Significance
Alpha-synuclein plays an important role in the pathophysiology of Parkinson’s disease (PD), however the molecular mechanisms related to α-synuclein in neurodegeneration of PD remain unknown. We show that α-synuclein specifically inhibits BDNF/TrkB signaling, leading to dopaminergic neuronal death. The disruption of this interaction rescues TrkB signaling, preventing α-Syn–induced dopaminergic neuronal death and restoring motor functions. This study reveals the mechanism related to α-synuclein–induced neurotoxicity of PD via regulation of TrkB neurotrophic signaling.
Keywords: neurodegenerative diseases, dopamine, Lewy bodies, substantia nigra
Abstract
BDNF/TrkB neurotrophic signaling is essential for dopaminergic neuronal survival, and the activities are reduced in the substantial nigra (SN) of Parkinson’s disease (PD). However, whether α-Syn (alpha-synuclein) aggregation, a hallmark in the remaining SN neurons in PD, accounts for the neurotrophic inhibition remains elusive. Here we show that α-Syn selectively interacts with TrkB receptors and inhibits BDNF/TrkB signaling, leading to dopaminergic neuronal death. α-Syn binds to the kinase domain on TrkB, which is negatively regulated by BDNF or Fyn tyrosine kinase. Interestingly, α-Syn represses TrkB lipid raft distribution, decreases its internalization, and reduces its axonal trafficking. Moreover, α-Syn also reduces TrkB protein levels via up-regulation of TrkB ubiquitination. Remarkably, dopamine’s metabolite 3,4-Dihydroxyphenylacetaldehyde (DOPAL) stimulates the interaction between α-Syn and TrkB. Accordingly, MAO-B inhibitor rasagiline disrupts α-Syn/TrkB complex and rescues TrkB neurotrophic signaling, preventing α-Syn–induced dopaminergic neuronal death and restoring motor functions. Hence, our findings demonstrate a noble pathological role of α-Syn in antagonizing neurotrophic signaling, providing a molecular mechanism that accounts for its neurotoxicity in PD.
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, with typical movement abnormalities that include resting tremor, rigidity, bradykinesia, and postural instability as major clinical manifestation. PD is characterized by selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and a corresponding decrease in dopaminergic innervation of the striatum, along with the accumulation of intraneuronal protein aggregates [Lewy bodies (LBs) and Lewy neurites (LNs)] primarily composed of the protein alpha-Synuclein (α-Syn) (1, 2). The physiological functions of α-Syn remain unclear, but several studies have demonstrated that it might be implicated in dopamine (DA) biosynthesis, synaptic plasticity, and vesicular dynamics (3, 4). A pathogenic role for α-Syn in PD is supported by various genetic data. For instance, multiplications of the gene encoding α-Syn (SNCA) and various point mutations in this gene (e.g., A53T, A30P, and E46K) result in dominant familial parkinsonism (1, 5, 6). Moreover, certain polymorphisms in SNCA are major risk factors for sporadic PD (7). The abnormal accumulation of α-Syn, resulting from an unbalanced production and/or degradation of the protein, is thought to trigger DAergic neuronal death in both familial and sporadic cases of PD (8). So far, several cellular mechanisms, such as aberrant protein folding, oxidative stress, and mitochondrial dysfunction have been linked to the development and progression of PD, however the exact molecular mechanisms of how α-Syn exerts neurotoxicity remain elusive.
Neurotrophins (NTs) are growth factors that regulate the development and maintenance of the peripheral and the central nervous systems. Brain-derived neurotrophic factor (BDNF) is a member of the NT family, which includes nerve growth factor (NGF), NT-3, and NT-4/5. BDNF, like the other NTs, exerts its biological functions on neurons through two transmembrane receptors: the p75 neurotrophin receptor (p75NTR) and the TrkB receptor tyrosine kinase (NGF binds to TrkA, BDNF and NT-4/5 bind to TrkB, and NT-3 preferentially binds to TrkC) (9). TrkB is one of the most widely distributed neurotrophic receptors (NTRs) in the brain and is highly enriched in the neocortex, hippocampus, striatum, and brainstem. BDNF binding to TrkB triggers its dimerization through conformational changes and autophosphorylation of tyrosine residues in its intracellular domain (ICD), resulting in activation of the three major signaling pathways involving mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and phospholipase C-g1 (PLC-γ1), mediating neural differentiation, survival, and neurogenesis. BDNF colocalizes with DAergic neurons in the SN (10), and promotes DAergic neuronal survival in vivo (11). PD patients show markedly decreased levels of BDNF (12–14), indicating that reduced levels of this neurotrophic factor may be involved in the etiology and pathogenesis of PD. Transplantation of modified fibroblasts that express BDNF into either the striatum or the midbrain attenuates 6-hydroxydopamine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal degeneration (15). Also, BDNF can modulate dopaminergic neurotransmission in nigrostriatal neurons, as shown by elevated rotational behavior and increased turnover of DA in the striatum (16). BDNF can promote functional recovery from 6-OHDA lesions following adeno-associated virus (AAV)-mediated BDNF overexpression within striatal medium spiny neurons (17). Together, these observations strongly support an essential role of BDNF signaling for the survival of DAergic neurons, which when lost may contribute to the pathology observed in PD.
In this study, we report that α-Syn directly binds to the kinase domain on TrkB receptors and blocks BDNF/TrkB neurotrophic signaling pathways by suppressing the lipid raft distribution of the BDNF/TrkB complex, as well as the endocytosis and trafficking of the TrkB receptors, ultimately leading to DAergic neurodegeneration. Notably, α-Syn overexpression elicits a prominent reduction in TrkB protein levels, which is mediated by an increase in TrkB ubiquitination. Disruption of α-Syn/TrkB complex formation with the monoamine oxidase B (MAO-B) inhibitor rasagiline rescues BDNF signaling and prevents α-Syn–induced DAergic neuronal loss and the corresponding motor impairment. Hence, this innovative finding provides insight into a pathological role(s) of α-Syn in mediating BDNF/TrkB signal transduction and may represent an unappreciated mechanism by which a-Syn contributes to PD pathogenesis.
Results
α-Synuclein Interacts with TrkB.
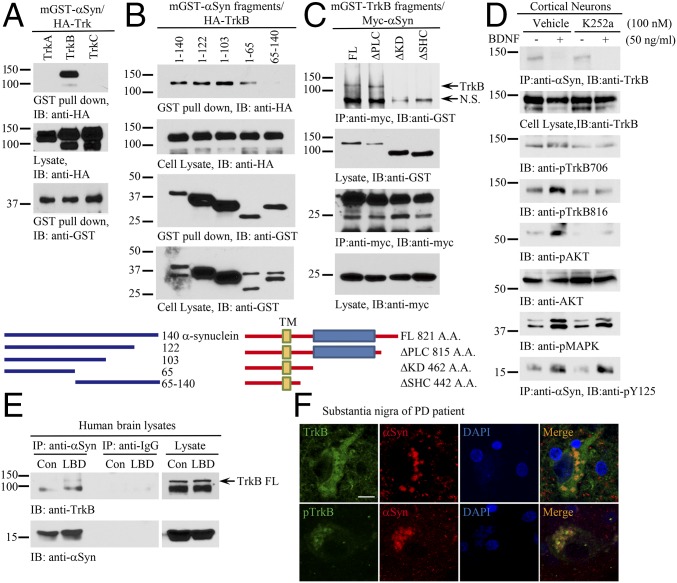
Due to the well-documented correlation between aberrant α-Syn accumulation and nigrostriatal degeneration, the association between reduced BDNF signaling and nigrostriatal degeneration, and the numerous studies revealing the cross-talk between α-Syn and BDNF (18–20), we hypothesized that pathological α-Syn might directly impinge on BDNF/TrkB pathway in PD. To test this hypothesis, we conducted a coimmunoprecipitation assay and found that α-Syn selectively interacted with the TrkB, but not TrkA or TrkC, receptor in cotransfected HEK293 cells (Fig. 1A). A truncation assay revealed that the N terminus of α-Syn was essential for binding to TrkB (Fig. 1B). Further, mapping studies demonstrated that the TrkB kinase domain on the intracellular motif was necessary for α-Syn binding to the TrkB receptor (Fig. 1C). To explore whether the kinase activity is necessary for mediating the interaction between TrkB and α-Syn, we pretreated rat primary cortical neurons with the Trk receptor inhibitor K252a, followed by BDNF treatment. Remarkably, BDNF completely disrupted the formation of the α-Syn/TrkB complex, and inhibition of TrkB barely affected the interactions (Fig. 1D, Top). As expected, BDNF-stimulated phosphorylation of TrkB (p-TrkB), and its downstream p-Akt and p-MAPK signals, was strongly blunted by K252a. Notably, BDNF strongly elicited α-Syn Y125 phosphorylation regardless of the treatment with K252a (Fig. 1D, Bottom). Interestingly, we found that α-Syn also bound to TrkB in human cortex samples from brains of patients with Lewy body dementia (LBD) but not in control brain tissue samples (Fig. 1E). Immunofluorescent staining also verified that TrkB colocalized with α-Syn in LBs in PD patients with p-TrkB signals much dimmer than total TrkB levels (Fig. 1F). Hence, α-Syn interacts with TrkB, which can be inhibited by BDNF treatment.
Fig. 1.
