Abstract
Merkel cell carcinoma (MCC) tumor cells express several markers detected in normal Merkel cells, a non-proliferative population of neuroendocrine cells which arise from epidermis. MCCs frequently contain Merkel cell polyomavirus (MCPyV) DNA and express viral transforming antigens, sT and tLT, but the role of these putative oncogenes in MCC development, and this tumor’s cell of origin, are unknown. Using a panel of pre-term transgenic mice, we show that epidermis-targeted co-expression of sT and the cell fate determinant atonal bHLH transcription factor 1 (Atoh1) leads to development of widespread cellular aggregates with histology and marker expression mimicking that of human intraepidermal MCC. The MCC-like tumor phenotype was dependent on the FBXW7-binding domain of sT, but not the sT-PP2A binding domain. Co-expression of MCPyV tLT did not appreciably alter the phenotype driven by either sT or sT combined with Atoh1. MCPyV sT, when co-expressed with Atoh1, is thus sufficient to initiate development of epidermis-derived MCC-like tumors in mice.
Keywords: melanoma/skin cancers, animal models of cancer, human tumor viruses, viral T antigens, Atoh1, cell lineage
Introduction
Merkel cell carcinoma (MCC) is a rare and potentially lethal skin tumor that arises either sporadically, or more commonly, in association with clonally-integrated Merkel cell polyomavirus (MCPyV) [reviewed in (1,2)]. Although MCPyV appears to play a causal role in MCC, our understanding of how viral proteins contribute to early stages of MCC development is incomplete. A major obstacle to progress in this area has been the lack of an MCPyV-based mouse model that yields MCC-like tumors, despite efforts by several groups (3–5).
MCPyV-positive MCCs express two putative viral oncoproteins: small and large T antigen (sT and LTAg), and in all MCCs examined, truncating mutations are present in LT yielding a shorter protein (tLT) [reviewed in (6)]. Polyomavirus TAgs contribute to transformation largely by modulating the expression or function of key cellular proteins, including tumor suppressors [reviewed in (7)]. In keeping with this paradigm, MCPyV tLT binds and inhibits the function of RB transcriptional corepressor 1 (RB1), and MCPyV sT binds the tumor suppressors protein phosphatase 2A (PP2A) and F-box and WD repeat domain containing 7 (FBXW7), a component of the Skp, Cullin, F-box containing (SCF) ubiquitin ligase complex [reviewed in (6)]. Although tLT and sT appear to be required for proliferation of established MCC cell lines (8,9), the potential role of MCPyV TAgs, either alone or in combination, has not been examined in early stages of MCC development.
Histologically, MCCs are small blue cell tumors due to scant cytoplasm in tumor cells. MCC tumor cells are characteristically located within the deeper, dermal compartment of skin, but in some cases tumor cells are present both in dermis and overlying epidermis, or restricted to just the epidermis (intraepidermal MCC) [reviewed in (10)]. This suggests that at least a subset of MCCs may arise from epidermal progenitors, in keeping with the epidermal origin of normal Merkel cells (11,12). MCC tumor cells express many of the same markers as normal Merkel cells, but in contrast to Merkel cells, nearly all of which are post-mitotic (13,14), MCC tumor cells are proliferative; in addition, the keratin filament network in MCC cells is typically reorganized into dot-like paranuclear aggregates highly characteristic of this malignancy (15).
To test the involvement of MCPyV TAgs in early stages of MCC development, we engineered nine transgenic mouse models expressing various combinations of tLT, sT, sT mutants, and Atoh1, which drives formation of ectopic Merkel cells (16). Our findings establish a central role for sT, but not tLT, in the initiation of MCC tumorigenesis; implicate the sT domain that binds FBXW7 in MCC development; support the notion that the Merkel cell lineage is self-sustaining once it is specified; and point to an epidermal cell of origin for at least a subset of MCCs.
