Abstract
In an attempt to combat the possibility of bacterial infection and insufficient bone growth around metallic, surgical implants, bioactive glasses may be employed as coatings. In this work, silica-based and borate-based glass series were synthesized for this purpose and subsequently characterized in terms of antibacterial behavior, solubility and cytotoxicity. Borate-based glasses were found to exhibit significantly superior antibacterial properties and increased solubility compared to their silica-based counterparts, with BRT0 and BRT3 (borate-based glasses with 0 and 15 mol% of titanium dioxide incorporated, respectively) outperforming the remainder of the glasses, both borate and silicate based, in these respects. Atomic Absorption Spectroscopy confirmed the release of zinc ions (Zn2+), which has been linked to the antibacterial abilities of glasses SRT0, BRT0 and BRT3, with inhibition effectively achieved at concentrations lower than 0.7 ppm. In vitro cytotoxicity studies using MC3T3-E1 osteoblasts confirmed that cell proliferation was affected by all glasses in this study, with decreased proliferation attributed to a faster release of sodium ions over calcium ions in both glass series, factor known to slow cell proliferation in vitro.
Keywords: Biomedical engineering, Materials science
1. Introduction
Titanium and some of its alloys (e.g. Ti6Al4V and Ti6Al7Nb) is a common implant material due to its ability to create a permanent bond to bone via osseointegration [1, 2]. It has also been incorporated in bioactive glasses [3, 4, 5], to influence crystallization mechanics of borosilicate glasses and to amend the solubility of both phosphate and silicate glasses for medical applications. Titanium has also been used in prosthetics, specifically, in direct skeletal attachment (DSA) [1], the method in which metallic implants (typically made out of titanium or a titanium alloy) are attached directly to the bone at the residual limb. After healing, the implant becomes the attachment mechanism between the prosthesis and the body [6]. Concerns regarding DSA include infection and skin irritation [7, 8, 9], with different approaches taken towards re-designing DSA devices for improving patient outcomes. These approaches usually involve modification of the surface by sandblasting, plasma-spraying with either titanium or hydroxyapatite (HA), coating the implant with a titanium dioxide (TiO2), or applying a coating made from bioactive glass [10, 11, 12]. HA coatings have been used clinically for more than 20 years due to its ability to promote bone ingrowth [13, 14, 15]. However, HA coatings have no mechanism to retard bacterial or biofilm colonization at the implant site. To address this deficiency, some coatings have been produced based on chlorhexidine and silicone with ammonia couplings [16, 17], but these have little clinical applicability as the active compounds erode as they migrate to the surface. Bioactive glasses, on the contrary, have shown encouraging results when used as coatings [11].
The development of 45S5 Bioglass® in the 1960s led to the potential of bioactive glasses being used as coating materials [18], with Bioglass® being the first synthetic material to chemically adhere to both hard and soft tissue [18, 19]. In situ degradation of such materials makes them desirable for clinical applications owing to the release of beneficial ions into the physiological environment, which can promote antibacterial behavior, bone formation and growth, and tissue healing [20, 21, 22]. Though bioactive glasses have been used for coating metals, compositions have contained aluminum [23, 24], associated with defective bone mineralization and neurotoxicity [25], while other compositions have been deficient in zinc [26, 27, 24, 28], an antibacterial component [22, 29, 30] that aids the healing process by inhibiting the growth of bacteria, including caries-related bacteria Streptococcus mutans [31]. Although virtually all materials facilitate bacterial ingress which can lead to biofilm formation, bacteria attach less readily to glass [32], making them a suitable option for coating surgical implants. As bioactive glasses can be formulated to influence genetic expression, differentiation and cell proliferation by the release of ions [21, 33, 34, 35], control of the biological response through dissolution products creates an opportunity for innovation. The current work considers two glass series, one based on silica (SiO2) and one on borate (B2O3), with increasing amounts of titanium dioxide (TiO2) incorporated at the expense of silica and borate, respectively. B2O3 has been shown to reduce the coefficient of thermal expansion (CTE) of glasses [36], so borate glasses have CTEs that better match the CTE of the metallic substrate to be coated (typically Ti6Al4V, with a CTE of 10.6 × 10−6/ °C in the range of 0–650 °C [37]). Coating such glasses into the metallic substrates (e.g. through enameling [26, 38], plasma spraying [39] or electrophoretic deposition [40]) requires heat treatment at temperatures high enough to allow for the glass to react with the substrate surface thus creating a chemical bond [41, 42]. After the bond has formed and the assembly brought back to room temperature, a difference in CTE between the glass and metal will induce residual stresses, evidenced by cracks appearing in the glass or at the glass/substrate interface. For this reason, a borate-based glass series is proposed, to evaluate the effect of B2O3 on its coating capability by means of a reduced CTE compared to silica-based glasses, which means that residual stresses at the glass-substrate interface would be reduced since both components would be subjected to comparable amounts of shrinkage during the cool off stage of enameling. A silica-based glass series is also proposed to allow for the evaluation of the effect of B2O3 versus SiO2 on resultant properties of the coating. Furthermore, TiO2 is incorporated to help promote a more stable chemical bond when coating onto Ti6Al4V [36]; limited literature is currently available on the effect of TiO2 inclusion in borate-based glass structures. Calcium oxide (CaO) and phosphorus pentoxide (P2O5) are also incorporated into the glasses as Ca and P are the main elements in the inorganic phase of bone, hence helping in bone formation and resorption [43]. Sodium oxide (Na2O) is included in the formulation as it has been proven to reduce glass transition and crystallization temperature [44], facilitating lower enameling temperatures, thus reducing the risk of α to β transformation of the titanium substrate, a transformation which can hinder corrosion resistance [37].
This study evaluates the characteristics of two new glass series, one silica-based and one borate-based, in terms of their degradative behavior (including solubility and ion release profiles), cytotoxicity, and in vitro antibacterial capabilities. For the latter studies, inhibition zones were measured in media containing Staphylococcus epidermidis and Escherichia coli.
2. Materials and methods
2.1. Glass preparation
Silica-based and borate-based glasses (Table 1) in this study were previously synthesized, and characterized structurally and mechanically as bulk and coating material [45, 46]. TiO2 was added at the expense of SiO2 for the SRT series and at the expense of B2O3 for the BRT series. The glasses were prepared by weighing out appropriate amounts of analytical grade reagents (Fisher Scientific, Ottawa, ON, Canada & Sigma-Aldrich, Oakville, ON, Canada), firing in silica crucibles (1400–1500°C for 1 h for the silica-based glasses, 1200°C for 1 h for borate-based glasses), and shock quenching in water. The resulting frit was then ball-milled, and sieved to retrieve glass particulates ≤20 μm.
Table 1.
