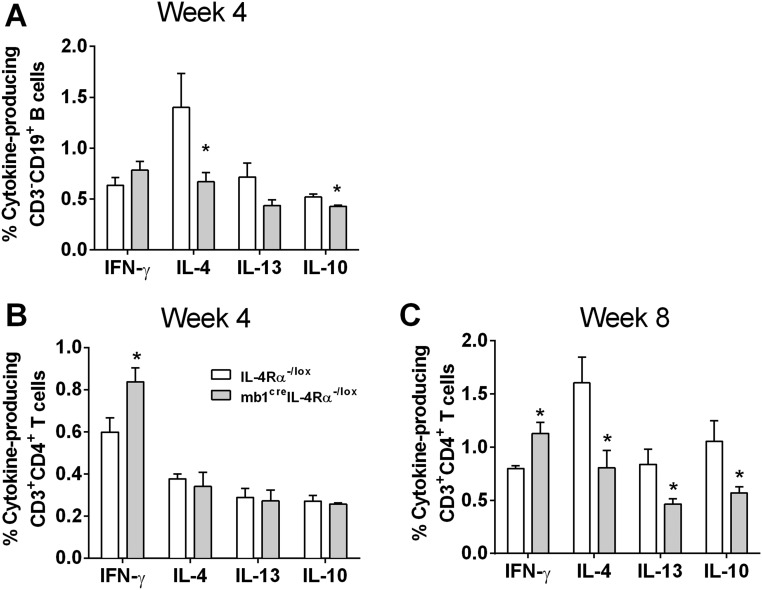
The number of B220+CD19+ B cells and follicular B cells were unaltered in the LNs of infected mb1creIL-4Rα–/lox BALB/c mice compared with control IL-4Rα–/lox mice (Fig. S5), suggesting that effector B cell populations developed independently of IL-4Rα responsiveness by B cells. As a result of the increased Th1 and significantly reduced Th2 responses, L. major LV39- and IL81 (IL81)-infected mb1creIL-4Rα–/lox BALB/c mice presented with a dominant type 1 B cell antibody response with strikingly reduced antigen (SLA)-specific IgG1 and total IgE and increased antigen-specific IgG2b (Fig. 3 A–C and Fig. S4 G–I). At week 4 postinfection, intracellular B cell cytokine production for IFN-γ was similar between infected mb1creIL-4Rα–/lox BALB/c mice and control IL-4Rα–/lox BALB/c mice but reduced for IL-4 and IL-10 in B cell-specific IL-4Rα–deficient mice, measured by intracellular FACS staining in B220+CD19+ B cells (Fig. S6A). By 8 wk postinfection, the strong reduction in B cell-derived IL-4, IL-13, and IL-10 cytokines was even more pronounced in mb1creIL-4Rα–/lox BALB/c mice (Fig. 3D), with similar IFN-γ production to control IL-4Rα–/lox BALB/c mice. Of interest, cytokine production by CD4+CD3+ Th cells from mb1creIL-4Rα–/lox BALB/c mice presented with increased IFN-γ secretion at 4 wk postinfection and a clear down-regulation of the type 2 response with reduced IL-4, IL-13, and IL-10 at 8 wk postinfection (Fig. S6 B and C). These results suggest that abrogation of IL-4Rα expression on B cells in mb1creIL-4Rα–/lox BALB/c mice not only impaired type 2 antibody responses but also induced a general reduction in type 2 cytokines from B cells. These data suggest that the healing response in mb1creIL-4Rα–/lox BALB/c at 8 wk postinfection was not due to differences in IFN-γ levels on B cells, but rather by their ability to down-regulate the type 2 B cell-derived cytokine response.
Fig. S5.
Normal recruitment and expansion of B cell populations in mb1creIL-4Rα–/lox mice infected with L. major LV39. (A–C) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with 2 × 106 L. major LV39 promastigotes into the hind footpad. Draining LN cells were FACS-stained and gated (A) for recruitment of CD19+B220+ B cells (B) and (C) expansion of follicular B cells (FO, CD19+B220+CD21+CD23+) by flow cytometry. A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice.
Fig. 3.
Type 2 effector B cell responses are reduced in IL-4Rα–deficient B cells during L. major infection and prevent L. major-induced cutaneous leishmaniasis. (A–C) mb1creIL-4Rα–/lox BALB/c and control mice were infected s.c. with 2 × 106 L. major LV39 promastigotes into the hind footpad. At week 8 postinfection, total IgE (A), antigen-specific IgG1 (B), and IgG2b (C) antibody isotypes were quantified from infected sera by ELISA. (D) B cell-specific (CD19+B220+) cytokines were measured in LNs by intracellular cytokine staining at week 8 after LV39 infection. (E–G) At day 0 and day 1–3 post-L. major LV39 infection, B220+CD19+CD3− B cells were FACS-sorted from the LNs (∼99% purity), and mRNA expression of il-4 (E), il-10 (F), and ifn-γ (G) transcripts were determined by quantitative real-time PCR. Expression was normalized against the housekeeping gene HPRT. (H and I) Recipient µMT mice were adoptively transferred with B cells purified from the LN of LV39-infected WT BALB/c, IL-4Rα−/−, or IL-4−/−/IL-4Rα−/− mice. B cell-reconstituted and control µMT mice were infected with 2 × 106 L. major LV39 to determine footpad swelling (H) and parasite burden in footpads and draining LNs (I) by limiting dilution assay 8 wk after infection. A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice as significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001).
Fig. S6.
Cytokine production by B cells and CD4+ T cells in mb1creIL-4Rα–/lox mice infected with L. major LV39. (A) B cell-specific (CD19+B220+) cytokines were measured in LNs by intracellular cytokine staining at week 4 after LV39 infection. (B and C) Th cell-specific (CD3+CD4+) cytokines were measured in LNs by intracellular cytokine staining at week 4 (B) and week 8 (C) after L. major LV39 infection in the footpad. A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to littermate IL-4Rα–/lox BALB/c control mice as significant (*P ≤ 0.05).
Early IL-4–Producing B Cells Drive Type 2 Immune Responses, Leading to Susceptibility in Cutaneous Leishmaniasis.