Materials and Methods
Generation of transgenic mice
The K5-sTAg-IRES-tdTomato (K5-sT), K5-sTL142A, and K5-sT91-95A transgenic cassettes have been described previously (3). A tLT sequence used to generate a K5-tLTAg1 (K5-tLT)1 transgenic cassette was cloned from an MCPyV-positive human MCC. Following RNA isolation and RT-PCR, sequencing revealed a mutation introducing a stop codon at nts 1362–1364 of the MCPyV genome (GenBank EU375803). This sequence, predicted to produce a 245aa protein, was cloned into the pBK5 transgenic cassette (17) following addition of Not1 and SnaBI restriction sites via PCR, upstream and downstream respectively. A K5-tLTAg2 (K5-tLT2) cassette was generated following de novo synthesis (GenScript, Piscataway, NJ) of a cDNA sequence based on MCCw168 (GenBank KC426954; join nts 1–234; 666–1259), which encodes a 275aa protein. An upstream Kozak sequence (GCCACC), a 5′ SnaBI and 3′ NheI restriction enzyme site were included for cloning into the pBK5 cassette. The K5-tLT1 and K5-tLT2 transgenes yielded indistinguishable results and both were therefore designated K5-tLT. The K5-Atoh1-IRES-GFP (K5-Atoh1) transgenic cassette was generated following de novo synthesis (GenScript) of a fragment containing the Mus musculus atonal homolog 1 (Atoh1; GenBank Accession No. NM_007500.4; nts 178–1233), including an upstream Kozak sequence and a downstream IRES-eGFP sequence flanked by 5′ NotI and 3′ NheI restriction sites. The NotI-Atoh1-IRES-eGFP-NheI fragment was cloned into the pBK5 vector. The IRES sequence (nts 2842–3416 from Clontech pLVX-IRES-tdTomato vector No. 631238) followed by an eGFP sequence (GenBank Accession U55761; nts 97–816) was included as a transgene reporter. Following sequence verification, BssHII-digested fragments of each of the above constructs were gel purified and used for single, double or triple co-injections (Supplementary Table S1) into (C57BL/6 X SJL) F2 oocytes in the University of Michigan Transgenic Core.
Analysis of pre-term embryos
Given the likelihood of peri-natal lethal phenotypes for some of the transgenes, all K5-driven transgenic founders were harvested as pre-term embryos at 18 days of embryogenesis. Transgenic founders were identified by PCR using transgene-specific primers as follows i) transgene-specific rabbit β-globin intron: nts 1106–1271 (1106F: TGCATATAAATTCTGGCTGGCG, 1271R: GCATGAACATGGTTAGCAGAGGG) (GenBank V00882); ii) sT primers: nts 354–590 (354F: GGAATTGAACACCCTTTGGA, 590R: CTACAATGCTGGCGAGACAA) and iii) tLT primers: nts 1054–1208 (1054F: CTGGGTATGGGTCCTTCTCA, 1208R: ATTGGGTGTGCTGGATTCTC) of the MCPyV genome (GenBank EU375803; isolate350); and iv) Atoh1 primers: nts 554–842 (554F: GCAAGCTGAAGGGTGGGGTT, 842R: GGCGGTTGCTCTCCGACATT) of Mus musculus atonal homolog 1 (GenBank NM_007500). We screened a total of 913 potential embryonic founders from single, double or triple co-injections of transgene combinations. All 117 transgene-positive founders determined by PCR were further analyzed for transgene expression in dorsal skin at the single-cell level by immunostaining for RFP (sT), LT, and GFP (Atoh1), identifying a total of 69 expressing founders. Individual founders for each single, double or triple co-injection are listed in Supplementary Table S1, including a detailed analysis of transgene expression and phenotype severity for each transgenic founder. Between three and 14 transgenic mice were analyzed for each injected transgene or combination of transgenes. All mice were housed and maintained according to University of Michigan IACUC guidelines under animal protocol #PRO00006657.
Tissue collection and immunostaining
All tissues were fixed overnight at room temperature in 10% neutral buffered formalin, transferred to 70% EtOH, processed, and paraffin embedded. For immunohistochemical (IHC) and immunofluorescent (IF) staining, tissue was sectioned at 4–5 μm and deparaffinized and rehydrated prior to antigen retrieval in citrate-based buffer (0.01 mol/L citric acid, pH 6.8) for 15 minutes at 100°C. Endogenous peroxidases were quenched with 3% H2O2 followed by blocking in 5% goat or donkey serum for 1 hour, and overnight incubation with primary antibodies at 4°C in a humidified chamber. Staining for Keratin 20 included an additional 10 minutes digestion step with pepsin (Thermo Fisher Scientific, IL) diluted 1:2 in phosphate buffered saline (PBS) following antigen retrieval. Primary antibodies and dilutions are listed in Supplementary Table S2. Bound antibodies were detected using the VectaStain ABC peroxidase or M.O.M. fluorescein or peroxidase Immunodetection Kits (Vector Laboratories, CA). SigmaFast diaminobenzidine was used as peroxidase substrate and nuclei counterstained with hematoxylin followed by mounting with Permount (Thermo Fisher Scientific, IL). For immunofluorescent staining AlexaFluor594, AlexaFluor555, and AlexaFluor488 (Jackson ImmunoResearch, PA), or AlexaFluor647 (No. A-31571, Thermo Fisher Scientific, IL) secondary antibodies were used at 1:300. Sections were mounted with ProLong Diamond or Gold Antifade Reagent with DAPI (Invitrogen, OR) for IF visualization.