Glass formulations (mol%).
| Silica-based glass |
Borate-based glasses |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reagent | SRT0 | SRT1 | SRT2 | SRT3 | BRT0 | BRT1 | BRT2 | BRT3 |
| SiO2 | 52 | 47 | 42 | 37 | 0 | 0 | 0 | 0 |
| B2O3 | 0 | 0 | 0 | 0 | 52 | 47 | 42 | 37 |
| CaO | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| P2O5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Na2O | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| ZnO | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| TiO2 | 0 | 5 | 10 | 15 | 0 | 5 | 10 | 15 |
2.1.1. Discs preparation
Approximately 200 mg of each glass were pressed using a hydraulic press with pressure ranging between 17–20 MPa. The pressed discs were then heat treated to promote the coalescence of glass particles and create a solid mass for biocompatibility testing.
2.2. Solubility analysis
Three glass discs (n = 3) for each glass composition were placed in separate containers filled with 25 mL of deionized water, and incubated at 37°C for 1, 7 and 30 days. The discs were weighted prior to incubation, and re-weighed after drying after each incubation period; the percentage difference from the initial mass of the disc was then computed as a function of time, per Eq. (1), as follows:
| (1) |
where %ml(t) is the percentage of mass loss, m0 is the mass at time zero (before incubation) and m(t) is the mass at time t (where t = 1, 7 and 30 days).
2.3. Ion release through Atomic Absorption Spectroscopy (AAS)
Samples for ion release profiles were prepared as described in subsection 2.2 and measured using Atomic Absorption Spectroscopy (AAS), model PinAAcle 500 Flame Atomic Absorption Spectrometer (PerkinElmer, Waltham, MA, USA). AAS calibration standards for titanium and zinc ions at concentrations of 0.00 (blank sample), 0.50, 1.00, 5.00 and 10.00 ppm were prepared from stock solutions on a gravimetric basis. At 1, 7 and 30 days, samples were tested, with the AAS measuring absorbance levels, which were automatically translated into concentration levels by the Syngistix Touch software (PerkinElmer, Waltham, MA, USA) based on the ion calibration curves.
2.4. pH change
Samples for pH change were prepared as described in subsection 2.2. At 1, 7 and 30 days, samples were tested using an Omega PHH222 pH meter (Omega, Laval, QC, Canada) and compared to reference samples at t = 0 days to determine how pH varied in the presence of the glass discs.
2.5. In vitro antibacterial analysis
The antimicrobial properties of the SRT and BRT glass discs were evaluated on agar plates against prokaryotic species Escherichia coli (E.coli) and Streptococcus epidermidis (S. epidermidis) against bacterial lawns, spread on Tryptic Soy Agar (TSA) (3 g/L Tryptic Soy Broth, 15 g/L agar). All chemicals were purchased from Fisher Scientific (Ottawa, ON, Canada). Bacterial cultures were grown to an exponential phase (12–16 h), diluted in Physiological Saline Solution (9 g/L NaCl) to 106 cells/mL and spread onto TSA. Antimicrobial properties were quantified on the bacteria by measuring and comparing the zones of growth inhibition. One disc of each glass was added to each bacterial plate (4 discs per plate, evenly spaced on the lawn). Each plate had a single microbial species, and each species was repeated in triplicate for statistical comparisons. The diameters of the bacterial inhibition zones were measured at 1, 7 and 30 days, and the means and standard deviations of triplicate samples were compared with the post-hoc Tukey analysis of variance.
2.6. Cytotoxicity study
Bone metabolic activity assays were conducted to determine in vitro bioactivity of the glasses. Pre-osteoblastic MC3T3-E1 cells (ATCC CRL-2593, ATCC, Manassas, VA, USA) from passage 3–5 were used for this study and were maintained in αMEM media supplemented with 10% FBS and 1% (2 mM) L-glutamine (Cambrex, MD, USA) within a cell culture incubator at 37° C/5% CO2/95% air atmosphere. 24 well plates were seeded with cells at a density of 5,000 cells/cm2 and incubated for 24 hours prior to testing. Culture media (1 ml) was then further supplemented with 100 μl of liquid extract (from the solubility samples at 30 days for all glasses; n = 3 per sample well) and then incubated for 24 h at 37° C/5% CO2. 0.1 ml of MTT was added to the culture media. The cultures were then re-incubated for 2 h (37° C/5% CO2) after which they were removed from the incubator and the resultant formazan crystals dissolved by adding an amount of MTT Solubilisation Solution (10% Triton x-100 in Acidic Isopropanol (0.1 n HCl)) equal to the original culture medium volume. Once the crystals were fully dissolved, the absorbance was measured at a wavelength of 570 nm. Cells seeded (at the same density) on tissue culture plastic (n = 3) were used as controls.
2.7. Statistical methods
One-way analysis of variance (ANOVA) was employed to analyze the data to determine significance in mean difference across the gathered data when p < 0.05. Post-hoc Tukey and Dunnett tests were used on MiniTab 17 (MiniTab Inc., State College, PA, USA). The Tukey test assumes equal variance in the data sets being analyzed to determine the significance in mean difference across all factors (i.e. all glasses in both series); the Dunnett test also assumes equal variance in the data, and it is employed when a control group is used to compare against the data sets. Results from the solubility analysis, pH change studies, AAS measurements and in vitro antibacterial studies were analyzed using post-hoc Tukey; cytotoxicity results were analyzed using post-hoc Dunnett.