α-Synuclein selectively interacts with TrkB receptors. (A) α-Syn specifically interacts with TrkB receptors. GST pull-down assay was conducted from HEK293 cells cotransfected with mammalian GST–α-Syn and HA-Trks. (B) α-Syn N terminus is implicated in binding TrkB. Different mGST-tagged α-Syn truncated were cotransfected with HA-TrkB into HEK293 cells. A GST pull-down assay was performed, and coprecipitated proteins were analyzed by immunoblotting with anti-HA (Top). Schematic diagram of α-Syn truncations (Bottom). (C) TrkB kinase domain is indispensable for α-Syn to interact with TrkB. (Top) Mapping assay for TrkB ICD required for binding to α-Syn. (Bottom) Schematic diagram of TrkB domains. (D) BDNF inhibits α-Syn/TrkB association. Cortical neurons were pretreated with K252a (100 nM) for 15 min, followed by BDNF treatment (50 ng/mL) for 30 min. Coimmunoprecipitation was performed with anti–α-Syn, and the coprecipitated proteins were analyzed by immunoblotting with anti-TrkB (Top). Cell lysates were probed with various antibodies (second through bottom panels). (E) α-Syn associates with TrkB in LBD human patient brains. Brain lysates from LBD patients were immunoprecipitated with control IgG or anti–α-Syn, and the coprecipitated proteins were analyzed by immunoblotting with anti-TrkB. (F) TrkB colocalizes with α-Syn in the LBs of PD patients. Immunofluorescent costainings with anti-TrkB or p-TrkB 706 (Green) and α-Syn (Red) were conducted with human PD brain sections. The nuclei were stained with DAPI. (Scale bar, 20 μm.)
α-Synuclein Inhibits BDNF-Mediated TrkB Signaling.
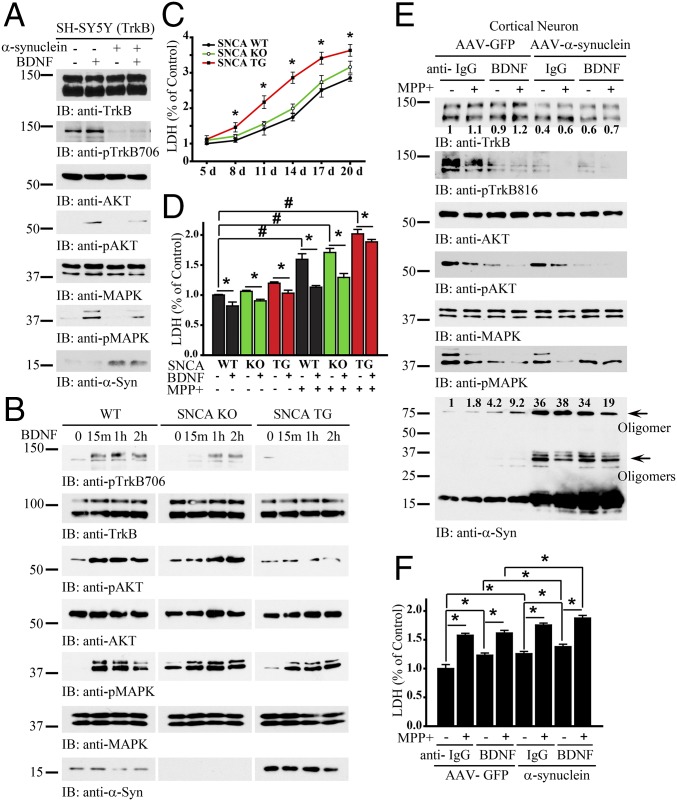
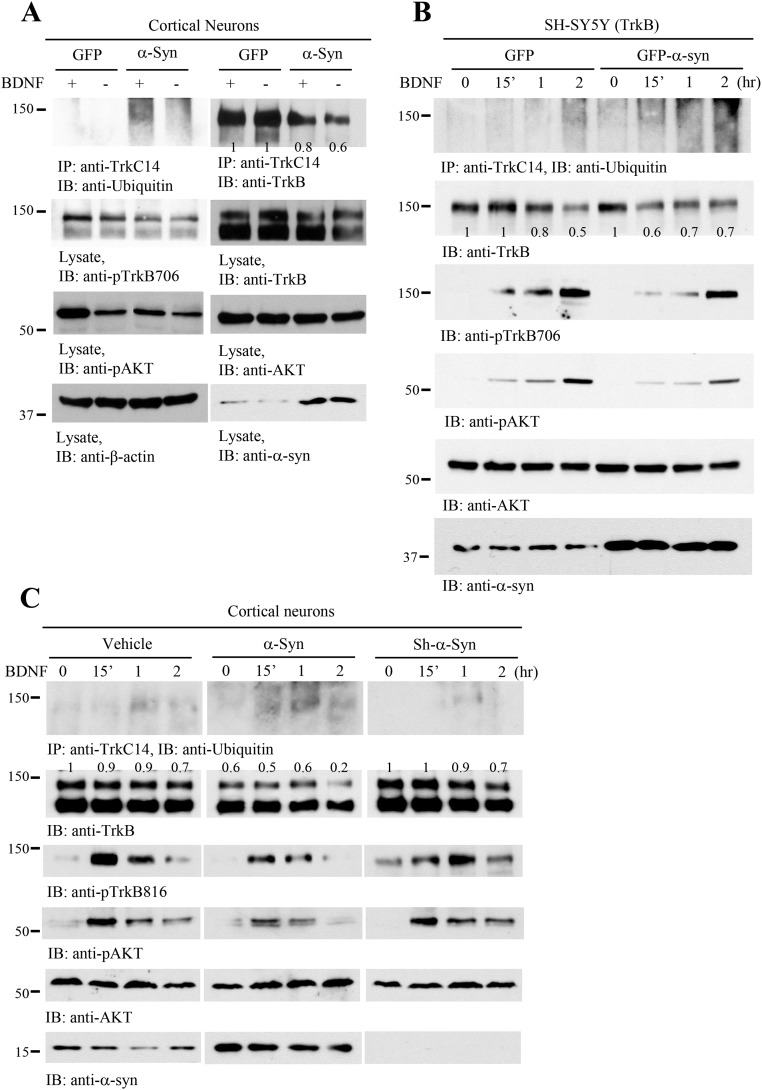
To examine the biological effect of α-Syn on BDNF/TrkB signaling, we transfected BR6 cells (SH-SY5Y cells stably transfected with the TrkB receptor) with α-Syn, followed by BDNF stimulation. BDNF-induced phosphorylation of TrkB and its downstream effectors was blocked by α-Syn overexpression (Fig. 2A). We also extended the experiment into primary cortical neurons from SNCA transgenic overexpressing mice or SNCA KO mice. In wild-type neurons, BDNF elicited prominent p-TrkB/p-Akt/p-MAPK signaling, which was significantly suppressed in SNCA transgenic neurons. In SNCA KO neurons, the BDNF-triggered signaling cascade remained intact, though the temporal onset of TrkB activation was delayed (Fig. 2B). One of the key physiological functions of the BDNF/TrkB pathway is to promote neuronal survival. Consequently, we performed a lactate dehydrogenase (LDH) release cytotoxicity assay to quantify spontaneous cell death. The LDH assay demonstrated that SNCA transgenic neurons exhibited much higher spontaneous cell death compared with wild-type or SNCA KO neurons (Fig. 2C). Treatment of primary neurons with BDNF significantly repressed neuronal cell death regardless of genotype (Fig. 2D). To investigate how α-Syn affects BDNF-mediated neuroprotection in the face of PD-associated pathology, we next treated primary neurons with the neurotoxicant 1-methyl-4-phenylpyridinium (MPP+) and analyzed LDH release. MPP+-induced LDH release was strongly reduced in both wild-type and SNCA KO neurons by BDNF treatment. However, the neuroprotective effect of BDNF was much weaker in SNCA transgenic neurons (Fig. 2D). To further investigate the biological role of α-Syn in BDNF-mediated neuronal survival, we transduced primary cortical neurons with AAV-expressing human α-Syn. And neurons underwent BDNF deprivation (by treatment with an anti-BDNF antibody) either in the presence or absence of MPP+. Within neurons transduced with the AAV-GFP control vector, depletion of BDNF diminished p-TrkB. MPP+ also reduced p-TrkB, and the combination of both BDNF depletion and MPP+ treatment resulted in an additive effect to further decrease p-TrkB (Fig. 2E, second panel, left four lanes). Strikingly, AAV-mediated overexpression of α-Syn strongly repressed BDNF-induced phosphorylation of TrkB. The combination of α-Syn overexpression with MPP+ treatment completely abolished p-TrkB levels, and importantly this effect was independent of the presence or absence of BDNF. Moreover, total TrkB levels were reduced following α-Syn overexpression (Fig. 2E, top two panels). The downstream phosphorylation of Akt followed the p-TrkB pattern. P-MAPK displayed similar effects, except that p-MAPK 42 was not further diminished in the AAV–α-Syn-infected neurons treated with anti-BDNF (Fig. 2E, panels 3–6). Immunoblotting of α-Syn showed increased α-Syn, which was prominently displayed as oligomers in the presence of MPP+ and anti-BDNF. Overexpression of α-Syn induced notable oligomerization (Fig. 2E, Bottom). We next analyzed cytotoxicity within these treatment groups. The LDH assay revealed an inverse correlation between levels of p-TrkB/p-Akt and LDH release. Neurons transduced with AAV–α-Syn exhibited more cell death than those transduced with AAV-GFP. Further, BDNF deprivation also significantly increased cell death. Finally, maximal cytotoxicity was observed following the combination of MPP+ and BDNF deprivation (Fig. 2F). These data indicate that overexpression of α-Syn reduces TrkB protein levels as well as p-TrkB signaling, thereby inhibiting the neurotrophic effects of BDNF, resulting in much more robust neuronal death in the face of PD-associated toxicity.
Fig. 2.
