CXCR7 is expressed on CD14+CD16+ monocytes in PBMC from uninfected and HIV infected individuals; CCX771 specifically blocks CXCL12-mediated transmigration of these cells.
Keywords: CXCR4, CCX771, CD14+CD16− monocytes, human, chemotaxis
Abstract
CD14+CD16+ monocytes transmigrate into the CNS of HIV-positive people in response to chemokines elevated in the brains of infected individuals, including CXCL12. Entry of these cells leads to viral reservoirs, neuroinflammation, and neuronal damage. These may eventually lead to HIV-associated neurocognitive disorders. Although antiretroviral therapy (ART) has significantly improved the lives of HIV-infected people, the prevalence of cognitive deficits remains unchanged despite ART, still affecting >50% of infected individuals. There are no therapies to reduce these deficits or to prevent CNS entry of CD14+CD16+ monocytes. The goal of this study was to determine whether CXCR7, a receptor for CXCL12, is expressed on CD14+CD16+ monocytes and whether a small molecule CXCR7 antagonist (CCX771) can prevent CD14+CD16+ monocyte transmigration into the CNS. We showed for the first time that CXCR7 is on CD14+CD16+ monocytes and that it may be a therapeutic target to reduce their entry into the brain. We demonstrated that CD14+CD16+ monocytes and not the more abundant CD14+CD16− monocytes or T cells transmigrate to low homeostatic levels of CXCL12. This may be a result of increased CXCR7 on CD14+CD16+ monocytes. We showed that CCX771 reduced transmigration of CD14+CD16+ monocytes but not of CD14+CD16− monocytes from uninfected and HIV-infected individuals and that it reduced CXCL12-mediated chemotaxis of CD14+CD16+ monocytes. We propose that CXCR7 is a therapeutic target on CD14+CD16+ monocytes to limit their CNS entry, thereby reducing neuroinflammation, neuronal damage, and HIV-associated neurocognitive disorders. Our data also suggest that CCX771 may reduce CD14+CD16+ monocyte-mediated inflammation in other disorders.
Introduction
HIV enters the CNS within 4–8 d after peripheral infection [1, 2], ultimately contributing to cognitive deficits in 40–70% of infected individuals [3–5]. These deficits develop despite ART and regardless of viral load [5–7]. They are mediated, in part, by transmigration of monocytes across the BBB into the CNS [3, 8–10]. HIV-infected, as well as uninfected, monocytes transmigrate into the CNS in response to chemokines [11–14]. Once within the CNS, these monocytes elaborate additional inflammatory mediators, viral proteins, and virus and may also differentiate into macrophages that contribute to the initial establishment and continued seeding of viral reservoirs within the brain [15–17]. These processes lead to infection of other CNS cell types, neuroinflammation, and neuronal damage that even in the presence of ART, may result in cognitive deficits [18–21].
Monocytes are heterogeneous and are distinguished based on their surface CD14 (the LPS receptor) and CD16 (the FcγIIIR) [22]. The majority of monocytes in healthy people are classic CD14+CD16− monocytes, whereas 5–10% of monocytes are mature and are CD14+CD16+ [23]. CD14+CD16+ monocytes are key to the inflammatory response and during HIV infection, are significantly increased in the peripheral blood, comprising 20–60% of monocytes in infected individuals despite successful ART [23–25]. In addition, CD14+CD16+ monocytes are susceptible to HIV infection [17, 26] and are found in the CNS of HIV-infected people [12, 13]. Thus, these cells are proposed to facilitate HIV entry into the CNS, contributing to the processes that lead to HIV-associated neurocognitive disorders [3, 23].
CD14+CD16+ monocytes transmigrate across the BBB in response to chemokines, such as CCL2 and CXCL12, which are each elevated in the brains of HIV-infected individuals [3, 8, 27–30]. CXCL12 is also expressed constitutively in the CNS and among many other functions, mediates T cell infiltration [31–33]. Our laboratory showed that CXCL12 induces the transmigration of CD14+CD16+ monocytes across an in vitro human BBB model [27]. Leukocyte migration to CXCL12 was believed to be solely mediated by chemokine receptor CXCR4 until the identification of another CXCL12 receptor, the atypical chemokine receptor 3 (CXCR7) [34]. With the use of the CXCR7 antagonist CCX771 and the CXCR4 antagonist AMD3100, several studies showed that CXCR7 scavenges excess CXCL12 to enable cell movement or found that CXCR7 directly mediates cell migration [31, 35–37]. CXCR7 is similar in structure to CXCR4, although CXCR7 signals through β-arrestin recruitment and MAPK activation rather than through conventional G protein signaling [35, 38, 39]. The expression of CXCR7 on different leukocyte subsets was unclear, and the role of CXCR7 in monocyte transmigration was undetermined [31, 34, 40–42]. Two studies reported CXCR7 on human monocytes, but another study showed that CXCR7 was not detectable on these cells [41, 43, 44]. These studies examined CXCR7 on the total population of peripheral blood monocytes; however, its expression and function had not been determined on mature CD14+CD16+ monocytes. As CD14+CD16+ monocytes are increased in the peripheral blood in HIV infection and in many inflammatory disorders [45] and enter the CNS [12, 13], it is essential to understand the potential involvement of CXCR7 in the transmigration of mature CD14+CD16+ monocytes across the BBB.
In this study, we show that CXCR7 is expressed on CD14+CD16+ monocytes from both healthy and HIV-infected individuals and that it mediates CXCL12-induced transmigration across the BBB of CD14+CD16+ monocytes but not of CD14+CD16− monocytes. This suggests that the mechanism by which CXCL12 induces the transmigration of CD14+CD16+ monocytes differs from that of CD14+CD16− monocytes. We also showed that the CXCR7 antagonist CCX771 blocks specifically CD14+CD16+ monocytes from transmigration across the BBB, suggesting that it will reduce their entry into the CNS, thereby reducing HIV infection of the brain. We propose that CXCR7 is a potential therapeutic target to reduce inflammation in HIV-associated neurocognitive disorders and that it may also be a therapeutic target for other inflammatory disorders in which CD14+CD16+ monocytes contribute to pathogenesis.
MATERIALS AND METHODS
Materials
The CXCR7-specific antagonist CCX771 was obtained from ChemoCentryx (Mountain View, CA, USA), CXCR4 antagonist AMD3100 was from Sigma-Aldrich (St. Louis, MO, USA), and CXCL12 from R&D Systems (Minneapolis, MN, USA). Cells were pretreated with 10 μM CCX771, 10 μM AMD3100, or DMSO (diluent) for 20 min before functional assays.
Cell isolation and culture
Blood was obtained from HIV-seronegative people or from leukopaks from the New York Blood Center, according to established protocols at the Albert Einstein College of Medicine. PBMCs were isolated by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. To obtain mature CD14+CD16+ monocytes by culture, monocytes were isolated from PBMCs by magnetic bead-positive selection using the CD14 EasySep separation kit (Stemcell Technologies, Vancouver, BC, Canada). Freshly isolated CD14+ monocytes were then cultured nonadherently for 3 d in Teflon-coated flasks at 2 × 106 cells/ml in supplemented RPMI in the presence of 10 ng/ml M-CSF (PeproTech, Rocky Hill, NJ, USA) to facilitate monocyte maturation and yield “mature CD14+CD16+ monocytes,” as described previously [8, 26].
Blood from ART-treated, HIV-seropositive people from the MHBB and WIHS (Bronx location) was obtained. Patients gave written, informed consent for the provision of blood for the purposes of research. The Institutional Review Board at Montefiore Medical Center, Albert Einstein College of Medicine, and the Mount Sinai Program for the Protection of Human Subjects Institutional Review Board approved the protocols under which these samples were obtained. Demographics and virologic characteristics of individuals from the 2 cohorts are in Table 1. PBMCs were isolated within 2–4 h of blood draw, phenotyped by flow cytometry, and also used for transmigration assays.
TABLE 1.
Characteristics of the HIV-infected study participants (n = 38)
| Characteristics of the WIHS and MHBB study participants | ||
|---|---|---|
| Cohort characteristics | WIHS (n = 29) Median [IQR] or n (%) | MHBB (n = 9) Median [IQR] or n (%) |
| Age, yr | 49 [40, 58] | 53 [48, 58] |
| Sex | ||
| Female | 29 (100.0) | 8 (88.9) |
| Male | 0 (0.0) | 1 (11.1) |
| Race | ||
| Black | 20 (69.0) | 3 (33.3) |
| Hispanic | 6 (20.7) | 3 (33.3) |
| White | 1 (3.5) | 3 (33.3) |
| Other | 2 (6.9) | 0 (0.0) |
| CD4 T cell count, cells/mm3 | 609 [302, 916] | 565 [277, 853] |
| Nadir CD4 T cell count, cells/mm3 | 357 [68, 646] | 49 [0, 237] |
| ART | ||
| Yes | 17 (58.6) | 9 (100.0) |
| No | 12 (41.4) | 0 (0.0) |
| Plasma HIV RNA, log10 copies/ml | 1.7 [1.3, 3.4] | 1.3 [1.3, 1.8] |
| Viral load undetectable | 12 (41.4) | 6 (66.7) |
| Drug user | ||
| Yes | 7 (24.1) | 6 (66.7) |
| No | 22 (75.9) | 3 (33.3) |
| HCV | ||
| Positive | 7 (24.1) | 2 (22.2) |
| Negative | 22 (75.9) | 7 (77.8) |
IQR, Interquartile range; HCV, hepatitis C virus.
