Significance
Hydroxyapatite (HA) nanocrystals are key constituents of the bone extracellular matrix and thus likely to influence the pathogenesis of breast cancer skeletal metastasis. However, there is currently an insufficient understanding of HA nanocrystal properties at sites prone to bone metastasis formation. Here we report a novel application of X-ray scattering and Raman imaging to characterize HA nanostructure in mouse models of breast cancer. Our results suggest that bone regions linked with the initiation of metastasis contain less-mature HA nanocrystals and that mammary tumors enhance HA nanocrystal immaturity in these regions even prior to secondary tumor formation. Insights from this work will significantly advance the development of mineralized culture models to investigate how the bone microenvironment regulates breast cancer metastasis.
Keywords: breast cancer, bone metastasis, bone mineral nanostructure, X-ray scattering, Raman imaging
Abstract
Skeletal metastases, the leading cause of death in advanced breast cancer patients, depend on tumor cell interactions with the mineralized bone extracellular matrix. Bone mineral is largely composed of hydroxyapatite (HA) nanocrystals with physicochemical properties that vary significantly by anatomical location, age, and pathology. However, it remains unclear whether bone regions typically targeted by metastatic breast cancer feature distinct HA materials properties. Here we combined high-resolution X-ray scattering analysis with large-area Raman imaging, backscattered electron microscopy, histopathology, and microcomputed tomography to characterize HA in mouse models of advanced breast cancer in relevant skeletal locations. The proximal tibial metaphysis served as a common metastatic site in our studies; we identified that in disease-free bones this skeletal region contained smaller and less-oriented HA nanocrystals relative to ones that constitute the diaphysis. We further observed that osteolytic bone metastasis led to a decrease in HA nanocrystal size and perfection in remnant metaphyseal trabecular bone. Interestingly, in a model of localized breast cancer, metaphyseal HA nanocrystals were also smaller and less perfect than in corresponding bone in disease-free controls. Collectively, these results suggest that skeletal sites prone to tumor cell dissemination contain less-mature HA (i.e., smaller, less-perfect, and less-oriented crystals) and that primary tumors can further increase HA immaturity even before secondary tumor formation, mimicking alterations present during tibial metastasis. Engineered tumor models recapitulating these spatiotemporal dynamics will permit assessing the functional relevance of the detected changes to the progression and treatment of breast cancer bone metastasis.
Eighty percent of advanced breast cancer patients develop metastases in bone (1). Following dissemination to the skeleton, metastatic breast cancer cells intimately interact with bone cells to facilitate seeding and expansion while disrupting homeostatic bone remodeling (1–3). The early-stage colonization of disseminated tumor cells appears to depend on active osteogenesis and adhesion to osteoblasts (4), whereas the eventual transition to macrometastasis involves a vicious cycle that promotes osteolytic activity through the aberrant activation of osteoclasts (1–3). While most studies have focused on identifying the cellular and molecular mechanisms underlying bone metastasis (1–4), very little is known about how breast cancer and bone metastasis alter the physical properties of the mineralized bone extracellular matrix (ECM).
The basic building block of the bone ECM is a nanocomposite of collagen fibrils and coaligned mineral crystals that underlie a unique hierarchical structure (5). Primarily composed of carbonated hydroxyapatite [Ca5(PO4,CO3)3(OH)] (HA), these mineral crystals are elongated platelets with thicknesses of ∼2–7 nm and with lengths on the order of 15–200 nm (5, 6). The physicochemical properties of HA (i.e., crystallinity, chemical composition, size, aspect ratio, and arrangement) dictate bone mechanical properties (5) and can vary as a function of disease, diet, age, and anatomical location (7–12). Notably, HA crystal size and orientation increase with bone tissue maturity (10–12). These variations, in turn, may modulate tumor progression. Studies with synthetically defined cell culture substrates have suggested that breast cancer cell adhesion, proliferation, and osteolytic factor expression are regulated by the materials properties of HA nanocrystals (13–15). However, the physiological relevance of these in vitro observations is unclear as it remains unresolved whether bone metastasis-relevant sites feature specific nanoscale HA materials properties and if these properties vary with cancer.
Previous in vivo studies of bone metastasis have used microcomputed tomography (µCT) or dual-energy X-ray absorptiometry to assess various bone indices such as bone mineral density, bone volume fraction, and trabecular number or thickness (16, 17). While these techniques have yielded important insights, they are not capable of resolving bone mineral properties at the nanometer length scale. We sought to address this gap in knowledge by using X-ray scattering, a nondestructive technique which can quantitatively assess the size (thickness via the T-parameter and length via the L-parameter) and arrangement (orientation via the ρ-parameter) of bone mineral nanocrystals (10, 18) (Fig. S1). To account for the spatial heterogeneity of bone mineral (5), X-ray scattering instruments can be programmed to scan larger micrometer-scale regions of interest. This technique has greatly contributed to the understanding of bone biomineralization, as it has been widely used to characterize HA nanocrystal structure across a range of organisms (e.g., humans, mice, rats, baboons, minipigs, and turkeys) (5) and has shown that even very small changes to these nanostructural features are linked to pathological conditions (19). Here, we have combined X-ray scattering with complementary imaging techniques that include large-area Raman imaging, backscattered electron microscopy (BSE), histology, and µCT. This multiscale approach allowed us to correlate HA nanostructural differences with bone tissue chemical composition and mineral crystallinity as well as cellularity and global changes in mineral distribution (Fig. 1). These studies provide the most-detailed-to-date assessment of bone hierarchical structure as it pertains to breast cancer and bone metastasis.
Fig. S1.
X-ray scattering analysis setup. (A and B) Bone specimens are exposed to highly collimated X-rays in a stepwise manner on the x and y plane perpendicular to the direction of the incident beam. The scattered light registers on a 2D detector and can then be used to derive information about mineral nanostructure. Laboratory-based SAXS analysis (A) provides information about mineral orientation (ρ) and thickness (T). Synchrotron-based SAXS/WAXS analysis (B) provides enhanced spatial resolution and, in addition to orientation and thickness, information about mineral nanocrystal length (L) derived from the width of the 002 peak.
Fig. 1.
Experimental setup for multiscale analysis of bone hierarchical structure. Different stages of human breast cancer were modeled in immunocompromised nude mice. To achieve bone metastasis, luciferase-labeled BoM1-2287 cells were intracardially injected, while injection into cleared mammary fat pads resulted in localized mammary tumors without overt metastasis. Mouse tibiae harvested after 5 or 7 wk were embedded in PMMA and subjected to μCT, histological analysis, and backscattered BSE to assess macro- to microscale changes in bone structure. SAXS and WAXS as well as large-area Raman imaging were used to characterize bone nanostructure and physicochemical composition.
Results
In Tibiae of Healthy Mice, HA Nanocrystals Are More Immature in Regions Prone to Metastasis.
