Graphical abstract
Keywords: Antinutritious, Digestibility, Fermentation, Proteins, Mineral
Highlights
-
•
Traditional food.
-
•
Microbial effects.
-
•
Biochemical characteristics.
-
•
Fermentation.
Abstract
Phaseolus vulgaris L. beans have been characterized as a nearly perfect food in all around the world. It is consider functional in terms of providing nutrients and energy to sustain daily life. Red bean contain high protein, mineral, fibres and chemically diverse micronutrient composition, which can be affected by processing. The main aim of this work is to investigate the effect of open and controlled fermentation on the proximate composition, mineral elements, antinutritional factors and flatulence- causing oligosaccharides of a domesticated bean (Anger, conscope and Loko). Open fermentation was done using endogenous microorganism present in the seed of beans, while controlled fermentation was done on autoclaved beans flour inoculated with thermophilic lactic culture which is a mixed strain culture containing Lactobacillus acidophilus, Bifidobacterium and Streptococcus thermophillus. The result shows that the open and controlled fermentation increased the protein digestibility up to 90% in all samples and 4 days of fermentation time, loko red bean was found to more suitable. The chemical compositions and mineral contain was also high as compared to remaining two verities.
1. Introduction
Red bean (Phaseolus vulgaris) is an herbaceous annual plant of the family leguminosae [1]. It is domesticated independently in ancient Mesoamerica and the Andes; although widely cultivated in hot climate throughout the world. It is available in white, black and reddish colour varieties in kidney shaped [2]. The bean plants (legumes) belong to the genus Vignasavi, and the family Leguminosae-papilionoidae and the tribe Phaseoleae which is made up of about 80–100 species [3]. They grow in the tropics and Asia Common bean usually refers to food legumes of the genus Phaseolus, family Leguminosae, subfamily Papilionoideae, tribe Phaseoleae, and subtribe Phaseolinae. The genus Phaseolus contains some 50 wild-growing species distributed only in the Americas (Asian Phaseolus have been reclassified as Vigna) [4]. Beans in general, are important sources of macronutrients, micronutrients and antioxidant compounds with a great potential for human and animal nutrition [5]. .However, they contain several anti-nutritional factors which limit their consumption and affect the digestibility and bioavailability of nutrients [6]. The cooked beans are generally used in flavorful foodstuff throughout the world, some other species of red beans and cow peas were consumed as vegetables in some parts of the country [7]. The large quantity of red beans are widely consumed, even though they are poorly digested and cause flatulence due to the presence of several biologically active compounds (phytochemicals) such as phytic acid, tannins, saponins and lectins which could limit their consumption [8]. The flatulence associated with red beans is mainly caused by oligosaccharides, particularly raffinose, stachyose and verbascose. Plant foods are fermented to enhance or create unique flavors, to change textural properties and to improve quality and digestibility. Fermented foods are an essential part of the human diet in many parts of the world, especially in Southeast Asia, the Near East and parts of Africa [9].
Microorganisms used for fermentation process of food products are capable of growing on a wide range of substrates. That can produce a remarkable spectrum of products and bioactive components, which enhance the biofunctionality of food products [10]. The relatively recent advent of in vitrogenetic manipulation has extended the range of products that may be produced by microorganisms and has provided new methods for increasing the yields of existing ones [11]. Thus fermentation in food processing is defined as the conversion of carbohydrates to alcohol and carbon dioxide or organic acids using yeast and/or bacteria, under anaerobic conditions [12]. The fermentation technology depends on the microbial components and produces different molecules from small laboratory scale to large industrial scale. Fermentation of beans has not been extensively studied but it has been shown to produce a reduction in bioactive compounds (phytochemicals), positive effects on protein digestibility, texture and aroma and an improvement in the biological value of legumes [13]. In spite of all these studies, there is still little formation concerning the use of open and controlled fermentation in P vulgaris in the developing country [14]. Especially on the eastern and great lakes part of Africa, where such beans are consumed in large quantities. In order to improve the nutritional quality of the red beans produced in developing regions as food/feed resources, removal/reduction of unwanted components from beans using different processing methods like fermentation is a desirable and often the easiest method.
The main aim of the work is to determine the reduction or elimination of flatulence-causing sugars and the enhancements of in vitro protein digestibility by natural and controlled fermentation. The study focus on the change in chemical composition after fermentation of red beans.
2. Materials and methods
2.1. Materials for fermentation process
Phaseolus vulgaris L. Anger, Canscope and Loko red kidney beans was purchased from framer of Ethiopia. The seeds were cleaned and the extraneous materials carefully removed by hand sorting. All verities of red beans are taken 500 g were soaked in distilled water for 2 h, then dried in an oven at 50 °C. The red bean seeds were sealed and placed in plastic bags, and stored at 5 °C before use. All analytical grade chemicals (hydrochloric acid and sodium hydroxide) and reagents (Sorbic acid) were used for experimental work.