3. Results
3.1. Solubility analysis
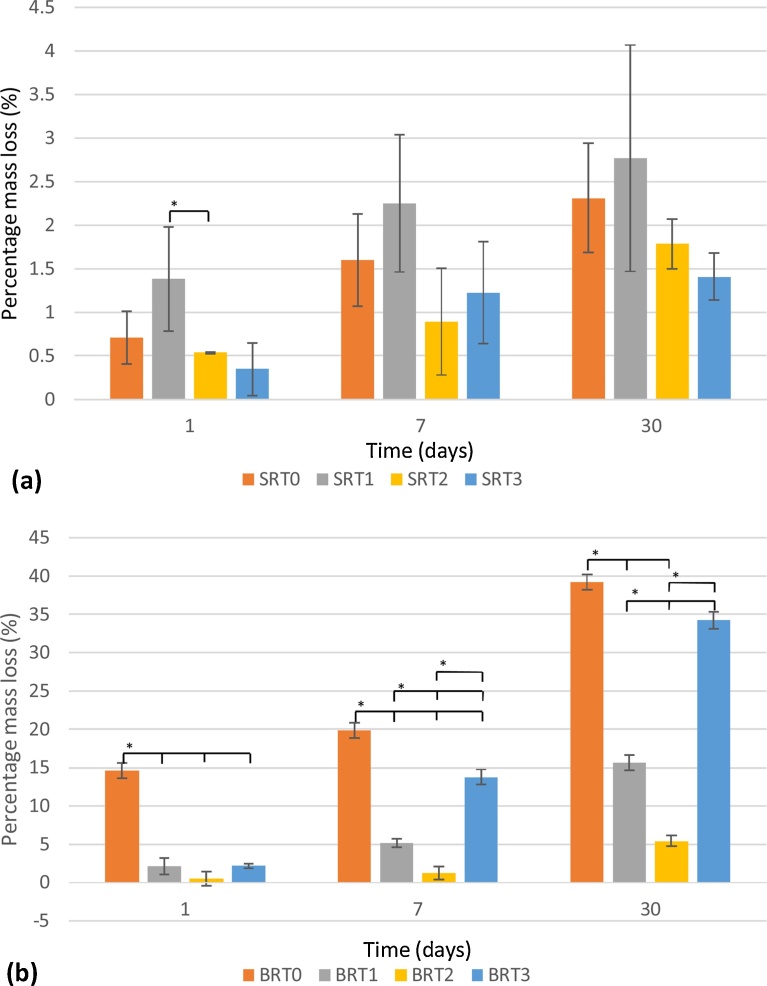
Evaluation of the mass loss at different incubation periods was undertaken, with results shown in Fig. 1 (a) for the SRT glass series, and Fig. 1 (b) for the BRT glass series. As a function of incubation period, it is observed that mass loss steadily increased from day 1 to day 30, with SRT1 and BRT0 experiencing the greatest amount of degradation within their respective series. Borate-based glasses exhibited much higher degradation behavior than their silica-based counterparts; additionally, crystallinity in the silica-based glasses may only account for the reduced solubility of these glasses. As a function of incubation period, for the SRT series, only SRT0 and SRT3 exhibited a significant increase in solubility between 1 and 30 days (vs. all other SRT glasses), and SRT0 and SRT3 between 1 and 7 days and between 7 and 30 days did not exhibit a significant difference in solubility; for the BRT series, all weight changes for all glasses were statistically different, except for BRT0 and BRT2 between 1 and 7 days.
Fig. 1.
Solubility study for SRT (a) and for BRT (b) glass series. n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
As a function of the amount of TiO2, no significant change in solubility is observed as the amount of TiO2 is increased to 15 mol% for the silica-based glasses. Similarly, for the borate-based glasses, a decrease in solubility is observed, but only up to 10 mol% TiO2, with a significant increase for BRT3 (15 mol% TiO2) at 7 and 30 days.
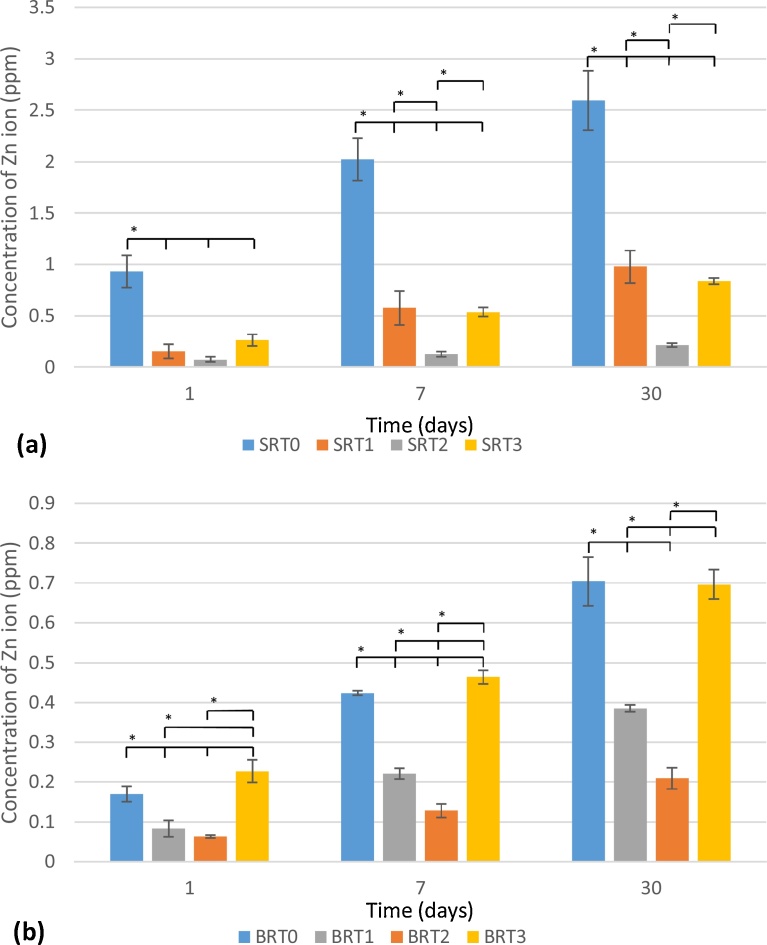
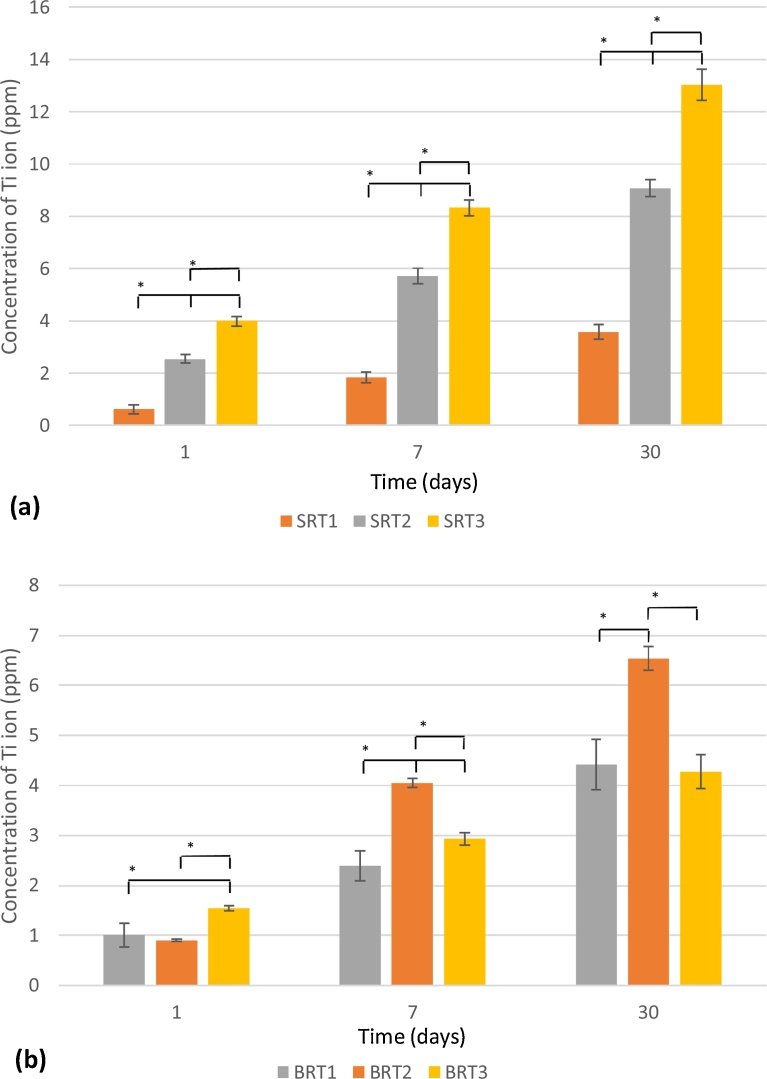
3.2. Ion release through Atomic Absorption Spectroscopy (AAS)