Overexpression of α-Syn blocks BDNF/TrkB signaling. (A) α-Syn inhibits BDNF/TrkB signaling. TrkB stably transfected SH-SY5Y (BR6) cells were transfected with α-Syn, followed by treatment with BDNF for 10 min. p-TrkB and its downstream effectors, p-Akt and p-MAPK, were analyzed in the cell lysates. (B) BDNF/TrkB signaling is blocked in SNCA overexpressing transgenic neurons. Wild-type, SNCA KO, and SNCA transgenic neurons were treated with BDNF for 15 min, 1 h, or 2 h, and the pTrkB signaling cascade was probed with various antibodies. (C) LDH assay of SNCA transgenic, SNCA KO, and wild-type dopaminergic neurons. (D) α-Syn overexpression decreases the neuroprotective effects of BDNF against MPP+-induced neuronal cell death. MPP+ sensitized α-Syn–induced neuronal cell death. Shown are SNCA transgenic, SNCA KO, or wild-type dopaminergic neurons in the presence or absence of BDNF, treated with MPP+ (200 μM) or not for 24 h. LDH assay was conducted with cell medium. (E and F) Overexpression of α-Syn decreases TrkB levels and elevates neuronal cell death. Primary cortical cultures were infected with AAV virus expressing α-Syn or GFP control, followed by treatment with anti-BDNF or anti-IgG, then treated with MPP+ (200 μM) for 24 h. Immunoblotting analysis of cell lysates with various antibodies (E) and LDH assay of the treated cells (F). Data are shown as mean + SEM. n = 3 each group. *P < 0.05, #P < 0.01. The relative intensities of the band that were quantified with Image J were indicated in the immunoblots.
α-Synuclein Blocks TrkB Signaling by Inhibiting Its Internalization and Lipid Raft Distribution.
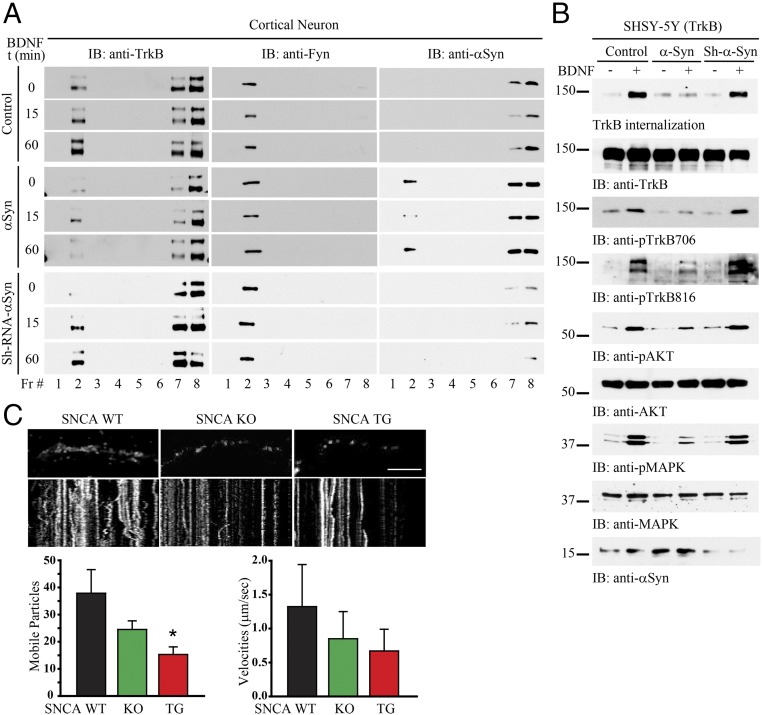
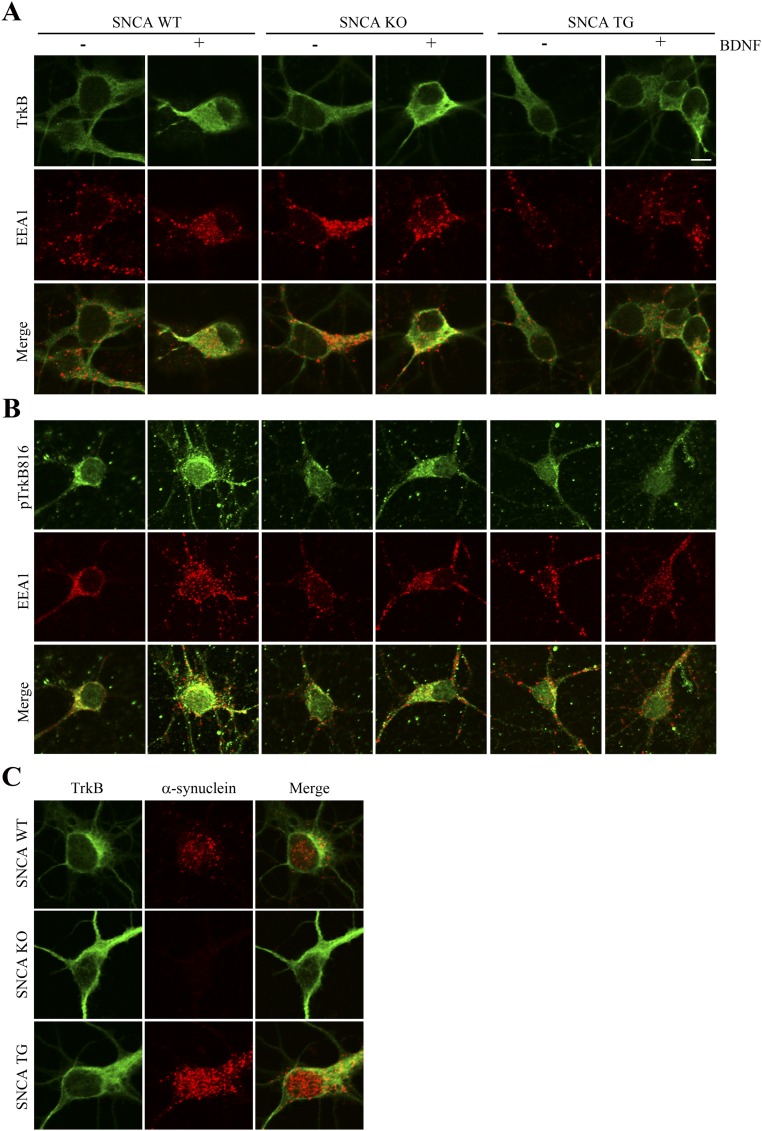
TrkB receptors reside within lipid rafts, and this localization is mediated by Fyn tyrosine kinase. Disrupting lipid rafts prevents the full activation of TrkB (21). To test whether α-Syn mediates TrkB cellular localization, we conducted a subcellular fractionation experiment. We prepared primary cortical neuronal cultures and transduced the cells with AAV overexpressing α-Syn or a short-hairpin RNA (shRNA) targeting endogenous α-Syn, followed by BDNF treatment for 0, 15, or 60 min. In control neurons, BDNF treatment resulted in a TrkB enrichment in fraction #2, where Fyn also eluted (i.e., this fraction also contained lipid rafts, as Fyn is also used as a marker for these; Fig. 3A). In addition, TrkB and α-Syn coeluted in fraction #7 and #8. When α-Syn was overexpressed, it distributed into fraction #2 as well. Notably, TrkB levels in lipid raft fractions were clearly reduced following α-Syn overexpression, whereas Fyn levels remained intact. Following α-Syn knockdown, TrkB levels in fraction #2 were increased upon BDNF stimulation, similar to control cells (Fig. 3A). The NT–Trk complex internalization is essential for the signal transduction that initiates the cellular response to target-derived NTs, and this process is regulated through clathrin-mediated endocytosis, leading to the formation of signaling endosomes (22, 23). Next, we examined whether α-Syn also regulates TrkB internalization in BR6 cells. BDNF strongly stimulated TrkB internalization in control cells and α-Syn shRNA-treated cells, associated with robust p-TrkB, p-Akt, and p-MAPK signals. Nonetheless, these effects were substantially antagonized by α-Syn overexpression (Fig. 3B). To further assess the effect of α-Syn on TrkB endocytosis, we performed immunofluorescent staining to monitor the subcellular localization of TrkB as well as the colocalization of TrkB with EEA1, a marker for early endosomes. BDNF induced the internalization of both TrkB and p-TrkB as indicated by colocalization with EEA1. These effects were reduced in SNCA transgenic neurons (Fig. S1). The trafficking of NT/Trk complexes within the endosome plays a critical role in neurotrophic signaling cascade. Accordingly, we performed a live-cell imaging assay using wild-type, SNCA transgenic, and SNCA KO dopaminergic neurons. We found that the mobility of TrkB fluorescent particles within the axon of SNCA overexpressing transgenic neurons was significantly impaired compared with that observed in wild-type or SNCA KO cells (Fig. 3C), fitting with previous reports that formation of α-Syn LN-like aggregates in axons impedes the transport of distinct endosomes (24).
Fig. 3.
α-Syn decreases TrkB lipid raft distribution, internalization, and axonal transportation. (A) Lipid raft distribution assay. Primary cortical cultures were infected with various AAV constructs overexpressing α-Syn or depleting α-Syn, followed by treatment with BDNF for 0, 15, and 60 min, respectively. The samples were subsequently subjected to subcellular fractionation. TrkB was dispensed in fraction #2, #7, and #8. Fyn, a well-characterized lipid raft resident, was distributed in fraction #2, with TrkB. (B) α-Syn decreases TrkB internalization and its downstream signaling. BR6 cells were transduced with the indicated virus, followed by treatment with BDNF for 15 min. TrkB internalization was conducted and monitored by immunoblotting. (C) Live-cell imaging. Different genotypes of dopaminergic neurons were transfected with GFP-TrkB and imaged 7 d later. Images were captured every 1 s for 3 min. Neurons were treated with BDNF for 30 min before imaging, and BDNF was included in the imaging media. Images are from movies captured every 1 s for 3 min. (Scale bar, 10 μm.) Kymographs shown below were generated as visual representations of distance traveled over time. Of the whole particles, the percentages of anterograde and retrograde mobile particles were quantified (Left). The speed of mobile TrkB-GFP particles was measured in BDNF-treated neurons (Right). Data are shown as mean + SEM. n = 6–9 axons each group. *P < 0.05.
Fig. S1.
TrkB endocytosis is reduced in SNCA transgenic neurons. (A and B) BDNF elicits prominent TrkB endocytosis in both wild-type and SNCA KO neurons but not in SNCA transgenic neurons. Hippocampal neurons from wild-type, SNCA KO, and SNCA transgenic mice were cultured and treated with BDNF for 15 min. The neurons were fixed and stained with anti-TrkB or p-TrkB (green) or anti-EEA1 (red) for the early endosomes. (Scale bar, 20 μm.) (C) Immunofluorescent staining of TrkB and α-Syn in primary wild-type, SNCA KO, and SNCA transgenic neurons.