3. Methods
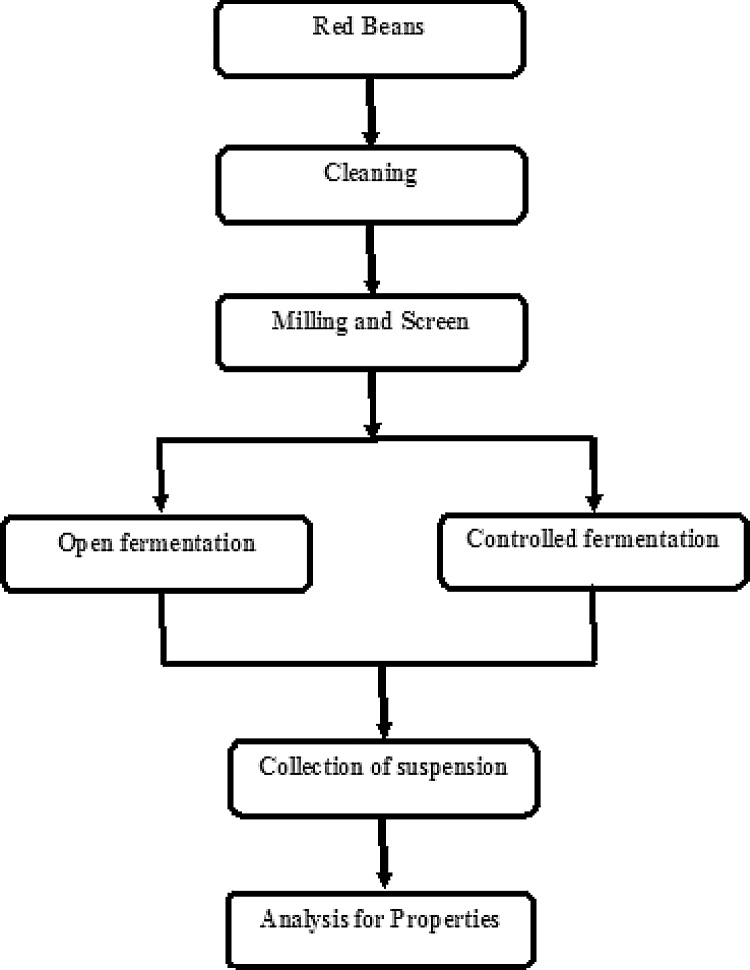
The experiment has been carried by natural fermentations and controlled fermentation on flatus-producing compounds, phytates, tannins, protease inhibitors, saponins and invitro protein digestibility of bean flour were studied. The method for fermentation process was categorized into natural and controlled fermentation. The experimental steps fallowed for fermentation is shown in Fig. 1.
Fig. 1.
Experimental steps on fermentation process of Phaseolus vulgaris (French bean).
3.1. Natural fermentation
Each variety of test bean samples was cleaned and sorted out by size, colour and appearance, and absence of foreign or abnormal odors and living or dead insects. The bean samples were finely ground in appropriate analytical mill and sieved through a 0.5 mm mesh screen. Suspensions of red bean flour in sterilized tap water were prepared in 250 ml glass containers at a concentration of 1:12 dilution (w/v). The flour slurry was allowed to ferment naturally with only the micro organisms borne on or inside the seeds at room temperature (20 °C) for 24, 48, 72 and 96 h in 15 glass containers based on the method by Dablado et al. [15]. Sorbic acid (0.5%) was added to all samples to control the growth of moulds. Titratable acidity was determined daily according to AOAC by titrating with 0.1N NaOH and expressed as a percentage of lactic acid (grams of lactic acid per 100 g dry matter) [16]. During the fermentation process, the pH was also measured daily on the sample homogenates using a pH meter. After fermentation, the slurries were collected daily and freeze-dried for subsequent analysis of bioactive compounds concentration, flatulence-producing factors and protein digestibility of fermented beans. For succeeding experimental analysis, the fermented freeze-dried samples were milled to pass through 0.5 mm sieve and stored in glass jars at 5 °C until used [17].
3.2. Controlled fermentation
Thermophilic lactic culture was obtained from Chr. Hansen’s (Denmark). These microorganisms are commonly used for fermented milk production. In order to obtain sterile flour, beans were rinsed four times in distilled water, drained and dried with a cloth to remove any adhering dust and foreign materials. The beans were then dried at 45–50 °C, and ground in a sample mill, then sieved through 50–250 μm mesh screens and the fractions were collected for the next analysis. Bean flour was placed in a 250 ml glass container covered and suspended in distilled water at 1:12 dilution (w/v). The suspensions were subsequently subjected to heat treatment process in an autoclave (1.5 kg cm−2) at 121 °C for 15 min. The autoclaved samples were then aseptically inoculated with 2% ABT-4 CHR Hansen’s DVS freeze-dried starters which contains 5 × 10 × 11 colony forming units(cfu) per mL. After mixing with a sterile spatula, the glass container was incubated for 4 days at 42 °C. Titratable acidity and pH of the bean suspension samples were collected daily and measured on daily basis from the initial to the last day of fermentation. After fermentation the samples were freeze-dried and ground in a mill through 1 mm mesh screen, placed into plastic containers and stored at 4 °C for the succeeding analyses [6].