Acquisition of human tumor
Human tissue samples were collected from patients who provided written informed consent for the use of surplus tissue according to a protocol approved by the University of Michigan Institutional Review Board (IRB Study ID: HUM00050085) in adherence to Helsinki Guidelines.
Results and Discussion
MCPyV sT expression in ectopic Merkel cells drives development of intraepidermal MCC-like tumors
We generated a panel of mouse models using the K5 promoter to coordinately express multiple transgenes, enabling us to study responses to MCPyV sT and tLT, alone or in combination, targeted either to epidermal cells or epidermal cells reprogrammed to a Merkel cell lineage by Atoh1 (Table 1, Supplementary Table S1, Supplementary Fig. S1). IRES-driven GFP and RFP (tdTomato) were used as reporters for Atoh1 and sT transgene expression, respectively (Supplementary Fig. S1), since antibodies for reliable detection of these proteins in formalin-fixed tissue sections are not available.
Table 1.
Transgene details and associated phenotypes
| Transgene Designation(s) | Transgenic Construct(s) | Effector(s); targeted cells | Phenotype |
|---|---|---|---|
| sT | K5-sTAg-IRES-RFP | sTAg; epidermis | epidermal hyperplasia/dysplasia |
| tLT | K5-tLTAg | tLTAg (MCC-specific);epidermis | none apparent |
| sT+tLT | K5-sTAg-IRES-RFP + K5-tLTAg | sTAg + tLTAg; epidermis | epidermal hyperplasia/dysplasia |
| Atoh1 | K5-Atoh1-IRES-GFP | Neuroendocrine cell fate determinant Atoh1; epidermis | ectopic Merkel cells |
| sT+Atoh1 | K5-sTAg-IRES-RFP +K5-Atoh1-IRES-GFP | sTAg + Atoh1; ectopic Merkel cells | MCC-like tumors |
| tLT+Atoh1 | K5-tLTAg + K5-Atoh1-IRES-GFP | tLTAg + Atoh1; ectopic Merkel cells | ectopic Merkel cells |
| sT+tLT+Atoh1 | K5-sTAg-IRES-RFP + K5-tLTAg + K5-Atoh1-IRES-GFP | sTAg + tLTAg + Atoh1; ectopic Merkel cells | MCC-like tumors |
| sTL142A+tLT+Atoh1 | K5-sTAgL142A-IRES-RFP + K5-tLTAg + K5-Atoh1-IRES-GFP | sTAgL142A (PP2A binding mutant) + tLTAg + Atoh1; ectopic Merkel cells | MCC-like tumors |
| sT91-95A+tLT+Atoh1 | K5-sTAg91-95A-IRES-RFP + K5-tLTAg + K5-Atoh1-IRES-GFP | sTAg91-95A (FBXW7 binding mutant) + tLTAg + Atoh1; ectopic Merkel cells | ectopic Merkel cells |
See Supplementary Table S1 for full details.