α-Synuclein Blocks the Prosurvival Effects of BDNF/TrkB in Dopaminergic Neurons.
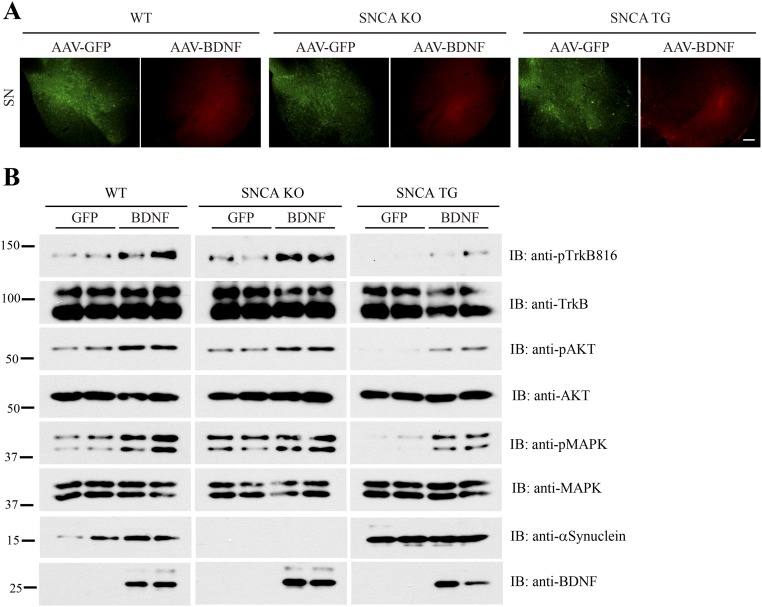
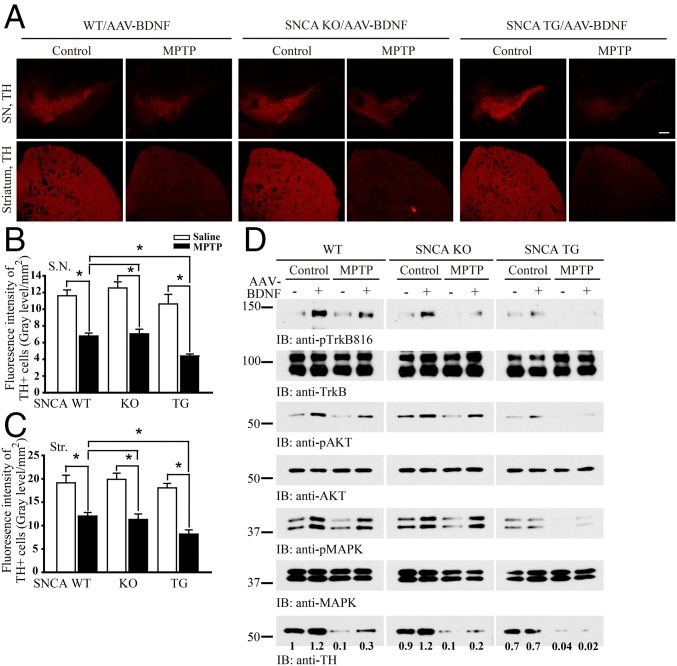
To explore the biologic effect of α-Syn–mediated blocking of BDNF/TrkB neurotrophic activities in DAergic neuronal survival, we stereotaxically delivered AAV-GFP into the left SNpc and AAV-BDNF into the right SNpc of WT SNCA KO or SNCA transgenic overexpressing mice. Mice then received a daily i.p. injection of MPTP (30 mg/kg) treatment for 5 d. Immunofluorescent staining demonstrated that GFP and BDNF were appropriately expressed in the respective SNpc regions (Fig. S2A). BDNF– expression induced the p-TrkB/p-Akt/p-MAPK signaling cascade in WT and SNCA KO mice, however this effect was suppressed in SNCA transgenic mice (Fig. S2B). As expected, MPTP administration induced substantial DAergic neurodegeneration in all of the genotypes, but tyrosine hydroxylase (TH)-positive cell loss was significantly greater in SNCA transgenic mice compared with WT or SNCA KO mice (Fig. 4 A–C). Immunoblotting analysis demonstrated that TH immunoreactivity was reduced in SNCA transgenic mice compared with wild-type or SNCA KO mice, and MPTP treatment almost completely eliminated TH immunoreactivity in SNCA transgenic mice (Fig. 4D). Hence, α-Syn inhibits TrkB signaling, rendering DAergic neurons more vulnerable to the neurotoxin MPTP.
Fig. S2.
BDNF virus induces TrkB signaling in SN. (A) Immunofluorescent staining to verify that BDNF is injected and expressed in the SN regions in different genotypes of mice. (Scale bar, 200 μm.) (B) BDNF-induced TrkB signals are blocked in SNCA transgenic mice. Immunoblotting analysis of SN from mice injected with AAV-GFP or AAV-BDNF with various indicated antibodies. BDNF elicited similar signaling cascades in both wild-type and SNCA KO tissues, which was impaired in SNCA transgenic tissues.
Fig. 4.
α-Syn overexpression accelerates dopaminergic neuronal loss following MPTP administration. (A) α-Syn overexpression sensitizes TH neuronal loss induced by MPTP. SNCA transgenic, SNCA KO, or wild-type mice were injected with AAV-BDNF virus. After 2 wk, MPTP (30 mg·kg·d) was injected for 5 d. TH immunoreactivity within the SNpc and striatum was analyzed by immunofluorescent staining. (Scale bar, 200 μm.) (B and C) Quantification of TH-positive fluorescent signaling in SNpc (B) and striatum (Str) (C). Overexpression of α-Syn exacerbated MPTP-induced dopaminergic neuronal loss, which was reduced by BDNF. Data are shown as mean + SEM. n = 6 sections each group. *P < 0.05. (D) Immunoblotting analysis of SNpc tissue lysates from the above animals. The relative intensities of TH that were quantified with Image J were indicated under the immunoblots.
Disruption of α-Syn/TrkB Association Promotes Dopaminergic Neuronal Survival.
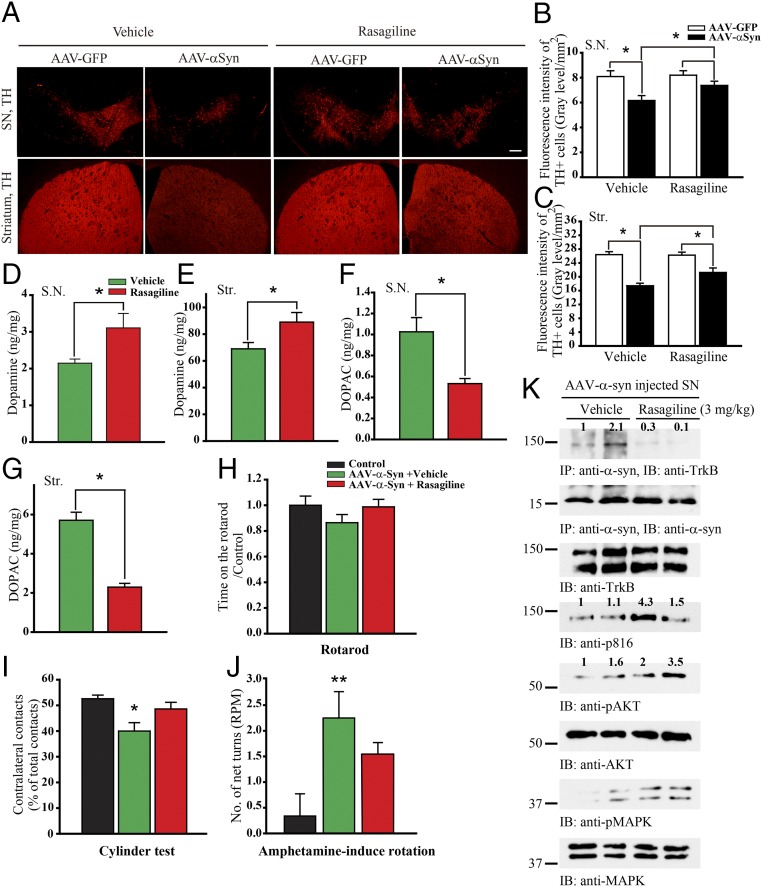
The DA metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) induces α-Syn binding to TrkB, leading to suppression of TrkB neurotrophic signaling and escalation of DAergic neurodegeneration (Fig. S3). Next, we tested whether the inhibition of DOPAL by MAO-B inhibitor (rasagiline) could block α-Syn/TrkB association, promoting DAergic neuron survival in vivo as well as in BR6 cells (Fig. S4). We injected mice with AAV-GFP or AAV–α-Syn into the left and right substantial nigra (SN) of the same mice, respectively, followed by rasagiline administration. As expected, overexpression of α-Syn elicited DAergic neurodegeneration in the SN and nigrostriatal denervation of the striatum compared with the expressing hemisphere. This effect was alleviated by rasagiline treatment (Fig. 5 A–C). Consequently, DA concentrations were significantly elevated in both SN and striatum (Fig. 5 D and E). Since MAO-B was potently inhibited by rasagiline, its oxidation product DOPAC was substantially reduced (Fig. 5 F and G). Motor behavioral assays indicated that α-Syn–induced motor dysfunctions were rescued by rasagiline (Fig. 5 H–J). Moreover, coimmunoprecipitation assays showed that rasagiline strongly inhibited the association between α-Syn and TrkB, restoring BDNF/TrkB neurotrophic signaling (Fig. 5K). Conceivably, rasagiline disrupts α-Syn/TrkB complex via inhibiting MAO-B–produced DOPAL.
Fig. S3.