Zn2+ release for the SRT series is shown in Fig. 2 (a), while results for the BRT glass series are shown in Fig. 2 (b); Ti4+ release is shown in Fig. 3(a) and Fig. 3 (b)for the SRT and BRT glass series, respectively. For both glass series, the inclusion of TiO2 up to 10 mol% resulted in a significant drop in the concentration of Zn2+ released; however, at 15 mol% an increase in Zn2+ release was observed. In terms of incubation time, a significant increase in Zn2+ is observed for both glass series at all incubation times, except for SRT2 between 1 and 7 days. With respect to Ti4+ release, for the SRT series an increase in release is observed with an increase in the TiO2 content, which may be associated with a decrease in crystallinity for this series as TiO2 is increased (SRT3 is amorphous, while SRT1 and SRT2 exhibit crystallinity) [45]; Ti4+ release in the BRT glasses increased with TiO2 content incorporated into the glass, with a decrease observed for BRT3, glass which exhibited a secondary crystal phase of TiO2 in XRD [45] explaining the decrease in Ti4+ release. As a function of incubation time, all glasses exhibited statistically different Ti4+ concentrations at each time.
Fig. 2.
Zn2+ release from SRT (a) and BRT (b) glass series. n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
Fig. 3.
Ti4+ release from SRT (a) and BRT (b) glass series. n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
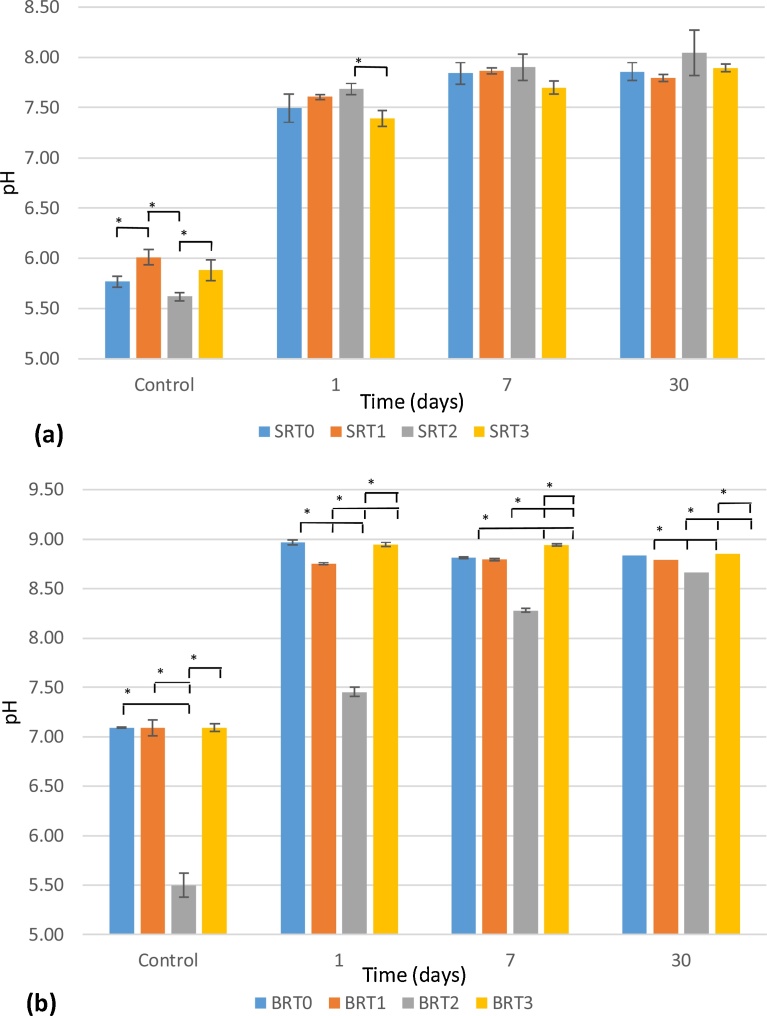
3.3. pH change
Changes in the pH of deionized water were evaluated for the SRT and BRT glasses, with results shown in Fig. 4. After 30-days incubation, no significant difference is observed as a function of the amount of TiO2 incorporated for the SRT series, with the SRT glasses averaging a pH of 7.90; due to the small standard deviations in the measurements for the BRT series, only the pH at 30 days for BRT0 and BRT3 are not significantly different. pH changes due to the BRT glasses ranged between 8.67 and 8.85.
Fig. 4.