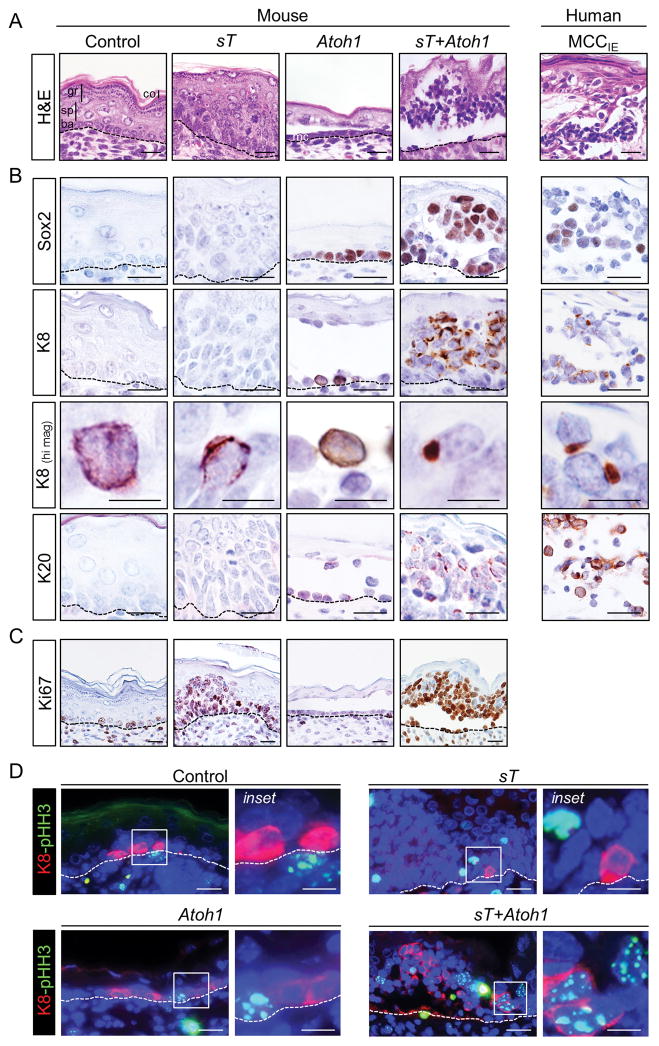
In keeping with previous studies, expression of MCPyV sT alone led to epidermal hyperplasia and dysplasia (3,5) while expression of Atoh1 yielded ectopic Merkel cells and a hypoplastic epidermis (16) (Fig. 1A). In striking contrast, co-expression of MCPyV sT and Atoh1 was sufficient to drive formation of small blue cell tumors resembling human intraepidermal MCC (Fig. 1A; Supplementary Table S1). Skin of sT+Atoh1 mice contained numerous collections of cells which expressed the MCC markers Sox2, K8, and K20 (Fig. 1B). Although these markers are also detected in the rare Merkel cells seen in control epidermis and in ectopic Merkel cells in mice expressing just Atoh1, in the latter cases Merkel cells are present as single cells or a single layer of cells (Fig. 1A,B). In contrast, cells expressing MCC markers in sT+Atoh1 mice are typically organized in multi-layered, tumor-like aggregates (Fig. 1A). Importantly, K8 and K20 expression in MCC-like tumors in sT+Atoh1 mice is frequently distributed in a clumped or dot-like pattern typically detected in MCC tumor cells but not normal or ectopic Merkel cells (Fig. 1B). Finally, a subset of MCC-like cells in sT+Atoh1 mice are proliferating, based on immunostaining for Ki67 as well as the mitosis marker pHH3 (Fig. 1C,D). Collectively, these phenotypic changes are strikingly similar to those observed in human MCC tumor cells.
Figure 1. MCPyV sT expression in ectopic Merkel cells initiates MCC-like tumor development in mice.
A) Histology of epidermis from control and pre-term transgenic mouse embryos expressing MCPyV sT, Atoh1 or sT+Atoh1 driven by the Keratin 5 promoter compared to human intraepidermal MCC (MCCIE). Control epidermis contains a single layer of basal cells (ba) with several layers of differentiating spinous (sp), granular (gr) and cornified (co) cells. Expression of sT alone leads to epidermal hyperplasia and dysplasia, Atoh1 alone drives ectopic Merkel cells (mc) within a hypoplastic epidermis, whereas co-expression of sT and Atoh1 leads to MCC-like tumors. B) Immunohistochemical staining for Merkel cell and MCC markers Sox2, K8 and K20. Appearances of tumor-like aggregates of cells and of K8 in paranuclear dots is restricted to mice expressing sT+Atoh1. C) Ki67 immunostaining shows high proliferative activity in multiple layers of epidermis only in sT-expressing mice. D) Co-immunofluorescence for phospho-Histone H3 (pHH3) and K8 indicates mitotic activity of K8-positive cells largely restricted to MCC-like tumor cells in sT+Atoh1 mice. Scale bars = 25 μm; scale bars for K8 (hi mag) = 10 μm.