Flow cytometry
Analysis was performed on PBMCs using antibodies specific for human CD14 (Clone M5E2), CD16 (Clone 3G8), and CD3 (Clone HIT3a; BD Biosciences, San Jose, CA, USA); CXCR7-PE (Clone 11G8; 0.3–0.45 μg/test depending on the lot; R&D Systems); and CXCR4-PE (Clone 12G5; 0.3 μg/test; eBioscience, San Diego, CA, USA) or isotype-matched control antibodies. CXCR7 and CXCR4 antibodies were titered to determine optimal staining conditions, and CD14, CD16, and CD3 were previously titered and optimized depending on the fluorophore used. Cells (2 × 105 in 100 μl) were stained in the dark on ice for 30 min, washed once, and fixed with 2% paraformaldehyde in 1× PBS. At least 10,000 monocytes or T cells were acquired with the BD FACSCanto II flow cytometer. Forward- and side-scatter and CD14- and CD3-positive signal based on isotype-matched control staining were used to gate on monocytes and T cells, respectively. Forward-scatter area vs. forward-scatter height was used to gate on single cells. Analysis was performed using FlowJo software (v. 10.0.8; Tree Star, Ashland, OR, USA). MFI for CXCR7 and CXCR4 was calculated after subtracting the MFI of the isotype-matched control antibody.
Antibody adsorption assay
MCF-7 cells (2 × 106), a human breast cancer cell line of epithelial cells derived from metastatic mammary gland tissue that expresses surface CXCR7 [46], were resuspended in 1× PBS with 1% BSA. Cells were incubated for 1 h at room temperature with CXCR7 antibody at 2× the concentration normally used for flow cytometry staining. After 1 h, cells were spun, and the unbound antibody in the supernatant was collected. This was repeated one more time. MCF-7 cells and mature CD14+CD16+ monocytes were then stained with the remaining unbound CXCR7 antibody in the supernatant (the adsorbed antibody) and acquired and analyzed as described in Flow cytometry above.
Human BBB model
The BBB model consists of human BMVECs (Applied Cell Biology Research Institute, Kirkland, WA, USA) and human cortical astrocytes isolated from tissue as part of an approved research protocol at the Albert Einstein College of Medicine. BMVECs and astrocytes were cocultured on opposite sides of a gelatin-coated tissue-culture insert with 3 μm pores (BD Falcon; Becton Dickinson, Franklin Lakes, NJ, USA), as described previously [8, 11]. In brief, astrocytes were seeded on the basolateral side (CNS side) of the insert and BMVECs added to the apical side (peripheral blood side). Cocultures were grown to confluence for 3 d, at which time, astrocytic foot processes penetrate the insert and contact the BMVEC layer to induce BBB properties, including impermeability to albumin [8, 11].
BBB transmigration assay
BBB coculture inserts were placed in wells of a 24-well plate, with BSA (vehicle) or CXCL12 (R&D Systems) added to the wells on the basolateral side of the inserts (bottom). For BBB transmigration experiments that included the antagonists, PBMCs were treated with DMSO (diluent), CCX771 (10 μM), or AMD3100 (10 μM) for 20 min before the transmigration assay. PBMCs (4 × 105) were then added to the apical side of the coculture inserts (top). Each transmigration condition was assayed with 4 replicate inserts. After 24 h, the transmigrated cells were collected from the bottom of the wells on the basolateral side of the inserts. Collected cells were stained for human CD14 (Clone M5E2), CD16 (Clone 3G8), and CD3 (Clone HIT3a; BD Biosciences); fixed with 2% paraformaldehyde; and quantified by flow cytometry. The number of each leukocyte subset that transmigrated was determined and quantified, or the percent of each subset that transmigrated was determined relative to the total absolute cell number of that subset (i.e., the percent of cells relative to their input).
The optimal transmigration duration and CXCL12 concentration were determined by kinetic and dose response analysis, respectively. Transmigration assays with cells from HIV-infected individuals were performed with cells from individuals from the WIHS cohort, as we did not receive enough blood to perform these assays with cells from the MHBB cohort.
Chemotaxis assay
Directed cell movement was assayed using a 48-well microchemotaxis chamber (Neuro Probe, Gaithersburg, MD, USA), as we performed previously [8, 47]. Mature CD14+CD16+ monocytes (5 × 105) were resuspended in chemotaxis medium (RPMI 1640, supplemented with 10% FBS) in the presence of CCX771 (10 μM) or DMSO (diluent) and incubated at 37°C for 20 min. Cells were then placed in the upper wells of the microchemotaxis chamber, separated from the lower wells by a polycarbonate filter with 5 μm pores (Neuro Probe). The lower wells of the microchemotaxis chamber contained chemotaxis medium alone or CXCL12 (5 ng/ml) resuspended in chemotaxis media. Duplicate chambers were incubated at 37°C with 5% CO2 for 30 or 60 min to enable cell chemotaxis. The filters were then removed, fixed, and stained using the Siemens Diff Quick Stain Set (Thermo Fisher Scientific, Waltham, MA, USA). Migrated cells were quantified using densitometry with the computer imaging software UN-SCAN-IT (Silk Scientific, Orem, UT, USA). Two time points were examined for each experiment, as the response to CXCL12 varies widely with primary human cells. The maximal fold change of the 30 and 60 min time points was determined for each experiment, and the time point with the strongest response to CXCL12 for each experiment was included in the final quantification and graph.
Statistical analysis
Statistical analyses were performed using Prism 6.0g (GraphPad Software, San Diego, CA, USA). Mann-Whitney U tests were used for paired nonparametric measures and Wilcoxon for nonpaired, nonparametric measures. Measures with P < 0.05 were considered statistically significant.
RESULTS
CD14+CD16+ monocytes transmigrate preferentially across the BBB in response to CXCL12 compared with CD14+CD16− monocytes or T cells
CXCL12 is expressed constitutively in the CNS and is increased in the brains of HIV-infected individuals with CNS pathology [29, 30, 48–50]. We [8, 23] and others [13, 24, 51] showed that CD14+CD16+ monocytes are significantly increased in the peripheral blood of HIV-infected individuals and that these cells are key mediators of HIV CNS pathogenesis. However, transmigration across the BBB of different monocyte populations in response to CXCL12 had not been characterized extensively. We examined CXCL12-mediated transmigration of the CD14+CD16+ and CD14+CD16− monocyte subsets using a human BBB model. As CXCL12 is known to be a potent T cell chemoattractant [31–33], we also examined transmigration of unstimulated T cells.
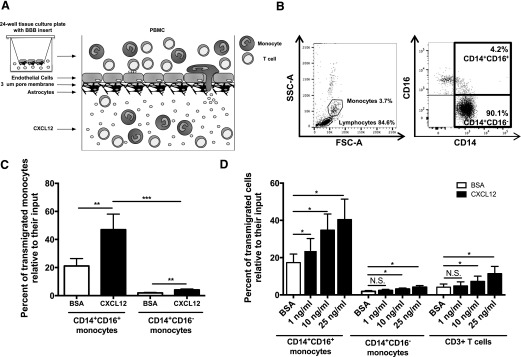
We added freshly isolated PBMCs obtained from HIV-negative individuals to the top of the in vitro model of the human BBB (Fig. 1A). Cells were allowed to transmigrate in response to different concentrations of CXCL12 for 24 h, and the input population was analyzed using flow cytometry (FACS). Monocytes and lymphocytes were identified using forward- and side-scatter. Monocyte subsets were then analyzed by CD14 and CD16 expression (Fig. 1B) and T cells by CD3. CXCL12 (25 ng/ml) induced transmigration of significantly higher numbers of CD14+CD16+ monocytes than CD14+CD16− monocytes relative to their input from 8 independent individuals, although both CD14+CD16+ and CD14+CD16− monocytes transmigrated in significantly greater numbers in response to CXCL12 than to vehicle (Fig. 1C). In addition, with cells from 6 of the 8 independent individuals, we determined that CD14+CD16+ monocytes transmigrated across the BBB in significantly greater numbers relative to their input and in response to lower concentrations of CXCL12 than did either CD14+CD16− monocytes or unstimulated CD3+ T cells (Fig. 1D). These data indicate that CD14+CD16+ monocytes are the population of cells that selectively transmigrate in greater numbers across the BBB in response to CXCL12 compared with their input numbers in comparison to CD14+CD16− monocytes and T cell transmigration compared with their own input numbers. The data also suggest that CD14+CD16+ monocytes would transmigrate in response to the low levels of CXCL12 that are present during basal homeostatic conditions in the CNS [49, 50], which may contribute to early infection of the CNS.
Figure 1. CD14+CD16+ monocytes preferentially transmigrate across the BBB in response to CXCL12.