pH Measurements for SRT (a) and for BRT (b) glass series. Control refers to samples prior to incubation n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
3.4. In vitro antibacterial analysis
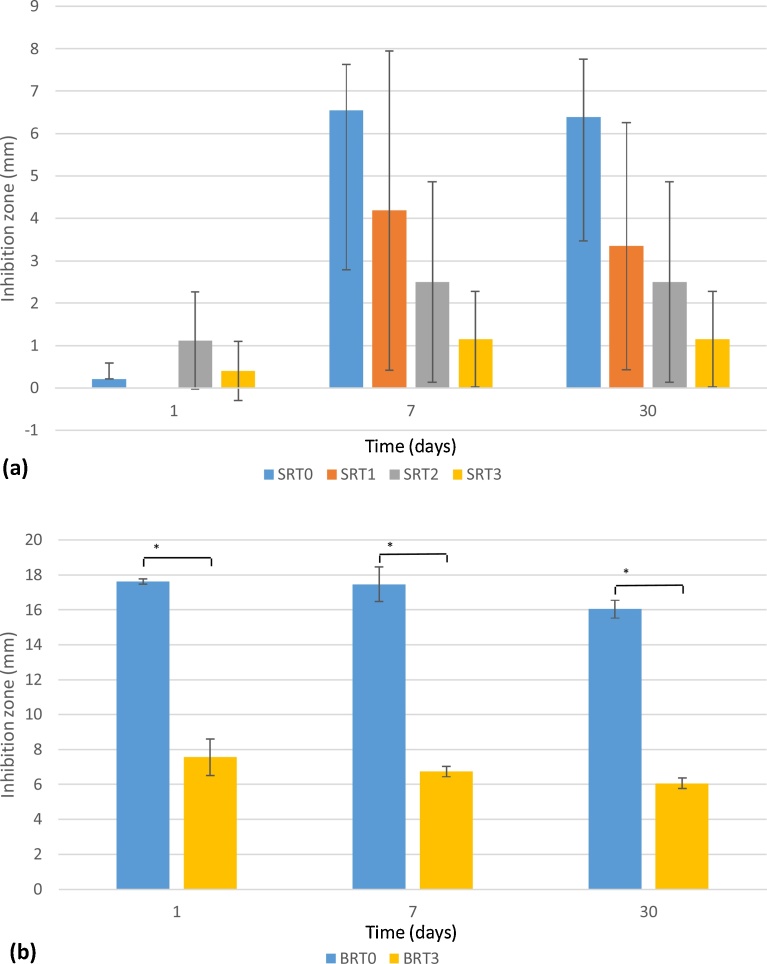
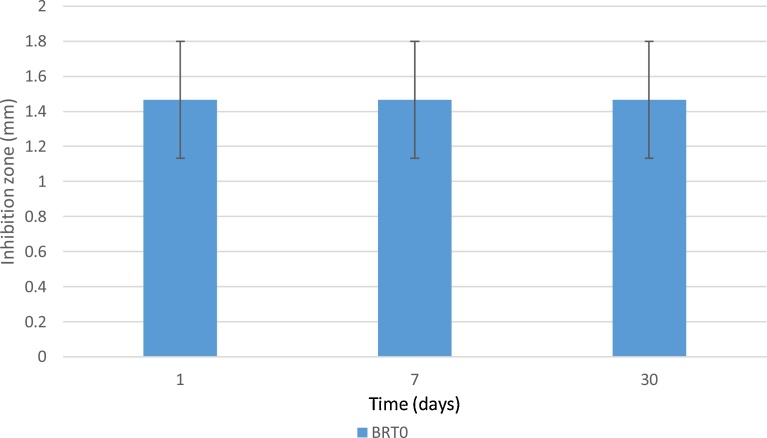
Inhibition zones were measured for cultures of S. epidermidis and E. coli after 1, 7 and 30 days of incubation with SRT and BRT glass discs. Results are shown in Fig. 5 for S. epidermidis inhibition by the SRT and BRT glasses, and in Fig. 6 for E. coli inhibition by the BRT glasses. No inhibition zones were observed in the E. coli cultures, with the exception of BRT0, which showed a small zone after 1 day, but with the presence of bacteria in-growth in the zone, indicating resistance of the E. coli bacteria to the antibacterial effect of BRT0.
Fig. 5.
Inhibition zones (in mm) for S. epidermidis for SRT (a) and BRT (b) glasses. BRT1 and BRT2 did not inhibit bacterial growth. n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
Fig. 6.
Inhibition zones (in mm) for E. coli for BRT glasses. BRT1 to BRT3 did not inhibit bacterial growth. n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
Regarding S. epidermidis, the SRT glasses exhibited inhibition zones that remained constant as a function of incubation time (with the exception of SRT1, which started exhibiting antibacterial activity after 7 days, and SRT0 significantly increased from day 1 to day 7). Greater inhibition zones were observed for the BRT glasses, namely BRT0 and BRT3, with BRT0 proving to be the best antibacterial glass from both glass series. BRT1 and BRT2 exhibited no inhibition zone. Statistically, for S. epidermidis, all SRT glasses are significantly equivalent, whereas for the BRT glasses only BRT1 and BRT2 (both glasses which exhibited no antibacterial behavior) are significantly equivalent. For the BRT glasses, which exhibited significant difference in antibacterial behavior with the addition of TiO2, it was observed that including TiO2 in the glass network prevented the bacterial growth inhibition, with the exception of BRT3, which inhibited the growth of Gram-positive bacteria S. epidermidis.
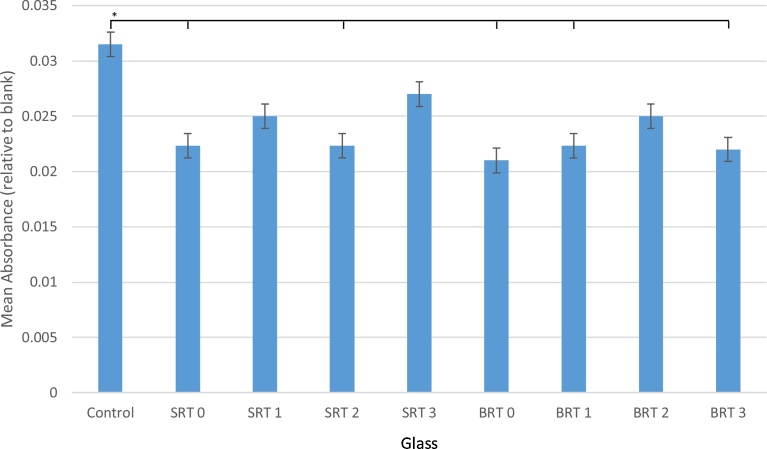
3.5. Cytotoxicity study
Cell viability was assessed through an MTT assay for cytotoxicity, with results reported in Fig. 7. Overall, a decrease in absorbance was observed for all glasses when compared with the control, indicating a decrease in proliferation in the presence of the glasses; nonetheless, glasses SRT1, SRT3 and BRT2 were found to be statistically equivalent to the controls. Contrasting the SRT and BRT series, the performance of both series could be described as similar, with SRT3 (10 mol% TiO2 incorporated) exhibiting higher proliferation opposed to the other glasses; however, all glasses were statistically equal in terms of cell viability among themselves.
Fig. 7.
MTT cytotoxicity assay results using 30-day incubation liquid extracts from SRT and BRT glasses. n = 3; scatter bars indicate one standard deviation from the mean. Stars and bars show statistical significance (p < 0.05).