Transgene-driven Atoh1 leads to sustained expression of Merkel cell markers
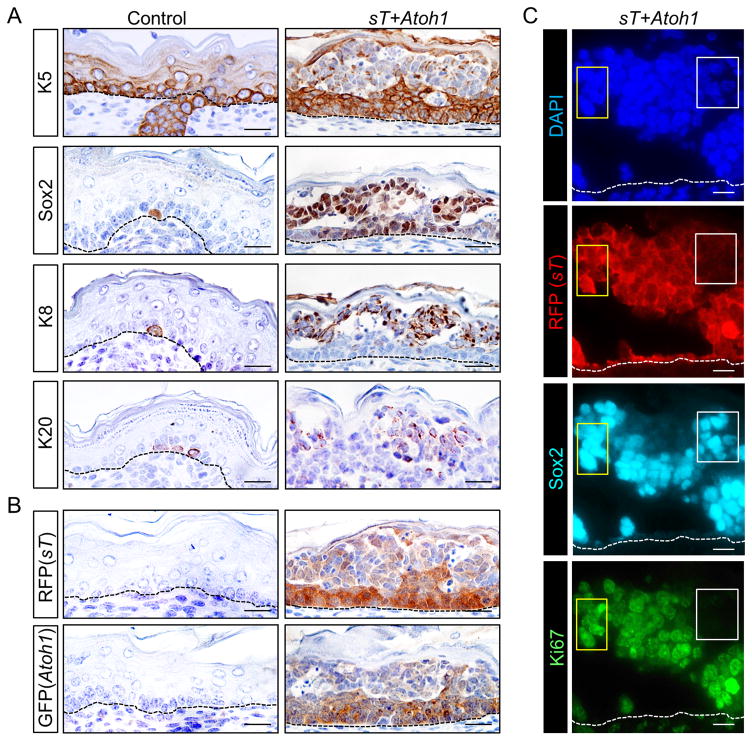
Atoh1-driven formation of ectopic Merkel cells from epidermis is accompanied by a switch from an epidermal to neuroendocrine pattern of lineage markers, leading to down-regulation of the epidermal keratin K5 and upregulation of the Merkel cell markers K8 and Sox2 (16). In MCC-like tumors arising in sT+Atoh1 mice, the early marker Sox2 (16,18) is first detectable in the expanded basal layer compartment that still expresses K5, whereas the later-stage markers K8 and K20 are most highly expressed in more superficially-located cells with markedly reduced levels of K5 (Fig. 2A). When residual K5 is detected in MCC-like tumor cells it is frequently co-expressed with K8 in paranuclear dots (Supplementary Fig. S2), which mimics the pattern also seen in human MCC (D. Mangelberger et al, in preparation).
Figure 2. Sustained expression of Merkel cell markers in MCC-like mouse tumor cells with reduced levels of transgene expression.
A) Immunohistochemical staining shows markedly reduced expression of K5 in suprabasal epidermis of sT+Atoh1 mice where MCC-like tumor cells express Merkel cell markers Sox2, K8 and K20. Isolated normal Merkel cells in control skin also express these markers (left panels). B) K5-driven expression of sT (RFP) and Atoh1 (GFP) transgenes is reduced in suprabasal regions of MCC-like tumors. C) Co-immunostaining shows robust Sox2 expression in regions with high (yellow boxes) as well as low (white boxes) sT (RFP) expression. In contrast, proliferative activity, based on Ki67 expression, is negligible in Sox2-positive cells with low-level sT (RFP) expression (white boxes). Scale bars = 25 μm.
In keeping with the reduced expression of K5 protein (Fig. 2A), K5 promoter-driven expression of GFP (Atoh1) and RFP (sT) is reduced in suprabasal compartments with MCC-like foci (Fig. 2B). Sox2 and K8 are highly expressed in these regions (Fig. 2A), in keeping with the concept that exogenous ATOH1 can trigger endogenous Atoh1 and Sox2 gene expression to maintain neuroendocrine cell fate via a feed-forward loop (16,19,20). In contrast, proliferative activity of Sox2-positive cells is typically robust only in cells with high-level RFP (sT) transgene expression (Fig. 2C). Together, these data support the concept that while transient expression of Atoh1 leads to sustained reprogramming of epidermal cells into the neuroendocrine lineage, proliferation of MCC-like tumor cells requires sT expression above a critical threshold.