(A) Schematic representation of the human BBB tissue-culture model. Inserts with human BMVECs and human cortical astrocytes cultured on opposite sides of 3 μm (um) pore membranes were placed in 24-well plates. PBMCs (4 × 105/insert) were added to the apical side of BBB inserts (“periphery”) and CXCL12 to the bottom of the wells on the basolateral side of the inserts (“CNS”). Cells were allowed to transmigrate across the BBB in response to CXCL12 for 24 h, after which cells in the bottom of the wells were collected, analyzed, and counted by FACS. (B) PBMCs from HIV-negative individuals were stained with CD14-allophycocyanin- and CD16-PE-coupled antibodies. Forward (FSC-A)- and side-scatter area (SSC-A) were used to determine monocyte and lymphocyte gating. CD14 and CD16 were then used to identify CD14+CD16+ and CD14+CD16− monocytes. Representative plots from a single donor are shown. (C) PBMCs from 8 independent individuals were allowed to transmigrate across the BBB for 24 h in response to BSA (vehicle; open bars) and 25 ng/ml CXCL12 (solid bars). The percentages of CD14+CD16+ and CD14+CD16− monocytes that transmigrated across the BBB, relative to the number of cells for that monocyte subset that was added to the apical side of the BBB model, were calculated. (D) PBMCs from 6 of the 8 independent individuals were allowed to transmigrate in response to BSA (vehicle; open bars) and 1, 10, and 25 ng/ml CXCL12 (solid bars). The percentages of CD14+CD16+ monocytes, CD14+CD16− monocytes, and CD3+ T cells that transmigrated across the BBB, relative to the number of cells for each cell subset that was added to the apical side of the BBB model, were calculated. Data are represented as means ± sem. Significance was determined by Wilcoxon signed-rank test (paired measures) and Mann-Whitney U test (unpaired measures). Significance is compared with baseline unless indicated otherwise. *P < 0.05; **P < 0.01; ***P < 0.001. N.S., Nonsignificant.
CD14+CD16+ monocytes express CXCR7
To determine whether CXCR7 participates in CXCL12-induced transmigration across the BBB of CD14+CD16+ monocytes, we first examined surface CXCR7 and CXCR4 on monocytes from healthy individuals. Studies from other groups had evaluated CXCR7 on the total population of freshly isolated monocytes that are 90–95% CD14+CD16− monocytes [23]. Whereas 2 of these studies found CXCR7 to be minimally expressed on monocytes [43, 44], another group demonstrated that the total population of monocytes does not express CXCR7. This group also showed, using a CXCR7-transfected human breast cancer cell line, that only the CXCR7 antibody from Clone 11G8 specifically detects CXCR7 [41]. We examined receptor expression on CD14+CD16+ and CD14+CD16− monocytes using the CXCR7 (11G8 clone) antibody that others showed to be specific [41]. We confirmed this specificity using an antibody adsorption assay, as described in Materials and Methods and in Supplemental Fig. 1.
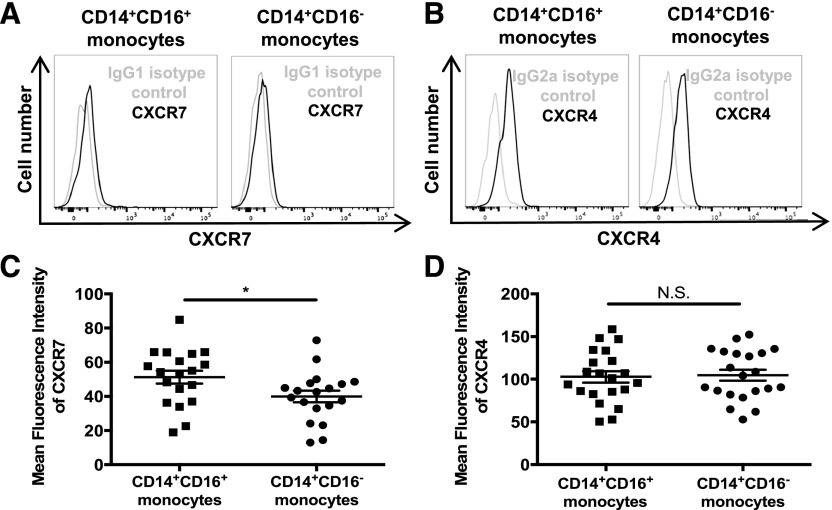
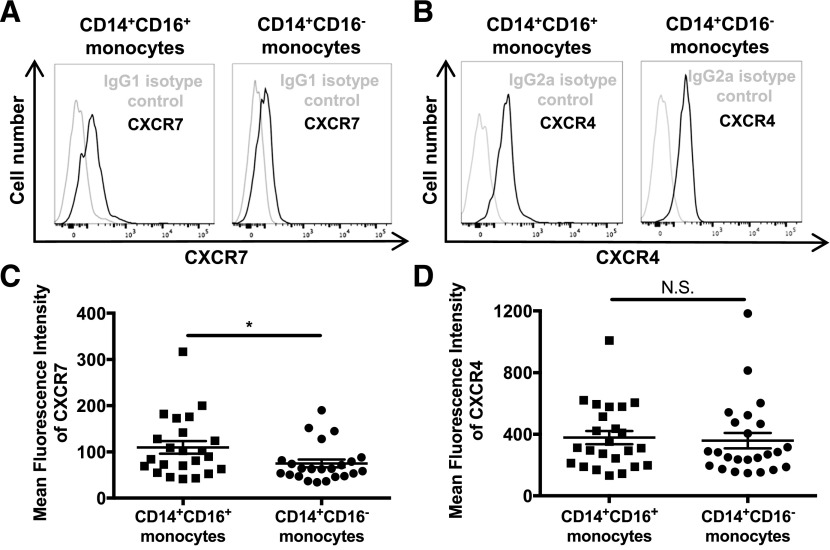
PBMCs were isolated from fresh whole blood from healthy people; stained for CD14, CD16, and CXCR7 or CXCR4; and analyzed by FACS. Monocyte subsets were identified by their CD14 and CD16 expression (Fig. 1B). Surface CXCR7 and CXCR4 were determined on both monocyte subsets by subtracting the IgG isotype-matched control MFI from the CXCR7 or CXCR4 MFI (Fig. 2A and B). We found that CD14+CD16+ monocytes expressed CXCR7 at significantly higher levels (MFI: 51.3 ± 16.4) than CD14+CD16− monocytes, which had less expression of the receptor (MFI: 40.0 ± 14.8; P < 0.05; Fig. 2C). CXCR4 expression was similar between CD14+CD16+ (MFI: 102.8 ± 30.6) and CD14+CD16− monocytes (MFI: 104.8 ± 29.4; Fig. 2D), showing that its expression is generally relatively equivalent on both monocyte subsets in PBMCs from healthy people. This is in agreement with some studies [52, 53] but in contrast with a few others that found that CXCR4 on the CD16+ subset was either decreased [17, 54] or increased [55] compared with CD14+CD16− monocytes. Although CXCR7 expression was, in general, low level, and expression differences between the 2 monocyte subsets were small, the data were highly reproducible with cells from the majority of individuals having an increase in surface CXCR7 on CD14+CD16+ monocytes compared with that on CD14+CD16− monocytes from the same individual. Moreover, this increased expression of CXCR7 on CD14+CD16+ monocytes compared with CD14+CD16− monocytes may explain, in part, a significant functional difference between these 2 subsets that we demonstrated in the following section.
Figure 2. CXCR7 surface expression is higher on CD14+CD16+ than on CD14+CD16− monocytes.
CXCR7 and CXCR4 surface expression on freshly isolated PBMCs from HIV-negative individuals were analyzed by flow cytometry. (A) Histograms from 1 donor representative of 19 independent individuals show surface CXCR7 on CD14+CD16+ and on CD14+CD16− monocytes. An IgG1 isotype-matched control antibody was used. (B) Histograms from 1 donor showing surface CXCR4 and IgG2a isotype control staining on CD14+CD16+ and CD14+CD16− monocytes representative of 21 independent individuals. (C) CXCR7 MFI for 19 independent donors was obtained by subtracting the IgG1 isotype-matched control MFI from the CXCR7 MFI. (D) CXCR4 MFI for 21 independent donors was obtained by subtracting the IgG2a isotype-matched control MFI from the CXCR4 MFI. Data are represented as means ± sem. Significance was determined by Mann-Whitney U test. *P < 0.05. N.S., Nonsignificant.
CXCR7 antagonist CCX771 specifically reduces CD14+CD16+ monocyte transmigration across the BBB
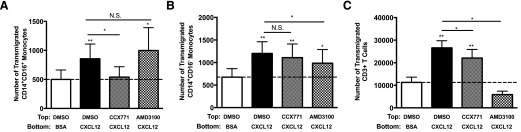
To demonstrate whether CXCR7 is functional and contributes to transmigration across the BBB in response to CXCL12, we examined the transmigration properties of CD14+CD16+ monocytes, CD14+CD16− monocytes, and CD3+ T cells using PBMCs isolated from HIV-negative individuals. Freshly isolated PBMCs were pretreated for 20 min with DMSO (diluent), 10 μM CCX771 (CXCR7 antagonist), or 10 μM AMD3100 (CXCR4 antagonist) added to the human BBB model and allowed to transmigrate in response to BSA (vehicle) or CXCL12 (25 ng/ml) for 24 h. Transmigrated cells were recovered from the bottom of the wells, stained for CD14, CD16 and CD3, and analyzed by FACS to quantify absolute cell numbers.