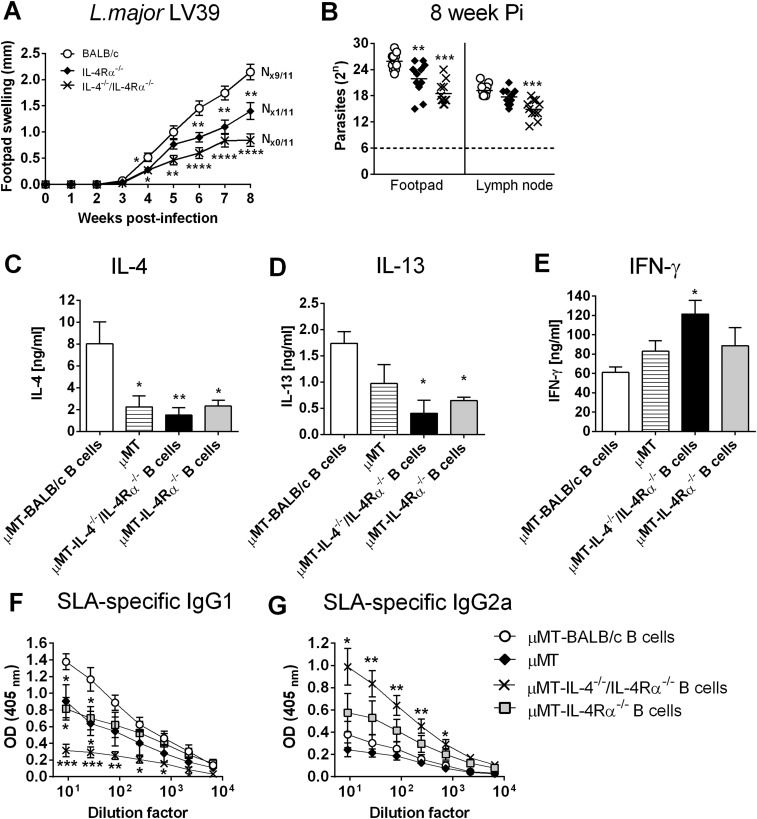
Studies have shown that early IL-4 production or α-IL-4 antibody within the first days can alter cutaneous disease outcome in BALB/c mice (28). Hence, a possible early dichotomy in B cell cytokine secretion, due to impaired IL-4Rα expression on B cells, might have influenced the immunological decision-making process. To address that, mb1creIL-4Rα–/lox BALB/c mice were infected with L. major LV39, and at days 1, 2, and 3 postinfection, singlet cells (FSC-A vs. FSC-H) were gated for B220+CD19+CD3− B cells from the dLN and isolated by FACS sorting (>99% purity). Following mRNA extraction, the expression of il-4, il-10, and ifn-γ transcripts were quantified by real-time PCR in comparison with the housekeeping gene hprt. Whereas before infection (day 0) negligible cytokine expression was detected in B cells, B cell-derived il-4 and il-10 was induced in the nonhealer BALB/c mice at day 1 and day 2 and barely induced in mb1creIL-4Rα–/lox and C57BL/6 mice (Fig. 3 E and F). In contrast, ifn-γ was induced in B cells from resistant mb1creIL-4Rα–/lox BALB/c mice and to a lesser extent in B cells from nonhealer BALB/c mice, starting at day 1 and gradually declining over the next 3 d (Fig. 3G). While the absence of B cells does not confer protection against L. major infection (Fig. 1 D and E), the results above suggest that antigen-primed, cytokine-producing B cells could modulate the immune response to infection in normal mice. We therefore hypothesized that early IL-4Rα–responsive and IL-4–competent B cells could directly confer susceptibility to L. major infection by directing Th cells toward predominant Th2 responses. To investigate this, we adoptively transferred 1 × 106 B cells (B220+CD19+CD3−) isolated from the dLN of L. major-infected IL-4Rα−/−, IL-4−/−/IL-4Rα−/−, or WT mice (all BALB/c) into naïve B cell-deficient (µMT) BALB/c mice. Three days later, animals were infected with L. major LV39. At the time of infection, despite the residual B cell population in µMT mice as previously reported (29), whole blood from all reconstituted mice contained a comparable percentage of repopulated B cells. Before B cell reconstitution, donor BALB/c mice expressed a susceptible phenotype, while IL-4Rα−/− and IL-4−/−/IL-4Rα−/− BALB/c animals contained acute infection (Fig. S7 A and B). Accordingly, transfer of L. major-primed IL-4Rα–responsive B cells from susceptible BALB/c mice augmented acute cutaneous leishmaniasis by week 6 postinfection, demonstrated by increasing footpad swelling (Fig. 3H) and increased parasite loads (Fig. 3I) in µMT mice (µMT-BALB/c B cells). In contrast, L. major-primed B cells deficient for the IL-4Rα chain, competent in IL-4 production but unable to respond to IL-4 (µMT-IL-4Rα−/− B cells), partially reduced footpad swelling and parasite loads in naïve µMT recipients (Fig. 3 H and I) compared with µMT recipients receiving BALB/c B cells. Of importance, µMT animals reconstituted with antigen-primed B cells unable to respond to IL-4 (as in µMT-IL-4Rα−/− B cell mice) but also unable to produce IL-4 (µMT-IL-4−/−/IL-4Rα−/− B cells) showed control of disease with significant reduced footpad swelling by week 6 postinfection and reduced parasite load (Fig. 3 H and I). We observed that disease progression was slightly delayed following adoptive transfer of antigen-primed B cells into µMT mice, which has been previously documented (14). However, footpad pathology, in terms of ulceration and necrosis, was completely absent in µMT mice reconstituted with IL-4/IL-4Rα−/− B cells (N0/12), confirming control of disease in these animals. In contrast, the high degree of ulceration and necrosis as seen in µMT mice reconstituted with WT BALB/c B cells (N8/11) indicates exacerbated disease as a consequence of exaggerated immunopathology, tissue destruction, apoptosis, and parasite replication. Disease control in µMT animals reconstituted with IL-4−/−/IL-4Rα−/− BALB/c B cells could be further explained by increased IFN-γ and reduced IL-4 and IL-13 production in LN cells when restimulated with α-CD3 (Fig. S7 C–E). The reduction of IL-4 was further reflected in a dominant type 1 antibody response and reduced type 2 (Fig. S7 F and G). These results confirm our previous observations that IL-4Rα–responsive B cells contribute to host susceptibility to L. major (Fig. 1) and further suggest that IL-4 produced by B cells is responsible for promoting disease progression.