3.3. Analyses methods
The phytic acid content was estimated by the procedure of Albarracín et al., 2013 [18]. The total phenolics were measured by spectrophotometer at 650 nm using Folin Ciocelteau reagent in alkaline medium, and were expressed as cafeic acid equivalents. Tannins were determined using the Vanillin-HCL method. The trypsin inhibitor activity (TUI mg)1) was assayed according to the AOAC method [16]. One trypsin inhibitor unit was defined as an increase of 0.01 absorbance unit, which occurred for each 10-ml reaction solution at 410 nm. In vitro protein digestibility of raw and processed samples was measured according to the method of Minekus et al., 2014 [19]. About 250 mg sample was suspended in 15 ml of 0.1 N HCl containing 1.5 mg pepsin (1:10,000) in a 100 ml conical flask. The mixture was incubated at 37 °C for 3 h. The mixture was then neutralized with 0.5 N NaOH and treated with 4 mg pancreatin in 7.5 ml of 0.2 M phosphate buffer (pH 8.0), containing 0.005 M sodium azide. The mixture solution was incubated at 37 °C for 24 h. Ten milliliters of 10% trichloroacetic acid (TCA) were added to the mixture to stop the reaction. The mixture was then centrifuged at 5000 rpm for 5 min. About 5.0 ml aliquots from the supernatant were pippetted and analyzed for nitrogen content.
| Protein digestibility (%) = Nin supernant- enzyme N/N in sample × 100 |
3.4. Proximate analysis
Proximate analyses: The proximate analyses of sample for moisture, crude fat, crude fibre and total ash were carried out in triplicate according to the methods of Association of Official Analytical Chemists [16]. Nitrogen was determined by the micro-Kjeldahl method and the percentage nitrogen converted to crude protein by multiplying by 6.25. The total carbohydrate content was determined by difference according to given in AOAC [16].
3.5. Mineral analysis
All the metals were determined by Atomic Absorption Spectrophotometer (Solar 969 Unicam) with exception of sodium and potassium that were determined using a flame photometer (Model 405, Coring UK)
3.6. Statistical analysis
Data were analyzed using analysis of variance (ANOVA) followed by least significant difference (LSD) for multiple comparison among treatment means at 5% level of significance. Analyses were performed in triplicate. The results were analyzed using one-way analysis of variance followed by Duncan’s multiple range test comparisons among means with significance level of 5%. Regression models and parameter estimation was performed by multiple regressions with a significance level of 5%. Pearson correlation coefficient was used to determine correlations among means with a significance level of 5%.
4. Results and discussion
4.1. Microbial effect on pH change
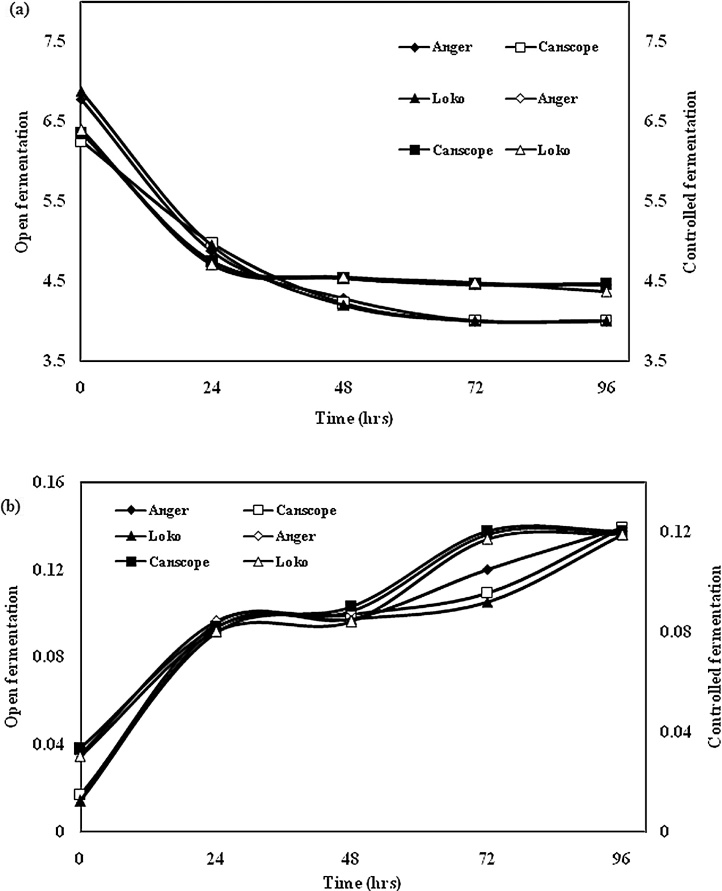
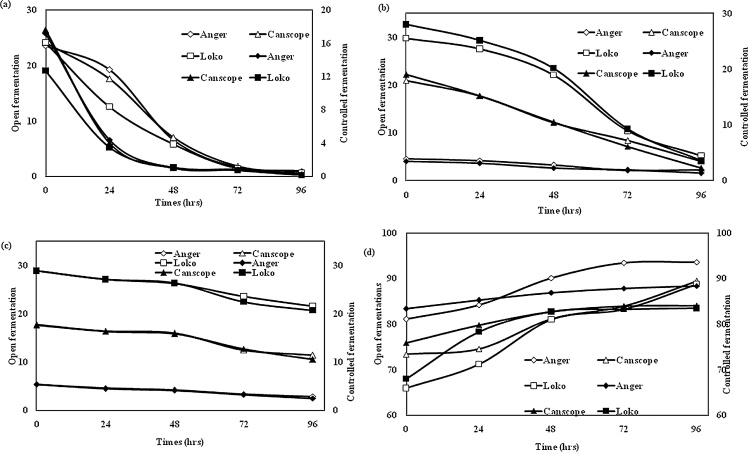