CXCL12 induced significant transmigration across the BBB for all cell types compared with vehicle (Fig. 3). Transmigration across the BBB of CD14+CD16+ monocytes was blocked by CCX771, whereas their transmigration was not reduced by AMD3100 (Fig. 3A). In contrast, CD14+CD16− monocyte transmigration was not blocked by CCX771 but was reduced significantly by AMD3100 (Fig. 3B). This suggests that transmigration of CD14+CD16+ monocytes is mediated by CXCR7 and not, or only minimally, by CXCR4, whereas the transmigration of CD14+CD16− monocytes, in response to CXCL12, is mediated by CXCR4. Although the number of cells that transmigrated in response to CXCL12 was relatively similar for CD14+CD16+ and CD14+CD16− monocytes (Fig. 3A and B), CD14+CD16+ monocytes represent only 5–10% of monocytes, whereas CD14+CD16− monocytes are 90–95% of monocytes. This shows that a significantly greater percentage of CD14+CD16+ monocytes responded to CXCL12 than did CD14+CD16− monocytes. Thus, relative to input, a much greater number of CD14+CD16+ monocytes transmigrated than did the CD14+CD16− monocytes. As CD14+CD16+ monocytes are preferentially infected and are key to HIV-associated cognitive deficits, these data indicate the importance in characterizing CXCR7 as a target to block CNS entry of specifically CD14+CD16+ monocytes. CCX771 reduced CXCL12-mediated transmigration of unstimulated T cells, although their transmigration remained significantly higher than baseline. Unstimulated T cells were shown previously to respond to CXCL12 through CXCR4 [56, 57], and in our assay, their transmigration was blocked significantly to below baseline by AMD3100 (Fig. 3C). This suggests that whereas CXCR7 may contribute minimally to the transmigration of T cells to CXCL12, their transmigration is mainly mediated by CXCR4.
Figure 3. CXCR7 is critical for transmigration of CD14+CD16+ monocytes but not for that of CD14+CD16− monocytes.
(A–C) Freshly isolated PBMCs from HIV-negative individuals were pretreated with diluent, 10 μM CCX771, or 10 μM AMD3100 for 20 min before being added to the BBB model and allowed to transmigrate to 25 ng/ml CXCL12 for 24 h. PBMCs were stained with CD14, CD16, and CD3, and transmigrated cells were collected from the bottom of the wells and analyzed by FACS. The number of (A) CD14+CD16+ monocytes, (B) CD14+CD16− monocytes, and (C) CD3+ T cells that transmigrated across the BBB was quantified. DMSO was used as a diluent and control. Transmigrations were performed with PBMCs from 10 independent donors, except for AMD3100 (n = 6). Data are represented as means ± sem. Significance was determined by Wilcoxon signed-rank test. Significance is compared with baseline unless indicated otherwise. *P < 0.05; **P < 0.01. N.S., Nonsignificant.
Monocytes matured in vitro with M-CSF express CXCR7
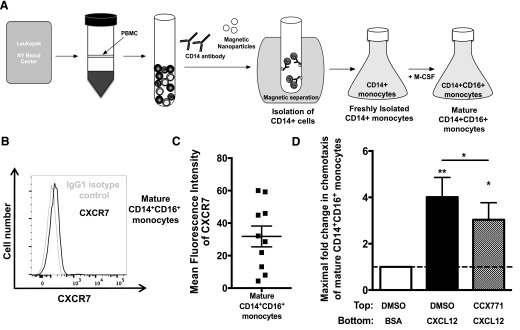
The endothelial cells of the BBB express CXCR7, which regulates the presentation of CXCL12 on the BBB [31]. In a publication from another group, CCX771 did not affect CXCL12 presentation or block CXCR7 on HUVECs when it was used to examine the role of CXCR7 in transendothelial migration of cancer cells [58]. Based on these findings, we did not expect endothelial cell CXCR7 to affect our monocyte transmigration studies. However, to exclude a potential effect of CCX771 on endothelial CXCR7/CXCL12 and to examine the role of CXCR7 in CD14+CD16+ monocyte migration in the absence of endothelial cells, we used an in vitro monocyte maturation system, as described previously [8, 26]. We use this monocyte maturation system to obtain sufficiently high numbers of primarily CD14+CD16+ monocytes, such that we are able to examine chemotaxis, as total monocytes from healthy individuals only contain 5–10% CD14+CD16+ monocytes, which would not enable us to determine specifically the effect of CCX771 on that population. In brief, for monocyte maturation in culture, we obtained freshly isolated CD14+ monocytes from PBMCs of HIV-negative individuals using Ficoll-Paque density gradient centrifugation and immunomagnetic CD14-positive selection. The CD14+ cells were then cultured nonadherently in Teflon-coated flasks in the presence of M-CSF to obtain large numbers of mature CD14+CD16+ monocytes (Fig. 4A). After 3 d, cultures were enriched significantly for mature CD14+CD16+ monocytes, comprising the vast majority of cells (70–75%, n > 300; data not shown). Our laboratory has shown previously that the cultured, mature CD14+CD16+ monocytes resemble CD14+CD16+ monocytes in PBMCs and that they can be used as a model to study their function further [8, 26].
Figure 4. CXCR7 is expressed on mature CD14+CD16+ monocytes and mediates their chemotaxis to CXCL12.
(A) Schematic representation of the isolation and maturation of CD14+ monocytes in culture to obtain high numbers of mature CD14+CD16+ monocytes. PBMCs were isolated from leukopaks obtained from the New York Blood Center, and monocytes were isolated using CD14+ magnetic bead separation. CD14+ monocytes were then cultured nonadherently in Teflon-coated flasks for 3 d in the presence of 10 ng/ml M-CSF to obtain mature CD14+CD16+ monocytes. (B) Histograms of cells obtained from 1 leukopak representative of 10 independent leukopaks show CXCR7 surface expression and IgG1 isotype control antibody staining on mature CD14+CD16+ monocytes. (C) CXCR7 MFI on mature CD14+CD16+ monocytes from 10 independent donors was obtained by subtracting the IgG1 isotype-matched control MFI from the CXCR7 MFI. (D) Mature CD14+CD16+ monocytes were pretreated with diluent or 10 μM CCX771 for 20 min before being added to the wells of a microchemotaxis chamber with a 5 μm polycarbonate membrane, as described in Materials and Methods. Cells chemotaxed to 5 ng/ml CXCL12 for 30 or 60 min at 37°C. Filters were then fixed and stained. Densitometry was used to determine the number of migrated cells per well, and fold change for each condition over baseline, which was set to 1 as indicated by the dotted line, was calculated for 8 independent experiments. The maximal chemotaxis to CXCL12 that was determined at 30 or 60 min for each independent experiment is presented and quantified. Data are represented as means ± sem. Significance was determined by Wilcoxon signed-rank test. Significance is compared with baseline unless indicated otherwise. *P < 0.05; **P < 0.01.
With the use of cells obtained with this maturation system, we were able to examine the role of CXCR7 specifically on mature CD14+CD16+ monocytes. We stained the cultured mature CD14+CD16+ monocytes for CD14, CD16, and CXCR7 and analyzed them by FACS. We found that the mature CD14+CD16+ monocytes express CXCR7 in a wide range, with expression levels varying depending on the leukopak donor (MFI range: 4.4–60.0, with most ranging from 20.0–60.0; Fig. 4B and C).
CCX771 reduces CXCL12-mediated chemotaxis of mature CD14+CD16+ monocytes
To determine the role of CXCR7 in CXCL12-mediated CD14+CD16+ monocyte migration, we performed chemotaxis. This assays the directed cell movement component of the multistep BBB transmigration process without leukocyte–endothelial interactions. Cultured, mature CD14+CD16+ monocytes were allowed to chemotax in the presence and absence of CCX771 to determine whether CXCR7 mediates CD14+CD16+ monocyte migration. CXCL12 (5 ng/ml) was added to the lower wells of a microchemotaxis chamber, and 5 × 105 mature CD14+CD16+ monocytes cultured from leukopaks from 8 independent donors were added to the upper wells of the chamber. Mature CD14+CD16+ monocytes were pretreated for 20 min with diluent or 10 μM CCX771 before adding the cells to the chemotaxis chamber. CCX771 significantly reduced CXCL12-mediated chemotaxis of CD14+CD16+ monocytes (Fig. 4D). This indicates that CXCR7 contributes to CD14+CD16+ monocyte migration in response to CXCL12.
CXCR7 is increased on CD14+CD16+ monocytes from PBMCs of HIV-infected individuals
We used cells from HIV-negative people to show for the first time that CXCR7 is expressed on CD14+CD16+ monocytes and that it mediates their transmigration and chemotactic response to CXCL12. To determine whether CXCR7 can be a therapeutic !target for disease, we focused on the role of CXCR7 on CD14+CD16+ monocytes from individuals with HIV. CD14+CD16+ monocytes are increased significantly in the periphery of HIV-infected people and are key mediators of HIV-associated cognitive deficits despite ART. In addition, CD14+CD16+ monocytes comprise the major monocyte subset that is HIV infected, and this monocyte subpopulation preferentially transmigrates across the BBB in response to chemokines, including CCL2 and CXCL12 [3, 8, 12, 13, 23, 24, 27]. Moreover, CXCL12 is elevated in the brains of HIV-infected people [29, 30]. We hypothesized that CXCR7 contributes to CD14+CD16+ monocyte transmigration across the BBB to CXCL12 in HIV-infected individuals. This would result in neuroinflammation and neuronal damage that may lead to the development of HIV-associated cognitive deficits for which there currently are no therapies.