In experimental mouse models of breast cancer bone metastasis, the tibia is a common site of secondary tumor formation (20, 21). Within the tibia, disseminated cancer cells appear to preferentially localize to the metaphysis rather than the diaphysis (20, 21), a phenomenon that has been attributed to specific cellular properties of metaphyseal blood vessels and an enrichment of chemoattractants (22). What is unclear, however, is whether the physical nature of bone mineral also varies between the metaphysis, which is largely comprised of trabecular bone, and the diaphysis, which is primarily dense cortical bone (23). To address this question, we applied laboratory-based small-angle X-ray scattering (SAXS) analysis (Fig. S1A) to establish a baseline of differences in HA nanocrystal thickness and orientation (Fig. 2A) between metaphyseal and diaphyseal bone in the tibiae of disease-free mice. We observed that mineral crystals in the metaphysis were significantly thinner (indicated by decreased T-parameter) and less oriented (indicated by decreased ρ-parameter) than those in the diaphysis (Fig. 2 B–D). In fact, crystals that were thinner were also less oriented (Fig. 2B), suggesting that the development of HA thickness and orientation occur in parallel. Our results are in strong agreement with previous studies that identified HA nanostructural heterogeneity in murine long bones (10, 24). The relative immaturity of metaphyseal HA crystals is consistent with their close proximity to the ossification center and the high turnover rate of metaphyseal trabecular bone (23). As breast cancer cells can adhere and proliferate better on smaller and less-perfect HA nanoparticles (13), the detected differences in metaphyseal versus diaphyseal HA nanostructure may be functionally relevant to the preferential metastatic tumor cell colonization of the proximal tibia.
Fig. 2.
Laboratory-based SAXS analysis of tibiae from healthy mice shows that HA nanocrystals in the metaphysis are more immature than ones in the diaphysis. (A) X-ray scattering techniques provide information on bone mineral nanostructure. (B) Correlation analysis between T-parameter and ρ-parameter as determined by simple linear regression. Data points represent individual measurements. The data are color-partitioned into two regions of interest (Diph, diaphysis; Mtph, metaphysis). (C and D) Spatial representation of quantitative data. Representative ρ-parameter (mean mineral crystal orientation) (C) and T-parameter (mean mineral crystal thickness) (D) data are overlaid on corresponding BSE images. Color scales: Warmer colors indicate greater crystal orientation (C) or thickness (D). Box-and-whisker plots of all ρ-parameter (C) and T-parameter (D) data from the regions of interest. Whiskers represent the 5th percentile and the 95th percentile. Outlier data points are depicted as dots. *P < 0.05, **P < 0.001.
Metastasis Initiation in the Proximal Tibial Metaphysis Alters the Bone ECM.
We next performed a multiscale characterization of tibial metastasis to identify tumor-mediated changes of bone ECM in these sites. To achieve bone metastasis, we injected luciferase-expressing BoM1-2287 breast cancer cells, a bone metastatic subpopulation of the MDA-MB-231 cell line (21), into the left ventricle of the mouse heart. As expected, this approach resulted in the reliable formation of macrometastasis in the proximal tibiae of immunocompromised BALB/c nude mice after 5 wk (21) (Fig. S2A). μCT scans (Fig. 3 A and C), Movat’s pentachrome staining (Fig. 3 B and D and Fig. S3), and BSE images (Fig. S4) revealed massive degradation of metaphyseal trabecular bone. Viewed at these length scales, macrometastatic outgrowths were largely contained in the metaphysis, did not occur in the epiphysis (Fig. S3), and left diaphyseal cortical bone unaffected (Fig. S4). Growth plate integrity was severely compromised in bone metastasis versus control samples, as suggested by the presence of fibrous tissue (25) rather than the organized arrays of chondrocyte columns (Fig. S3). These observations support the possibility that disseminated tumor cells preferentially entered the tibiae through sinusoidal vessels supplying the metaphyseal trabecular bone (22) and that the growth plate acted as a barrier preventing tumor cell advancement to the epiphysis.
Fig. S2.
Mouse models of advanced breast cancer. To determine localization via bioluminescence imaging, luciferase-expressing bone metastatic breast cancer cells (BoM1-2287) were used in both conditions. (A) Injection of tumor cells into the left ventricle of the heart consistently resulted in metastasis to the hind limbs and to the brain. The animal is shown in dorsal recumbency. Tibiae were harvested 5 wk postsurgery. (B) Injection of tumor cells into a pair of contralateral mammary fat pads resulted in localized tumors only. No macrometastatic colonies were observed using this method. The animal is shown in ventral recumbency. Tibiae were harvested 7 wk postsurgery. (C) Average body weights 1 d before bone harvest. Ctrl, control mice; Tumor, mammary fat pad-injected mice. In the Tumor group, the weight of the tumor was subtracted from the total weight. P = 0.41. (D) Average growth plate thicknesses in the proximal tibiae. P = 0.73. (C and D) Data are means ± SD (n = 4). Student’s t test was used to confirm statistical significance between groups. Statistical analysis was performed in Graphpad Prism 5 (GraphPad Software).
Fig. 3.
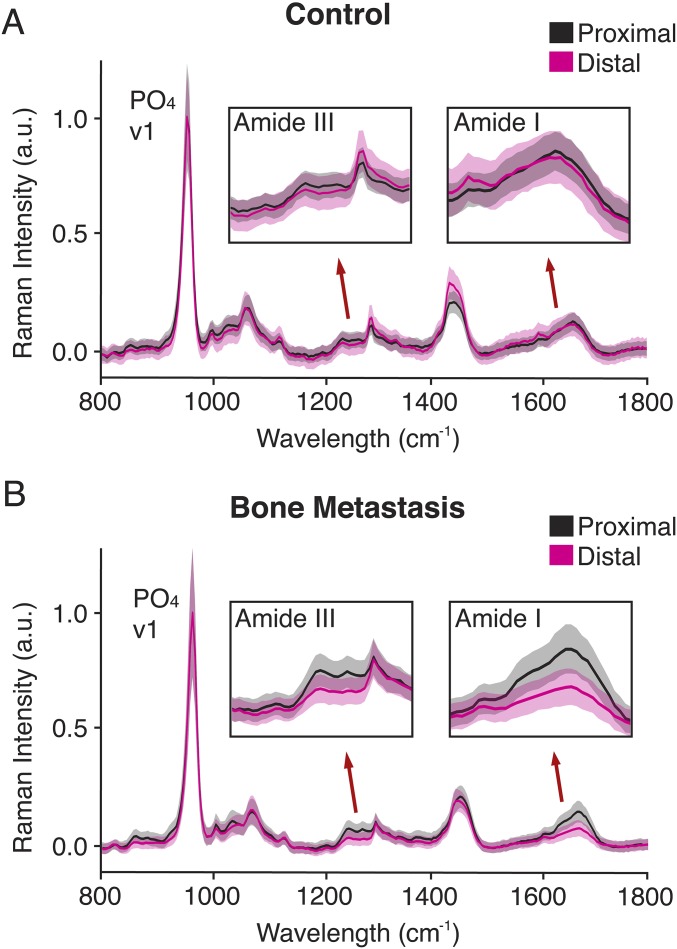
Multiscale characterization of the bone metastatic site identifies changes in the ECM associated with secondary tumor formation. (A–D) Macro- to microscale qualitative analysis: Representative μCT images (A and C) and Movat’s pentachrome (MP)-stained cross-sections (B and D). Color legend of the MP stain: yellow, bone; green, calcified cartilage; black, nuclei; intense red, muscle; red/pink, fibrous tissue. The metastasized tumor is highlighted by the black asterisk and outlined with dashed lines (D). (E–J) Nanoscale quantitative analysis: large-area Raman imaging of defined regions (black insets) (E and H). ‟Prox” and ‟Dist” (E, F, H, and I) indicate bone on the proximal (i.e., the epiphysis) and distal (i.e., the metaphysis) sides of the growth plate, respectively. The metastasized tumor is highlighted by the black asterisk and outlined with dashed lines (H). False color heat maps (F and I): Blue and red depict the intensities of the PO4 v1 and amide I peaks, respectively. Mean Raman spectra (bold line: means; fill areas: SD) (G and J) of the indicated bone regions with insets (range: 1,600–1,700 cm−1) highlighting the amide 1 peak (1,677 cm−1).