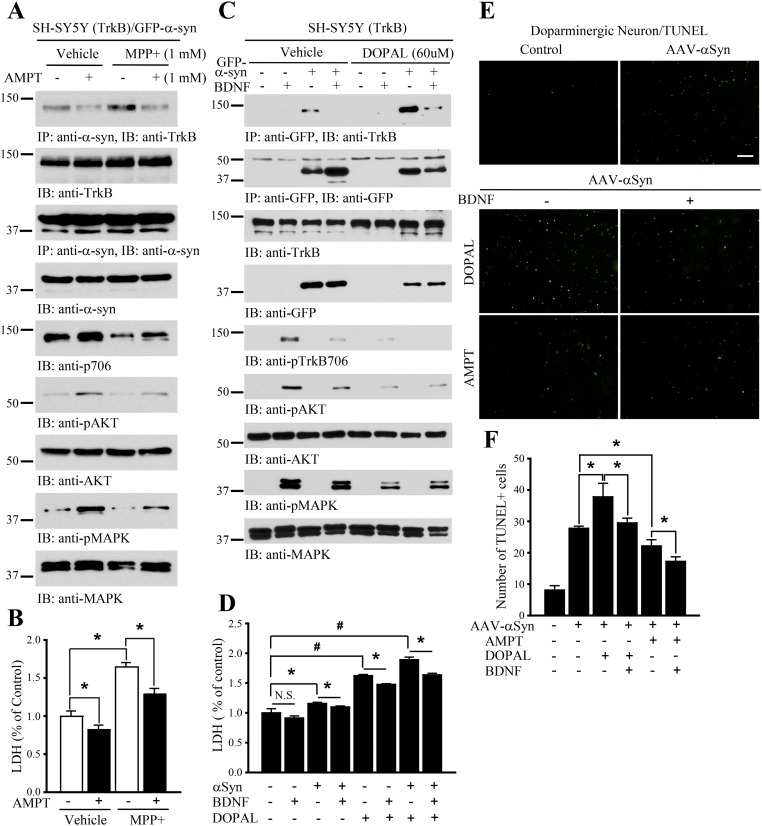
DA and its metabolite DOPAL augment α-Syn/TrkB association. (A and B) Inhibition of DA by AMPT antagonizes the interaction between α-Syn and TrkB, reducing α-Syn and MPP+ neurotoxicity. Data are shown as mean + SEM. n = 3 each group. *P < 0.05. (C) DOPAL increases TrkB/α-Syn association. BR6 cells were transfected with GFP–α-Syn, followed by DOPAL treatment in the presence or absence of BDNF. DOPAL induced α-Syn/TrkB complex formation and inhibited TrkB signals. (D) DOPAL increases α-Syn–induced neuronal cell death. LDH assay with the above-indicated cell medium. Data are shown as mean + SEM. n = 3 each group. *P < 0.05, #P < 0.01. (E and F) DA and DOPAL mediate dopaminergic neurons induced by α-Syn. TUNEL assays were conducted on dopaminergic neurons, infected with AAV–α-Syn, and treated with DOPAL or TH inhibitor AMPT, in the presence or absence of BDNF. Data are shown as mean + SEM. n = 3 each group. *P < 0.05. (Scale bar, 200 μm.) N.S., not significant.
Fig. S4.
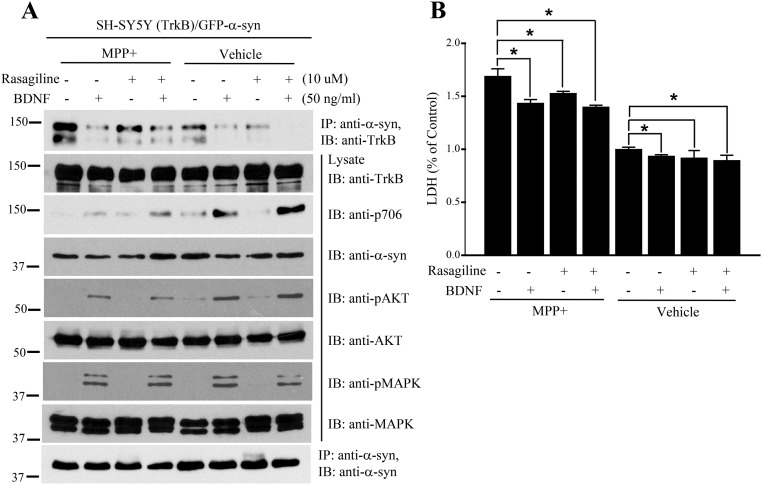
MAO inhibitor inhibits MPP+-stimulated α-Syn/TrkB association. (A) Rasagiline dissociates MPP+-triggered α-Syn/TrkB complex. BR6 cells were transfected with GFP–α-Syn, followed by rasagiline (10 μM) treatment in the presence or absence of BDNF, stimulated with MPP+ for 24 h. α-Syn was immunoprecipitated and the coprecipitated TrkB was monitored by immunoblotting (Top). The cell lysates were analyzed by immunoblotting with the indicated antibodies. Rasagiline restored TrkB signals inhibited by α-Syn. (B) Rasagiline rescues MPP+ and α-Syn–induced cell death. Shown is the LDH assay with the medium from the above indicated cells. Data are shown as mean + SEM. n = 3 each group. *P < 0.05.
Fig. 5.
Rasagiline disrupts α-Syn/TrkB complex and rescues dopaminergic neurons from α-Syn–induced cell death. (A) Rasagiline reduces TH loss induced by α-Syn. C57BL/6 mice were injected with AAV-GFP or AAV–α-Syn into the left and right SN, respectively, followed by rasagiline (3 mg·kg·d) treatment for 10 d. TH expression in SN and striatum was analyzed by immunofluorescent staining. (Scale bar, 200 μm.) (B and C) Quantification of TH-positive fluorescent signaling in SN (B) and striatum (Str) (C). Data are shown as mean + SEM. n = 6 sections each group. *P < 0.05. (D and E) DA concentrations in SN and striatum were increased by rasagiline in α-Syn–overexpressed mice. (F and G) DA metabolite DOPAC concentrations in SN and striatum were decreased by MAO-B inhibitor, rasagiline, in α-Syn–overexpressed mice. Data are shown as mean + SEM. n = 3 each group. *P < 0.05. (H–J) Motor behavioral assays. Overexpression of α-Syn induced motor impairment, and rasagiline significantly improved the motor functions. Data are shown as mean + SEM. n = 8 each group. *P < 0.05, **P < 0.01. (K) Rasagiline disrupts α-Syn/TrkB complex and restores p-TrkB signaling. Coimmunoprecipitation assay with anti–α-Syn from SN tissues treated with or without rasagiline and coprecipitated proteins were analyzed by immunoblotting. SN lysates were probed by various indicated antibodies.
Discussion
In the current study, we provide compelling evidence that α-Syn directly interacts with the TrkB receptors. This interaction is in turn negatively regulated by BDNF and Fyn tyrosine kinase activity, resulting in the phosphorylation of α-Syn on Y125, causing it to dissociate from TrkB receptors (Fig. S5). Strikingly, α-Syn binds TrkB and completely suppresses its neurotrophic activities, increasing the vulnerability of DA neurons to degeneration. Moreover, α-Syn potently reduces TrkB protein levels by stimulating TrkB ubiquitination (Fig. S6). The data presented herein suggest that α-Syn blocks TrkB signaling by diminishing TrkB endocytosis, internalization, and axonal transport. Though α-Syn overexpression in neurons from SNCA overexpressing transgenic mice greatly inhibits BDNF/TrkB neurotrophic signaling, the absence of α-Syn in neurons of SNCA KO mice has little effects on these events. Here, we observed that α-Syn KO exhibit a slight delay in the kinetics of BDNF-induced phosphorylation of TrkB and its downstream effectors. This delay did not affect the neurotrophic activity of BDNF, as the effects of BDNF treatment were comparable in both wild-type and SNCA KO DA neurons in the face of MPP+-induced neurotoxicity (Fig. 2 C–F). These observations indicate that SNCA is not required for BDNF/TrkB neurotrophic activities, despite the finding that increased levels of α-Syn can inhibit BDNF neurotrophic signaling. Previous studies indicate that BDNF stimulates the association between endogenous TrkB and Fyn. In neurons derived from Fyn knockout mice, the translocation of TrkB to lipid rafts in response to BDNF is compromised, while inhibiting TrkB translocation to lipid rafts prevents the full activation of TrkB and downstream signals (21). In the current study, we replicated the finding that BDNF treatment translocates TrkB to lipid rafts and extended these findings to show that α-Syn overexpression diminishes TrkB lipid raft residency, whereas α-Syn depletion does not affect TrkB subcellular distribution. Using TrkB stably transfected dopaminergic SH-SY5Y cells, we showed that BDNF-triggered TrkB internalization is abolished by α-Syn overexpression. These findings are consistent with previous reports that BDNF-induced TrkB accumulation at lipid rafts is prevented by blocking the internalization of TrkB (21). Conceivably, BDNF treatment activates Fyn, which subsequently phosphorylates α-Syn on Y125, resulting in a dissociation from TrkB, allowing TrkB lipid raft translocation. α-Syn interacts with complex I of the mitochondrial respiratory chain and interferes with its function, promoting the production of reactive oxygen species (ROS) (25). Accumulating evidence suggests that the toxic interaction between DA, DA metabolites, and α-Syn might promote an oxidative environment within DAergic neurons. Oxidative modification of α-Syn by DA metabolites has been proposed to be responsible for the selective vulnerability to DAergic neurons (26, 27). DA-modified α-Syn tends to form protofibrillar intermediates but not large fibrils (27). Such “oligomeric” α-Syn has been suggested to represent the primary toxic species responsible for DAergic neurotoxicity (28). Consistent with these reports, we found that DA and its metabolite, DOPAL, strongly stimulate α-Syn binding to TrkB receptors, completely blocking TrkB neurotrophic signaling, a phenomenon which was partially attenuated by BDNF. Accordingly, α-Syn overexpression-induced neuronal cell death was further escalated by DOPAL. Moreover, blocking DA biosynthesis by α-methyl-p-tyrosine (AMPT), which inhibits TH activity, significantly attenuates α-Syn–elicited neuronal cell death (Fig. S3). Elevation of DOPAL levels may contribute to the specific vulnerability of DAaergic neurons to complex I inhibition (29). DOPAL covalently modifies α-Syn and stimulates its aggregation and is neurotoxic in vivo (30). Fitting with in vitro results (Fig. S4), we found that rasagiline strongly dissociates the α-Syn/TrkB complex in the SNpc of mice injected with AAV–α-Syn or MPTP, resulting in a subsequent up-regulation of p-TrkB/p-Akt/p-MAPK signaling. Consequently, rasagiline significantly attenuated α-Syn–induced DAergic neuron death in the SN with the concomitant preservation of DA terminals within the striatum and improvements in motor functions. These findings are consistent with previous reports that rasagiline promotes regeneration of SN dopaminergic neurons in post–MPTP-induced parkinsonism via activation of tyrosine kinase receptor signaling pathway (31, 32).