We first examined surface CXCR7 on monocytes from HIV-infected individuals. Their demographics and virological characteristics are in Table 1. In brief, PBMCs were isolated from fresh whole blood of HIV-infected individuals; stained for CD14, CD16, CXCR7, and CXCR4; and analyzed by FACS. Monocytes were identified by forward- vs. side-scatter, and by CD14 and CD16 expression (Fig. 1B). As shown in other studies, we found that the percentage of CD14+CD16+ monocytes in the total population of peripheral blood monocytes is increased in HIV-infected individuals (data not shown). Expression of CXCR7 and CXCR4 on CD14+CD16+ and CD14+CD16− monocytes was examined by subtracting the IgG isotype-matched control MFI from the CXCR7 or CXCR4 MFI (Fig. 5A and B). We found that surface CXCR7 was increased significantly on CD14+CD16+ monocytes from HIV-infected people (MFI: 109.8 ± 65.5; P < 0.05) compared with that on CD14+CD16− monocytes from those individuals (MFI: 75.2 ± 40.7; Fig. 5C). This suggests that CXCR7 may also mediate differences in transmigration across the BBB of CD14+CD16+ monocytes and CD14+CD16− monocytes from HIV-infected people. Surface CXCR4 was not significantly different between CD14+CD16+ monocytes (MFI: 358.9 ± 244.1) and CD14+CD16− monocytes (MFI: 378.5 ± 208.8; Fig. 5D), which is similar to what we found for HIV-negative individuals (Fig. 2). Although 31.6% of all subjects were ART naïve, we did not detect a difference in CXCR7 or CXCR4 between those that were ART naïve or those that were prescribed therapy (data not shown). However, in a direct comparison of our CXCR7 data between both monocyte subsets from HIV-infected individuals and HIV-negative people, we found that CXCR7 is significantly higher on both monocyte subsets from HIV-infected people compared with the expression on those cells from HIV-negative individuals (Supplemental Fig. 2).
Figure 5. CXCR7 expression is higher on CD14+CD16+ monocytes than on CD14+CD16− monocytes from PBMCs of HIV-infected individuals.
(A and B) CXCR7 and CXCR4 surface expression on freshly isolated PBMCs from HIV-positive individuals were analyzed by FACS. Histograms from 1 donor representative of 23–24 independent individuals show (A) surface CXCR7 and IgG1 isotype control antibody staining and (B) surface CXCR4 and IgG2a isotype control antibody staining on CD14+CD16+ and CD14+CD16− monocytes. (C) CXCR7 MFI was obtained by subtracting the IgG1 isotype-matched control MFI from the CXCR7 MFI. (D) CXCR4 MFI was obtained by subtracting the IgG2a isotype-matched control MFI from the CXCR4 MFI. Data are from 23–24 independent individuals and are represented as means ± sem. Significance was determined by Mann-Whitney U test. *P < 0.05. N.S., Nonsignificant.
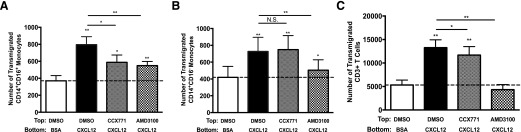
CCX771 reduces transmigration across the BBB of CD14+CD16+ monocytes from PBMC of HIV-infected people
To determine whether increased surface CXCR7 on CD14+CD16+ monocytes from HIV-infected people compared with their CD14+CD16− monocytes contributes to a functional difference between these 2 monocyte subsets, we used CCX771 to examine the role of CXCR7 in transmigration. All samples used for transmigration were from subjects that were on ART. We found that CXCL12 induced significant transmigration across the BBB for all cell types compared with baseline (Fig. 6). CXCL12-mediated transmigration of CD14+CD16+ monocytes was reduced significantly by CCX771. Unexpectedly, their transmigration was also reduced by AMD3100 (Fig. 6A). In contrast, transmigration of CD14+CD16− monocytes was not blocked by CCX771, but AMD3100 did significantly reduce their transmigration (Fig. 6B). This suggests that CD14+CD16+ monocytes from HIV-infected individuals use both CXCR7 and CXCR4 in the transmigration process, whereas CD14+CD16− monocytes use CXCR4 only. T Cells were blocked significantly by AMD3100 to below baseline, whereas CCX771 also reduced their transmigration, but it remained significantly higher than baseline (Fig. 6C). This may indicate that CXCR7, as was found with cells from HIV-negative individuals, may contribute to the transmigration of T cells to CXCL12 but that it is likely that their transmigration is mainly mediated by CXCR4.
Figure 6. CCX771 reduces transmigration across the BBB of CD14+CD16+ monocytes but not of CD14+CD16− monocytes from HIV-infected individuals.
(A–C) Freshly isolated PBMCs from HIV-positive individuals were pretreated with diluent, 10 μM CCX771, or 10 μM AMD3100 for 20 min before being added to the BBB model and allowed to transmigrate to 25 ng/ml CXCL12 for 24 h. PBMCs were stained with CD14, CD16, and CD3, and transmigrated cells were collected from the bottom of the wells and analyzed by FACS. The number of (A) CD14+CD16+ monocytes, (B) CD14+CD16− monocytes, and (C) CD3+ T cells that transmigrated across the BBB was quantified. DMSO was used as diluent and control. Transmigrations were performed with PBMCs from 8 independent donors. Data are represented as means ± sem. Significance was determined by Wilcoxon signed-rank test. Significance is compared with baseline unless indicated otherwise. *P < 0.05; **P < 0.01. N.S., Nonsignificant.
DISCUSSION
Cognitive deficits as a result of HIV infection of the brain are mediated, in part, by entry of CD14+CD16+ monocytes into the CNS [3, 13, 24, 59]. Although ART has significantly improved the lives of people with HIV, it has not decreased the incidence of HIV-associated neurocognitive disorders [5, 7]. In addition, there are still no therapies that decrease neuroinflammation and neurodegeneration mediated by HIV infection of the CNS. Whereas some therapeutic targets have been suggested, these do not prevent entry of CD14+CD16+ monocytes specifically into the brain [23, 60–62]. We demonstrated in this study that CXCR7 is expressed on the cell surface of CD14+CD16+ monocytes, that it is higher in expression on CD14+CD16+ monocytes than on CD14+CD16− monocytes, and that it is increased on the surface of CD14+CD16+ monocytes from HIV-infected individuals compared with those cells from uninfected people. In addition, we showed that CD14+CD16+ monocytes responded to low levels of CXCL12 and transmigrated in greater numbers relative to their input to CXCL12 than did CD14+CD16− monocytes or CD3+ T cells. Importantly, CCX771 reduced CD14+CD16+ monocyte but not CD14+CD16− monocyte transmigration across the BBB. This demonstrates a functional difference between CD14+CD16+ and CD14+CD16− monocyte transmigration to CXCL12 that is mediated by CXCR7. It also suggests that to our knowledge, CXCR7 is the first potential therapeutic target on CD14+CD16+ monocytes that could reduce the entry of this specific monocyte subset into the CNS. This would reduce the neuroinflammation potentially contributing to the cognitive deficits in HIV-infected people.
Monocytes are heterogeneous, and the subpopulations are classified based on their CD14 and CD16 expression [22, 26, 63, 64]. CD14+CD16+ monocytes are increased in the peripheral blood during HIV infection and contribute to HIV infection of the CNS [12, 13, 23, 24]. We showed that CD14+CD16+ monocytes transmigrate to low levels of CXCL12 and transmigrate in significantly greater numbers relative to input across the BBB than do CD14+CD16− monocytes or CD3+ T cells. This suggests that early after peripheral infection, even with low constitutive levels of CXCL12 in the brain, HIV-infected and uninfected CD14+CD16+ monocytes may enter the CNS, leading to early viral seeding and initiation of neuroinflammation.
Transmigration to CXCL12 may be mediated by CXCR7 and CXCR4. CXCR4 has been established for decades as essential to cell movement, whereas CXCR7 was only shown in the past 10 yr to be important in the migration of certain cell types [32–34]. To determine whether CXCR7 could be involved, we examined its expression on both monocyte subsets. As the expression of CXCR7 on monocytes is controversial [41, 43, 44], we used a directly fluorophore-coupled 11G8 antibody clone and showed with an antibody adsorption assay that this antibody is specific to CXCR7, as described previously [41]. We showed that CXCR7 is expressed on CD14+CD16+ monocytes and found that CD14+CD16− monocytes minimally express surface CXCR7. The conflicting results on CXCR7 from prior studies may be a result of the isolation method of the cells of interest. In 1 study, CD14+ monocytes isolated by magnetic beads did not express CXCR7 [41]. In our experiments, this method of isolation masked CXCR7 (data not shown), which may account for that negative finding. In other studies that did not use magnetic bead isolation, CXCR7 expression was found on the total monocyte population [43, 44]. The variations we showed in CXCR7 expression between CD14+CD16+ monocytes and CD14+CD16− monocytes may result in significant differences in their functional properties, supported by our finding that there is a difference in the CXCL12-mediated transmigration mechanisms of the 2 monocyte subsets.