Fig. S7.
Infection kinetics in BALB/c, IL-4Rα−/−, and IL-4−/−/IL-4Rα−/− mice to generate L. major LV39-primed B cells. (A and B) Mice were infected s.c. with 2 × 106 promastigote L. major LV39 parasites into the hind footpad to determine footpad swelling (A) and parasite burdens in homogenized footpads and in draining LNs (B) at week 8 after infection (n = 11 mice per group). (C–E) Total LN cells were restimulated with anti-CD3 or SLA for 72 h, and cell supernatants were analyzed for the production of IL-4 (C), IL-13 (D), and IFN-γ (E) by ELISA. (F and G) Antigen-specific IgG1 (F) and IgG2a (G) antibody isotypes were quantified from infected sera by ELISA. A representative of two individual experiments is shown with mean values ± SEM. Statistical analysis was performed defining differences to WT BALB/c control mice as significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
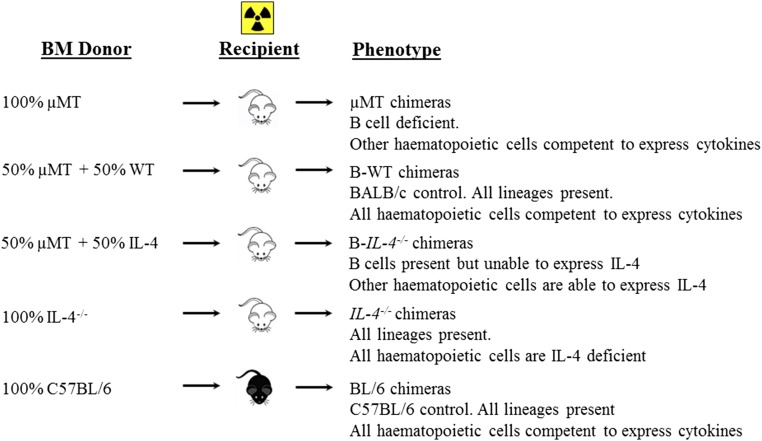
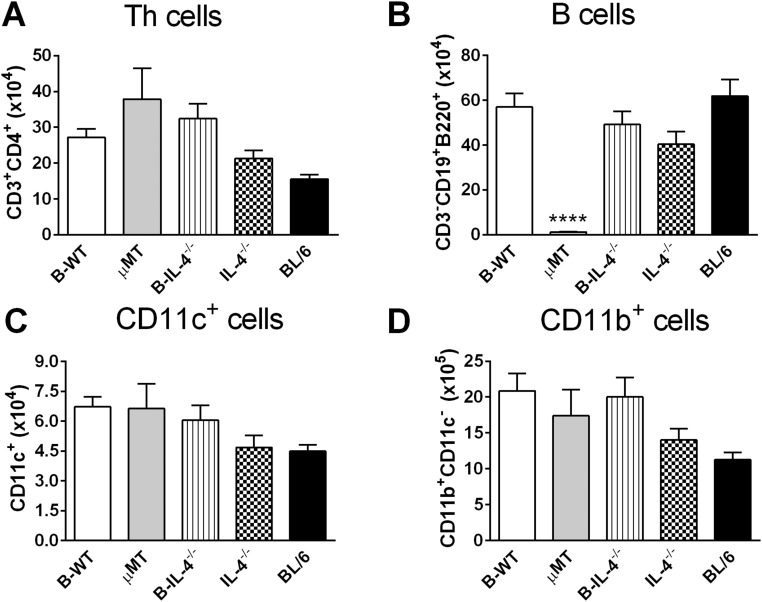
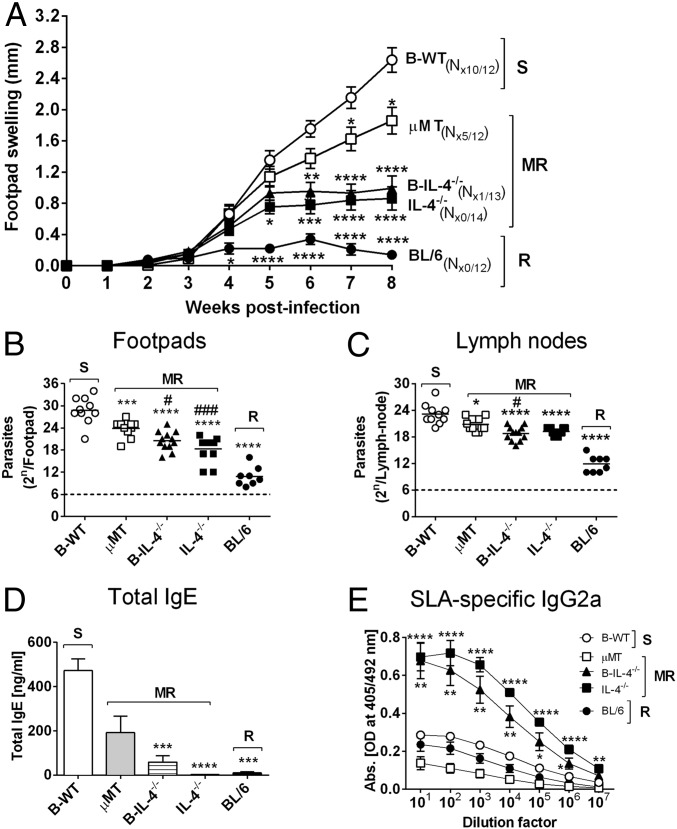
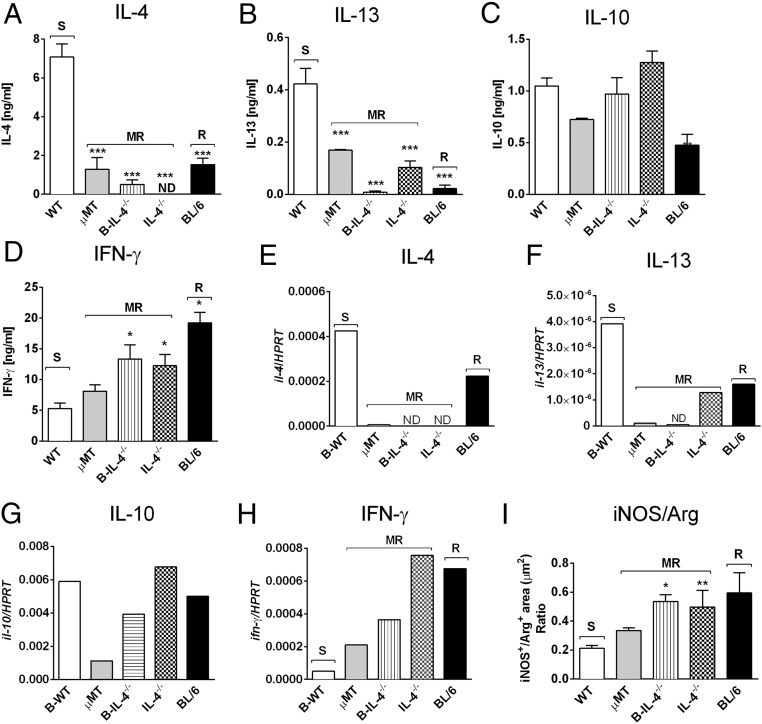
We further strengthened this conclusion by generating mixed bone-marrow (BM) chimeras as reported (2) using irradiated recipient µMT BALB/c mice with either 1:1 µMT BM and BALB/c BM (B-WT chimeras) or µMT BM and IL-4−/− BM (B-IL-4−/− chimeras). As controls, 100% BL/6 and 100% IL-4−/− chimeras were created (Fig. S8). At 8 wk postinjection, we confirmed successful reconstitution in all chimeric groups, demonstrated by similar cell number and cell-type distribution (Fig. S9). Importantly, apart from µMT animals, all chimeric groups maintained equivalent repopulation of CD19+B220+ B cells (Fig. S9B). Mice were infected with 2 × 106 promastigotes of L. major LV39 strain for 8 wk. As anticipated, control 100% BL/6 chimeras healed and 100% IL-4−/− chimeras contained acute leishmaniasis with low footpad swelling, the absence of acute cutaneous leishmaniasis, demonstrated by the absence of necrosis and ulceration, as well as lowered footpad (1.6-fold) and LN burden (Fig. 4 A–C). As expected, this was due to a beneficial dominant type 1 immune response (Figs. 4 D and E and 5 A–F), as previously shown by us in IL-4–deficient BALB/c mice (18). In stark contrast, µMT B-WT chimeras developed typical acute cutaneous leishmaniasis followed by µMT chimeras (Fig. 4 A–C) due to detrimental type 2 responses (Figs. 4 D and E and 5 A–F). Confirming the adoptive transfer results (Fig. 3 H–J), µMT mice reconstituted with IL-4–impaired B cells (B-IL-4−/− chimeras) efficiently controlled cutaneous leishmaniasis (only 1 of 14 mice developed ulceration on lesions), with reduced pathogen burden in the footpad (1.5-fold) followed by the dLN (1.2-fold) compared with B-WT animals (Fig. 4 A–C). We observed that parasite replication in the footpad of B-IL-4−/− and IL-4−/− chimeras was controlled to a greater degree than in the LN compared with µMT B-WT chimeras. The footpad has a higher frequency of iNOS-secreting macrophages and DCs to host parasites than the LN and is capable of killing intracellular parasites (25, 27), which might account for a slower but effective control in the LN. This was further accompanied by strikingly reduced detrimental type 2 antibody (Fig. 4 D and E) and Th2 immune responses (Fig. 5 A–C) and up-regulated IFN-γ responses (Fig. 5D), leading to efficient NO killing effector responses (Fig. 5I and Fig. S3B). The absence of IL-4 on B cells and IL-4 globally in B-IL-4−/− and 100% IL-4−/− chimeras was further confirmed by quantitative RT-PCR on B cells FACS-sorted from the dLN of chimeric mice at 8 wk after infection (Fig. 5E). B cell-derived IL-13 was also reduced in the absence of IL-4 on B cells and IL-4 globally (Fig. 5F). Surprisingly, levels of B cell-derived IL-10 remained unaltered (Fig. 5G), while B cell-derived IFN-γ (Fig. 5H) marginally increased. Together, these data demonstrate that early IL-4Rα–responsive B cells producing IL-4 influence early Th cell polarization toward detrimental Th2 responses, which leads to L. major-induced cutaneous leishmaniasis.