Fig. S3.
Representative Movat’s pentachrome (MP)-stained cross-sections show massive destruction of metaphyseal trabecular bone and severe disruption of the epiphyseal chondrocytes in tibiae with metastasis. (A and B) Color legend of the MP stain: yellow, bone; green, calcified cartilage; black, nuclei; red/pink, fibrous tissue. “GP” refers to the location of the growth plate. Prox and Dist indicate bone on the proximal (i.e., the epiphysis) and distal (i.e., the metaphysis) sides of the growth plate, respectively. The metastasized tumor (B) is highlighted by the black asterisk and outlined with dashed lines.
Fig. S4.
The metaphysis experiences severe degradation during bone metastasis while the diaphysis remains unaffected. Representative BSE images of the proximal tibia (A–D) and diaphysis (E and F) from control mice (A and C) and mice with bone metastasis (B and D). C and D show the regions defined by the red insets in A and B, respectively. GP labels the growth plate.
We were specifically interested in characterizing the compositional and structural properties of tumor-interfacing bone remnants of what was once a mesh of metaphyseal trabecular bone (Figs. S3 and S4). To this end, we used large-area Raman imaging to compare the chemical signatures of trabecular bone proximal (i.e., the epiphysis) and distal (i.e., the metaphysis) to the growth plate in control and metastasis conditions (Fig. 3 E–J). Interestingly, in both control and tumor-bearing tibiae we observed that the intensity of the PO4 v1 peak (960 cm−1) was comparable between the metaphysis and the epiphysis (Fig. 3 F and I). This result suggests that the immediate presence of a tumor has little effect on the mineral content of the remaining bone (26). In contrast, we found pronounced spectral differences in the amide I peak (1,677 cm−1), a proxy for collagen type I content (26), between the metaphysis and epiphysis of metastasis-associated bones (Fig. 3 I and J). Because the intensity of the amide I peak is polarization-dependent and may be affected by polarization orientation of the incident beam, we also assessed the intensity of the polarization-independent amide III peak (1,256 cm−1) (26, 27), which exhibited a concomitant decrease (Fig. S5B). In contrast, the amide I and III peaks were virtually unaffected in the tibial metaphysis of control mice (Fig. 3 F and G and Fig. S5A). Hence, a significant increase in the phosphate-to-amide (PO4/Amide I) ratio (P < 0.05) was observed in the bone metastasis condition versus the control condition (Fig. S6). Collectively, these data suggest a decrease in collagen relative to mineral content within bone that is in physical contact with the secondary tumor.
Fig. S5.
Collagen type I content is decreased in metaphyseal bone that is interfacing with the secondary tumor. Raman spectroscopy of bone proximal (i.e., the epiphysis) and distal (i.e., the metaphysis) to the growth plate in control (A) and bone metastasis (B) conditions. Mean Raman spectra with insets (1,200–1,330 cm−1; 1,600–1,700 cm−1) highlighting the amide III (1,256 cm−1) and amide I (1,677 cm−1) peaks, respectively. The bold lines represent the means and the fill areas represent the respective SDs.
Fig. S6.
Large-area Raman imaging indicates that the phosphate-to-amide ratio (PO4/Amide I) is increased in metaphyseal bone interfacing with a secondary tumor. (A) PO4/Amide I as a function of distance from the growth plate. The bold lines represent the means and the fill areas represent the respective SDs. (B) PO4/Amide I visualized by heat maps of the region highlighted in the red insets. Bone Mets, bone metastasis; Ctrl, control. BSE images: GP labels the growth plate. The metastasized tumor is indicated by the white asterisk and outlined with dashed lines. Raman heat maps (RAMAN): ‟Distance” refers to the proximity to the growth plate. Lighter colors indicate a greater phosphate-to-amide ratio.
While the mineral content in the remnant bone tissue may have not been affected by the immediate presence of a tumor (Fig. 3I and Figs. S4 and S6B), bone mineral nanostructure may still have been impacted (8, 9, 28). As such, we were also interested in assessing changes in HA nanostructure within metastasis-associated bone remnants. Targeting these bone remnants with X-ray scattering techniques proved technically infeasible, however. As an alternative, we derived mineral crystallinity—a measure of HA nanocrystal maturity—from the inverse of the Raman PO4 v1 peak FWHM (8, 29). This parameter positively correlates with the stoichiometric perfection and length of the crystallites along the c axis (29). Interestingly, we found that mineral crystallinity in the bone remnants was markedly decreased (P < 0.05) in the bone metastasis condition relative to the control (Fig. S7). Taken together, the immediate presence of a tumor may promote the decrease of mineral maturity along with reduced collagen content.
Fig. S7.
Large-area Raman imaging indicates that metaphyseal bone mineral crystallinity (FWHM−1 * 100 of the PO4 v1 peak) is decreased in mice with localized mammary tumors as well as in mice with bone metastasis. (A) Crystallinity as a function of distance from the growth plate. The bold lines represent the means and the fill areas represent the respective SDs. (B) Crystallinity visualized by heat maps of the region highlighted in the red insets. Bone Mets, bone metastasis; Ctrl, control; Mam Tumor, mammary tumor. BSE images: GP labels the growth plate. The metastasized tumor is indicated by the white asterisk and outlined with dashed lines. Raman heat maps (RAMAN): Distance refers to the proximity to the growth plate. Lighter colors indicate greater mineral crystallinity.
Metaphyseal HA Nanostructure is Altered by the Presence of a Localized Mammary Tumor.
Next, we asked if the bone ECM can be altered by the presence of a localized primary tumor. While previous studies of bone metastasis have shown that circulating biomolecules can modulate bone cell activity (30) and bone collagen remodeling (31), our focus here was to investigate the possibility of premetastatic changes in the mineral nanostructure of bone. To recapitulate a scenario of breast cancer without evident bone metastases, we implanted the same BoM1-2287 cells into the mammary fat pads of BALB/c nude mice, which, over a period of 7 wk, enabled primary tumor growth but did not result in metastatic outgrowth (32) (Fig. S2B) or the physical wasting associated with cachexia (Fig. S2C). Complementary techniques with resolutions spanning the macro- to microlength scales did not reveal primary tumor-mediated changes in metaphyseal bone. Histological staining, BSE imaging, and μCT analysis suggested that the cellular composition (Fig. S8A), mineral density (Fig. S8A), and trabecular bone structure (Fig. S8B) was similar between the tibial metaphysis of control mice and mice carrying primary mammary tumors.