We determined that CXCR7 mediates the transmigration of CD14+CD16+ monocytes to CXCL12 and that CXCR4 but not CXCR7 mediates the transmigration of CD14+CD16− monocytes using a BBB model and antagonists for CXCR7 (CCX771) and CXCR4 (AMD3100). These antagonists specifically block their respective receptors and have been used to examine the function of each receptor in different physiologic processes. AMD3100 was examined in HIV-infected individuals as an antagonist to block interaction between HIV and its coreceptor CXCR4 [65, 66]. However, it failed to be approved as a result of its causing a large increase in WBC counts by mobilizing cells from the bone marrow. It now is in clinical use as Plerixafor to mobilize hematopoietic stem cells in individuals with cancer, with minor adverse effects reported [67]. CCX771 has not been tested yet in clinical trials, and its toxicity remains to be determined. Nevertheless, other studies showed the therapeutic potential of CCX771 for several different inflammatory diseases and cancer [31, 42, 58, 68]. With the use of these antagonists in our study, we showed a distinct difference in the transmigration mechanism of CD14+CD16+ and CD14+CD16− monocytes. This functional difference in transmigration adds to data from some other studies that indicated that CD14+CD16+ and CD14+CD16− monocytes may have different functional abilities [8, 26, 63, 64]. One of these showed that CD14+CD16+ monocytes have increased phagocytic capacity compared with CD14+CD16− monocytes [64]. The functional variation in the transmigration mechanism to CXCL12 may be a result of the interaction between this chemokine and its chemokine receptors on these monocyte subsets. Other groups found that depending on the cell type, CXCL12/CXCR7/CXCR4 interactions can potentiate or prevent downstream signaling [31, 34, 35, 68]. This may depend on whether there is homo- or heterodimerization of CXCR7 and CXCR4 [36, 37, 69], as previous studies showed that T cells and monocytes chemotax more to CXCL12 when both receptors are present than when only CXCR7 or CXCR4 is available [36, 44, 70, 71]. One study showed that increasing amounts of CXCR7 transfected into CXCR4-expressing cancer cells mediated a dose-dependent increase in chemotaxis to CXCL12 [36]. This may explain, in part, why increased surface CXCR7 on CD14+CD16+ monocytes led to increased sensitivity of those cells to CXCL12, and why antagonism of CXCR7 by CCX771 specifically decreased CD14+CD16+ monocyte transmigration to CXCL12. CD14+CD16− monocyte transmigration may not be affected by CCX771, as CXCR7 may not be functional on CD14+CD16− monocytes. It could also be that there may be little or no heterodimerization of CXCR7 on CD14+CD16− monocytes, and this could prevent downstream signaling and transmigration. Additionally, CXCR7 expression may be too low on CD14+CD16− monocytes and may therefore not reach the threshold needed for signaling to occur, or any combination of these possibilities. Future studies will examine mechanisms that mediate the differences in the CXCL12/CXCR7/CXCR4 axis of these 2 monocyte subsets.
To examine in more detail the role of CXCR7 in directed cell movement of CD14+CD16+ monocytes, we used a chemotaxis assay and highly enriched mature CD14+CD16+ monocytes obtained with our previously described culture system. We found that chemotaxis of mature CD14+CD16+ monocytes to CXCL12 is significantly decreased in the presence of CCX771, showing that CCX771 directly impacts the CXCL12–CXCR7 interaction of mature CD14+CD16+ monocytes. The partial reduction in the chemotactic response of these cells to CXCL12 compared with the reduction to baseline of CD14+CD16+ monocyte in CXCL12-induced transmigration across the BBB may be a result of several reasons. The presentation of CXCL12 to CD14+CD16+ monocytes may be different in the presence of endothelial cells in the BBB model compared with chemotaxis in which there is no endothelial–leukocyte interaction. This may cause a differential effect of CCX771 on the CXCR7–CXCL12 axis and its antagonism. It may also be that the numerous steps in the multistep BBB transmigration process may be affected by CCX771, whereas in chemotaxis-only directed cell movement, a single step of the multistep BBB transmigration process is assayed and affected. All of our data collectively indicate that CXCR7 plays a role in CD14+CD16+ monocyte transmigration and not in that of CD14+CD16− monocytes. They also suggest that CXCR7 may be a potential therapeutic target to reduce specifically CD14+CD16+ monocyte entry into the CNS.
Chemokines and cytokines are increased in the plasma and CSF of HIV-infected individuals even on ART, as are chemokine receptors on leukocyte subsets [17, 23, 28, 72, 73]. With the use of CCX771, we showed that CXCL12-induced transmigration of CD14+CD16+ monocytes from HIV-negative people is mediated by CXCR7 only and that transmigration of CD14+CD16+ monocytes from HIV-infected people is by both CXCR7 and CXCR4. In contrast, we found with PBMCs from both HIV-infected and HIV-negative individuals that transmigration of their CD14+CD16− monocytes in response to CXCL12 is not mediated by CXCR7 but rather by CXCR4. As CXCR7 is expressed more highly on CD14+CD16+ monocytes from HIV-positive individuals than from those who are HIV negative, we used higher concentrations of CCX771 in some additional experiments. We found that higher CCX771 concentrations reduced CD14+CD16+ monocyte transmigration to baseline for cells from infected individuals in a way similar to what we showed at 10 μM CCX771 with cells from HIV-negative people, whereas it did not reduce their CD14+CD16− monocyte transmigration (data not shown). The use of higher CCX771 concentrations in HIV-infected individuals may be warranted as a result of increased CXCR7 on their CD14+CD16+ monocytes. CCX771, as a therapeutic to reduce CD14+CD16+ monocyte infiltration specifically, could be used to mitigate their entry into the CNS and other tissues in HIV-infected individuals. We and others propose that it could also be used to treat additional inflammatory diseases in which CD14+CD16+ monocyte infiltration contributes to pathology, such as rheumatoid arthritis and atherosclerosis [13, 68, 74–76]. CCX771 could potentially be an alternative to other therapeutics, as it does not affect cell trafficking of CD14+CD16− monocytes and only minimally of T cells. It thus may pose less of a risk for development of progressive multifocal leukoencephalopathy, which occurred with rituximab therapy that reduced all immune surveillance and could possibly occur with use of natalizumab and other integrin inhibitors that do not specifically target 1 monocyte subset [61, 77, 78].
HIV infection remains a major problem worldwide, and with the success of ART, people with HIV are living longer. However, ART has led to an increased incidence of age-related comorbidities at a younger age in HIV-infected people, including cognitive deficits [79–81]. In the current ART era, accelerated aging is commonly seen in individuals infected with HIV [80]. HIV infection is associated with increased early incidence of cardiovascular disease, cancer, neurocognitive deficits, and other comorbidities that may be mediated, in part, by chronic inflammation and immune activation defined as “inflammaging” [79–81]. Our characterization of CXCR7 on CD14+CD16+ monocytes and our data on the reduction of CXCL12-mediated CD14+CD16+ monocyte transmigration across the BBB in the presence of CCX771 suggest that CCX771 may limit the entry of HIV-infected and uninfected CD14+CD16+ monocytes into the CNS, thereby reducing viral seeding, neuroinflammation, and neuronal damage that may ameliorate the cognitive deficits related to HIV. In addition, it may reduce CD14+CD16+ monocyte transmigration into tissues in many other inflammatory diseases, including rheumatoid arthritis, diabetes, atherosclerosis, and stroke [45, 68, 75, 76, 82].
AUTHORSHIP
M.V., D.W.W., T.M.C., and J.W.B. designed the experiments. K.A. and S.M. provided samples and cohort data. M.V., D.W.W., and T.M.C. performed the experiments and analyzed the results. M.V. and J.W.B. wrote the manuscript. D.W.W., T.M.C., K.A., and S.M. participated in discussion and editing of the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) Grants R01MH075679, R21MH102113-01A1, and R01MH090958 (all to J.W.B.); U24MH100931 (Manhattan HIV Brain Bank); P30AI124414 (Einstein-Rockefeller-CUNY Center for AIDS Research); and TL1TR001072 [Einstein-Montefiore Clinical and Translational Science Awards (CTSA)] and by the eCLIPSE program fellowship, supported by the Burroughs Wellcome Foundation grant program “Unifying Population and Laboratory Based Sciences.” Data in this manuscript were collected by the WIHS (U01A1035004) with the Bronx WIHS principal investigator, K.A. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute on Drug Abuse, and National Institute of Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, and NIH Office of Research on Women’s Health. The authors thank Dr. James Campbell from ChemoCentryx for providing CCX771. The authors thank Matias Jaureguiberry-Bravo and Lillie Lopez for their help with experiments and for thoughtful discussions; the New York Blood Center, patients, and staff of the MHBB and WIHS; and Dr. Lydia Tesfa, of the FACS facility at Einstein.
Glossary
- AMD3100
CXCR4 antagonist
- ART
antiretroviral therapy
- BBB
blood brain barrier
- BMVEC
brain microvascular endothelial cell
- CCX771
small molecule CXCR7 antagonist
- MCF-7
human epithelial breast cancer cell line
- MFI
mean fluorescence intensity
- MHBB
Manhattan HIV Brain Bank
- WIHS
Women’s Interagency HIV Study
Footnotes
The online version of this paper, found at www.jleukbio.org, contains supplemental information.
SEE CORRESPONDING EDITORIAL ON PAGE 1155
DISCLOSURES
The authors declare no conflict of interest. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH.