Fig. S5.
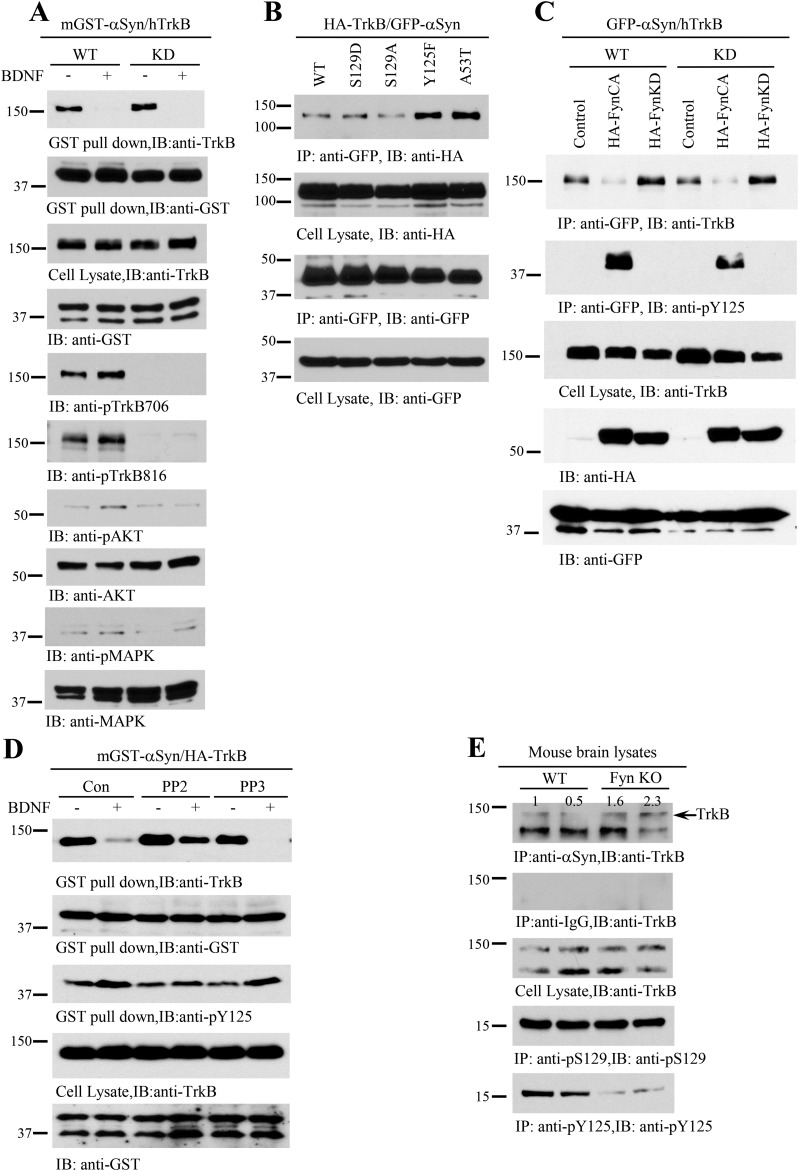
Fyn but not TrkB tyrosine kinase activity is implicated in α-Syn/TrkB association. (A) TrkB kinase activity is not required for α-Syn/TrkB association. TrkB wild-type or kinase-dead (K576A) mutant was cotransfected with mGST–α-Syn into HEK293 cells. A GST pull-down assay was performed, and coprecipitated proteins were analyzed by immunoblotting for TrkB (Top). Cell lysates were analyzed by immunoblotting with various antibodies (second through bottom panels). (B and C) α-Syn Y125 phosphorylation inhibits its interaction with TrkB. Various GFP-tagged α-Syn mutants were cotransfected with HA-TrkB; α-Syn was immunoprecipitated with anti-GFP and analyzed by immunoblotting with anti-HA. The nonphosphorylatable Y125F and the PD-associated A53T mutant forms of α-Syn displayed increased interaction with TrkB compared with WT α-Syn (B). Constitutively active Fyn CA but not kinase-dead Fyn KD disrupted α-Syn/TrkB interaction (C). (D) Fyn inhibition increases the interaction between α-Syn andTrkB. HEK293 cells cotransfected with α-Syn and TrkB were pretreated with the Fyn inhibitor PP2, or its inactive analog (PP3) with phosphorylation of α-Syn at Y125 was potently blocked by PP2 but not PP3 (Bottom). (E) Depletion of Fyn increases the association between α-Syn/TrkB. Wild-type and Fyn KO brain lysates were immunoprecipitated with anti–α-Syn, and coprecipitated proteins were analyzed by immunoblotting for TrkB (Top). Brain lysates from Fyn KO mice showed a reduction in α-Syn–TrkB complex formation with a corresponding decrease in α-Syn pY125 content (Bottom).
Fig. S6.
α-Syn enhances TrkB ubiquitination and reduces TrkB protein levels. (A) α-Syn overexpression escalates BDNF-induced TrkB ubiquitination. Primary cortical cultures were infected with AAV-GFP or AAV–α-Syn, followed by BDNF treatment. TrkB was immunoprecipitated and monitored by immunoblotting with anti-ubiquitin. TrkB levels were reduced in α-Syn–overexpressed cells (top two panels). (B) α-Syn escalates TrkB ubiquitination. BR6 cells were treated with BDNF for different time points, and TrkB receptors were immunoprecipitated and examined by immunoblotting with anti-ubiquitin (Top). Cell lysates were monitored by different antibodies, and TrkB levels and its p-TrkB/p-Akt signals were reduced, when α-Syn was overexpressed. (C) Depletion of α-Syn does not affect TrkB ubiquitination or its protein levels. The relative intensities of TrkB that were quantified with Image J were indicated in the immunoblots.
Here we show that α-Syn binds to, and inhibits, TrkB neurotrophic activities. The current study provides a pathological function for α-Syn in the binding of the TrkB receptor and resultant inhibition of BDNF/TrkB neurotrophic signaling. This interaction is strongly up-regulated by DA’s metabolite DOPAL, resulting in increased dopaminergic neuronal death. This discovery provides a model for the underlying molecular etiology of α-Syn–mediated neurotoxicity and DAergic neuronal loss in PD.
Methods
Animals.
All mice were obtained from the Jackson Laboratory. Eight- to 12-wk-old C57BL/6 were used as controls. SNCA null mice (B6;129 × 1-Sncatm1Rosl, stock no. 003692) or human SNCA overexpressing mice [B6.Cg-Tg(SNCA)OVX37Rwm Sncatm1Rosl/J, stock no. 023837] on pure genetic background were backcrossed. Genotyping was performed by PCR using genomic DNA isolated from the tail tip. PCR was performed using mutant primers F (5′TCA GCC ACG ATA AAA CTG AGG3′), R (5′GCC TGA AGA ACG AGA TCA GC3′) and transgene primers F (5′CCT CCT GTT AGC TGG GCT TT3′), R (5′ACC ACT CCC TCC TTG GTT TT3′). Animal care and handling were performed according to the Declaration of Helsinki and Emory Medical School guidelines. Investigators were blinded to the group allocation during the animal experiments. The protocol was reviewed and approved by the Emory Institutional Animal Care and Use Committee.
Human Tissue Samples.
Postmortem brain frozen samples of LBD patients (n = 3) and paraffin-embedded sections of PD patients (n = 3) were provided from the Emory Alzheimer’s Disease Research Center. The study was approved by the biospecimen committee at Emory University. LBD and PD cases were clinically diagnosed and neuropathologically confirmed. Informed consent was obtained from all subjects.
Plasmid Clones and Viral Genomes.
AAV viral genome contained either the human α-syn or the GFP coding sequence controlled by the hybrid chicken beta-actin/cytomegealovirus promoter. AAV2/5 and LV pseudotyped with VSV-G was produced as described previously (33).
Statistical Analysis.
Statistical analysis was performed using either Student’s t test (two-group comparison) or one-way ANOVA followed by LSD post hoc test (more than two groups), and differences with P values less than 0.05 were considered significant.
SI Methods
Immunoprecipitation.
The mouse brain tissue samples or cultured cells were lysed in lysis buffer and centrifuged for 15 min at 16,000 g. The supernatant was incubated with anti–α-synuclein or GFP antibody and protein A/G-agarose overnight at 4 °C. After extensive washing, the bound proteins were eluted from the beads by boiling in sample buffer and subjected to Western blot analyses.
Primary Neuron Cultures.
Primary rat ventral midbrain or cortical neurons in E18 were cultured. Neurons were dissected and suspended by pipetting in DMEM containing 5% FCS and 5% horse serum. The cell suspension was then centrifuged at 250 × g for 5 min. This operation was repeated again. Cells were seeded into polyethyleneimine-coated 10-cm dishes and 12-well plates, including coated coverslips, and incubated at 37 °C in 5% CO2/95% air. After 3 h, the culture medium was changed to Neurobasal containing a B-27 supplement (Invitrogen), and a half medium was changed to fresh Neurobasal/B-27 every 4 d. Neurons cultured 7 d in vitro (DIV 7) were transduced with AAV-GFP, AAV–α-syn, or Lenti-Sh–α-syn. After 2–3 d, cells were treated by MPP (20 µM), AMPT (1 mM), or DOPAL (60 µM) for 24 h. BDNF was pretreated for 15 min before cell lysis. For Western blot experiments, cells were collected, lysed, and denatured with 4× sample buffer at 95 °C for 5 min. The neurons were fixed in 4% formaldehyde, permeabilized, and immunostained with anti-TH antibody and TUNEL. The toxicity was analyzed by counting TUNEL-positive cells.