Fig. S8.
Macro- to microscale analysis of metaphyseal bone suggests no structural differences between control mice and mice with mammary tumors. (A) Representative images of Movat’s pentachrome-stained cross-sections and BSE images of comparable regions of trabecular bone in the proximal tibia. (B) μCT analysis of the trabecular bone highlighted in dark purple. Graphs represent the means ± SD of BV/TV, Tb.Th, and Tb.Sp data.
We then used X-ray scattering analysis to examine potential primary tumor-mediated changes in bone nanostructure. Laboratory-based SAXS did not indicate differences in HA nanocrystal orientation and thickness in metaphyseal bone between control and mammary tumor groups (Table S1). However, since tumor-mediated differences in the mineral phase could conceivably occur on extremely small length scales, we hypothesized that the spatial resolution of laboratory-based SAXS (Fig. S1A) may have been insufficient to detect these possible changes in HA. Therefore, we next analyzed the samples using scanning synchrotron-based SAXS and wide-angle X-ray scattering (WAXS) (Fig. S1B). In addition to providing information on mineral crystal thickness and orientation from SAXS, this approach also enables the derivation of the mineral crystal length (L-parameter) from WAXS (Fig. S1B). Given that the growth plate is a region of high metabolic activity and mineralization (23), we focused our analysis on the HA mineral within 50 µm of the growth plate cartilage. Here, we observed tumor-mediated changes in mineral crystal nanostructure, which were dependent on the location of the scanned mineral (Fig. 4). More specifically, HA mineral crystals immediately distal to the growth plate were significantly shorter in tibiae from mice with mammary tumors versus control mice (Fig. 4B). A downward, though nonsignificant, trend was also detected in both mineral crystal orientation (P = 0.33) (Fig. 4C) and thickness (P = 0.56) (Fig. 4D). This observed decrease in HA nanocrystal size was corroborated with Raman imaging measurements of corresponding bone regions, which suggested that mineral crystallinity was decreased (P < 0.05) in the mammary tumor condition relative to that of the control (Fig. S7). Furthermore, these differences mimicked measurements of mineral crystallinity in the bone remnants physically adjoined to a metastatic tumor mass (Fig. S7). During murine bone development HA crystals undergo large increases in length while thickness stays relatively constant (10), which could suggest that HA length is more susceptible to aberrant perturbations. Interestingly, these tumor-mediated changes were only observed in the tissue distal but not proximal to the growth plate (Fig. 4 B–D), which is consistent with the preferential initiation of secondary tumors in the metaphysis rather than in the epiphysis (Fig. 3 and Fig. S3).
Table S1.
Summary of ρ- and T-parameter data from laboratory-based SAXS analysis
| Parameter | Region | Control (mean ± SD) | Mammary tumor (mean ± SD) | P value (control vs. tumor) |
| ρ, a.u. | Diaphysis | 0.587 ± 0.066 | 0.522 ± 0.102 | 0.30 |
| Metaphysis | 0.246 ± 0.003 | 0.283 ± 0.036 | 0.40 | |
| T, nm | Diaphysis | 2.268 ± 0.052 | 2.279 ± 0.016 | 0.66 |
| Metaphysis | 2.131 ± 0.027 | 2.091 ± 0.064 | 0.26 |
Fig. 4.
Scanning synchrotron-based SAXS/WAXS analysis of metaphyseal tissue bordering the growth plate reveals shorter HA nanocrystals in mice carrying mammary tumors. (A) Representative BSE images of the region of interest. Prox and Dist indicate the proximal and distal sides of the growth plate, respectively. (B–D) Spatial representation of quantitative data. Box-and-whisker plots summarize all L-parameter (mean mineral crystal length) (B), ρ-parameter (mean mineral crystal orientation) (C), and T-parameter (mean mineral crystal thickness) (D) data. Whiskers represent the 5th and the 95th percentile. Outlier data points are depicted as dots. *P < 0.05. n.s. indicates nonsignificance. Representative L-parameter (B), ρ-parameter (C), and T-parameter (D) data are overlaid on corresponding BSE images. Color scales: Warmer colors indicate greater crystal length (B), orientation (C), or thickness (D).
To explore possible mechanisms by which a mammary tumor could remotely alter metaphyseal bone mineral, we assessed markers of bone matrix remodeling in tibiae harvested from mice systematically conditioned with daily i.p. injections of tumor-derived media over the course of 3 wk. Picrosirius Red-stained cross-sections of the proximal tibiae revealed a significant increase in the collagen content of metaphyseal trabecular bone (Fig. S9A) while tartrate‐resistant acid phosphatase (TRAP) staining of this same region revealed no difference in the number of osteoclasts (Fig. S9B). These data suggest that in a skeletal region prone to metastasis, circulating tumor-derived factors can lead to an increase in local osteogenesis, while leaving bone resorption unaffected. Accordingly, culturing bone marrow-derived mesenchymal stem cells (MSCs) in the same tumor-derived media resulted in elevated matrix calcification via Alizarin Red S (ARS) staining compared with controls (Fig. S9C), which is indicative of tumor factor-mediated osteogenic differentiation. Importantly, this trend was more pronounced (P < 0.05) when MSCs were cultured in media from the bone metastatic cells used in our bone mineral characterization studies (BoM1-2287) relative to the parental cell line (Fig. S9C). Taken together, these results imply that factors secreted from bone metastatic breast cancer cells can aberrantly promote local osteogenesis by altering the fate of bone marrow-residing MSCs. Increased osteogenesis may explain the enhanced HA immaturity at metastatic sites, as newly formed bone is typically composed of smaller, less-perfect, and less-oriented HA crystals (6).
Fig. S9.
Tumor-secreted factors can promote osteogenic activity. (A) Representative Picrosirius Red-stained cross-sections of metaphyseal trabecular bone in tibiae harvested from control mice (Control) or mice injected with media conditioned by parental MDA-MB-231 (TCM) cells. The growth plate border is indicated by the white dotted line. Graphs represent the back-transformed predicted means with 95% confidence interval of green and red integrated density measurements for thin/immature and thick/mature collagens, respectively. Data are normalized to regions of diaphyseal bone. *P < 0.05. (B) Representative TRAP-stained (pink) cross-sections (with hematoxylin counterstain) of either control or TCM tibiae. Graphs represent quantification (mean ± SD) of number of osteoclasts normalized to bone perimeter (N.Oc/B.Pm). (C) Representative images of ARS in bone marrow-derived MSCs treated with control media (Control) or conditioned media from parental MDA-MB-231 breast cancer cells (TCM) or BoM1-2287 (Bone-TCM). Red color indicates positive stain for matrix calcification. Graphs represent mean ± SD of ARS extracted from stained matrices. *P < 0.05.