REFERENCES
- 1.Witwer K. W., Gama L., Li M., Bartizal C. M., Queen S. E., Varrone J. J., Brice A. K., Graham D. R., Tarwater P. M., Mankowski J. L., Zink M. C., Clements J. E. (2009) Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One 4, e8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valcour V., Chalermchai T., Sailasuta N., Marovich M., Lerdlum S., Suttichom D., Suwanwela N. C., Jagodzinski L., Michael N., Spudich S., van Griensven F., de Souza M., Kim J., Ananworanich J.; RV254/SEARCH 010 Study Group (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 206, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW (2014) CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol. Neuroimmunol. Neuroinflamm. 1, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valcour V. G., Ananworanich J., Agsalda M., Sailasuta N., Chalermchai T., Schuetz A., Shikuma C., Liang C. Y., Jirajariyavej S., Sithinamsuwan P., Tipsuk S., Clifford D. B., Paul R., Fletcher J. L., Marovich M. A., Slike B. M., DeGruttola V., Shiramizu B.; SEARCH 011 Protocol Team (2013) HIV DNA reservoir increases risk for cognitive disorders in cART-naïve patients. PLoS One 8, e70164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton R. K., Clifford D. B., Franklin D. R. Jr., Woods S. P., Ake C., Vaida F., Ellis R. J., Letendre S. L., Marcotte T. D., Atkinson J. H., Rivera-Mindt M., Vigil O. R., Taylor M. J., Collier A. C., Marra C. M., Gelman B. B., McArthur J. C., Morgello S., Simpson D. M., McCutchan J. A., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T. L., Wong J., Grant I.; CHARTER Group (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cysique L. A., Brew B. J. (2011) Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J. Neurovirol. 17, 176–183. [DOI] [PubMed] [Google Scholar]
- 7.Heaton R. K., Franklin D. R., Ellis R. J., McCutchan J. A., Letendre S. L., Leblanc S., Corkran S. H., Duarte N. A., Clifford D. B., Woods S. P., Collier A. C., Marra C. M., Morgello S., Mindt M. R., Taylor M. J., Marcotte T. D., Atkinson J. H., Wolfson T., Gelman B. B., McArthur J. C., Simpson D. M., Abramson I., Gamst A., Fennema-Notestine C., Jernigan T. L., Wong J., Grant I.; CHARTER Group; HNRC Group (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams D. W., Calderon T. M., Lopez L., Carvallo-Torres L., Gaskill P. J., Eugenin E. A., Morgello S., Berman J. W. (2013) Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 8, e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. (1986) Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA 83, 7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. (1986) Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 233, 1089–1093. [DOI] [PubMed] [Google Scholar]
- 11.Eugenin E. A., Osiecki K., Lopez L., Goldstein H., Calderon T. M., Berman J. W. (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 26, 1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdo T. H., Soulas C., Orzechowski K., Button J., Krishnan A., Sugimoto C., Alvarez X., Kuroda M. J., Williams K. C. (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 6, e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer-Smith T., Croul S., Sverstiuk A. E., Capini C., L’Heureux D., Régulier E. G., Richardson M. W., Amini S., Morgello S., Khalili K., Rappaport J. (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol. 7, 528–541. [DOI] [PubMed] [Google Scholar]
- 14.Clay C. C., Rodrigues D. S., Ho Y. S., Fallert B. A., Janatpour K., Reinhart T. A., Esser U. (2007) Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J. Virol. 81, 12040–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avalos C. R., Price S. L., Forsyth E. R., Pin J. N., Shirk E. N., Bullock B. T., Queen S. E., Li M., Gellerup D., O’Connor S. L., Zink M. C., Mankowski J. L., Gama L., Clements J. E. (2016) Quantitation of productively infected monocytes and macrophages of simian immunodeficiency virus-infected macaques. J. Virol. 90, 5643–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis M. L., Meltzer M. S. (1993) Induction of IFN-alpha by HIV-1 in monocyte-enriched PBMC requires gp120-CD4 interaction but not virus replication. J. Immunol. 151, 2208–2216. [PubMed] [Google Scholar]
- 17.Ellery P. J., Tippett E., Chiu Y. L., Paukovics G., Cameron P. U., Solomon A., Lewin S. R., Gorry P. R., Jaworowski A., Greene W. C., Sonza S., Crowe S. M. (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 178, 6581–6589. [DOI] [PubMed] [Google Scholar]
- 18.Buscemi L., Ramonet D., Geiger J. D. (2007) Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol. Dis. 26, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson A. M., Fennema-Notestine C., Umlauf A., Taylor M. J., Clifford D. B., Marra C. M., Collier A. C., Gelman B. B., McArthur J. C., McCutchan J. A., Simpson D. M., Morgello S., Grant I., Letendre S. L.; CHARTER Group (2015) CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. J. Neurovirol. 21, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson A. M., Harezlak J., Bharti A., Mi D., Taylor M. J., Daar E. S., Schifitto G., Zhong J., Alger J. R., Brown M. S., Singer E. J., Campbell T. B., McMahon D. D., Buchthal S., Cohen R., Yiannoutsos C., Letendre S. L., Navia B. A.; HIV Neuroimaging Consortium (2015) Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 69, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meulendyke K. A., Ubaida-Mohien C., Drewes J. L., Liao Z., Gama L., Witwer K. W., Graham D. R., Zink M. C. (2014) Elevated brain monoamine oxidase activity in SIV- and HIV-associated neurological disease. J. Infect. Dis. 210, 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J., Liu Y. J., MacPherson G., Randolph G. J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J. M., Lutz M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80. [DOI] [PubMed] [Google Scholar]
- 23.Williams D. W., Anastos K., Morgello S., Berman J. W. (2015) JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV-infected individuals. J. Leukoc. Biol. 97, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulliam L., Gascon R., Stubblebine M., McGuire D., McGrath M. S. (1997) Unique monocyte subset in patients with AIDS dementia. Lancet 349, 692–695. [DOI] [PubMed] [Google Scholar]
- 25.Kim W. K., Sun Y., Do H., Autissier P., Halpern E. F., Piatak M. Jr., Lifson J. D., Burdo T. H., McGrath M. S., Williams K. (2010) Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J. Leukoc. Biol. 87, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckner C. M., Calderon T. M., Willams D. W., Belbin T. J., Berman J. W. (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell. Immunol. 267, 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams D. W., Veenstra M., Gaskill P. J., Morgello S., Calderon T. M., Berman J. W. (2014) Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr. HIV Res. 12, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamat A., Lyons J. L., Misra V., Uno H., Morgello S., Singer E. J., Gabuzda D. (2012) Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J. Acquir. Immune Defic. Syndr. 60, 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostasy K., Egles C., Chauhan A., Kneissl M., Bahrani P., Yiannoutsos C., Hunter D. D., Nath A., Hedreen J. C., Navia B. A. (2003) SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J. Neuropathol. Exp. Neurol. 62, 617–626. [DOI] [PubMed] [Google Scholar]
- 30.Langford D., Sanders V. J., Mallory M., Kaul M., Masliah E. (2002) Expression of stromal cell-derived factor 1alpha protein in HIV encephalitis. J. Neuroimmunol. 127, 115–126. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Orengo L., Holman D. W., Dorsey D., Zhou L., Zhang P., Wright M., McCandless E. E., Patel J. R., Luker G. D., Littman D. R., Russell J. H., Klein R. S. (2011) CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J. Exp. Med. 208, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bleul C. C., Farzan M., Choe H., Parolin C., Clark-Lewis I., Sodroski J., Springer T. A. (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833. [DOI] [PubMed] [Google Scholar]
- 33.Poznansky M. C., Olszak I. T., Foxall R., Evans R. H., Luster A. D., Scadden D. T. (2000) Active movement of T cells away from a chemokine. Nat. Med. 6, 543–548. [DOI] [PubMed] [Google Scholar]
- 34.Burns J. M., Summers B. C., Wang Y., Melikian A., Berahovich R., Miao Z., Penfold M. E., Sunshine M. J., Littman D. R., Kuo C. J., Wei K., McMaster B. E., Wright K., Howard M. C., Schall T. J. (2006) A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203, 2201–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zabel B. A., Wang Y., Lewén S., Berahovich R. D., Penfold M. E., Zhang P., Powers J., Summers B. C., Miao Z., Zhao B., Jalili A., Janowska-Wieczorek A., Jaen J. C., Schall T. J. (2009) Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J. Immunol. 183, 3204–3211. [DOI] [PubMed] [Google Scholar]
- 36.Décaillot F. M., Kazmi M. A., Lin Y., Ray-Saha S., Sakmar T. P., Sachdev P. (2011) CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J. Biol. Chem. 286, 32188–32197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levoye A., Balabanian K., Baleux F., Bachelerie F., Lagane B. (2009) CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 113, 6085–6093. [DOI] [PubMed] [Google Scholar]
- 38.Rajagopal S., Kim J., Ahn S., Craig S., Lam C. M., Gerard N. P., Gerard C., Lefkowitz R. J. (2010) Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. USA 107, 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Libert F., Parmentier M., Lefort A., Dumont J. E., Vassart G. (1990) Complete nucleotide sequence of a putative G protein coupled receptor: RDC1. Nucleic Acids Res. 18, 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu S., Brown M., Sengupta R., Penfold M. E., Meucci O. (2011) CXCR7 protein expression in human adult brain and differentiated neurons. PLoS One 6, e20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berahovich R. D., Zabel B. A., Penfold M. E., Lewén S., Wang Y., Miao Z., Gan L., Pereda J., Dias J., Slukvin I. I., McGrath K. E., Jaen J. C., Schall T. J. (2010) CXCR7 protein is not expressed on human or mouse leukocytes. J. Immunol. 185, 5130–5139. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez-Martín L., Sánchez-Mateos P., Cabañas C. (2013) CXCR7 impact on CXCL12 biology and disease. Trends Mol. Med. 19, 12–22. [DOI] [PubMed] [Google Scholar]