4. Discussion
From Fig. 1, it can be observed that the inclusion of TiO2 to both the silica-based and the borate-based glass structures resulted in a decrease in solubility. For the silica-based glasses, at 30 days, decreasing solubility with increasing TiO2 content to up to 15 mol% can be observed, whereas for the borate-based glasses this trend is observed only up to 10 mol% TiO2. BRT3 exhibited degradation behavior comparable to that of the control BRT0, which may be attributed to TiO2 in BRT3 partially existing as a separate phase from the glass phase [45], which may explain the increased solubility of these particular glass samples. In terms of overall solubility, the borate-based glasses exhibited higher solubility compared to the silica-based glasses, which was expected since borate-based glasses have been shown to degrade faster than their silica-based counterparts due to their lower chemical durability [48, 49, 47], making them a suitable option in applications where faster dissolution of the coating is required. Degradation is also confirmed through AAS, which recorded larger quantities of Zn2+ released from the control glasses (SRT0 and BRT0) as opposed to their titanium-containing counterparts. Borate-based structures allow for the release of the modifier ions more readily than silica-based structures. Moreover, for BRT3, an increase in degradation is observed when compared to BRT2, the amount of Ti4+ released did not follow this trend, which, again, is to be attributed to the occurrence of TiO2 as a separate phase in the glass, a phase which, due to its crystallinity, does not allow for titanium to be released [50].
As expected, exposure of the glass samples to deionized water resulted in an increase in pH due to the release of ions altering the acidity of the media. With the greater dissolution of the BRT series, pH was observed to increase more than in the SRT series; however, at 30 days, it can be seen that the pH of the media for all BRT glasses reached a maximum of 8.85. For the SRT series, which exhibited much lower solubility, the pH reached a maximum of 8.05 for SRT2 after 30 days incubating. A higher pH raise in borate-based glasses compared to silica-based glasses has been previously reported [20]; furthermore, the substitution of SiO2 with TiO2 did not significantly affect pH, and substitution of B2O3 with TiO2 did not result in a substantial change in pH after 30 days.
As an antibacterial agent, the amounts of Zn2+ measured through AAS at day 1 are linked to the larger bacterial inhibition zones in BRT0 and BRT3 in the BRT series and SRT0 in the SRT series for Gram-positive bacteria S. epidermidis, with the BRT series significantly outranking the SRT series. The reduction in solubility due to the addition of TiO2 translated into a reduction in release of Zn2+, and therefore the lack of antibacterial behavior of glasses BRT1 and BRT2, with the sudden increase in antibacterial behavior observed in BRT3 attributed to the occurrence of TiO2 as a separate phase [45]. Additionally, the SRT glasses that exhibited antibacterial behavior also showed signs of bacterial recolonization, evidencing the weak antibacterial nature of these glasses. The proliferation of Gram-negative bacteria E. coli was not significantly affected by the presence of any of the glasses. Gram-positive bacterial growth is more likely to be inhibited when compared to Gram-negative bacterial growth given that Gram-negative bacteria have a layer of lipopolysaccharide (LPS) molecules, not present in Gram-positive bacteria, acting as an impermeable layer against Zn2+ ingress [51, 52]. It has been shown that, for the same concentration of zinc ions from zinc nanoparticles, inhibition zones for Gram-negative bacteria are 16–33% smaller than those of Gram-positive bacteria [53]. In fact, glass BRT0, which showed greater inhibition zones in the presence of gram-positive bacteria S. epidermidis, was the only glass composition in this study that exhibited an inhibition zone for Gram-negative E. coli. In the light of these results, it would be expected that BRT0 would exhibit antibacterial behavior when tested in vivo, tackling the issue of bacterial infection at the site of prosthetic implantation [7, 8, 9]. Further time-dependent tests in liquid or biofilm systems are necessary to corroborate these antibacterial results in scenarios closer to in vivo environments. When tested to determine the cell viability capacity of the glasses, it was determined that all glasses slowed down the proliferation of MC3T3-E1 osteoblasts, which was unexpected, as the levels of Zn2+ were not found to be toxic [54], indicating potential toxicity of the borate ion (BO3)3− for the BRT glasses, which has been found to occur in vitro in borate-based glasses [55], while this toxicity has not been reported under dynamic testing conditions [56]. Recognizing, though, that both glass series exhibited comparable cytotoxic behavior, then inhibition of cell proliferation for both glasses may be more likely explained by a faster release of sodium (alkali ion) over calcium (alkaline ion) [44, 57, 58], linked to the glass compositions possessing a higher Na2O than CaO (14 mol% vs. 12 mol%).
Once an implant is inserted in the human body, two processes start to occur: bacteria attempt to colonize it, while tissue integration from the surroundings occurs; this phenomenon is known as “the race for the surface” [59, 60]. The desirable outcome is that bacterial infection is inhibited, while tissue integration is promoted; the antibacterial results herein presented present BRT0 and BRT3 as potential candidates to eliminate bacteria at the implantation site, with their superior antibacterial conditions potentially allowing for bone cells to attach and proliferate around the implant in vivo.
5. Conclusions
Silica-based and borate-based glass series have been synthesized and their antibacterial, solubility and cytotoxicity characteristics evaluated to determine their potential for coating surgical implants. Overall, the borate-based glasses exhibited significantly superior antibacterial and solubility behavior to the silica-based glasses, with BRT0 and BRT3 (with 0 and 15 mol% of TiO2 incorporated, respectively) outperforming the remainder of the glasses. Atomic Absorption Spectroscopy confirmed the release of Zn2+ which is linked to the antibacterial inhibition observed from glasses SRT0, BRT0 and BRT3 at concentrations lower than 0.7 ppm, toxic to S. epidermidis and E. coli, with the reduction in solubility due to TiO2 presence accounting for the decrease in Zn2+ release in BRT1 and BRT2, preventing these glasses from exhibiting antibacterial behavior. In vitro cytotoxicity studies were conducted using MC3T3-E1 osteoblasts to evaluate how cell proliferation was affected by the proposed glasses, with results indicating a decrease in proliferation from glasses SRT0, SRT2, BRT0 and BRT1, which may be attributed to a faster release of sodium ion over calcium ion in both glass series for both glass series, factor known to slow cell proliferation in vitro. In vivo studies are now required to evaluate the effect of a dynamic environment, as surgical implants would be subjected to in the human body, on the cytotoxicity and antibacterial inhibitory characteristics of these glasses.
Declarations
Author contribution statement
Omar Rodriguez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Wendy Stone: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Emil Schemitsch, Paul Zalzal, Stephen Waldman and Marcello Papini: Conceived and designed the experiments.
Mark Towler: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by the Collaborative Health Research Project fund (#315694-DAN).
Additional information
No additional information is available for this paper.
Acknowledgments
The authors would like to thank Warren Tang for assisting with collecting the cytotoxicity data and Dr. Daniel Boyd for providing technical feedback throughout the elaboration of this manuscript.