Transfection and Viral Infection of the Cells.
HEK293 cells were transfected with plasmids encoding mGST-, Myc-, HA-, or GFP-tagged alpha-synuclein, point mutant alpha-synuclein, wild-type TrkB, or kinase dead TrkB using polyethylenimine (PEI). AAV–α-syn, AAV-GFP, or Lenti-ShRNA–α-syn were used for infection in primary neurons and SH-SY5Y cells. The neurotoxicity was analyzed using LDH assay (CytoTox 96 Non-Radio; Promega).
Lipid Rafts Isolation.
DIV 9–11 rat cortical neurons were infected by control, AAV–α-syn, or Lenti-Sh–α-syn for 3 d. BDNF was supplied 0, 15, or 60 min before cell lysis. Lysates were fractionated by discontinuous Optiprep Density Gradient Medium (sigma). The 35% Optiprep solution (mixture of 750 μL of lysates and 850 μL of a 70% Optiprep) was overlayed sequentially with 8 mL of 30% Optiprep in lysis buffer and 3.2 mL of lysis buffer. The gradients were centrifuged for 6 h at 36,000 rpm in a Beckman ultracentrifuge, using an SW41 Ti rotor. Eight fractions of 1.5 mL were then collected from the top, after discarding the first 1.1 mL. For lower concentration samples, the samples were concentrated using Amicon Ultra centrifugal filter tubes (Millipore) to increase the loading concentration of lysates.
Behavioral Test.
Loss of motor function due to DAergic neurodegeneration was tested 15 wk following the vector injection or 5 d following the MPTP injection. Behavioral test included the rotarod test, grid performance, cylinder test, and amphetamine-induced rotation. For the rotarod test, mice were trained for 3 sequential days on the rotarod. Each daily practice session consisted of placing the subject on the rotarod (San Diego Instruments) at a slow rotational speed (5 rpm) for a maximum of 10 min. Mice were given three test trials on the test day. The rotational speed of rotarod was modulated from 0 rpm to a maximum 40 rpm. It was gradually increased during the trial at a rate of 0.1 rpm/s. Latency to fall down from the accelerating rotarod as well as the rotational speed were the primary dependent variables. For the grid performance test, the mice were placed on a horizontal grid. The grid was inverted, so the mice were hanging upside down by their paws. Animals were videotaped for up to 60 s, and then a 10-s segment scored for forepaw step distance as defined by the number of grids transversed with each step. For the cylinder test, mice were placed individually inside a glass cylinder (12 cm diameter, 22 cm height) and video-recorded. Video files were examined by an observer blinded to the animal’s treatment. Between 20 and 30 wall touches per animal (contacts with fully extended digits executed with the forelimb ipsilateral and contralateral to the lesion) were counted. For the amphetamine-induced rotation test, d-amphetamine (free base, 2 mg/kg in saline; Sigma) was injected i.p. The number of completed ipsilateral or contralateral circles was quantified.
Western Blot Analysis.
The mouse brain, human tissue samples, or cultured cells were lysed in lysis buffer (50 mM Tris, pH 7.4, 40 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 10 mM sodium β-glycerophosphate, supplemented with a mixture of protease inhibitors) and centrifuged for 15 min at 16,000 g. The supernatant was boiled in SDS loading buffer. After SDS/PAGE, the samples were transferred to a nitrocellulose membrane. Primary antibodies to the following targets were used: GST-HRP, alpha-tubulin, beta-actin (Sigma-Aldrich), HA, myc, GFP, PY99, anti–alpha-synuclein, S129, TrkB, TrkC14, Ubiquitin P4D1 (Santa Cruz), anti-Y125, His (GE healthcare), pMAPK/MAPK, pAKT S473/AKT, Fyn, PARP, (Cell Signaling), anti-TH (Sigma-Aldrich), alpha-synuclein LB509 (Thermo Fisher Scientific), and anti-BDNF (Novus Biologicals).
Immunostaining.
Paraffin-embedded human brain sections or free-floating mouse brain sections sliced by cryotom were treated with 0.3% H2O2 for 10 min. Sections were washed three times in PBS and blocked in 1% BSA, 0.3% Triton X-100, for 30 min, followed by overnight incubation with TH antibody (1:1,000) or anti–alpha-synuclein LB509 antibody (1:500) at 4 °C. The signal was developed using Histostain-SP kit (Invitrogen). For immunofluorescence, the sections were washed three times in PBS, after primary antibody incubation, and incubated with Alexa Fluor 555-conjugated anti-mouse IgG for 2 h at room temperature. The slides were washed three times in PBS and covered with a glass using mounting solution.
For TrkB localization, primary dopaminergic neurons from WT, SNCA KO, and SNCA TG mice were pretreated by BDNF (50 ng/mL) for 15 min. Cells were washed three times with ice-cold acid-salt solution (0.2 M acetic acid, pH 2.8, 0.5 M sodium chloride) and fixed by 4% formalin for 10 min at room temperature. Cells were permeabilized and blocked for 1 h at room temperature in PBS solution containing 10% goat serum, 1% BSA, and 0.3% Triton-X-100. The primary antibodies (anti-TrkB, p816 TrkB, or EEA1) were incubated overnight at 4 °C. After washing, slides were incubated with the secondary antibodies (anti-mouse Alexa 555 or anti-rabbit Alexa 488) for 2 h at room temperature. The slides were washed three times in PBS and then covered with a glass cover using mounting solution.
For TUNEL staining, slides were incubated at 37 °C using In Situ Cell Death Detection Kit (Roche). Image capture and analysis were examined under a fluorescence microscope (Olympus).
HPLC Analysis.
DA and DOPAC levels were determined by HPLC with coulometric detection. Brain samples were homogenized in 0.1 N HClO4 solution (containing 0.01% sodium metabisulfite and 25 ng/mL internal standard 3,4-dihydroxybenzylamine HBr) and centrifuged at 13,000 g for 15 min at 4 °C. Aliquots of supernatant fractions were filtered with a 0.2-μm HT Tuffryn membrane (Pall); then injected into an Ultrasphere 5 μm ODS column, 250 × 4.6-mm (Hichrom Limited); and separated with a mobile phase containing 0.1 M sodium phosphate, 0.1 mM EDTA, 0.30 mM sodium octyl sulfate, and 5% (vol/vol) acetonitrile, pH 3.2. The DA and DOPAC amount (ng per sample) was then quantified by comparison with internal standards, with a standard curve generated with 0.1–5 ng of DA standard. The protein level (mg per sample) was determined with Lowry protein assays with a standard curve generated with 0–95 μg BSA.
Cells Quantification.
TH immunoreactivity in the SN and striatum was estimated using fluorescence intensity quantified using Image J software. For each animal, three consecutive sections of the SN and striatum were analyzed. For the quantification of TUNEL-positive cells in dopaminergic neuron, three images were taken in each group using a 10× objective lens (Olympus). Positive cells were counted and calculated for the average from each group (n = 3, each group). The conditions of the analysis were blinded to the investigator.
SI Results
Fyn-Mediated Phosphorylation of α-Synuclein at Y125 Disrupts the Binding to TrkB.
To further explore whether the tyrosine kinase activity of TrkB is necessary for the α-Syn–TrkB complex formation, we used a TrkB kinase-dead (KD) K572A mutant. A coimmunoprecipitation assay indicated that both TrkB wild-type and KD robustly associated with α-Syn, which was totally inhibited by BDNF (Fig. S5A, Top). P-TrkB and the phosphorylation of downstream effectors was completely absent in conditions using the KD TrkB construct (Fig. S5A, five bottom panels). To test whether α-Syn mutations or phosphorylation status influences the interactions, we used various phosphomimetic or nonphosphorylatable mutants and found that the nonphosphorylatable Y125F and PD-associated A53T displayed much stronger binding affinity to TrkB than wild-type α-Syn (Fig. S5B). This finding indicates that Y125 phosphorylation, which is predominantly mediated by Fyn kinase, might antagonize the binding between α-Syn and TrkB. To further assess the role of Fyn in this process, we used constitutively active Fyn (Fyn-CA) and kinase-dead Fyn (Fyn-KD). Compared with control or Fyn-KD, Fyn-CA substantially repressed α-Syn–TrkB complex formation (Fig. S5C). BDNF-stimulated α-Syn Y125 phosphorylation was inversely correlated with the binding affinity between α-Syn and TrkB (Fig. 1D, Bottom), suggesting that BDNF-induced activation of Fyn might inhibit α-Syn–TrkB complex formation. Accordingly, we tested the effects of the Fyn tyrosine kinase inhibitor PP2 on α-Syn–TrkB complex formation. As expected, the Fyn inhibitor PP2, but not the inactive analog PP3, blocked the dissociation between α-Syn and TrkB by BDNF (Fig. S5D), underscoring that Fyn phosphorylation of α-Syn inhibits its interaction with TrkB. Supporting this, we observed increased binding between α-Syn and TrkB in Fyn knockout mouse brain compared with wild-type mouse brains (Fig. S5E). Taken together, these results indicate that Fyn tyrosine kinase prevents α-Syn/TrkB complex formation via phosphorylation of α-Syn at Y125.
Alpha-Synuclein Decreases TrkB Protein Level via Ubiquitination.