MCPyV tLT expression does not appreciably alter sT-driven MCC-like tumors
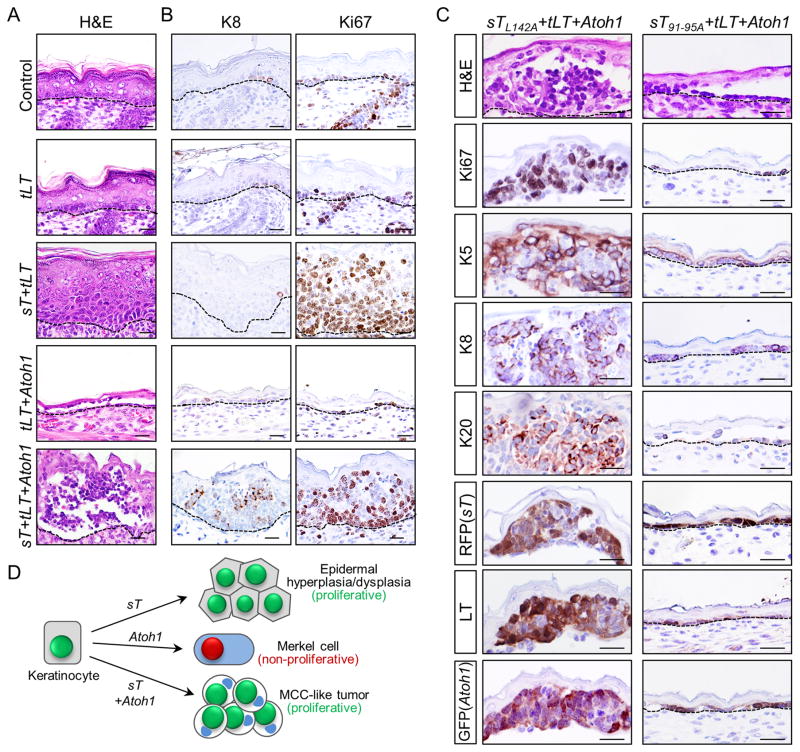
Because tLT and sT are co-expressed in human MCC [reviewed in (6)], we tested the epidermal response to tLT expressed either alone or together with the sT and Atoh1 transgenes (Table 1; Supplementary Table S1; Fig. 3). Despite the central role of LT from other polyomaviruses in driving neoplastic transformation [reviewed in (21)], we did not detect an overt phenotype in epidermis of transgenic mouse embryos expressing MCPyV tLT (Fig. 3A,B). Moreover, in co-expression studies, addition of tLT did not appreciably alter the phenotypic response to sT, Atoh1, or sT+Atoh1 (Fig. 3A,B; compare with Fig. 1A–C). Together, these data support the concept that sT is the major MCPyV-derived oncogenic driver in MCC, and establish that tLT is dispensable for initiation of MCC-like tumors in this model.
Figure 3. MCPyV tLT does not alter the MCC-like tumor phenotype, which is dependent on the FBXW7 binding domain of sT.
A) The epidermal phenotype of Control, sT, Atoh1, or sT+Atoh1 mice is not appreciably altered by the addition of tLT (compare with Fig. 1A). B) Expression of tLT expression does not cause gross alterations in expression of the Merkel cell/MCC marker K8 or proliferation marker Ki67 either in control mice or transgenics (compare to Fig. 1B,C). C) Epidermal histology from mice expressing a sT-PP2A binding mutant sTL142A+tLT+Atoh1; or mice carrying a mutation in the sT-FBXW7 binding domain sT91-95A+tLT+Atoh1. sTL142A+tLT+Atoh1 mice develop MCC-like tumors indistinguishable from those in sT+tLT+Atoh1 founders carrying wild-type sT, including expression of MCC markers, Ki67, and transgenes (left panels). In contrast, MCC-like tumor development is completely blocked in sT91-95A+tLT+Atoh1 mice, which produce a hypoplastic epidermis indistinguishable from Atoh1 (Fig. 1A) or tLT+Atoh1 (A) founders. D) Cartoon summarizing key phenotypes observed following transgene (co-) injections as described in Table 1. Redistribution of keratins to a paranuclear dot-like pattern is detected in MCC-like tumor cells. Scale bars in A–C = 25 μm.