References
- 1.Branemark R., Branemark P.I., Rydevik B., Myers R.R. Osseointegration in skeletal reconstruction and rehabilitation: a review. J. Rehabil. Res. Dev. 2001;38(2):175–182. [PubMed] [Google Scholar]
- 2.Branemark P.I., Hansson B.O., Adell R., Breine U., Lindstrom J., Hallen O., Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Scand. J. Plast. Reconstruct. Surg. 1997;11:1–52. [PubMed] [Google Scholar]
- 3.Kashif I., Soliman A.A., Farouk H., Sanad A.M. Effect of titanium addition on crystallization kinetics of lithium borosilicate glass. J. Alloys Compd. 2009;475(1):712–717. [Google Scholar]
- 4.Brauer D.S., Karpukhina N., Law R.V., Hill R.G. Effect of TiO2 addition on structure, solubility and crystallisation of phosphate invert glasses for biomedical applications. J. Non-Cryst. Solids. 2010;356(44):2626–2633. [Google Scholar]
- 5.Wren A.W., Laffir F.R., Kidari A., Towler M.R. The structural role of titanium in Ca–Sr–Zn–Si/Ti glasses for medical applications. J. Non-Cryst. Solids. 2011;357(3):1021–1026. [Google Scholar]
- 6.Pitkin M. Design features of implants for direct skeletal attachment of limb prostheses. J. Biomed. Mater. Res. Part A. 2013;101(11):3339–3348. doi: 10.1002/jbm.a.34606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster J.B., Chou T., Kenly M., English M., Roberts T.L., Bloebaum R.D. Perceptions and acceptance of osseointegration among individuals with lower limb amputations: a prospective survey study. J. Prosthet. Orthot. 2009;21(4):215–222. [Google Scholar]
- 8.Sullivan J., Uden M., Robinson K.P., Sooriakumaran S. Rehabilitation of the trans–femoral amputee with an osseointegrated prosthesis: the United Kingdom experience. Prosthet. Orthot. Int. 2003;27(2):114–120. doi: 10.1080/03093640308726667. [DOI] [PubMed] [Google Scholar]
- 9.Tillander J., Hagberg K., Hagberg L., Branemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin. Orthop. Related Res. 2010;468(10):2781–2788. doi: 10.1007/s11999-010-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendegrass C.J., Goodship A.E., Blunn G.W. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials. 2006;27(23):4183–4191. doi: 10.1016/j.biomaterials.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhari A., Braem A., Vleugels J., Martens J.A., Naert I., Cardoso M.V., Duyck J. Bone tissue response to porous and functionalized titanium and silica based coatings. PLoS One. 2011;6(9):e24186. doi: 10.1371/journal.pone.0024186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buser D., Schenk R.K., Steinemann S., Fiorellini J.P., Fox C.H., Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991;25(7):889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 13.Furlong R.J., Osborn J.F. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J. Bone Joint Surg. 1991;73(5):741–745. doi: 10.1302/0301-620X.73B5.1654336. British Volume. [DOI] [PubMed] [Google Scholar]
- 14.Havelin L.I., Engesæter L.B., Espehaug B., Furnes O., Lie S.A., Vollset S.E. The Norwegian arthroplasty register: 11 years and 73,000 arthroplasties. Acta Orthop. 2000;71(4):337–353. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- 15.Geesink R.G. Osteoconductive coatings for total joint arthroplasty. Clin. Orthop. Relat. Res. 2002;395:53–65. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Logghe C., Van Ossel C.H., D'Hoore W., Ezzedine H., Wauters G., Haxhe J.J. Evaluation of chlorhexidine and silver-sulfadiazine impregnated central venous catheters for the prevention of bloodstream infection in leukaemic patients: a randomized controlled trial. J. Hosp. Infect. 1997;37(2):145–156. doi: 10.1016/s0195-6701(97)90184-5. [DOI] [PubMed] [Google Scholar]
- 17.Gottenbos B., van der Mei H.C., Klatter F., Nieuwenhuis P., Busscher H.J. In vitro and in vivo antimicrobial activity of covalently coupled quaternary ammonium silane coatings on silicone rubber. Biomaterials. 2002;23(6):1417–1423. doi: 10.1016/s0142-9612(01)00263-0. [DOI] [PubMed] [Google Scholar]
- 18.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;5(6):117–141. [Google Scholar]
- 19.Baino F., Novajra G., Miguez-Pacheco V., Boccaccini A.R., Vitale-Brovarone C. Bioactive glasses: special applications outside the skeletal system. J. Non-Cryst. Solids. 2016;432:15–30. [Google Scholar]
- 20.Kaur G., Pandey O.P., Singh K., Homa D., Scott B., Pickrell G. A review of bioactive glasses: their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. Part A. 2014;102(1):254–274. doi: 10.1002/jbm.a.34690. [DOI] [PubMed] [Google Scholar]
- 21.Xynos I.D., Edgar A.J., Buttery L.D., Hench L.L., Polak J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000;276(2):461–465. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 22.Boyd D., Li H., Tanner D.A., Towler M.R., Wall J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci.: Mater. Med. 2006;17(6):489–494. doi: 10.1007/s10856-006-8930-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen M., Li W., Shen M., Zhu S., Wang F. Glass coatings on stainless steels for high-temperature oxidation protection: Mechanisms. Corros. Sci. 2014;82:316–327. [Google Scholar]
- 24.Peddi L., Brow R.K., Brown R.F. Bioactive borate glass coatings for titanium alloys. J. Mater. Sci.: Mater. Med. 2008;19(9):3145–3152. doi: 10.1007/s10856-008-3419-0. [DOI] [PubMed] [Google Scholar]
- 25.Flaten T.P., Alfrey A.C., Birchall J.D., Savory J., Yokel R.A. Status and future concerns of clinical and environmental aluminum toxicology. J. Toxicol. Environ. Health Part A. 1996;48(6):527–542. doi: 10.1080/009841096161050. [DOI] [PubMed] [Google Scholar]
- 26.Sola A., Bellucci D., Cannillo V. Enamelled coatings produced with low-alkaline bioactive glasses. Surf. Coat. Technol. 2014;248:1–8. [Google Scholar]
- 27.Saiz E., Goldman M., Gomez-Vega J.M., Tomsia A.P., Marshall G.W., Marshall S.J. In vitro behavior of silicate glass coatings on Ti6Al4V. Biomaterials. 2002;23(17):3749–3756. doi: 10.1016/s0142-9612(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 28.Gomez‐Vega J., Saiz E.T.A., Oku T., Suganuma K., Marshall G., Marshall S. Novel bioactive functionally graded coatings on Ti6Al4V. Adv. Mater. 2000;12:12:894–898. [Google Scholar]
- 29.Coughlan A., Scanlon K., Mahon B.P., Towler M.R. Zinc and silver glass polyalkenoate cements: an evaluation of their antibacterial nature. Bio-Med. Mater. Eng. 2010;20(2):99–106. doi: 10.3233/BME-2010-0620. [DOI] [PubMed] [Google Scholar]