The effect of fermentation on pH change was observed during open fermentation and controlled fermentation, which represents in Fig. 2 (a) and Fig. 2(b). The result indicates that after 48 h of fermentation the pH decreased significantly, but pH values reduced more in OF than in CF. Open fermentation caused a significant (P < 0.05) drop after 48 h and then remained steady to the end of the fermentation period (96 h), whereas controlled fermentation decreased its pH gradually at 72 h and then remained constant. These results were observed for all three processed varieties of fermented red beans flour. The decrease in pH is might be due to the production of lactic bacteria during open fermentation. The result found in this study is almost similar to one of the author was also found higher lactic acid production during open fermentation of lentils at 42 °C for 4-days [20]. The differences may be due to the use of different legume species and varieties. With the origin of the grain being very different, it is probable that the amount and nature of endogenous micro flora are also different. It was also observed that bean flour fermented naturally had a lower pH than those prepared by controlled fermentation. Therefore there was more and efficient acidic fermentation in open fermentation. Like in many traditional fermentation products the drop in pH results is a means for protection from many food pathogens. The removal of bioactive compounds and flatulence-causing factors through fermentation would serve as a major benefit to bean consumers [21].
Fig. 2.
(a): Effect of (a) open fermentation and (b) controlled fermentation on pH change of all three types of red beans.
4.2. Microbial effect on flatulence-producing compounds
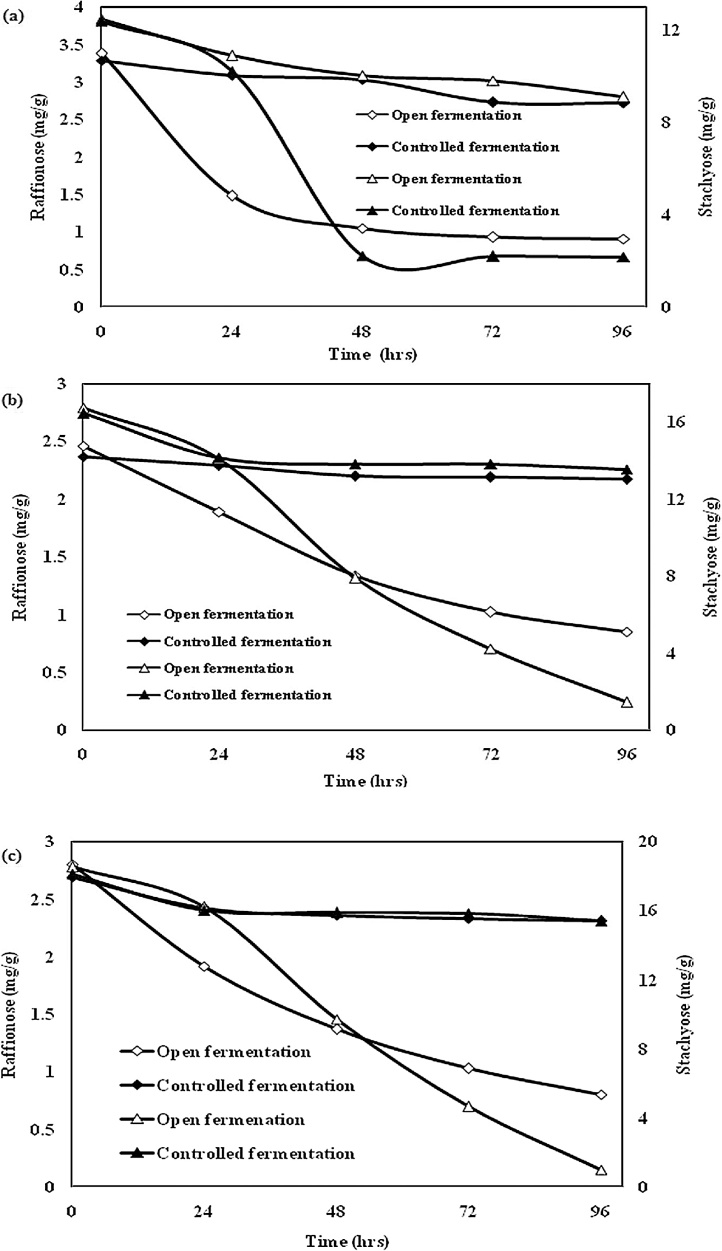
The microbial effect on flatulent producing compound (Stachyose and Raffinose) contents of red bean flours in open and controlled is presented in Fig. 3 (a) to (c). It was found that stachyose and raffinose are changed throughout the fermentation experiment. Raffinose content decreased significantly (P < 0.05) in open fermentation processing of red beans flour. After 72 h of open fermentation the concentration of raffinose for all bean varieties was decreased to an un-expectable level. However, there was no significant difference in the concentration of α-galactoside (flatulence) contents even after 96 h of fermentation for all bean varieties using controlled fermentation. Some lactic acid bacteria contain galactosidase and may utilize oligosaccharides [22]. Thus, elimination of oligosaccharides might be due to the ability of some of the lactic acid organisms to utilize raffinose and stachyose for their metabolism. These results indicated that the society of micro organisms present in open fermentation is able to produce enzymes that are capable of breaking down these oligosaccharides into an absorbable form. This was not the case for the organisms used in the controlled fermentation [23].