MCC-like tumor development is dependent on the FBXW7 binding domain of sT
We performed additional studies with mutants to test whether either the PP2A or FBXW7 binding domains of sT are required for MCC-like tumor development in mice that also express Atoh1 and tLT (Table 1, Supplementary Table S1). sTL142A+tLT+Atoh1 mice, which carry a PP2A binding mutant of sT, produce a phenotype indistinguishable from that seen in sT+tLT+Atoh1 mice (Fig. 3C), arguing that sT-PP2A interaction is not required for MCC-like tumor development in this model. In striking contrast, sT91-95A+tLT+Atoh1 mice, carrying a mutation in the sT FBXW7 binding domain, resemble tLT+Atoh1 or Atoh1 mice, with a single layer of ectopic neuroendocrine cells (Fig. 3C, Table 1, Supplementary Table S1). These findings establish a critical requirement for the FBXW7 binding domain of sT in MCC-like tumor development in this model, and are in keeping with previous studies implicating this domain in sT-driven fibroblast transformation in vitro (22) and epidermal tumorigenesis in vivo (3).
Based on the results reported here we conclude the following. 1) MCPyV sT, when expressed in keratinocytes reprogrammed to a Merkel cell fate by Atoh1, is sufficient to initiate MCC-like tumors in late-stage mouse embryos (Fig. 3D). 2) The MCC-like phenotype does not require the sT domain that binds PP2A, but is strictly dependent on the sT FBXW7 binding domain. 3) MCPyV tLT does not appear to influence the phenotype of either normal epidermis, sT-transformed epidermis, or MCC-like tumors driven by sT+Atoh1. However, further studies examining the role of tLT at later stages of MCC tumorigenesis will be required, given the proposed requirement for RB1 disruption in established MCCs either via MCPyV tLT interaction or, in virus-negative tumors, somatic mutation [(23) and references therein]. 4) Atoh1-reprogrammed epidermal cells retain Merkel cell fate even after transgene expression is down-regulated due to loss of K5 promoter activity. Together, these data support the notion that sT, via its FBXW7-interacting domain, is the primary oncogenic driver during initiation of MCC development, and that MCCs may arise from keratinocytes or other cell types in which expression of ATOH1 has been activated, even transiently, to drive cells into the Merkel cell lineage.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by NIH grants R01 CA189352 and R21 CA183084 (A.A. Dlugosz); R21 CA209166 (S.Y. Wong); P30 DK034933 (A.A. Dlugosz); P30 CA046592 (University of Michigan Comprehensive Cancer Center Support Grant) (A.A. Dlugosz, S.Y. Wong, T.L. Saunders); the American Skin Association Milstein Research Scholar Award Melanoma/Non-Melanoma Skin Cancer and the Helen L. Kay Charitable Trust (M.E. Verhaegen); and the Ronald P. and Joan M. Nordgren Cancer Research Funds (A.A. Dlugosz).
We thank Ehab Nazzal, Kristin Rybski, Jack Weick, Paul Baciu, and Haley Zabawa for additional technical assistance, José Jorcano for providing reagents, and Michael Imperiale for helpful comments throughout the course of these studies and critical reading of the manuscript. We gratefully acknowledge Wanda Filipiak and Galina Gavrilina of the Transgenic Animal Model Core of the University of Michigan’s Biomedical Research Core Facilities for production of transgenic mice.
