Among all the complex medical syndromes that intensivists face daily, perhaps the least understood and most costly in terms of mortality, morbidity and costs (personal and societal) is acute kidney injury (AKI).1 Over the last five years, our understanding of the mechanisms of AKI, its effect on failure of other organs, and particularly the complexity of subsequent kidney repair, has grown substantially. The extensive search for biological urinary markers that could identify cellular distress in the kidney prior to substantial functional decline had been disappointing. Most early biomarkers have not performed satisfactorily when externally validated in heterogeneous populations of critically ill patients where multiple comorbidities and other inflammatory syndromes, like sepsis, cause variability in biomarker measurement.2
In 2013, the Sapphire study, an international multicenter study of 728 critically ill patients, validated a new urinary biomarker, the product of tissue inhibitor of metalloproteinase and insulin-like growth factor-binding protein (TIMP2*IGFBP7), for the risk stratification of AKI. (TIMP2*IGFBP7) provided evidence of improved predictive performance in comparison to existing biomarkers.3 Subsequently, the Topaz study, which enrolled 420 critically ill patients within 24 hours of intensive care unit (ICU) admission, validated the predictive performance of a prespecified cutoff of the urine (TIMP2*IGFBP7) [0.3 (ng/mL)2/1,000] for the clinically adjudicated diagnosis of AKI.4 Patients with a urinary (TIMP2*IGFBP7) result above this threshold had seven times the risk for developing moderate to severe AKI within 12 hours of the test compared to patients with a result <0.3.
This study provided the clinical basis for subsequent U.S. Food and Drug Administration approval of the NephroCheck® Test (Astute Medical, Inc., San Diego, California, USA), the first urinary biomarker approved for risk stratification of AKI in critically ill patients. Both TIMP2 and IGFBP7 are inducers of G1 cell cycle arrest in response to an injurious trigger. Thus, their increase in patients at high risk of developing AKI may reflect an increase in the level of tubular cell distress, and their detection can be used as a warning signal before actual damage occurs.
Not surprisingly, many intensivists are left wondering how to incorporate this new tool into everyday practice, potentially enhancing the use of the tools already at our disposal. As one of the most important organs in maintaining homeostasis, the kidney is both vulnerable and resilient to systemic effects of critical illness; thus our approach to AKI has to accommodate this physiological complexity in order to be successful. Considering the high prevalence of AKI and the deleterious effect when it occurs, our effort must focus on AKI prevention, mitigation of further injury when AKI has already occurred, treatment of negative effects on other organs, and facilitation of renal recovery in patients with established AKI. This clinical pathway requires a medical team of experts rather than the lonely hero; we need to involve all primary healthcare providers who manage critically ill patients, from operating theaters to the ICU, backed up by bedside nurses, pharmacists and nephrologists. In the ICU, all critical steps of this process, including the identification of patients at risk for AKI, the assessment of tubular distress, subsequent identification of potential causes, and initiation of treatment, have to occur early—preferably within the first 12 to 24 hours of the ICU admission. Every patient admitted to the ICU needs to undergo a comprehensive and systematic assessment of kidney health following a new paradigm that differs substantially from the traditional “fluid-and-electrolytes” approach common on critical care rounds. This requires assessment of the patient’s renal reserve and susceptibility to new injury, the extent of exposure to an insult and the resulting distress or damage sustained by it. The determination of potential causes of AKI, initiation of treatment and reassessment of kidney distress in response to that therapy should follow promptly afterwards.
Assessment of renal resilience to acute injurious triggers requires looking for evidence of previous functional or structural kidney damage that may increase susceptibility to new injury. Chronic kidney disease (CKD) is a well-known risk factor not only for AKI but also for long-term longevity, hospital mortality and surgical outcomes.5 Review of the medical history should include diagnosis and staging of known CKD, evidence of previous AKI episodes and presence of risk factors for CKD. For each patient, calculation of estimated glomerular filtration rate (GFR) using reference serum creatinine and measurement of albuminuria using urine dipstick or random microalbumin/creatinine ratio tests can provide an inexpensive snapshot of baseline kidney health and be an early marker of kidney dysfunction.
The exposure to an insult should be determined at ICU admission and a review should be conducted of the preceding 24 to 48 hours for any history of hypotension, use of vasopressors or nephrotoxic medications, respiratory failure with hypoxia, sepsis, severe trauma, high-risk surgery, and any other conditions associated with a high risk for AKI that may be specific in different ICU units.
The assessment of tubular distress, as evident by the positive urinary biomarkers, concludes the kidney assessment at ICU admission. Patients with known CKD, as well as those with any degree of GFR decline or structural damage prior to ICU admission, are especially vulnerable for new tubular damage and would benefit from assessment of tubular distress using the urinary biomarker test regardless of the exposure level. Patients with normal kidney health and preserved renal reserve should undergo assessment of tubular distress only in the presence of a known exposure. The NephroCheck® Test (TIMP2*IGFBP7) performed at ICU admission can identify those patients with a urinary test result >0.3 who are at high risk for AKI and in whom immediate action is required. The patient identified as low risk can receive usual ICU care.