Fig. 3.
(a) Microbial effect on flatulence-producing compounds on (a) anger red bean, (b) conscope red bean and (c) loko red bean.
4.3. Microbial on bioactive compounds
The microbial effect on phytic acid, trypsin inhibitors and saponins (bioactive compound) was carried out at 25 °C for both open and controlled fermentations, which is shown in Fig. 4 (a) to (d). A significant (P < 0.05) reduction in phytic acid (phytate) content of red bean flour was observed, which is shown in Fig. 4(a). It was found that the control value (zero time) of phytic acid, trypsin inhibitors and saponins were different for open and controlled fermentation. This might be due to the samples for controlled fermentation was sterilized before inoculation, which attributes leading to decrease the heat labile antinutritional factors. Phytate content was approximately 25% less than the initial value for open fermentation. Controlled fermentation had significantly (P < 0.05) lowered the phytate content of the samples. Controlled and open fermentation had eliminated most of the phytic acid in red bean flours after 72 h of fermentation. This attribute to the inactivation of plant-based phytases during the sterilization process, an increase in phytase loss occurred during controlled fermentation. Furthermore, this can also be attributed to higher microbial phytases produced by the pure cultures [24].
Fig. 4.
(a) Microbial effect on reduction of (a) phytic acid, (b) tryspin, (c) tannin and (d) invitro protein in all three red beans. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Trypsin Inhibitor Activity (TIA) study is represented in Fig. 4(b). It was observed that trypsin inhibitor activity reduced significantly in open fermentation for all three bean varieties. Fermentation of lentils for 96 h brought about a decrease in trypsin inhibitor activity and a sharp drop in the ratio of tannin which is a desirable feature from a nutritional point of view. The digestibility of protein in the fermented samples was augmented compared to the non-fermented beans as a result of TIA diminution as noted later. The trypsin inhibitors were also shows the reduction with the fermentation time during the controlled fermentation [25].
Open and controlled lactic fermentation significantly (P < 0.05) reduced the tannin contents of red bean flour of all varieties, which is shown in Fig. 4 (c). It was measured in milligram of per gram of beans flour. From the result about 45, 36 and 28% reduction of tannins was observed in both open and controlled fermentation for all three red beans. It may be due to break down the polyphenols in the beans by organism. During the preparation of many fermented foods, tannins are reduced before the fermentation step because of their localization in the outer layers or seed coats of the raw ingredients. Dehulling and cooking eliminated more than 90% of the tannins in other food bean also because of their predominance in the seed coats. In several fermented foods the seed coat or testa is removed from the substrate before fermentation so the antinutritional potential caused by the presence of tannins is of little concern [26].
The effect of fermentation in vitro protein was study during open and controlled fermentation is representing in Fig. 4(d). It was observed that the anger red bean shows 92.50% maximum protein digestibility during open fermentation. It might be due to protein denaturation and inactivation of trypsin inhibitor by heat treatment. Micro flora produces some proteolytic enzymes during fermentation which are responsible for increased protein digestibility of fermented red beans [27]. In this study, phytic acid which is known to inhibit the proteolytic enzymes was eliminated in red bean flour samples fermented at room temperature. This also contributed to the improvement in protein digestibility as a significant (P < 0.05) negative correlation existed between phytic acid and protein digestibility (in vitro) of red bean. Similar correlation can also be made for the increase in protein digestibility with reduction in trypsin inhibitors as observed in this study [28].