References
- 1.Moshiri AS, Nghiem P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J Natl Compr Canc Netw. 2014;12(9):1255–62. doi: 10.6004/jnccn.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang Y, Moore PS. Merkel cell carcinoma: a virus-induced human cancer. Annu Rev Pathol. 2012;7:123–44. doi: 10.1146/annurev-pathol-011110-130227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhaegen ME, Mangelberger D, Harms PW, Vozheiko TD, Weick JW, Wilbert DM, et al. Merkel cell polyomavirus small T antigen is oncogenic in transgenic mice. J Invest Dermatol. 2015;135(5):1415–24. doi: 10.1038/jid.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spurgeon ME, Cheng J, Bronson RT, Lambert PF, DeCaprio JA. Tumorigenic activity of merkel cell polyomavirus T antigens expressed in the stratified epithelium of mice. Cancer Res. 2015;75(6):1068–79. doi: 10.1158/0008-5472.CAN-14-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuda M, Guastafierro A, Geng X, Shuda Y, Ostrowski SM, Lukianov S, et al. Merkel Cell Polyomavirus Small T Antigen Induces Cancer and Embryonic Merkel Cell Proliferation in a Transgenic Mouse Model. PLoS One. 2015;10(11):e0142329. doi: 10.1371/journal.pone.0142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell polyomavirus. Curr Opin Virol. 2015;11:38–43. doi: 10.1016/j.coviro.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCaprio JA, Garcea RL. A cornucopia of human polyomaviruses. Nat Rev Microbiol. 2013;11(4):264–76. doi: 10.1038/nrmicro2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houben R, Adam C, Baeurle A, Hesbacher S, Grimm J, Angermeyer S, et al. An intact retinoblastoma protein-binding site in Merkel cell polyomavirus large T antigen is required for promoting growth of Merkel cell carcinoma cells. Int J Cancer. 2012;130(4):847–56. doi: 10.1002/ijc.26076. [DOI] [PubMed] [Google Scholar]
- 9.Shuda M, Chang Y, Moore PS. Merkel cell polyomavirus-positive Merkel cell carcinoma requires viral small T-antigen for cell proliferation. J Invest Dermatol. 2014;134:1479–81. doi: 10.1038/jid.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czapiewski P, Biernat W. Merkel cell carcinoma - recent advances in the biology, diagnostics and treatment. Int J Biochem Cell Biol. 2014;53:536–46. doi: 10.1016/j.biocel.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol. 2009;336(1):76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van KA, Mascre G, Youseff KK, Harel I, Michaux C, De GN, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187(1):91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moll I, Zieger W, Schmelz M. Proliferative Merkel cells were not detected in human skin. Arch Dermatol Res. 1996;288(4):184–7. doi: 10.1007/BF02505222. [DOI] [PubMed] [Google Scholar]
- 14.Wright MC, Reed-Geaghan EG, Bolock AM, Fujiyama T, Hoshino M, Maricich SM. Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. J Cell Biol. 2015;208(3):367–79. doi: 10.1083/jcb.201407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holser GA, Patterson JW. Neural and neuroendocrine tumors. In: Patterson JW, editor. Weedon’s Skin Pathology. 4. Elsevier Limited; 2016. pp. 1062–65. [Google Scholar]
- 16.Ostrowski SM, Wright MC, Bolock AM, Geng X, Maricich SM. Ectopic Atoh1 expression drives Merkel cell production in embryonic, postnatal and adult mouse epidermis. Development. 2015;142(14):2533–44. doi: 10.1242/dev.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez A, Bravo A, Jorcano JL, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type- specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58(1):53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- 18.Perdigoto CN, Bardot ES, Valdes VJ, Santoriello FJ, Ezhkova E. Embryonic maturation of epidermal Merkel cells is controlled by a redundant transcription factor network. Development. 2014;141(24):4690–6. doi: 10.1242/dev.112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127(6):1185–96. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- 20.Bardot ES, Valdes VJ, Zhang J, Perdigoto CN, Nicolis S, Hearn SA, et al. Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. Embo j. 2013;32(14):1990–2000. doi: 10.1038/emboj.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An P, Saenz Robles MT, Pipas JM. Large T antigens of polyomaviruses: amazing molecular machines. Annu Rev Microbiol. 2012;66:213–36. doi: 10.1146/annurev-micro-092611-150154. [DOI] [PubMed] [Google Scholar]
- 22.Kwun HJ, Shuda M, Feng H, Camacho CJ, Moore PS, Chang Y. Merkel Cell Polyomavirus Small T Antigen Controls Viral Replication and Oncoprotein Expression by Targeting the Cellular Ubiquitin Ligase SCF(Fbw7) Cell Host Microbe. 2013;14(2):125–35. doi: 10.1016/j.chom.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesbacher S, Pfitzer L, Wiedorfer K, Angermeyer S, Borst A, Haferkamp S, et al. RB1 is the crucial target of the Merkel cell polyomavirus Large T antigen in Merkel cell carcinoma cells. Oncotarget. 2016;7(22):32956–68. doi: 10.18632/oncotarget.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.