BDNF treatment elicits TrkB receptor ubiquitination and degradation. To assess the effect of α-Syn on TrkB stability, we conducted a Trk immunoprecipitation and ubiquitination assay with primary cortical neurons transduced with AAV overexpressing a GFP control or the α-Syn transgene. α-Syn overexpression induced the spontaneous ubiquitination of Trk receptors, which was further enhanced with BDNF treatment (Fig. S6A, Top Left). TrkB levels were reduced following overexpression of α-Syn compared with GFP (Fig. S6A, top and second panels). Accordingly, p-TrkB/p-Akt signals were diminished when α-Syn was overexpressed. To further test the effect of α-Syn on TrkB ubiquitination, we performed a temporal analysis of the effects of BDNF exposure in TrkB-expressing SH-SY5Y (BR6 cells) and cortical neurons (Fig. S6B). Depletion of α-Syn with its shRNA did not affect TrkB levels or p-TrkB/p-Akt signaling cascade. Again, TrkB protein levels were highly reduced in both BR6 and primary neurons when α-Syn was overexpressed (Fig. S6 B and C, second panels). Conceivably, α-synuclein decreases TrkB protein levels via enhancing its ubiquitination.
DOPAL Induces Cell Death Through Increasing α-Syn/TrkB Association.
DA and its oxidized metabolites make the accumulated α-Syn selectively toxic to dopaminergic neurons (29). DOPAL is a metabolic intermediate formed by the oxidative deamination of DA, mainly catalyzed by MAO-B. Accordingly, we examined the effects of DA and its metabolite DOPAL on TrkB/α-Syn complex formation. We pretreated GFP–α-Syn–transfected BR6 cells with TH inhibitor AMPT to deplete DA, followed by MPP+ stimulation. MPP+-elicited α-Syn/TrkB complex was robustly disrupted by inhibition of DA, suggesting that DA might regulate the interaction between α-Syn and TrkB. Consequently, both α-Syn and MPP+-induced cell death was significantly reduced when DA was depleted (Fig. S3 A and B). To assess whether DA exerts the neurotoxicity through its oxidative metabolite DOPAL, we conducted binding assay in DAergic BR6 cells. DOPAL increased α-Syn/TrkB association, blocking p-TrkB and its downstream signaling (Fig. S3C). We next analyzed the cytotoxicity with LDH release assay and found that α-Syn–induced cell death was escalated by DOPAL, which in turn was inhibited by BDNF treatment, tightly fitting with p-TrkB/p-Akt signals (Fig. S3D). We made similar observations using DAergic neurons, in which apoptotic cells were labeled with terminal deoxynucleotidyl transferase (TUNEL). Noticeably, depletion of DA with the TH inhibitor AMPT diminished α-Syn’s neurotoxic activity, which was further decreased by BDNF (Fig. S3 E and F). Hence, DA via its oxidative metabolite DOPAL by MAO-B promotes α-Syn to bind TrkB and exerts neurotoxicities in DAergic neurons. To further explore DOPAL’s role in these events, we used the irreversible MAO inhibitor rasagiline (specific to MAO-B) in BR6 cells transfected with GFP–α-Syn (Fig. S4). The transfected cells were treated with BDNF with or without MAO inhibitors and in the presence or absence of MPP+. The association between TrkB/α-Syn was reduced in BR6 cells separately treated by BDNF or MAO inhibitor. The complex was completely disrupted when cells were exposed to both BDNF and rasagiline together. MPP+ treatment strongly increased the interactions between α-Syn and TrkB, which was inhibited by BDNF or rasagiline alone. Noticeably, BDNF displayed a more potent effect than rasagiline in disrupting the α-Syn/TrkB complex. Again, when α-Syn bound to TrkB, it blocked p-TrkB and its downstream signals. P-TrkB levels were inversely correlated with the binding activities between α-Syn and TrkB (Fig. S4A). LDH release assay supported the idea that rasagiline inhibited MPP+-induced cell death, which was further inhibited by BDNF, in alignment with the binding patterns between α-Syn and TrkB (Fig. S4B).
SI Discussion
Neurons of both the central and the peripheral nervous system are critically dependent on neurotrophic signals for their survival and differentiation. It has been well established that the signal must be retrogradely transported to the cell body to exert its trophic effect. Following receptor-mediated endocytosis, the NGF/activated TrkA signaling complexes are sorted into a subpopulation of endosomes to give rise to the signaling endosomes (22). It is believed that the signaling endosome serves as a retrograde vesicular carrier to maintain signal fidelity and to sustain the signaling of the BDNF/activated TrkB complex during their transit from the terminal to the soma via the axon. In PD and other synucleinopathies, the axonal accumulation of α-Syn into amyloid fibrils of distinct morphology, LNs, is potentially more detrimental to the neuron relative to LBs in the soma. Lee and colleagues showed that addition of α-Syn preformed fibrils (PFFs), generated from recombinant α-Syn protein, to primary neurons seeds the recruitment of normal, endogenously expressed α-Syn into pathological aggregates that, similar to LBs and LNs in synucleinopathies, are insoluble, hyperphosphorylated, ubiquitinated, and filamentous by electron microscopy (24). Using live-cell imaging, they reported that α-Syn aggregates perturb the axonal transport of Rab7-positive and TrkB-positive endosomes, as well as autophagosomes, whereas the transport of mitochondria and synaptic vesicle precursors remains relatively unimpeded (24). These findings are consistent with our observation that axonal transport of TrkB receptors is slowed in SNCA transgenic overexpressing neurons, which aligns with the observation of impaired neurotrophic signaling.
A number of studies have demonstrated that mitochondrial dysfunction and oxidative stress are linked to neuronal degeneration in PD. α-Syn protein has a noncanonical mitochondrial targeting sequence at its N terminus and is indeed translocated to mitochondria in human fetal dopaminergic neuronal culture and postmortem brain tissue from healthy individuals. The accumulation of α-Syn in the mitochondria is enhanced in PD brains (25). Presumably, DOPAL-stimulated binding of α-Syn to TrkB inhibits its neurotrophic activities, which may account for the neurotoxicity induced by DOPAL. By using the MAO-B inhibitor rasagiline to prevent DOPAL formation, we showed that induction of the α-Syn/TrkB complex was suppressed (Fig. S4). When combined with BDNF, rasagiline completely disrupts the α-Syn/TrkB complex. Notably, MPP+ robustly enhanced the association between α-Syn and TrkB, which was alleviated by BDNF or rasagiline or both. As a result, MPP+-induced DAergic neuronal death was repressed by rasagiline.
Acknowledgments
We thank Alzheimer’s Disease Research Center at Emory University for human PD and LBD patients and healthy control samples. This work was supported by M. J. FOX Foundation Grant 11137 and NIH Grant RF1, AG051538 (to K.Y.); National Key Basic Research Program of China Grant 2010CB945202 (to Y.E.S.); and NSFC Grants 81461138037, 31471029, and 31671055 (to J.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713969114/-/DCSupplemental.
References
- 1.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 4.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 5.Krüger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 7.Simón-Sánchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda T, Nakata Y, Mochizuki H. α-Synuclein and neuronal cell death. Mol Neurobiol. 2013;47:466–483. doi: 10.1007/s12035-012-8327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 10.Seroogy KB, et al. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- 11.Hagg T. Neurotrophins prevent death and differentially affect tyrosine hydroxylase of adult rat nigrostriatal neurons in vivo. Exp Neurol. 1998;149:183–192. doi: 10.1006/exnr.1997.6684. [DOI] [PubMed] [Google Scholar]
- 12.Parain K, et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport. 1999;10:557–561. doi: 10.1097/00001756-199902250-00021. [DOI] [PubMed] [Google Scholar]
- 13.Mogi M, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- 14.Howells DW, et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Holgado F, Doherty P, Williams G. Tandem repeat peptide strategy for the design of neurotrophic factor mimetics. CNS Neurol Disord Drug Targets. 2008;7:110–119. doi: 10.2174/187152708783885200. [DOI] [PubMed] [Google Scholar]
- 16.Altar CA, et al. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci USA. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- 18.Kohno R, et al. BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem Biophys Res Commun. 2004;318:113–118. doi: 10.1016/j.bbrc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 19.von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency for trkB and trkC receptors induces cell loss and accumulation of alpha-synuclein in the substantia nigra. FASEB J. 2005;19:1740–1742. doi: 10.1096/fj.05-3845fje. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y, et al. Overexpression of alpha-synuclein down-regulates BDNF expression. Cell Mol Neurobiol. 2010;30:939–946. doi: 10.1007/s10571-010-9523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira DB, Chao MV. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J Neurosci. 2007;27:4859–4869. doi: 10.1523/JNEUROSCI.4587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimes ML, et al. Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beattie EC, Howe CL, Wilde A, Brodsky FM, Mobley WC. NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. J Neurosci. 2000;20:7325–7333. doi: 10.1523/JNEUROSCI.20-19-07325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpicelli-Daley LA, et al. Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell. 2014;25:4010–4023. doi: 10.1091/mbc.E14-02-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, et al. Dopamine-dependent neurotoxicity of alpha-synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 27.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 28.Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamensdorf I, et al. 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res. 2000;868:191–201. doi: 10.1016/s0006-8993(00)02309-x. [DOI] [PubMed] [Google Scholar]
- 30.Burke WJ, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 31.Mandel SA, Sagi Y, Amit T. Rasagiline promotes regeneration of substantia nigra dopaminergic neurons in post-MPTP-induced parkinsonism via activation of tyrosine kinase receptor signaling pathway. Neurochem Res. 2007;32:1694–1699. doi: 10.1007/s11064-007-9351-8. [DOI] [PubMed] [Google Scholar]
- 32.Weinreb O, Amit T, Bar-Am O, Youdim MB. Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: New insights and implications for therapy. Ann N Y Acad Sci. 2007;1122:155–168. doi: 10.1196/annals.1403.011. [DOI] [PubMed] [Google Scholar]
- 33.Benskey MJ, Sandoval IM, Manfredsson FP. Continuous collection of adeno-associated virus from producer cell medium significantly increases total viral yield. Hum Gene Ther Methods. 2016;27:32–45. doi: 10.1089/hgtb.2015.117. [DOI] [PubMed] [Google Scholar]