4.4. Microbial effect on saponins
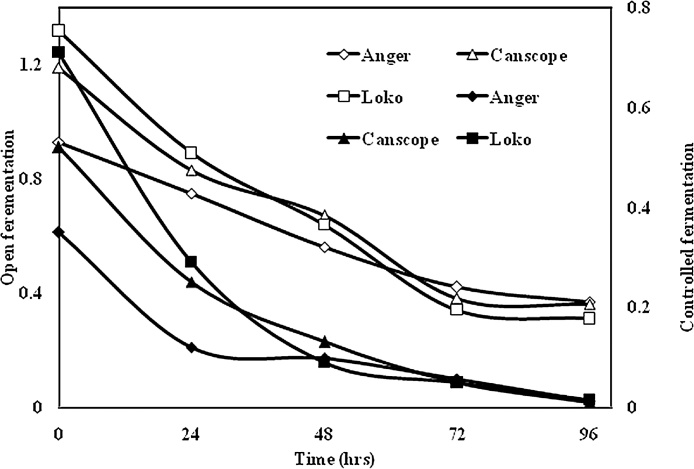
The effect of open and controlled fermentation was also studied for all three varieties of red beans. The result represent in Fig. 5. It was found that saponin content in red bean flour reduced during preparation of samples due to their heat-sensitive nature. After 48 h of controlled fermentation, the saponin contents were reduced to almost zero. The amount of bioactive compounds in some foods can be reduced by fermentation in varying extents. However, the mechanism by which these components are eliminated and the interaction of bioactive compounds with other components of the diet needs to be further studied to understand the role of bioactive compounds in human nutrition [29].
Fig. 5.
Microbial effect on reduction of saponins in all three red beans.
4.5. Proximate chemical compositions in red beans
The proximate chemical compositions of all varieties are shown in Table 1. The moisture, ash, crude protein, crude fat, crude fibre and carbohydrate content were reported in percentage of the dry weight. From the result it was found that loko red bean containing 3.4% of moisture, which is high as compared to remaining to variety, but low as compared to values obtained by obtained and reported by Olaofe et al. (2010) for red beans flour [30]. The low values of moisture contain remains an asset in storage and preservation of the nutrients. The high value of moisture content could lead to food spoilage through increasing due to microbial action. The percentage of ash contains is less in Loko red beans as compared to anger (2.2%) and conscope (2.1%). Audu and Aremu, found that after sprouting processing of red beans the ash contain is less as compared to fermentation and cooking [31]. The fat content in Loko was 11.2%, which is more as compared to other two verities, this may be due to metabolic activity is more in Loko red beans. Similarly the other chemical compositions are found to be more in Loko red beans. High carbohydrate indicates that it’s good supplement to scarce cereal grains as sources of energy. More energy means it can use for feed formulation as well as more fatty acid suggest that the oil may be edible and suitable for industrial purpose.
Table 1.
Chemical composition in all three varieties of red beans.
| S.No | Chemical Compositions | Anger | Conscope | Loko |
|---|---|---|---|---|
| 1 | Moisture | 3.1 ± 4.50 | 3.2 ± 2.50 | 3.4 ± 5.20 |
| 2 | Ash | 2.2 ± 1.90 | 2.1 ± 0.1 | 2.0 ± 0.50 |
| 3 | Fat | 10.56 ± 0.78 | 11.0 ± 0.3 | 11.2 ± 0.60 |
| 4 | Crude protein | 22.8 ± 2.25 | 23.9 ± 2.31 | 25.9 ± 1.59 |
| 5 | Crude fibre | 3.9 ± 0.51 | 4.2 ± 0.31 | 4.8 ± 0.45 |
| 6 | Carbohydrates | 44.3 ± 3.20 | 46.69 ± 4.10 | 49.7 ± 4.20 |
| 7 | Energy | 1620.23 | 1680 | 1700.56 |
| 8 | Fatty acid | 10.5 | 9.7 | 9.1 |
The mineral contain in all three varieties has been also determined, which is presented in Table 2. The Anger red bean has less percentage of calcium as compared to conscope and Loko. Mineral like calcium, phosphorus and magnesium are more suitable for born formation in children. The value for magnesium in the anger flour sample is higher than values reported by most authors for some legume [32], [33]. Magnesium is an activator of many enzyme systems and maintains the electrical potential in nerves [34]. Phosphorus is always found with calcium in the body both contributing to the blood formation and supportive structure of the body. Modern foods rich in animal protein and phosphorus can promote the loss of calcium in urine. This led to the concept of calcium phosphorus ratio. If the Ca/P ratio is low, calcium will be low and there will be high phosphorus intake which leads to calcium loss in the urine more than normal. If the Ca/P ratio of any food is above one that food is considered “good” and “poor” if the ratio is less that 0.5, Ca/P ratio above two helps to increase the absorption of calcium in the small intestine [35]. Sodium and potassium are required for the maintenance of osmotic balance of the body fluids, the pH of the body to regulate muscles and nerves irritability, control glucose absorption and enhance normal retention of protein during growth. The sodium to potassium ratio in the body is of great concern for the prevention of high blood pressure.
Table 2.