Fig. S8.
Generation of mixed BM chimeras to assess the in vivo role of IL-4–producing B cells during cutaneous leishmaniasis. Recipient B cell-deficient mice (μMT) were lethally irradiated with a total of 1,000 rad (500 rad, two doses, 3 h apart). Twenty-four hours later, irradiated mice were reconstituted with 1 × 107 total BM cells per recipient mouse to generate the following chimeras: 50% µMT BM and 50% B-WT, 50% µMT BM, and 50% IL-4−/− BM (B-IL-4−/−). As controls, μMT recipient mice were reconstituted with 100% µMT BM (µMT), 100% IL-4−/− BM (IL-4−/−), while C57BL/6 recipient mice were reconstituted with 100% C57BL/6 BM (BL/6). Chimeric mice were allowed to reconstitute for 8 wk before repopulation was analyzed.
Fig. S9.
Successful reconstitution of chimeric mice with donor BM cells following irradiation. Irradiated µMT mice were reconstituted with 50% µMT BM and 50% WT BM (B-WT chimeras), 50% µMT BM and 50% IL-4−/− BM (B-IL-4−/−), and 50% µMT BM. As controls, µMT mice were reconstituted with 100% µMT BM (µMT), 100% IL-4−/− BM (IL-4−/−), and C57BL/6 recipient mice with 100% C57BL/6 BM (BL/6). At 8 wk postreconstitution, mouse blood was collected and leukocytes FACS-stained for total numbers of CD3+CD4+ Th cells (A), CD19+B220+ B cells (B), CD4−CD11chi dendritic-like cells (C), and CD11bhiCD11c−CD3− macrophage-like cells (D). Data are representative of two independent experiments (n = 6/7 mice/group). Statistical analysis was performed defining differences to B-WT mice as significant (****P ≤ 0.0001).
Fig. 4.
IL-4–producing B cells influence susceptibility to L. major infection in BALB/c mice. (A) Respective chimeric mice (Fig. S8) were infected with 2 × 106 L. major LV39 (MRHO/SV/59/P) promastigote parasites into the hind footpad, and footpad swelling was measured weekly. (B and C) Parasite burden was determined by limiting dilution of homogenized footpads (B) and draining LNs (C) at 8 wk after infection. (D and E) Total IgE (D) and SLA-specific IgG2a (E) antibody isotype production was measured from infected sera by ELISA. Data represent a pool of two independent experiments (n = 12–14 mice per group). Statistical analysis was performed defining differences to B-WT mice (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001) and µMT as significant (#P ≤ 0.05, ###P ≤ 0.001). N#/12–14, where # represents number of mice in a group of 12–14 showing necrosis/ulceration. MR, moderately resistant; R, resistant; S, susceptible.
Fig. 5.
IL-4–producing B cells modulate type 2 immune responses and killing effector functions during L. major-induced cutaneous leishmaniasis. (A–D) At week 8 after L. major LV39 infection, draining LN CD4+ T cells were restimulated for 72 h with fixed antigen presenting cells and SLA. The production of IL-4 (A), IL-13 (B), IL-10 (C), and IFN-γ (D) was determined in culture supernatants by ELISA. Data represent one of two independent experiments with n = 6/7 mice per group. (E–H) mRNA expression of il-4 (E), il-13 (F), il-10 (G), and ifn-γ (H) transcripts in CD19+B220+ B cells sorted from 8 wk-infected LNs of chimeric mice by quantitative real-time RT-PCR. Expression was normalized against the housekeeping gene HPRT. Data represent one of two independent experiments of pooled LN cells per group. (I) Quantification of iNOS and arginase 1-positive areas in multiple sections of formalin-fixed footpads by immunohistochemical staining. Results are expressed as a ratio of iNOS+/Arg-1+, serving as a proxy for classical versus alternative activation. Data represent a pool of two independent experiments with n = 16 sections per group. Statistical analysis was performed defining differences to B-WT mice as significant (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001). MR, moderately resistant; R, resistant; S, susceptible.
S. mansoni Eggs Induce Early IL-4 in B Cells.
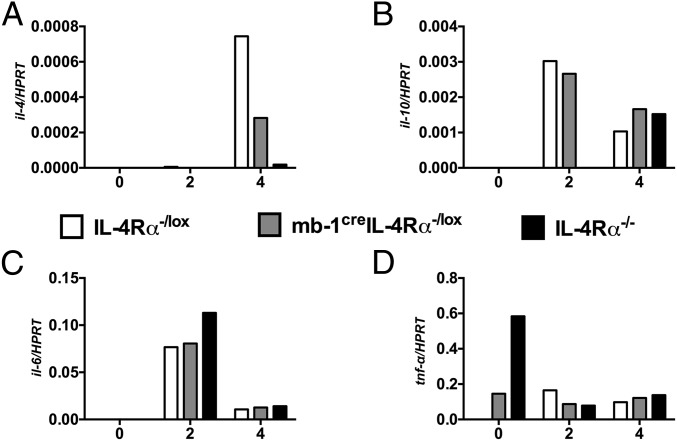
The question remained if B cell regulation of T cell dichotomy is rather an exception of this specific disease or a more general mechanism. To directly test this in a time-controlled manner, we injected 2,500 S. mansoni eggs into the hind footpad of animals to elicit a robust and swift type 2 immune response, as previously described (30, 31). Subsequently, we sorted for B220+CD19+CD3− B cells by flow cytometry at day 0, 2, and 4 post-egg challenge, extracted mRNA, and quantified cytokine transcripts by qRT-PCR. Similar to the findings obtained from L. major-infected B cells (Fig. 3G), IL-4Rα–responsive B cells induced higher il-4 transcripts at day 4 post-egg challenge than B cells impaired of IL-4Rα signaling (mb1creIL-4Rα–/lox and IL-4Rα−/− mice) (Fig. 6A). We detected transcripts for il-10, il-6, and tnf-α, although they were not differentially expressed between the different strains (Fig. 6 B–D). Interestingly, we found that tnf-α transcripts were highly expressed by B cells from IL-4Rα−/− mice at day 0 but were significantly reduced after egg challenge similarly to IL-4Rα–/lox littermate control mice and mb1creIL-4Rα–/lox mice (Fig. 6D). Conclusively, the absence of IL-4Rα–responsive B cells reduces early IL-4 transcripts following exposure to S. mansoni eggs.
Fig. 6.
Early cytokine responses from S. mansoni egg-challenged mice. (A–D) IL-4Rα–/lox, mb1creIL-4Rα–/lox, and IL-4Rα−/− mice were challenged with 2,500 S. mansoni eggs in the left hind footpad and killed at day 2 and 4 postchallenge. Single-cell suspension was prepared from popliteal lymph node (pLN), and B220+CD19+ B cells were sorted to ∼99% purity using the FACS Aria. RNA was extracted using the Qiagen kit and cDNA synthesized using the cDNA synthesis kit. The levels of il-4 (A), il-10 (B), il-6 (C), and tnf-α (D) mRNA transcripts were determined by real-time PCR. Expression was normalized against the housekeeping gene HPRT Data represent two independent experiments.
B Cell-Specific IL-4Rα–Deficient Mice Are Susceptible to Acute Schistosomiasis with Diminished Type 2 Immune Responses.