- 30.Murphy S., Wren A.W., Towler M.R., Boyd D. The effect of ionic dissolution products of Ca–Sr–Na–Zn–Si bioactive glass on in vitro cytocompatibility. J. Mater. Sci.: Mater. Med. 2010;21(10):2827–2834. doi: 10.1007/s10856-010-4139-9. [DOI] [PubMed] [Google Scholar]
- 31.Foley J., Blackwell A. Ion release from copper phosphate cement and influence on Streptococcus mutans growth in vitro: a comparative study. Caries Res. 2002;37(6):416–424. doi: 10.1159/000073393. [DOI] [PubMed] [Google Scholar]
- 32.Donlan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. J. 2002;8(9):881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xynos I.D., Hukkanen M.V.J., Batten J.J., Buttery L.D., Hench L.L., Polak J.M. Bioglass® 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000;67(4):321–329. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 34.Xynos I.D., Edgar A.J., Buttery L.D., Hench L.L., Polak J.M. Gene‐expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001;55(2):151–157. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 35.Asselin A., Hattar S., Oboeuf M., Greenspan D., Berdal A., Sautier J.M. The modulation of tissue-specific gene expression in rat nasal chondrocyte cultures by bioactive glasses. Biomaterials. 2004;25(25):5621–5630. doi: 10.1016/j.biomaterials.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Sola A., Bellucci D., Cannillo V., Cattini A. Bioactive glass coatings: a review. Surf. Eng. 2011;27(8):560–572. [Google Scholar]
- 37.Welsch G., Boyer R., Collings E.W., editors. ASM international; Materials Park: 1993. Materials Properties Handbook: Titanium Alloys. [Google Scholar]
- 38.Matinmanesh A., Rodriguez O., Towler M., Zalzal P., Schemitsch E., Papini M. Quantitative evaluation of the adhesion of bioactive glasses onto Ti6Al4V substrates. Mater. Des. 2016;97:213–221. 20016. [Google Scholar]
- 39.Schrooten J., Helsen J.A. Adhesion of bioactive glass coating to Ti6Al4V oral implant. Biomaterials. 2000;21(14):1461–1469. doi: 10.1016/s0142-9612(00)00027-2. [DOI] [PubMed] [Google Scholar]
- 40.Mehdipour M., Afshar A., Mohebali M. Electrophoretic deposition of bioactive glass coating on 316L stainless steel and electrochemical behavior study. Appl. Surf. Sci. 2012;258(24):9832–9839. [Google Scholar]
- 41.Brow R.K., Watkins R.D. Sandia National Labs; Albuquerque: 1987. Reactions and Bonding Between Glasses and Titanium, in (No. SAND-87-0541C; CONF-871207-18) [Google Scholar]
- 42.Saha S.K., Jain H., Goldstein J.I., Miller A.C., Brow R.K. Reaction between titanium and B2O3 melt/glass. Phys. Chem. Glasses. 1998;39(2):118–121. [Google Scholar]
- 43.Hoppe A., Güldal N., Boccaccini A. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Wallace K., Hill R., Pembroke J., Brown C., Hatton P. Influence of sodium oxide content on bioactive glass properties. J. Mater. Sci.: Mater. Med. 1999;10(12):697–701. doi: 10.1023/a:1008910718446. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez O., Curran D., Papini M., Placek L., Wren A., Schemitsch E., Zalzal P., Towler M. Characterization of silica-based and borate-based, titanium-containing bioactive glasses for coating metallic implants. J. Non-Cryst. Solids. 2015;433C:95–102. [Google Scholar]
- 46.Rodriguez O., Matinmanesh A., Phull S., Schemitsch E., Zalzal P., Clarkin O., Papini M., Towler M. Silica-based and borate-based, titania-containing bioactive coatings characterization: critical strain energy release rate, residual stresses, hardness, and thermal expansion. J. Funct. Biomater. 2016;7(4):32. doi: 10.3390/jfb7040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao A., Wang D., Huang W., Fu Q., Rahaman M., Day D. In vitro bioactive characteristics of borate‐based glasses with controllable degradation behavior. J. Am. Ceram. Soc. 2007;90(1):303–306. [Google Scholar]
- 48.Rahaman M.N., Day D.E., Bal B.S., Fu Q., Bonewald J.S.B.L.F., Tomsia A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorustovich A.A., López J.M.P., Guglielmotti M.B., Cabrini R.L. Biological performance of boron-modified bioactive glass particles implanted in rat tibia bone marrow. Biomed. Mater. 2006;1(3):100. doi: 10.1088/1748-6041/1/3/002. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., Coughlan A., Laffir F., Pradhan D., Mellott N., Wren A. Investigating the mechanical durability of bioactive glasses as a function of structure, solubility and incubation time. J. Non-Cryst. Solids. 2013;380:25–34. [Google Scholar]
- 51.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 52.McDevitt C., Ogunniyi A., Valkov E., Lawrence M., Kobe B., McEwan A., Paton J. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 2011;7(11):e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azam A., Ahmed A., Oves M., Khan M., Habib S., Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomed. 2012;7(7):6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aina V., Perardi A., Bergandi L., Malavasi G., Menabue L., Morterra C., Ghigo D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem. Biol. Interact. 2007;167(3):207–218. doi: 10.1016/j.cbi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Brown R., Rahaman M., Dwilewicz A., Huang W., Day D., Li Y., Bal B. Effect of borate glass composition on its conversion to hydroxyapatite and on the proliferation of MC3T3‐E1 cells. J. Biomed. Mater. Res. Part A. 2009;88(2):392–400. doi: 10.1002/jbm.a.31679. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X., Jia W., Gu Y., Xiao W., Liu X., Wang D., Zhang C., Huang W., Rahaman M., Day D., Zhou N. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials. 2010;31(22):5865–5874. doi: 10.1016/j.biomaterials.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Kansal I., Reddy A., Mu-oz F., Choi S., Kim H., Tulyaganov D., Ferreira J. Structure, biodegradation behavior and cytotoxicity of alkali-containing alkaline-earth phosphosilicate glasses. Mater. Sci. Eng. C. 2014;44:159–165. doi: 10.1016/j.msec.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Tilocca A. Sodium migration pathways in multicomponent silicate glasses: Car-Parrinello molecular dynamics simulations. J. Chem. Phys. 2010;133(1):014701. doi: 10.1063/1.3456712. [DOI] [PubMed] [Google Scholar]
- 59.Gristina A.G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 60.Odekerken J., Welting T., Arts J., Walenkamp G., Emans P. Modern Surface Engineering Treatments. INTECH; 2013. Modern orthopaedic implant coatings — their pro's, con's and evaluation methods; pp. 45–73. [Google Scholar]