Discussion
Interactions between breast cancer cells and the bone microenvironment are critical to the pathogenesis of skeletal metastases, but the associated nanostructural changes of bone mineral remain unclear. Gaining an improved understanding of this potential relationship is critical as HA nanocrystal properties can significantly alter cellular phenotypes (13–15) and, thus, conceivably affect malignancy. Using a combination of high-resolution analytical techniques to characterize tibiae from mouse models of bone metastatic and localized breast cancer, we show that the nanostructure of HA crystals varies between anatomical regions that are more and less prone to metastatic colonization, and that primary tumors may reinforce these variations even before metastatic outgrowth.
The observation that breast cancer cells preferentially colonize the proximal tibial metaphysis may have broader pathological relevance. Adjacent to a secondary ossification center and enriched with trabecular bone (23), the tibial metaphysis of young mice can exemplify a distinct anatomical site of high bone turnover (i.e., resorption of old bone followed by formation of new bone). Clinicians have long observed preferential metastatic localization to sites of active remodeling (33), such as the trabecular bone-rich pelvis and spinal vertebral bodies (2, 33). As the nanostructure of human and murine bone mineral is similar (5, 10), it is likely that HA crystals in these metabolically more active human skeletal sites are also more immature. Future studies will need to confirm this possibility and assess the functional role of HA materials properties in driving secondary tumor formation in these sites.
Furthermore, our SAXS/WAXS and Raman data point to a potential mammary tumor-mediated increase of local osteogenesis in the initial trabecular network before secondary tumor formation. This finding supports experimental evidence by others that primary tumors can activate premetastatic remodeling of the bone, and that these changes ultimately promote metastatic dissemination (30, 31). While these previous studies of mammary tumor-mediated changes focused on bone cell activity (30) and circulating markers of bone collagen remodeling (31), our data now additionally suggest that structural changes occur in the mineral phase of bone. More specifically, we observed a decrease in mineral nanocrystal size in the metaphyseal bone tissue bordering the growth plate. Since these changes occurred at a bone growth front, these data indicate that a localized mammary tumor may be affecting the mineralization of new matrix. In bone, the deposition of HA results from a thermodynamically driven cascade that may involve the initial formation of disordered, less stable nanoparticulate phases that become more crystalline and increase in size as they progress to the final mineral phase (34, 35). As such, a mammary tumor could conceivably decrease general mineral maturity by releasing factors that stimulate osteogenic cells to increase their production of new bone (Fig. S9), which typically consists of smaller, less-perfect and less-oriented HA crystals (6). Alternatively, the physiological maturation of bone mineral could also be directly inhibited by soluble factors secreted by the mammary tumor. The glycoprotein osteopontin is a potential candidate, as it is highly expressed by the BoM1-2287 cells (21) used in this study and is known to inhibit the growth of HA mineral (36). Furthermore, changes in HA nanostructure may have been due to mammary tumor-mediated effects on the longitudinal bone growth of the young mice (37) used in our studies. However, given the similarities in growth plate thicknesses (Fig. S2D) and trabecular network properties (Fig. S8 A and B) between control tibiae and mammary tumor-associated tibiae, the metabolic program of growth plate chondrocytes and the consequent rate of endochondral ossification (38) appear to be unaffected by the primary tumors. Taken together, our results suggest that the primary mammary tumor could induce the formation of less-mature bone mineral in the absence of a secondary tumor, likely without changing overall skeletal growth.
Premetastatic, primary tumor-mediated changes in nanoscale bone mineral properties may be an underappreciated mechanism that could play a role in the initial establishment and survival of a secondary tumor colony in the bone. Because metastatic breast cancer cells exhibit increased proliferation and adhesion on smaller and less-perfect HA nanocrystals (13), these early-stage changes of bone mineral may promote the initial seeding and survival of disseminated tumor cells. Furthermore, the deposition of a more immature mineral phase that is more susceptible to osteolytic degradation (39, 40) could facilitate the vicious cycle of bone metastasis, thus promoting tumor outgrowth. Complemented by our previous work suggesting that nanoscale HA materials properties can influence breast cancer malignant progression (13–15), we propose a modified view of the vicious cycle of bone metastasis in which HA materials properties are functionally linked with the pathogenesis of breast cancer bone metastasis (Fig. 5). Detailed in vivo studies will be needed to conclusively define the functional consequences of our observations. Nevertheless, our results bring to light that nanostructural parameters of the bone metastatic site are currently underappreciated but should be considered when studying the microenvironmental complexities that influence bone metastasis.
Fig. 5.
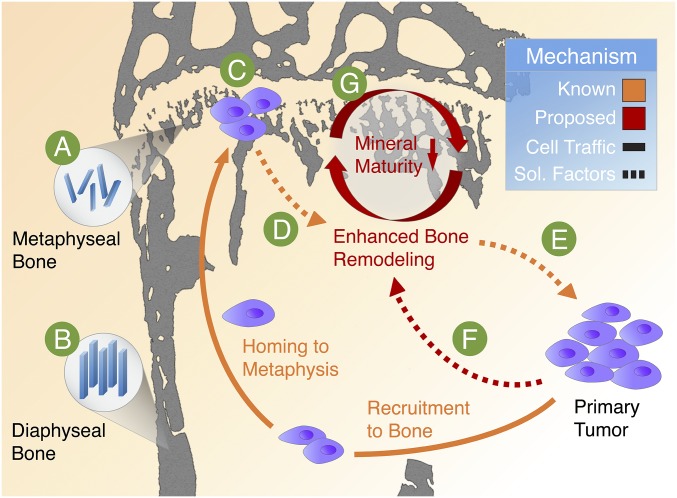
Proposed functional relationship between HA mineral characteristics and breast cancer bone metastasis. In mice, breast cancer cells typically colonize the metaphysis in the metabolically active proximal tibia. We show that mineral in metaphyseal bone (A) is less mature (i.e., smaller, less-perfect, and less-oriented) relative to other skeletal locations (B) not prone to initiation of metastasis. Because previous in vitro findings show that tumor cells preferentially adhere to less-mature crystals, we propose that this difference in mineral properties may be functionally relevant to the establishment of a tumor colony (C). The metastasized tumor cells can then disrupt typical bone remodeling processes (D) to result in the aberrant activity of bone cells and the increased release of tumor-recruiting soluble factors (E). Furthermore, breast cancer cells located within primary tumors can stimulate bone remodeling by secreting circulating factors (F) that enhance local osteogenesis and, thus, alter the composition and structure of the bone ECM. Here, we propose that these premetastatic changes lead to the deposition of a less-mature mineral phase (G) which may be essential in driving the vicious cycle of osteolytic bone metastasis.
While others have previously reported that breast cancer can remotely engender bone destruction (16, 30, 31), we now further advance this field of work by quantifying tumor-mediated changes in HA nanostructure in skeletal locations associated with metastasis. The findings here may inform future therapeutic strategies. For instance, adjuvant prescription of antiresorptive drugs such as bisphosphonates (BPs) is currently largely palliative and does not improve patient survival rate (1). BPs, which are taken up by bone-resorbing osteoclasts, localize to mineralized tissue by selectively binding to HA (1). As molecular simulation studies show that BP–HA binding energies are dependent on HA nanocrystal size (41), BP therapeutic efficacy may be affected by tumor-induced changes in HA nanostructure. Understanding how nanoscale variations in HA structure affect BP binding may aid the development of derivatives and protein conjugates (42) that are more effective in the treatment of bone metastasis.