Mineral contained in all three varieties of red beans (per 100 g of flour).
| S.No | Minerals | Anger | Conscope | Loko |
|---|---|---|---|---|
| 1 | Calcium (Ca) | 53.5 | 54.1 | 54.5 |
| 2 | Nickel (Ni) | 2.9 | 2.8 | 2.7 |
| 3 | Zinc (Zn) | 2.3 | 2.1 | 2.2 |
| 4 | Copper (Cu) | 0.7 | 0.5 | 0.2 |
| 5 | Iron (Fe) | 6.9 | 7.1 | 7.3 |
| 6 | Chromium (Cr) | 1.8 | 1.9 | 2.0 |
| 7 | Sodium (Na) | 20.5 | 21.6 | 23.26 |
| 8 | Potassium (K) | 12.5 | 13.2 | 13.9 |
| 9 | Phosphorus (P) | 2.9 | 3.0 | 3.5 |
| 10 | Magnesium (Mg) | 730 | 710 | 690 |
5. Conclusions
Fermentation of processed pearl millet grains caused significant reduction in antinutritional factors of the grains, which was accompanied by significant improvement in the protein digestibility. The open and controlled fermentation increased the protein digestibility to about 90% in all samples. This result was consistent for Loko, with an initial protein digestibility value of 65% and was increased to around 84% after the fermentation process. Red bean fermentation resulted in the reduction of phytates, trypsin inhibitor activity (TIA), saponins, tannins and raffinose oligosaccharides. Fermentation offers unique nutritional advantages for making the protein of coarse-grained red beans more digestible by reducing its bioactive compounds. The tannins were more difficult to breakdown by fermentation and a maximum of 45% reduction was achieved. Open fermentation had a better effect on the breakdown of the oligosaccharides, raffinose and stachyose. Furthermore, it does not require the use of pure cultures which might be costly even if they are available. It is also concluding that 96 h is sufficient time for fermentation. The chemical compositions are found to less for Loko red beans after fermentation, but mineral contain is more as compared to anger red beans. The outcome of this investigation is that open fermentation process is one of the simple and feasible processes to improve the biochemical properties and mineral content of local available beans. The product obtained can be marketed as dried beans powder for extruded products that can be sold at eateries.
Conflict of interest
Author wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Choy S.Y., Prasad K.M.N., Wu T.Y., Ramanan R.N. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015;12(1):367–390. [Google Scholar]
- 2.Whitaker E.D. Taylor & Francis; 2017. The Trouble with Human Nature: Health, Conflict, and Difference in Biocultural Perspective. [Google Scholar]
- 3.Umdale S.D., Patil P.D., Malik S.K., Latha M., Rao S.R., Yadav S.R., Gaikwad N.B., Bhat K.V. Seed coat sculpture of subgenus Ceratotropis (Piper) verdc., genus Vigna Savi in India and its taxonomic implications. Botany Lett. 2017;164(1):63–78. [Google Scholar]
- 4.Morales P., Herrera P.G., González M.C.M., Hurtado M.C., de Cortes Sánchez Mata M. Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications. 2017. Wild greens as source of nutritive and bioactive compounds over the world; pp. 199–261. [Google Scholar]
- 5.Fernández-Ruiz V., Morales P., Ruiz-Rodríguez B.M., TorijaIsasa E. Wild Plants; Mushrooms and Nuts: Functional Food Properties and Applications: 2017. Nutrients and Bioactive Compounds in Wild Fruits Through Different Continents; pp. 263–314. [Google Scholar]
- 6.Fadahunsi I.F. The effect of soaking, boiling and fermentation with Rhizopus oligosporus on the water soluble vitamin content of Bambara groundnut. Pak. J. Nutr. 2009;8(6):835–840. [Google Scholar]
- 7.EARO . Ethiopian Agricultural Research Organization (EARO); Addis Ababa, Ethiopia: 2001. Food Science and Post Harvest Technology Research Program Strategy; pp. 12–17. [Google Scholar]
- 8.Udenta E.A. 2017. Effect Of Controlled Fermentation Of Some Traditional Foods On The Glycaemic Response Of Diabetic Adult Rats (Doctoral Dissertation) [Google Scholar]
- 9.Katz S.E. Chelsea Green Publishing; 2016. Wild Fermentation: The Flavor, Nutrition, and Craft of Live-Culture Foods. [Google Scholar]
- 10.Yadav H., Jain S., Rastamanesh R., Bomba A., Catanzaro R., Marotta F. Fermentation technology in the development of functional foods for human health: where we should head. Ferment. Technol. 2011;1:e102. doi: 10.4172/2167-7972.1000e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers P.J., Pretorius I.S. Fermenting knowledge: the history of winemaking, science and yeast research. Eur. Mol. Biol. Org. Rep. 2010;11(12):914–920. doi: 10.1038/embor.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Rahman M.A., Tashiro Y., Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013;31(6):877–902. doi: 10.1016/j.biotechadv.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Diana M., Quílez J., Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: a review. J. Funct. Foods. 2014;10:407–420. [Google Scholar]
- 14.Bigliardi B., Galati F. Innovation trends in the food industry: the case of functional foods. Trends Food Sci. Technol. 2013;31(2):118–129. [Google Scholar]
- 15.Doblado R., Frias J., Munoz R., Vidal-Valverde C. Anti-nutritional factors content of dry beans (Phasealus vulgaris) as affected by fermentation. Pol. J. Food Nutr. Sci. 2002;11(52):73–75. [Google Scholar]
- 16.AOAC Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International. In: William, H., (ed). vol. II, 17th ed. Food composition; additives; natural contaminants. Washington, D.C . Official method 942.15-982, 14, 2000, 12–42.