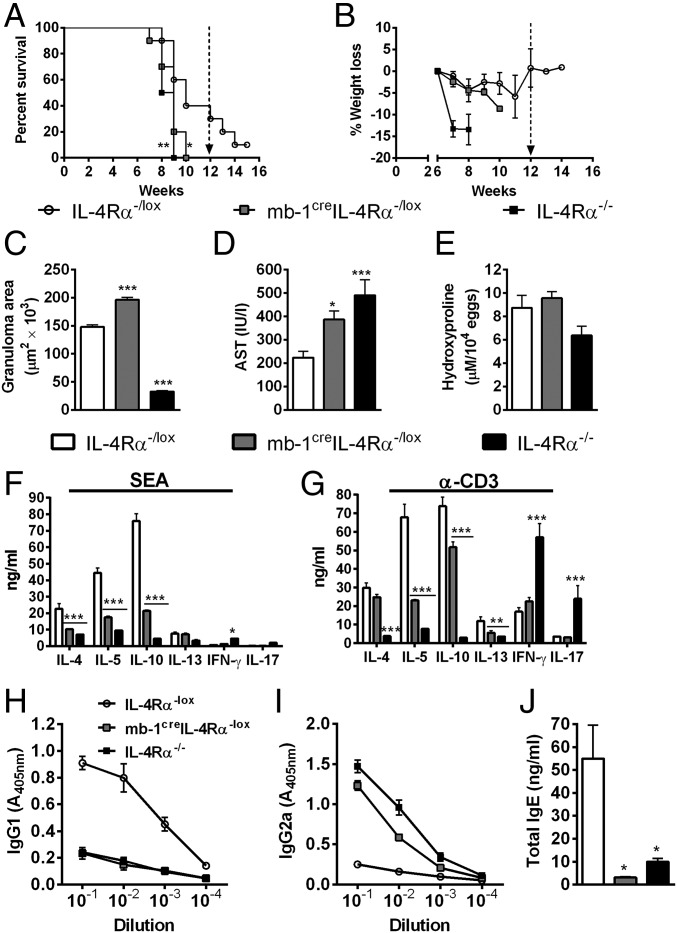
As we demonstrated above, regulation of T cell polarization by B cells appears to be a more general mechanism. Hence, we hypothesized that infection of mb1creIL-4Rα–/lox mice with S. mansoni may be detrimental to the host, contrary to our findings in L. major infection studies. Indeed, we found that mb1creIL-4Rα–/lox mice infected with S. mansoni quickly succumbed to infection similarly to the highly susceptible IL-4Rα−/− mice compared with infected IL-4Rα–/lox littermate control mice that died significantly later (Fig. 7A) without weight loss (Fig. 7B). S. mansoni-infected mb1creIL-4Rα–/lox mice presented with exacerbated liver pathology indicated by augmented granuloma formation (Fig. 7C) and increased hepatocellular damage shown by increased serum aspartate transaminase concentration (Fig. 7D) in comparison with IL-4Rα–/lox littermate control mice. However, the absence of IL-4Rα–responsive B cells in S. mansoni-infected mb1creIL-4Rα–/lox mice did not alter egg-induced fibrosis, measured as a hydroxyproline concentration in the tissue (Fig. 7E) compared with IL-4Rα–/lox littermate control mice. More importantly, S. mansoni-infected mb1creIL-4Rα–/lox mice showed strikingly reduced production of Th2 cytokines (IL-4, IL-5, and IL-10) by mesenteric LN cells stimulated with either S. mansoni soluble eggs antigen (SEA) or α-CD3 compared with IL-4Rα–/lox littermate control mice (Fig. 7 F and G). This was further confirmed by reduced type 2 antibody titres (IgG1 and total IgE) but increased type 1 antibody titres (IgG2a) compared with IL-4Rα–/lox littermate control mice (Fig. 7 H and I). Together, these results suggest that early IL-4Rα–responsive and IL-4–producing B cells are crucial for driving an efficient Th2 and Type 2 immune response necessary to confer protection against acute schistosomiasis.
Fig. 7.
IL-4Rα–responsive B cells are required for protection against acute schistosomiasis in mice. IL-4Rα–/lox, mb1creIL-4Rα–/lox, and IL-4Rα−/− mice were infected with 100 live S. mansoni cercariae, and survival was monitored over a 14-wk period. (A) Survival kinetics of mice infected percutaneously with 100 cercariae. (B) Percent body weight loss monitored on a weekly basis. Data represent two independent experiments (n = 8–10). Survival curves were compared using log-rank test. *P < 0.05 and **P < 0.01 vs. IL-4Rα–/lox mice. IL-4Rα–/lox, mb1creIL-4Rα–/lox, and IL-4Rα−/− mice were infected with 100 S. mansoni cercariae and analyzed 7 wk postinfection. (C) Granuloma area surrounding eggs quantified by microscopic analysis on H&E-stained sections. Twenty to 30 granulomas per mouse were included in the measurement of granuloma area. (D) Hepatocellular damage quantified as serum aspartate transaminase (AST) levels. (E) Liver fibrosis measured as hydroxyproline normalized to egg numbers. (F and G) Cytokine production by mesenteric LN cells restimulated with either SEA or α-CD3. (H–J) Antigen-specific Ig (IgG) and total IgE titers were detected by ELISA. Data are representative of three independent experiments. n = 4–6 mice. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. IL-4Rα–/lox mice using one-way ANOVA with Bonferroni’s posttest.
Discussion
The introduction of gene-deficient mouse models for IL-4Rα and its ligands, IL-4 and IL-13, challenged the basic premise that the IL-4/IL-13/IL-4Rα axis acted in isolation to regulate host susceptibility to the type 1 disease cutaneous leishmaniasis, caused by L. major (20), or host protection to the type 2 disease schistosomiasis, caused by S. mansoni (32). This led to paradigm shifts in the literature and suggested that both IL-4/IL-13–dependent and IL-4/IL-13–independent factors orchestrate disease outcome. Leading on from these investigations and others, crucial roles for alternate cytokines, Th cell lineages, and immune cell populations were revealed. In this study, we report on the contribution of IL-4–responsive B cell responses to acute cutaneous leishmaniasis and schistosomiasis in mice.
B lymphocytes are believed to play only a limited role in Th2-mediated susceptibility to Leishmania infection (14, 33, 34) and resistance to S. mansoni infection (15, 16), as the absence of B cells in B cell-deficient mice do not critically alter host ability to control either infection. This is not surprising given that BALB/c mice have a default Th2 pathway that is still activated upon Leishmania infection even in the absence of B cells (35). However, this conclusion changed substantially when we disrupted IL-4Rα expression on B cells in mb1creIL-4Rα–/lox BALB/c mice in experimental cutaneous leishmaniasis and acute schistosomiasis. As a result, nonhealer BALB/c mice with predominant and detrimental type 2 responses during L. major infection transformed to a healer strain with beneficial type 1 responses due to the absence of IL-4Rα–responsive B cells, bearing a similar phenotype to CD4+ T cell-specific IL-4Rα–deficient BALB/c mice (21). Of importance, the observed T cell shift was not limited to type 1 diseases, as subsequent infection of B cell-specific IL-4 receptor alpha (IL-4Rα)–deficient mice with S. mansoni resulted in diminished type 2 responses, which was detrimental to the host with early mortality and pathology, as seen before in S. mansoni-infected pan-T cell IL-4Rα–deficient BALB/c mice (22). Reconstitution studies in µMT B cell-deficient animals demonstrated that nonhealing disease to L. major could be controlled by the removal of IL-4Rα+ B cells. Moreover, removal of IL-4+ and IL-4Rα+ B cells together (IL-4/IL-4Rα−/− mice) had an added beneficial effect, highlighting that IL-4+ B cells play an essential exacerbatory role in cutaneous leishmaniasis. Indeed, chimeric mouse models confirmed that the selective absence of IL-4–responsive B cells is a key trigger for the generation of host-protective humoral and cellular immune responses to L. major. Thus, our study provides evidence that B cell IL-4 and IL-4Rα is critical in the nonhealing response to L. major.