Although limited by sample throughput, X-ray scattering analysis and large-area Raman imaging generate position-resolved information on the micro- to nanoscales that can be combined with a host of other techniques to provide a comprehensive assessment of bone materials properties in a variety of pathological contexts. Thus, similar approaches to the one described in this paper can be used to assess bone nanostructure in animal models of other cancers (e.g., prostate and lung) that also metastasize to bone (2). Insights from these analyses will inform the design of biomimetic culture systems (43) and high-throughput screening platforms (44) to investigate the functional role of HA mineral in bone metastasis as well as the development of tissue-engineered tumor models that recapitulate the bone microenvironment (45). Moreover, future work will also need to consider the effect of changing bone mineral properties in the context of the intimately associated collagen fibrils and adhesive proteins enriched in the metastatic site. Interdisciplinary collaborations between the fields of biomaterials, cancer biology, and oncology will be key to this promising new field of work in which materials science approaches will play an important role.
Materials and Methods
Detailed descriptions are provided in SI Materials and Methods.
Mouse Models of Breast Cancer.
Three-week-old, female BALB/c nude mice (Taconic) were used for animal studies. Intracardiac injections of luciferase-labeled BoM1-2287 cells led to tibial metastases while mammary fat pad injections generated localized mammary tumors. Tibiae were harvested at 5 (bone mets) or 7 wk (mam tumor), fixed in 70% EtOH (48 h), and embedded in polymethylmethracrylate (PMMA). All animal studies were performed in accordance with protocols approved by Cornell University's Institutional Animal Care and Use Committee.
Macro- to Microscale Analysis.
Samples were scanned via μCT (BrukerCT; Scanco) and trabecular bone outcomes (BV/TV, Tb.Th, and Tb.Sp) were compared (n = 3). Backscattered SEM (FEI) and Movat's pentachrome staining were performed on longitudinal cross-sections.
X-Ray Scattering.
Lab SAXS (Bruker) was performed across the proximal tibiae and on select diaphyseal bone of longitudinal sections while synchrotron SAXS/WAXS (BESSY II) was performed on bone bordering the growth plate and on select diaphyseal bone. The sample size was at least n = 3. The T-, ρ-, and L-parameters were calculated as previously described (10, 18). A mixed model was used for statistical analyses.
Large-Area Raman Imaging.
Raman imaging (WiTec) was performed on select regions of representative longitudinal sections. PO4 v1 (960 cm−1), amide I (1,677 cm−1), and amide III (1,256 cm−1) peaks were fitted with Gaussian functions and their intensities as well as FWHMs were extracted. After excluding outlier data, the means and SDs were calculated. A two-sample t test was used to assess differences between regions.
SI Materials and Methods
Tibiae from Mouse Xenograft Models of Breast Cancer.
Three-week-old, female BALB/c athymic nude mice from Taconic Biosciences were used for animal studies. To recapitulate either bone metastasis or localized breast cancer in these mice, two different surgical techniques were used. To generate secondary, bone-metastatic tumors, luciferase-expressing BoM1-2287 cells (105 cells in 100 μL PBS) were injected into the left ventricles of the hearts (one injection per mouse). Tibiae were harvested after 5 wk. To assess the effects of a primary mammary tumor, luciferase-expressing BoM1-2287 cells (0.756 cells in 20 μL of DMEM, 10% FBS, and 1% antibiotic) were injected into cleared and contralateral mammary fat pads (two injections per mouse). Tibiae were harvested after 7 wk. Tumor cell localization was determined by bioluminescent imaging: Mice were intraperitoneally injected with luciferin (150 mg/kg; Gold Biotechnology) and then imaged after a 5-min incubation period (Xenogen IVIS-200). Age-matched mice were used as controls. Immediately following harvest, tibiae were stored in 70% ethanol for 48 h and embedded in PMMA by the Analytical Microscopy Core Laboratory at the Hospital for Special Surgery. All animal studies were performed in accordance with Cornell University animal care guidelines and were approved by Cornell University's Institutional Animal Care and Use Committee (IACUC).
µCT.
Mouse tibia specimens were scanned using two quantitative µCT systems. Bone metastatic tibiae were scanned with the Skyscan 1172 (BrukerCT) while control and mammary tumor tibiae were scanned with the Scanco µCT35 (Scanco Medical AG). Tibiae were scanned at moderate isotropic resolutions of either 10 or 15 µm. To obtain high-contrast images, the X-ray source was set to a voltage of 55 kV and a current of 145 µA. To reduce the effects of beam hardening, a 0.5-mm aluminum filter was used. Avizo Fire 8.1 (FEI) was used to assess macroscopic architecture following 3D reconstruction of the 2D slices. A sample size of n = 3 was used to compare trabecular bone outcomes, which included BV/TV (bone volume fraction), Tb.Th (trabecular thickness), and Tb.Sp (trabecular separation), between control and mammary tumor groups.
BSE.
The PMMA sample blocks were cut to expose a longitudinal section of the entire bone. An environmental scanning electron microscope (FEI) was used under low-vacuum conditions in backscattered mode at a working distance of 10 mm with electron beam energies of 10 kV and 12.5 kV. Grayscale images of mineralized tissue were captured, with regions of high calcium content appearing brighter than regions of low calcium content.
Histological Studies.
Movat’s pentachrome staining was performed on 5-μm longitudinal sections of the PMMA-embedded samples. The following tissue types were stained: bone (yellow), cartilage (blue to green), and fibrous connective tissue (pink to purple). Representative specimens were imaged with an Axioskop 2 microscope (Carl Zeiss Microscopy GmbH).
SAXS Measurements.
Longitudinal sample sections ∼200 μm thick were mounted ∼600 mm from the detector and perpendicular to the X-ray beam path. An X-ray generator (Bruker AXS) with a rotating copper anode operating at 40 kV and 100 mA (Cu K-alpha radiation) produced a beam with a wavelength of 1.5418 Å. An X-ray radiograph was used to select points of measurement. For each sample, ∼80–100 points to sufficiently map the proximal tibiae were selected along with 5–10 points in the diaphysis. A two-pinhole setup with a focal diameter of 200 μm collimated the X-ray beam. The scattering signal was recorded for 3,600 s by a position-sensitive area detector (pixel size 105.26 × 105.26 μm2, HI-STAR; Bruker AXS). Calculation of the beam center was achieved by using a silver behenate standard. A sample size of n = 3 was used for both control and mammary tumor groups. Image files were analyzed with AutoFit (proprietary Fit2D-based software by the Max Planck Institute of Colloids and Interfaces). The T- (mean mineral crystal thickness) and ρ- (degree of alignment) parameters were calculated as previously described (10, 18). To consistently assess mineral nanostructure of the metaphyseal bone, all data points that were distally within 1,000 μm of the growth plate were considered for analysis.
Synchrotron Measurements (SAXS/WAXS).