- 17.Wandee Y., Uttapap D., Puncha-arnon S., Puttanlek C., Rungsardthong V., Wetprasit N. In vitro fermentabilities of raw and cooked canna starches and their derivatives. J. Funct. Foods. 2017;34:461–469. [Google Scholar]
- 18.Albarracín M., González R.J., Drago S.R. Effect of soaking process on nutrient bio-accessibility and phytic acid content of brown rice cultivar. LWT-Food Sci. Technol. 2013;53(1):76–80. [Google Scholar]
- 19.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T.O.R.S.T.E.N., Bourlieu C., Carriere F., Boutrou R., Corredig M., Dupont D., Dufour C. A standardised static in vitro digestion method suitable for food?an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- 20.Difo H.V., Onyike E., Ameh D.A., Ndidi U.S., Njoku G.C. Chemical changes during open and controlled fermentation of cowpea (Vigna unguiculata) flour. Int. J. Food Nutr. Saf. 2014;5(1):1–10. [Google Scholar]
- 21.Difo H.V., Onyike E., Ameh D.A., Njoku G.C., Ndidi U.S. Isolation of Aspergillus niger from three varieties of Bambara nuts for simultaneous production of phytase and tannase. J. Yeast Fungal Res. 2013;4:1–4. [Google Scholar]
- 22.Peres C.M., Peres C., Hernández-Mendoza A., Malcata F.X. Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria–with an emphasis on table olives. Trends Food Sci. Technol. 2012;26(1):31–42. [Google Scholar]
- 23.Limon R.I., Peñas E., Torino M.I., Martínez-Villaluenga C., Dueñas M., Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–352. doi: 10.1016/j.foodchem.2014.09.084. [DOI] [PubMed] [Google Scholar]
- 24.Madeira J.V., Macedo J.A., Macedo G.A. Detoxification of castor bean residues and the simultaneous production of tannase and phytase by solid-state fermentation using Paecilomyces variotii. Bioresour. Technol. 2011;102:7343–7348. doi: 10.1016/j.biortech.2011.04.099. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y., Ge C., Yuan W., Zhu R., Zhang W., Du L., Xue J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J. Sci. Food Agric. 2010;90(7):1194–1202. doi: 10.1002/jsfa.3947. [DOI] [PubMed] [Google Scholar]
- 26.Marathe S.A., Deshpande R., Khamesra A., Ibrahim G., Jamdar S.N. Effect of radiation processing on nutritional, functional: sensory and antioxidant properties of red kidney beans. Radiat. Phys. Chem. 2016;125:1–8. [Google Scholar]
- 27.Hayat I., Ahmad A., Masud T., Ahmed A., Bashir S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): an overview. Crit. Rev. Food Sci. Nutr. 2014;54(5):580–592. doi: 10.1080/10408398.2011.596639. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A., Kumari S., Wongputtisin P., Nout M.J.R., Sarkar P.K. Optimization of soybean processing into kinema, a Bacillus-fermented alkaline food, with respect to a minimum level of antinutrients. J. Appl. Microbiol. 2015;119(1):162–176. doi: 10.1111/jam.12826. [DOI] [PubMed] [Google Scholar]
- 29.Ndidi U.S., Ndidi C.U., Olagunju A., Muhammad A., Billy F.G., Okpe O. Proximate, antinutrients and mineral composition of raw and processed (boiled and roasted) sphenostylisstenocarpa seeds from Southern Kaduna, North-West Nigeria. ISRN Nutr. 2014;14:1–9. doi: 10.1155/2014/280837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olaofe O., Famurewa J.A., Ekuagbere A.O. Chemical and functional properties of kidney bean seed (Phaseolus vulgaris L.) flour. Int. J. Chem. Sci. 2010;3:51–69. [Google Scholar]
- 31.Audu S.S., Aremu M.O. Effect of processing on chemical composition of red kidney bean (Phaseolus vulgaris L.) flour. Pak. J. Nutr. 2011;10(11):1069–1075. [Google Scholar]
- 32.Aremu M.O., Olaofe O. Protein and amino acid composition of some legumes grown in Nigeria. A review. Int. J. Chem. Sci. 2008;1:128–139. [Google Scholar]
- 33.Aremu M.O., Olaofe O., Basu S.K., Abdulazeez G., Acharya S.N. Processed cranberry bean (Phaseolus coccineus L.) seed flour for African diet. Can. J. Plant Sci. 2010;90:719–728. [Google Scholar]
- 34.Singh Y., Chandra S. Evaluation of physical properties of kidney beans (Phaseolus vulgaris) Food Sci. Res. J. 2014;5(2):125–129. [Google Scholar]
- 35.Mohajerani S., Fazel M.A., Madani H., Lak S., Modhej A. Effect of the foliar application of humic acid on red bean cultivars (Phaseolus vulgaris L.) J. Exp. Biol. Agric. Sci. 2016;4(5):519–524. [Google Scholar]