How might IL-4Rα signaling on B cells and its ligand lead to activation of B cells and release of IL-4 to influence Th dichotomy? Functionally, we demonstrated that the loss of IL-4Rα+ B cells in both diseases altered the quality and quantity of cytokines secreted by effector B cells as early as day 1 after infection (Figs. 3 and 6), which in turn apparently influenced dendritic cells and early Th cells. This suggests that the early B cell-derived IL-4 could act on the IL-4Rα chain and activate the B cell population to sustain IL-4 production, thereby creating an autocrine loop, while also signaling IL-4Rα–responsive DCs and T cells in a paracrine manner, which together either exacerbate or control disease depending on the disease model. Another source of early IL-4 after L. major infection is attributed to the CD4+ T cells expressing the Vβ4Vα8 T cell receptor that recognizes the Leishmania antigen LACK (Leishmania homolog of receptors for activated C kinase) (36). Importantly, progressive infection in susceptible strains is attributed to sustained IL-4 production and an inability to redirect this early Th2 response toward a protective Th1 response (18, 20, 25). Collectively, B cells could become activated by directly responding to antigen, releasing this early IL-4, and in an autocrine loop, enhancing the response or signaling the IL-4 released by the Vβ4Vα8 T cells or other innate cells, such as mast cells, neutrophils, and eosinophils, in the same manner.
This is supported by the fact that B cells with their repertoire of natural antibodies are possibly the first cells of adaptive immunity to recognize and acquire foreign antigen through their BCR, independent of MHCII support. Besides BCR signaling, recent reports indicate that B cells can respond to antigen and become “innately” activated via alternative mechanisms such as activation of endosomal Toll-like receptors or direct peptide loading, which has been shown to lead to early B cell cytokine production, again mimicking an autocrine loop (37–40). Furthermore, B cells are competent APCs (4), which would allow the B cells to present the acquired antigen and, together with the cytokine it secretes, facilitate the aforementioned paracrine activation of neighboring immune cells, as previously demonstrated during the early stages of bacterial and viral infection (41). In contrast, naïve CD4+ T cells are “blind” and completely dependent on MHCII support to recognize foreign antigens presented on APCs through their T cell receptor. Differentiation into Th1 or Th2 cells depends on the cytokines secreted in their vicinity, which then regulates and modulates the disease outcome. Indeed, previous studies have confirmed that early blocking of IFN-γ or IL-4 production shifts Th cell polarization, altering the disease outcome (28, 42), strengthened by the selective recruitment of either IL-4Rα or the IFN-γ receptor to the immunological synapse in the absence of Th1- and Th2-inducing signals, respectively (43). Thus, naïve T cell precursors may be able to take advantage of cytokine-producing B cells as differentiation signals (44), strengthening the possibility that B cells can control and enhance inflammation and Th cell differentiation, not just as bystander cells but as direct participants.
A hallmark in vitro study by Harris et al. (8) first documented the presence of distinct cytokine-secreting B cell subsets. B cells that secreted higher amounts of IFN-γ and IL-12 were termed B effector 1 cells (Be1 cells), while B cells that produced more IL-4, IL-13, IL-10, and IL-2 were named Be2 cells. To date, these subsets of cytokine-producing B cells have been implicated in various mouse models of parasitic and bacterial infection (2, 7, 8, 45, 46), in models of ulcerative colitis (10) and autoimmunity (11, 47), and in human infectious and autoimmune diseases (45, 48, 49). However, with the exception of IL-10 and, recently, type I IFNs (40), the functional role of B cell-derived cytokines in human leishmaniasis and schistosomiasis have until now not been investigated. The distinct cytokine profiles of IL-4Rα–responsive and IL-4Rα–unresponsive B cells reported here corroborate CD4+ Th1 and Th2 cells, respectively. Indeed, the data from our adoptive B cell transfer experiments highlight that a clear phenotype in B cells is solely capable of altering the outcome of cutaneous leishmaniasis in B cell-deficient animals. Our study therefore supports the idea that the repertoire of early cytokine-producing B cells can regulate CD4+ Th cell dichotomy and together initiate and maintain the immune response elicited in type 1/type 2-controlled diseases.
Of note, the immune response mediated by IL-4–producing B cells tends to be pathogen-specific. While we show a relevance for IL-4–producing B cells in immunity to S. mansoni and susceptibility to L. major, it was previously shown to be dispensable for immunity to H. polygyrus (2) and Nippostrongylus brasiliensis (50). Indeed, the role of IL-4 itself in cutaneous leishmaniasis has been contentious, being shown to be either important for susceptibility (18, 19) or dispensable for disease progression (51). These discrepancies have been attributed to strain differences in the source of parasites, parasite dose, and the embryonic stem cells used to generate IL-4 knockout mice. Moreover, apart from a Th2-promoting role, IL-4 has also been reported to instruct DCs to secrete IL-12 for protective Th1 responses to L. major LV39 (25, 52) and shown to instruct protective immunity and successful chemotherapy to L. donovani infection (53). Altogether, these reports and the work presented here highlight that IL-4 plays a dynamic, multifaceted role in the spectrum of human leishmaniasis, and these differing roles must be considered in targeting this cytokine in a treatment or vaccine.
Importantly, previous work by us reported that IL-4−/− BALB/c mice are resistant to L. major due to a suppression of the nonhealing IL-4–driven Th2 response and induction of the protective IFN-γ response (18). Our present study expands on these findings, suggesting that resistance to L. major in IL-4−/− BALB/c mice may not be solely due to the absence of IL-4–producing T cells but also influenced by the absence of early and late IL-4–producing B cells. A separate study has reported that IL-10–producing B cells influence susceptibility to L. major (54). In agreement, L. major susceptibility in BALB/c mice was associated with increased B cell IL-10 and IL-4 transcription, whereas control of infection in mb1creIL-4Rα–/lox mice correlated with reduced B cell IL-10 mRNA transcripts. This suggested reciprocal roles for IL-4/IL-10 in the nonhealing response to L. major. However, this proved fallacious, as we found no change in IL-10 secretion following removal of IL-4+ B cells. Interestingly, the original paper on Be1 and Be2 cells reported similar IL-10 levels between these two populations (8). Following on, Wojciechowski et al. (2) also found no role for B cell IL-10 in H. polygyrus infection, and a recent study (55) suggests that T cell IL-10 plays a more crucial role in the nonhealing response to L. major and, in lieu of our data, acts independently of B cell IL-4.
In summary, we report that IL-4Rα signaling on B cells might play an essential role in the nonhealing response to L. major, similar to Th2 cells (21). We therefore conclude that early B cells modulate pathologic and protective immune responses during type 1- and type 2-controlled diseases by differentially regulating early cytokine production, which in turn influences the effector functions of other cell types, mainly CD4+ Th cells. Thus, vaccine and therapeutic development should aim to target both B and T cell immunity for optimal efficacy. Early B cell-specific IL-4Rα blocking may be a possible therapy for type 1 diseases, as recently demonstrated in IL-4–driven allergy (56) and others (57, 58), and reciprocally, early IFN-γ/IFN-γ receptor and IL-12 blocking may be beneficial for type 2 diseases. However, further research is required to determine if such a therapy might be applicable for all cutaneous Leishmania given the strain and model specificity reported for IL-4 in this disease.
Materials and Methods
Generation and Genotyping of mb1creIL-4Rα–/lox BALB/c Mice.