The previously prepared 200-μm-thick sections were further polished to obtain ∼50-μm-thick sections. Measurements were obtained at the BESSY II (Berliner Elektronenspeicherring-Gesellschaft fur Synchrotronstrahlung; Helmholtz-Zentrum Berlin). The instrument produced a 30-μm beam with a wavelength of 0.82656 Å. The samples were positioned ∼300 mm from the detector and perpendicular to the beam path. An X-ray radiograph was used to select points of measurement. For each sample, roughly 150 measurement points from mineralized tissue on both distal and proximal sides of the growth plate were selected along with five points in the diaphysis. The scattering signal was recorded for 90 s with a position sensitive CCD detector (pixel size 73.242 × 73.242 μm2, MarMosaic 225; Mar USA). Instrument calibration and beam center localization were determined by a quartz standard. The raw scattering data were then corrected for ring current variations. A sample size of n = 4 was used for both control and mammary tumor groups. Image files were analyzed with AutoFit. The T- (mean mineral crystal thickness), ρ- (degree of alignment), and L- (mean mineral crystal length) parameters were calculated as previously described (10, 18) and then normalized to the sample means of the corresponding parameter values for diaphyseal cortical bone to control for variability between animals. To investigate tumor-mediated effects at the growth plate region, all data points that were within 50 μm of the growth plate cartilage were considered for comparison with corresponding data points in control samples.
Statistical Analysis of X-Ray Scattering Data.
Because mouse tibiae were measured in multiple regions and repeatedly measured within these regions, a mixed model accounting for both fixed and random effects was used. The fixed effects were the (i) disease state, (ii) region, and (iii) interaction between the disease state and the region. The random effects were the (i) mouse and (ii) region nested within each mouse. Contrasts were subsequently run to compare either the regions of interest within each disease state or the disease state within each region of interest. A Bonferroni correction was applied to adjust for multiple comparisons. The correlation between T-parameter with ρ-parameter was performed using a simple linear regression (n = 148). P < 0.05 was considered statistically significant. JMP 12 (SAS Institute Inc.) and Graphpad Prism 5 (GraphPad Software) were used for all statistical analyses, which were performed in consultation with an independent statistician at the Cornell Statistical Consulting Unit.
Large-Area Raman Imaging.
Representative samples were chosen for supplementary analysis via Raman imaging. Raman spectra were collected using a WiTec Alpha300 Raman system. A continuous 532-nm laser beam was focused on select regions of 5-μm longitudinal sections. Each spectrum was acquired with an accumulation time of 0.33 s per point using a CCD behind a grated (600 g/mm) spectrometer (WITec UHTS raman spectrometer) with a spectral resolution of 4 cm−1. Raman maps were generated with a spatial resolution of 2 μm. The WiTec Project 4.1 software was used for image processing and analysis. To avoid the effect of cosmic rays and other aberrations when averaging spectra, data smaller or greater than 3σ from the mean were excluded, which constituted less than 3% of the data. Background resin spectra was subtracted from the raw data for better fitting. PO4 v1 (960 cm−1), amide I (1,677 cm−1), and amide III (1,256 cm−1) peaks (26, 27) were fitted with Gaussian functions and their FWHMs as well as intensities were extracted. Bone tissue with phosphate intensities above 120 CCD counts were used for further analysis. Phosphate peak FWHM−1 and phosphate-to-amide ratio (PO4/Amide I) were calculated at each point. For segmentation analysis, the metaphyseal bone was divided into 10 segments with increasing distance from the growth plate. After excluding outlier data, the means and SDs of each segment were calculated. A two-sample t test was used to assess differences in regions of interest.
Analysis of Mouse Tibiae from Cell-Free Tumor Conditioning.
Five-week-old, female NOD.SCID mice from Jackson Laboratories were used for animal studies. Animal studies were performed in accordance with Cornell University animal care guidelines. For 3 wk, mice received daily i.p. injections of tumor-derived media (TCM) or blank media control. To generate TCM for injections, MDA-MB-231 breast cancer cells (ATCC) at 90% confluency were incubated in low-serum DMEM supplemented with 1% FBS (Atlanta Biologicals) and 1% penicillin/streptomycin (Invitrogen) for 12 h. Conditioned media was collected, concentrated 10-fold in an Amicon centrifugal filter unit (molecular weight cutoff 3 kDa; EMD Millipore), and subsequently diluted fivefold with low-serum DMEM. Blank low-serum DMEM was processed similarly (control) for mice receiving control injections. Tibiae were harvested and fixed in 10% neutral buffered formalin for 48 h and decalcified in EDTA (10% EDTA, pH 7.4). TRAP staining for osteoclasts and Picrosirius Red staining for collagen were performed on paraffin-embedded sections. Images of TRAP-stained sections were captured using a Scanscope digital slide scanner (Aperio). TRAP+ osteoclasts were counted and normalized to bone perimeter (N.Oc/B.Pm). Metaphyseal and diaphyseal regions of Picrosirius Red-stained sections were imaged under cross-polarized light (Eclipse TE2000-S; Nikon), and green and red integrated density was measured using ImageJ to quantify thin/immature and thick/mature collagens, respectively. Metaphyseal measurements for each sample were normalized to respective diaphyseal measurements. Sample size was at least n = 5 for each group. Collagen content in mouse tibiae was measured over two sections with two images per section. The resulting data were analyzed using a mixed model with random effect of (i) mouse and (ii) section nested within mouse. The fixed effect was condition (between mouse variable). The response was log-transformed to better fit the assumptions of the model (normality of residuals, constant variance). Student’s t test was used to analyze TRAP staining data. P < 0.05 was considered statistically significant. JMP 12 (SAS Institute Inc.) was used for statistical analyses, which were performed in consultation with an independent statistician at the Cornell Statistical Consulting Unit.
In Vitro Assessment of Osteogenic Differentiation.
TCM derived from MDA-MB-231 and the BoM1-2287 subline (Bone-TCM) were collected after 24 h and processed as above but instead diluted fivefold into osteogenic induction media (low-serum DMEM supplemented with 50 μM ascorbic acid, 0.1 μM dexamethasone, and 10 mM β-glycerophosphate). Bone marrow-derived MSCs (3.1 × 103 cells per cm2; Lonza) were treated with the prepared TCM, Bone-TCM, or control for 3 wk, after which cells were fixed and stained with ARS to visualize and quantify matrix calcification colorimetrically following extraction of ARS with 10% acetic acid. Sample size was n = 3 for all groups. One-way ANOVA with Tukey’s multiple comparisons was used to analyze ARS data. P < 0.05 was considered statistically significant. Graphpad Prism 5 (GraphPad Software) was used for statistical analysis.
Acknowledgments
We thank C. Li, S. Siegel, and I. Zenke for assistance with SAXS/WAXS; and F. Vermeylen for consultation with statistical analysis. BoM1-2287 cells were a gift from J. Massagué. This work was funded by NIH Grant R01CA173083, National Cancer Institute Grants 1U54CA199081-01 and 1U54CA210184-01, an NSF fellowship (to F.H.), and an A. v. Humboldt fellowship (to C.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708161114/-/DCSupplemental.