- 43.Infantino S., Moepps B., Thelen M. (2006) Expression and regulation of the orphan receptor RDC1 and its putative ligand in human dendritic and B cells. J. Immunol. 176, 2197–2207. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Martín L., Estecha A., Samaniego R., Sánchez-Ramón S., Vega M. A., Sánchez-Mateos P. (2011) The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117, 88–97. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler-Heitbrock L. (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 81, 584–592. [DOI] [PubMed] [Google Scholar]
- 46.Boudot A., Kerdivel G., Habauzit D., Eeckhoute J., Le Dily F., Flouriot G., Samson M., Pakdel F. (2011) Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS One 6, e20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvallo L., Lopez L., Che F. Y., Lim J., Eugenin E. A., Williams D. W., Nieves E., Calderon T. M., Madrid-Aliste C., Fiser A., Weiss L., Angeletti R. H., Berman J. W. (2015) Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes. J. Immunol. 194, 3246–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Report of a Working Group of the American Academy of Neurology AIDS Task Force. (1991) Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Neurology 41, 778–785. [DOI] [PubMed] [Google Scholar]
- 49.Edwards K. R., Goyal J., Plavina T., Czerkowicz J., Goelz S., Ranger A., Cadavid D., Browning J. L. (2013) Feasibility of the use of combinatorial chemokine arrays to study blood and CSF in multiple sclerosis. PLoS One 8, e81007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tingjun C., Zhaohui L., Zhaocai J., Zihao L., Quangang X., Dehui H., Qing L., Shihui W. (2015) Changes of CXCL12, CXCL14 and PDGF levels in the brain of patients with idiopathic demyelinating optic neuritis and neuromyelitis optica. J. Neuroimmunol. 279, 1–6. [DOI] [PubMed] [Google Scholar]
- 51.Jaworowski A., Kamwendo D. D., Ellery P., Sonza S., Mwapasa V., Tadesse E., Molyneux M. E., Rogerson S. J., Meshnick S. R., Crowe S. M. (2007) CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J. Infect. Dis. 196, 38–42. [DOI] [PubMed] [Google Scholar]
- 52.Ancuta P., Rao R., Moses A., Mehle A., Shaw S. K., Luscinskas F. W., Gabuzda D. (2003) Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197, 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shantsila E., Tapp L. D., Wrigley B. J., Pamukcu B., Apostolakis S., Montoro-García S., Lip G. Y. (2014) Monocyte subsets in coronary artery disease and their associations with markers of inflammation and fibrinolysis. Atherosclerosis 234, 4–10. [DOI] [PubMed] [Google Scholar]
- 54.Ingersoll M. A., Spanbroek R., Lottaz C., Gautier E. L., Frankenberger M., Hoffmann R., Lang R., Haniffa M., Collin M., Tacke F., Habenicht A. J., Ziegler-Heitbrock L., Randolph G. J. (2010) Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82. [DOI] [PubMed] [Google Scholar]
- 56.Kawaguchi A., Orba Y., Kimura T., Iha H., Ogata M., Tsuji T., Ainai A., Sata T., Okamoto T., Hall W. W., Sawa H., Hasegawa H. (2009) Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I tax transgenic mice. Blood 114, 2961–2968. [DOI] [PubMed] [Google Scholar]
- 57.Mehling M., Frank T., Albayrak C., Tay S. (2015) Real-time tracking, retrieval and gene expression analysis of migrating human T cells. Lab Chip 15, 1276–1283. [DOI] [PubMed] [Google Scholar]
- 58.Zabel B. A., Lewén S., Berahovich R. D., Jaén J. C., Schall T. J. (2011) The novel chemokine receptor CXCR7 regulates trans-endothelial migration of cancer cells. Mol. Cancer 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis L. E., Hjelle B. L., Miller V. E., Palmer D. L., Llewellyn A. L., Merlin T. L., Young S. A., Mills R. G., Wachsman W., Wiley C. A. (1992) Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42, 1736–1739. [DOI] [PubMed] [Google Scholar]
- 60.Campbell J. H., Burdo T. H., Autissier P., Bombardier J. P., Westmoreland S. V., Soulas C., González R. G., Ratai E. M., Williams K. C. (2011) Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One 6, e18688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell J. H., Ratai E. M., Autissier P., Nolan D. J., Tse S., Miller A. D., González R. G., Salemi M., Burdo T. H., Williams K. C. (2014) Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 10, e1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lakritz J. R., Thibault D. M., Robinson J. A., Campbell J. H., Miller A. D., Williams K. C., Burdo T. H. (2016) α4 Integrin antibody treatment blocks monocyte/macrophage traffic to, vascular cell adhesion molecule-1 expression in, and pathology of the dorsal root ganglia in an SIV macaque model of HIV-peripheral neuropathy. Am. J. Pathol. 186, 1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zawada A. M., Rogacev K. S., Rotter B., Winter P., Marell R. R., Fliser D., Heine G. H. (2011) SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 118, e50–e61. [DOI] [PubMed] [Google Scholar]
- 64.Antonelli L. R., Leoratti F. M., Costa P. A., Rocha B. C., Diniz S. Q., Tada M. S., Pereira D. B., Teixeira-Carvalho A., Golenbock D. T., Gonçalves R., Gazzinelli R. T. (2014) The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog. 10, e1004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donzella G. A., Schols D., Lin S. W., Esté J. A., Nagashima K. A., Maddon P. J., Allaway G. P., Sakmar T. P., Henson G., De Clercq E., Moore J. P. (1998) AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4, 72–77. [DOI] [PubMed] [Google Scholar]
- 66.De Clercq E. (2009) Antiviral drug discovery: ten more compounds, and ten more stories (part B). Med. Res. Rev. 29, 571–610. [DOI] [PubMed] [Google Scholar]
- 67.Horwitz M. E., Long G., Holman P., Libby E., Calandra G. C., Schriber J. R. (2012) Efficacy and safety of hematopoietic stem cell remobilization with plerixafor+G-CSF in adult patients with germ cell tumors. Bone Marrow Transplant. 47, 1283–1286. [DOI] [PubMed] [Google Scholar]
- 68.Ma W., Liu Y., Ellison N., Shen J. (2013) Induction of C-X-C chemokine receptor type 7 (CXCR7) switches stromal cell-derived factor-1 (SDF-1) signaling and phagocytic activity in macrophages linked to atherosclerosis. J. Biol. Chem. 288, 15481–15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luker K. E., Gupta M., Luker G. D. (2009) Imaging chemokine receptor dimerization with firefly luciferase complementation. FASEB J. 23, 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar R., Tripathi V., Ahmad M., Nath N., Mir R. A., Chauhan S. S., Luthra K. (2012) CXCR7 mediated Giα independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Cell. Immunol. 272, 230–241. [DOI] [PubMed] [Google Scholar]
- 71.Balabanian K., Lagane B., Infantino S., Chow K. Y., Harriague J., Moepps B., Arenzana-Seisdedos F., Thelen M., Bachelerie F. (2005) The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 280, 35760–35766. [DOI] [PubMed] [Google Scholar]
- 72.Fischer-Smith T., Tedaldi E. M., Rappaport J. (2008) CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res. Hum. Retroviruses 24, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krishnan S., Wilson E. M., Sheikh V., Rupert A., Mendoza D., Yang J., Lempicki R., Migueles S. A., Sereti I. (2014) Evidence for innate immune system activation in HIV-1 infected elite controllers. J. Infect. Dis. 209, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer T., Wyatt C. M., D’Agati V. D., Croul S., McCourt L., Morgello S., Rappaport J. (2014) Mononuclear phagocyte accumulation in visceral tissue in HIV encephalitis: evidence for increased monocyte/macrophage trafficking and altered differentiation. Curr. HIV Res. 12, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amoruso A., Sola D., Rossi L., Obeng J. A., Fresu L. G., Sainaghi P. P., Pirisi M., Brunelleschi S. (2016) Relation among anti-rheumatic drug therapy, CD14(+)CD16(+) blood monocytes and disease activity markers (DAS28 and US7 scores) in rheumatoid arthritis: a pilot study. Pharmacol. Res. 107, 308–314. [DOI] [PubMed] [Google Scholar]
- 76.Rogacev K. S., Cremers B., Zawada A. M., Seiler S., Binder N., Ege P., Grosse-Dunker G., Heisel I., Hornof F., Jeken J., Rebling N. M., Ulrich C., Scheller B., Böhm M., Fliser D., Heine G. H. (2012) CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 60, 1512–1520. [DOI] [PubMed] [Google Scholar]
- 77.Carson K. R., Evens A. M., Richey E. A., Habermann T. M., Focosi D., Seymour J. F., Laubach J., Bawn S. D., Gordon L. I., Winter J. N., Furman R. R., Vose J. M., Zelenetz A. D., Mamtani R., Raisch D. W., Dorshimer G. W., Rosen S. T., Muro K., Gottardi-Littell N. R., Talley R. L., Sartor O., Green D., Major E. O., Bennett C. L. (2009) Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113, 4834–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwab N., Ulzheimer J. C., Fox R. J., Schneider-Hohendorf T., Kieseier B. C., Monoranu C. M., Staugaitis S. M., Welch W., Jilek S., Du Pasquier R. A., Brück W., Toyka K. V., Ransohoff R. M., Wiendl H. (2012) Fatal PML associated with efalizumab therapy: insights into integrin αLβ2 in JC virus control. Neurology 78, 458–467, discussion 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciccarelli N., Fabbiani M., Baldonero E., Fanti I., Cauda R., Di Giambenedetto S., Silveri M. C. (2012) Effect of aging and human immunodeficiency virus infection on cognitive abilities. J. Am. Geriatr. Soc. 60, 2048–2055. [DOI] [PubMed] [Google Scholar]
- 80.Schouten J., Wit F. W., Stolte I. G., Kootstra N. A., van der Valk M., Geerlings S. E., Prins M., Reiss P.; AGEhIV Cohort Study Group (2014) Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin. Infect. Dis. 59, 1787–1797. [DOI] [PubMed] [Google Scholar]
- 81.Nasi M., De Biasi S., Gibellini L., Bianchini E., Pecorini S., Bacca V., Guaraldi G., Mussini C., Pinti M., Cossarizza A. (2017) Ageing and inflammation in patients with HIV infection. Clin. Exp. Immunol. 187, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawanaka N., Yamamura M., Aita T., Morita Y., Okamoto A., Kawashima M., Iwahashi M., Ueno A., Ohmoto Y., Makino H. (2002) CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 46, 2578–2586. [DOI] [PubMed] [Google Scholar]