In patients at high risk for AKI, a set of parallel clinical action pathways focused on identification of the cause of AKI, initiation of preventive therapies and daily reassessment of the response to therapies (Figure 1) should begin. This process requires a set of specialized clinical, laboratory and imaging tools, including renal ultrasound and renal Doppler scans, and should be performed as a coordinated effort between the ICU and nephrology teams.6 This approach allows for a diagnosis of specific causes of AKI (e.g., acute obstruction, acute glomerular diseases and rare tubular diseases) that may require specialized treatments versus the more common acute tubular necrosis. For most causes of AKI (multifactorial, sepsis, nephrotoxic, and ischemic), strategies are focused on preventing further injury; this can include adjusting hemodynamic status, employing goal-directed management of fluid resuscitation, maximizing blood pressure, strictly avoiding nephrotoxic medications, monitoring drug levels, and avoiding side effects by adjusting drug dosing via estimation of kinetic GFR.7 The goal here is to prevent functional decline whenever possible and to avoid the negative consequence of fluid overload and drug toxicity in patients with unavoidable decline. Early renal replacement therapy may be appropriate for those with high levels of tubular distress and rapid functional decline where impaired handling of fluid resuscitation may endanger other organs. Future studies will clarify whether subsequent biomarker use can stratify risk regarding the potential for renal recovery once AKI is established.
Figure 1. Comprehensive Assessment of Kidney Health at Intensive Care Unit Admission.
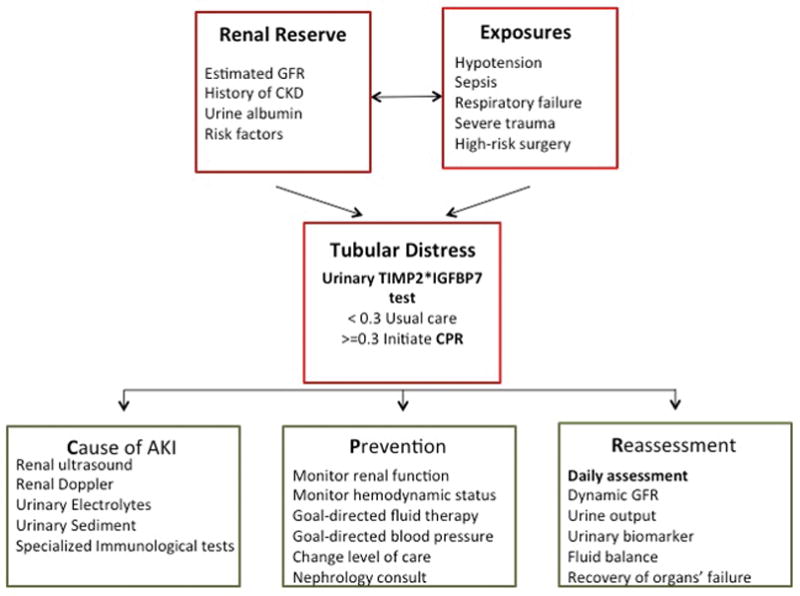
GFR = glomerular filtration rate; CKD = chronic kidney disease; TIMP2*IGFBP7 = tissue inhibitor of metalloproteinase and insulin-like growth factor-binding protein
Modified with permission from www.prisma-p.org.
ICU practitioners are urged to consider, in conjunction with nephrology colleagues, the development of institutional protocols to standardize the early care of critically ill patients at high risk for AKI. They should consider following the proposed clinical approach and modifying it to fit their own clinical practice. To translate the exciting discoveries in renal pathophysiology, AKI epidemiology and biomarker discovery into meaningful improvements in clinical outcomes, we need to change the current state of apathy about AKI. It must not be seen as a rare and unavoidable disease—when it is neither. Rather, we must put our newly acquired understanding of AKI to good use, implement our skills and new tools to standardize the early care of critically ill patients at high risk for AKI, and alleviate the burden of this complex disease in our patients.
Acknowledgments
Azra Bihorac, MD, MS, FCCM, is currently supported by the Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences and has received research grants from Astute Medical, Inc.
Footnotes
References and disclosures are available at www.sccm.org/criticalconnections.
References
- 1.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2014 May 30; doi: 10.1097/SLA.0000000000000732. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014;85(3):513–521. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189(8):932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 5.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 6.Faubel S, Patel NU, Lockhart ME, et al. Renal relevant radiology: use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014;9(2):382–394. doi: 10.2215/CJN.04840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol. 2013;24(6):877–888. doi: 10.1681/ASN.2012070653. [DOI] [PubMed] [Google Scholar]