References
- 1.Kozlow W, Guise TA. Breast cancer metastasis to bone: Mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 2.Ren G, Esposito M, Kang Y. Bone metastasis and the metastatic niche. J Mol Med (Berl) 2015;93:1203–1212. doi: 10.1007/s00109-015-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: A fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27:193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–2123. [Google Scholar]
- 6.Olszta MJ, et al. Bone structure and formation: A new perspective. Mater Sci Eng Rep. 2007;58:77–116. [Google Scholar]
- 7.Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt. 2005;10:031102. doi: 10.1117/1.1922927. [DOI] [PubMed] [Google Scholar]
- 8.Bi X, et al. Prostate cancer metastases alter bone mineral and matrix composition independent of effects on bone architecture in mice––a quantitative study using microCT and Raman spectroscopy. Bone. 2013;56:454–460. doi: 10.1016/j.bone.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke M, et al. The impact of metastasis on the mineral phase of vertebral bone tissue. J Mech Behav Biomed Mater. 2017;69:75–84. doi: 10.1016/j.jmbbm.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Lange C, et al. Fetal and postnatal mouse bone tissue contains more calcium than is present in hydroxyapatite. J Struct Biol. 2011;176:159–167. doi: 10.1016/j.jsb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Bigi A, et al. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J Inorg Biochem. 1997;68:45–51. doi: 10.1016/s0162-0134(97)00007-x. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn LT, et al. A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif Tissue Int. 2008;83:146–154. doi: 10.1007/s00223-008-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathi SP, Lin DDW, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32:5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S, Coonrod S, Estroff L, Fischbach C. Chemical and physical properties of carbonated hydroxyapatite affect breast cancer cell behavior. Acta Biomater. 2015;24:333–342. doi: 10.1016/j.actbio.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F, et al. Protein-crystal interface mediates cell adhesion and proangiogenic secretion. Biomaterials. 2017;116:174–185. doi: 10.1016/j.biomaterials.2016.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorpe MP, et al. Breast tumors induced by N-methyl-N-nitrosourea are damaging to bone strength, structure, and mineralization in the absence of metastasis in rats. J Bone Miner Res. 2011;26:769–776. doi: 10.1002/jbmr.277. [DOI] [PubMed] [Google Scholar]
- 17.Lynch ME, et al. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res. 2013;28:2357–2367. doi: 10.1002/jbmr.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pabisch S, Wagermaier W, Zander T, Li C, Fratzl P. Imaging the nanostructure of bone and dentin through small- and wide-angle X-ray scattering. In: De Yoreo JJ, editor. Methods in Enzymology. Academic; New York: 2013. pp. 391–413. [DOI] [PubMed] [Google Scholar]
- 19.Fratzl P, Gupta HA, Roschger P, Klaushofer K. Nanotechology. Vol 5. Wiley-VCH; Weinheim, Germany: 2009. Bone nanostructure and its relevance for mechanical performance, disease, and treatment; pp. 345–360. [Google Scholar]
- 20.Wright LE, et al. Murine models of breast cancer bone metastasis. Bonekey Rep. 2016;5:804. doi: 10.1038/bonekey.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 22.Mastro AM, Gay CV, Welch DR. The skeleton as a unique environment for breast cancer cells. Clin Exp Metastasis. 2003;20:275–284. doi: 10.1023/a:1022995403081. [DOI] [PubMed] [Google Scholar]
- 23.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesch W, et al. Orientation of mineral crystallites and mineral density during skeletal development in mice deficient in tissue nonspecific alkaline phosphatase. J Bone Miner Res. 2003;18:117–125. doi: 10.1359/jbmr.2003.18.1.117. [DOI] [PubMed] [Google Scholar]
- 25.Joiner DM, et al. Accelerated and increased joint damage in young mice with global inactivation of mitogen-inducible gene 6 after ligament and meniscus injury. Arthritis Res Ther. 2014;16:R81. doi: 10.1186/ar4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamsjaeger S, Kazanci M, Pachalis EP, Fratzl P. Raman application in bone imaging. In: Maher SA, editor. Raman Spectroscopy for Soft Matter Applications. Wiley; New York: 2009. pp. 227–267. [Google Scholar]
- 27.Kazanci M, Roschger P, Paschalis EP, Klaushofer K, Fratzl P. Bone osteonal tissues by Raman spectral mapping: Orientation-composition. J Struct Biol. 2006;156:489–496. doi: 10.1016/j.jsb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Bloebaum RD, Skedros JG, Vajda EG, Bachus KN, Constantz BR. Determining mineral content variations in bone using backscattered electron imaging. Bone. 1997;20:485–490. doi: 10.1016/s8756-3282(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 29.Yerramshetty JS, Akkus O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone. 2008;42:476–482. doi: 10.1016/j.bone.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Kelly T, et al. Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Res. 2005;65:5778–5784. doi: 10.1158/0008-5472.CAN-05-0749. [DOI] [PubMed] [Google Scholar]
- 32.Kuperwasser C, et al. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- 33.Togawa D, Lewandrowski DU. The pathophysiology of spinal metastases. In: McLain RF, editor. Cancer in the Spine. Humana; New York: 2006. pp. 17–24. [Google Scholar]
- 34.De Yoreo JJ, et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science. 2015;349:aaa6760–aaa6760. doi: 10.1126/science.aaa6760. [DOI] [PubMed] [Google Scholar]
- 35.Mahamid J, et al. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: A cryo-electron microscopy study. J Struct Biol. 2011;174:527–535. doi: 10.1016/j.jsb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 36.George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008;108:4670–4693. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piao J, et al. Sirt6 regulates postnatal growth plate differentiation and proliferation via Ihh signaling. Sci Rep. 2013;3:3022. doi: 10.1038/srep03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagano M, Nakamura T, Kokubo T, Tanahashi M, Ogawas M. Differences of bone bonding ability and degradation behaviour in vivo between amorphous calcium phosphate and highly crystalline hydroxyapatite coating. Biomaterials. 1996;17:1771–1777. doi: 10.1016/0142-9612(95)00357-6. [DOI] [PubMed] [Google Scholar]
- 40.Barry AB, Baig AA, Miller SC, Higuchi WI. Effect of age on rat bone solubility and crystallinity. Calcif Tissue Int. 2002;71:167–171. doi: 10.1007/s00223-001-1071-5. [DOI] [PubMed] [Google Scholar]
- 41.Wright JE, Zhao L, Choi P, Uludag H. Simulating hydroxyapatite binding of bone-seeking bisphosphonates. Adv Exp Med Biol. 2004;553:139–148. doi: 10.1007/978-0-306-48584-8_11. [DOI] [PubMed] [Google Scholar]
- 42.Gittens SA, Bansal G, Zernicke RF, Uludağ H. Designing proteins for bone targeting. Adv Drug Deliv Rev. 2005;57:1011–1036. doi: 10.1016/j.addr.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Murphy WL, Hsiong S, Richardson TP, Simmons CA, Mooney DJ. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303–310. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 44.Barney LE, et al. A cell-ECM screening method to predict breast cancer metastasis. Integr Biol. 2015;7:198–212. doi: 10.1039/c4ib00218k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seib FP, Berry JE, Shiozawa Y, Taichman RS, Kaplan DL. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials. 2015;51:313–319. doi: 10.1016/j.biomaterials.2015.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]