Generation, characterization, and genotyping of mb1creIL-4Rα–/lox BALB/c mice are previously described (17). All mice were housed in specific pathogen-free barriers at the University of Cape Town animal facility. All mouse experiments were approved by the Animal Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town and were conducted in accordance with the recommendations of the South African national guidelines and University of Cape Town practice of laboratory animals.
L. major Infection.
L. major LV39 (MRHO/SV/59/P) and L. major IL81 (MHOM/IL/81/FEBNI) strains were maintained by continuous passage in BALB/c mice and prepared for infection as previously described (19). Anesthetized mice were inoculated s.c. into the left hind footpad with 2 × 106 (LV39) or 2 × 105 (IL81) stationary phase metacyclic promastigotes. Footpad swelling was measured weekly using a Mitutoyo caliper (Brütsch).
Detection of Viable Parasite Burden in L. major-Infected Mice.
Detection of viable parasite burden from infected footpad and draining LNs was estimated by twofold limiting dilution assay in Schneider’s culture medium (Sigma) as previously described (19).
Live S. mansoni Infection of Mice.
Mice were percutaneously infected with 100 live cercariae (acute infection) of a Puerto Rican strain of S. mansoni obtained from infected Biomphalaria glabrata (a gift from Adrian Mountford, University of York, York, UK). The mice were weighed weekly.
Footpad S. mansoni Egg Model.
S. mansoni egg challenge was conducted as previously described (30, 31). Briefly, 2,500 S. mansoni eggs were injected s.c. into the hind footpad, and mice were killed at day 7 postchallenge.
Antibodies and Flow Cytometry.
The following antibodies comprising the B cell antibody panel were used: B220, CD19, CD124 (IL-4Rα), CD23, CD21, CD24, and MHCII (BD Bioscience). The T cells panel consisted of the following antibodies: CD4, CD3, CD62L, and CD44 (BD Bioscience). For intracellular cytokine staining, single-cell suspensions were stimulated at 37 °C with 50 ng/mL phorbal myristate acetate (PMA), 250 ng/mL ionomycin, and 200 µM monensin in IMDM/10% FCS (all Sigma-Aldrich). Cells were stained with extracellular markers, fixed in 2% (wt/vol) paraformaldehyde, permeabilized with 0.5% saponin buffer, and stained with PE-labeled anti-mouse antibodies and isotype controls (BD Biosciences). iNOS production was analyzed in CD11chiMHCIIhiCD11b+Ly6chi inflammatory DCs by staining for expression of intracellular iNOS using rabbit anti-mouse iNOS antibody (Abcam) followed by goat anti-rabbit PE (Abcam). Cells were acquired on a FACS Fortessa (BD Immunocytometry), and data were analyzed using Flowjo software (Treestar).
Ex Vivo Restimulation.
Single-cell suspensions were stimulated with plate-bound α-CD3 (20 µg/mL) or with 50 µg/mL SLA or 20 µg/mL soluble SEA. Supernatants were collected after 72 h, and cytokines were measured by sandwich ELISA as previously described (19). Antigen-specific restimulation of CD4+ T cells from infected mice was performed as previously reported (25).
Cell Sorting.
Total LN cells from L. major- and S. mansoni-infected animals were labeled with specific mAbs for B cells (CD19+B220+CD3−) and isolated by cell sorting on a FACS Vantage machine. Purity was determined by flow cytometry and was at least >98%.
Quantitative RT-PCR.
Total RNA was extracted from sorted B cells using Tri reagent (Applied Biosystems) and minielute columns (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized with Transcriptor First Strand cDNA synthesis kit (Roche), and real-time PCR was performed by using Lightcycler FastStart DNA Master PLUS SYBR Green I reaction mix (Roche) on a Lightcycler 480 II (Roche). See Table S1 for primers used.
Table S1.
Primers used in qRT-PCR
| Gene | Forward primer, 5′–3′ | Reverse primer, 5′–3′ |
| ifn-γ | GCTCTGAGACAATGAACGCT | AAAGAGATAATCTGGCTCTGC |
| il-4 | TCGGCATTTTGAACGAGGTC | GAAAAGCCCGAAAGAGTCTC |
| il-10 | AGCCGGGAAGACAATAACTG | CATTTCCGATAAGGCTTGG |
| il-6 | GTTCTCTGGGAAATCGTGGA | TGTACTCCAGGTAGCTATGG |
| tnf-α | TCTCATCAGTTCTATGGCCC | GGGAGTAGACAAGGTACAAC |
| HPRT | GGCCATGAGGCTGGATCTC | GGCCATGAGGCTGGATCTC |
Enzyme-Linked Immunosorbent Assays (ELISAs).
Cytokines in cell supernatants were measured by sandwich ELISA as previously described (19). For antibody ELISAs, titres of antigen-specific IgG1, IgG2a, IgG2b, and total IgE were determined as described (19).
Hydroxyproline Assay.
Hydroxyproline content as a measure of collagen production was determined using a modified protocol (59). Briefly, weighed liver samples were hydrolyzed and added to a 40-mg Dowex/Norit mixture. The supernatants were neutralized with 1% phenolphthalein and titrated against 10 M NaOH. An aliquot was mixed with isopropanol and added to chloramine-T/citrate buffer solution (pH 6.5). Erlich’s reagent was added, and absorbance was read at 570 nm. Hydroxyproline levels were calculated using 4-hydroxy-l-proline (Calbiochem) as a standard, and results were expressed as µmoles of hydroxyproline per weight of tissue that contained 104 eggs.
Histology.
Liver, gut, footpad, and LN samples were fixed in 4% (vol/vol) formaldehyde, embedded in wax, and processed. S. mansoni-infected liver and gut sections (5–7 µm) were stained with hematoxylin and eosin (H&E) and aniline blue solution (CAB) and counterstained with Wegert’s hematoxylin for collagen staining. Micrographs of liver granuloma were captured using a Nikon 5.0 mega pixel color digital camera (DCT DS-SMc). The diameter of each granuloma containing a single egg was measured with the ImageJ 1.34 software. An average of 25 granulomas per mouse was included in the analyses. Formalin-fixed footpads of L. major-infected mice were cut into 3 μm-thick sections and stained with rabbit anti-mouse iNOS (Abcam) or goat anti-mouse arginase (Santa Cruz Biotechnology) followed by HRP-labeled anti-rabbit iNOS (Dako) or HRP-labeled anti-mouse arginase. 3,3′-diaminobenzidine substrate (DAB) was used for chromogenic detection of iNOS and arginase deposits. Complete footpad sections were scanned at 2× objective lens on a Nikon Eclipse 90i microscope (Nikon Instruments Inc.) and quantification performed by measuring iNOS+- or Arg+-positive staining areas in the full scan using object count function of the Nikon-NIS Elements AR v.4.0 advanced research imaging software from at least n = 16 sections (4 mice per group).
NO Production ex Vivo by Total Footpad Cells.
Footpad cells collected at week 6 after infection (25) were restimulated with LPS (10 ng/mL; Sigma-Aldrich) for 48 h. Following stimulation, supernatants were collected for quantification of NO (60).
Adoptive Transfer of L. major-Primed B Cells into B Cell-Deficient µMT Mice.
B cells were purified from LN cells of 8-wk infected BALB/c, IL-4Rα−/−, or IL-4−/−/IL-4Rα−/− mice using MACS beads (Miltenyi Biotec), according to the manufacturer’s instructions. The purity of B cells was >99% as determined by flow cytometry analysis using anti-CD19 and anti-CD3 antibodies. BALB/c µMT mice were reconstituted with 107 purified B cells i.v. Following 3 d for reconstitution, repopulation of B cells was confirmed in mouse blood leukocytes by flow cytometry followed by infection with 2 × 106 L. major LV39 promastigotes into the hind footpad.
Generation of Mixed-BM Chimeric Mice.
Recipient B cell-deficient (μMT) BALB/c mice were lethally irradiated with a 60Cs source with a total of 1,000 rad (500 rad, two doses, 3 h apart) and reconstituted with 107 total BM cells 24 h later. See Fig. S8 for a description of the generation of mixed BM chimeras used. Mixed BM chimeric mice were allowed to reconstitute for 8 wk before infection. Before infection, chimeric mice were checked for equivalent reconstitution of immune cell populations in mouse blood by flow cytometry (Fig. S9 for reconstitution profiles).
Statistics.
Data were analyzed using the unpaired Student’s t test or two-way ANOVA with Bonferroni’s posttest via GraphPad Prism 4 software (www.prism-software.com). Values of P ≤ 0.05 were considered significant.
Acknowledgments
We thank the genotyping staff, the animal facility staff, Rodney Lucas, as well as Jennifer Claire Hoving, Lizette Fick, and Ronnie Dreyer for excellent technical assistance. This work was supported by SAMRC (Unit on Immunology of Infectious Diseases), the National Research Foundation (NRF), and the South African Research Chair Initiative (SARCHi) (F.B.); postdoctoral fellowships from the NRF and Claude Leon Foundation (CLF) (R.H.); postdoctoral fellowships from the Clinical Infectious Diseases Research Initiative (CIDRI) under Wellcome Trust Grant 084323 (to H.H.N. and S.P.P.); and an NRF Doctoral Scholarship (M.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708125114/-/DCSupplemental.
References
- 1.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wojciechowski W, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Jensen PE. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp (Warsz) 2008;56:77–83. doi: 10.1007/s00005-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 4.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Lund FE, et al. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 6.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, et al. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris DP, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 9.Amu S, et al. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124.e8. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 11.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of IFN-gamma production by B effector 1 cells: Essential roles for T-bet and the IFN-gamma receptor. J Immunol. 2005;174:6781–6790. doi: 10.4049/jimmunol.174.11.6781. [DOI] [PubMed] [Google Scholar]
- 13.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: The development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol. 2005;175:7103–7107. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- 14.Ronet C, et al. Leishmania major-specific B cells are necessary for Th2 cell development and susceptibility to L. major LV39 in BALB/c mice. J Immunol. 2008;180:4825–4835. doi: 10.4049/jimmunol.180.7.4825. [DOI] [PubMed] [Google Scholar]
- 15.Ferru I, Roye O, Delacre M, Auriault C, Wolowczuk I. Infection of B-cell-deficient mice by the parasite Schistosoma mansoni: Demonstration of the participation of B cells in granuloma modulation. Scand J Immunol. 1998;48:233–240. doi: 10.1046/j.1365-3083.1998.00376.x. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic D, et al. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med. 1998;187:619–629. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoving JC, et al. B cells that produce immunoglobulin E mediate colitis in BALB/c mice. Gastroenterology. 2012;142:96–108. doi: 10.1053/j.gastro.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Kopf M, et al. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohrs M, et al. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- 20.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 21.Radwanska M, et al. Deletion of IL-4Ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 2007;3:e68. doi: 10.1371/journal.ppat.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewals B, et al. IL-4Ralpha responsiveness of non-CD4 T cells contributes to resistance in Schistosoma mansoni infection in pan-T cell-specific IL-4Ralpha-deficient mice. Am J Pathol. 2009;175:706–716. doi: 10.2353/ajpath.2009.090137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbert DR, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 24.Jankovic D, et al. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- 25.Hurdayal R, et al. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLoS Pathog. 2013;9:e1003699. doi: 10.1371/journal.ppat.1003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hölscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 27.De Trez C, et al. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathog. 2009;5:e1000494. doi: 10.1371/journal.ppat.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA. In susceptible mice, Leishmania major induce very rapid interleukin-4 production by CD4+ T cells which are NK1.1- Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 29.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–3471. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Dewals B, Hoving JC, Horsnell WG, Brombacher F. Control of Schistosoma mansoni egg-induced inflammation by IL-4-responsive CD4(+)CD25(-)CD103(+)Foxp3(-) cells is IL-10-dependent. Eur J Immunol. 2010;40:2837–2847. doi: 10.1002/eji.200940075. [DOI] [PubMed] [Google Scholar]
- 31.Wynn TA, et al. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 32.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 33.Kima PE, et al. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanasen N, Xin L, Soong L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int J Parasitol. 2008;38:417–429. doi: 10.1016/j.ijpara.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DR, Reiner SL. Polarized helper-T-cell responses against Leishmania major in the absence of B cells. Infect Immun. 1999;67:266–270. doi: 10.1128/iai.67.1.266-270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Launois P, Swihart KG, Milon G, Louis JA. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 37.Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jellison ER, Guay HM, Szomolanyi-Tsuda E, Welsh RM. Dynamics and magnitude of virus-induced polyclonal B cell activation mediated by BCR-independent presentation of viral antigen. Eur J Immunol. 2007;37:119–128. doi: 10.1002/eji.200636516. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 40.Silva-Barrios S, et al. Innate immune B cell activation by Leishmania donovani exacerbates disease and mediates hypergammaglobulinemia. Cell Rep. 2016;15:2427–2437. doi: 10.1016/j.celrep.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 41.Bao Y, et al. Identification of IFN-γ-producing innate B cells. Cell Res. 2014;24:161–176. doi: 10.1038/cr.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris L, Troutt AB, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–2721. [PubMed] [Google Scholar]
- 43.Maldonado RA, et al. Control of T helper cell differentiation through cytokine receptor inclusion in the immunological synapse. J Exp Med. 2009;206:877–892. doi: 10.1084/jem.20082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosmann T. Complexity or coherence? Cytokine secretion by B cells. Nat Immunol. 2000;1:465–466. doi: 10.1038/82707. [DOI] [PubMed] [Google Scholar]
- 45.Barr TA, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganapamo F, Dennis VA, Philipp MT. CD19(+) cells produce IFN-gamma in mice infected with Borrelia burgdorferi. Eur J Immunol. 2001;31:3460–3468. doi: 10.1002/1521-4141(200112)31:12<3460::aid-immu3460>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 47.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 48.Fillatreau S. Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis. 2013;72:ii80–ii84. doi: 10.1136/annrheumdis-2012-202253. [DOI] [PubMed] [Google Scholar]
- 49.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 50.Horsnell WG, et al. IL-4Rα-associated antigen processing by B cells promotes immunity in Nippostrongylus brasiliensis infection. PLoS Pathog. 2013;9:e1003662. doi: 10.1371/journal.ppat.1003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noben-Trauth N, Paul WE, Sacks DL. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol. 1999;162:6132–6140. [PubMed] [Google Scholar]
- 52.Biedermann T, et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2:1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 53.Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombacher F. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol. 2000;30:2935–2943. doi: 10.1002/1521-4141(200010)30:10<2935::AID-IMMU2935>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 54.Ronet C, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz T, et al. T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog. 2013;9:e1003476. doi: 10.1371/journal.ppat.1003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borish LC, et al. IL-4R Asthma Study Group Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107:963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 57.Bankaitis KV, Fingleton B. Targeting IL4/IL4R for the treatment of epithelial cancer metastasis. Clin Exp Metastasis. 2015;32:847–856. doi: 10.1007/s10585-015-9747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck LA, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 59.Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963;35:1961–1965. [Google Scholar]
- 60.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]