Human MAIT cells have the capacity to provide help to B cells through induction of plasmablast differentiation and antibody production.
Keywords: plasmablast, B cell help, MR-1, antibody
Abstract
Mucosal-associated invariant T (MAIT) cells are an innate-like T cell subset, restricted by the nonclassic MHC class I-related protein MR1 and enriched at mucosal sites. Human studies have shown an association between MAIT cells and pathogen-specific antibody responses. In this study, we investigate the effect of human MAIT cells on B cells ex vivo. We found that supernatants from microbe- or cytokine-stimulated MAIT cells, when added to purified autologous B cells, increase frequencies of plasmablasts and promote IgA, IgG, and IgM production. We found effects to be mostly MR1-dependent and that the increases in plasmablasts are likely a result of increased differentiation from memory B cells. Furthermore, microbe-activated MAIT cell supernatant contains multiple cytokines known to stimulate B cells, including IL-6, -10, and -21. This study thus provides the first direct evidence of a newly identified role of MAIT cells in providing help to B cells.
Introduction
To elicit effective antibody responses to pathogens and differentiate into a mature phenotype, B cells frequently require the help of T cells to provide secondary signals. T follicular helper cells are one of the most commonly recognized T cell subsets to become B cell helpers [1]; however, other T cell subsets, such as invariant natural killer T (iNKT) cells, have been shown to play a similar role, through both cognate and noncognate interactions with B cells [2].
Mucosal associated invariant T (MAIT) cells are a recently described subset of innate-like T cells that, similar to iNKT cells, use a semi-invariant TCR to recognize nonpeptide bacteria-derived ligands presented on the surface of nonclassic MHC molecules [3]. MAIT cells account for ∼5% of T cells in healthy humans [4] and are restricted by the nonpolymorphic MHC-related protein MR1, which presents intermediates of the riboflavin synthesis pathway common to several microorganisms [3]. The MAIT cell TCR is most commonly composed of an invariant TCR V α chain (TCR-α variable; TRAV1-2, alternatively referred to as Vα 7.2) paired with semi-invariant J α and more variable β chains [5–7]. MAIT cells express CD161, an NK-associated receptor, and in response to MR1-dependent activation are known to produce IFN-γ, TNF-α, IL-17, and granzyme b [8, 9]. In addition, MAIT cells are capable of MR1-independent responses to several cytokines, including IL-2, -7, -12, -15, and -18 [10–12].
Although B cells have been shown to modulate MAIT cell responses [13], the ability of MAIT cells to stimulate B cell responses has not been investigated. Several observations in human studies suggest that MAIT cells may be B cell helpers. We have observed that in acute Vibrio cholerae infection, V. cholerae LPS-specific IgA and IgG antibody responses correlate positively with changes in MAIT cell frequencies [14]. In addition, Le Bourhis et al. [15] showed that in humans vaccinated orally with an attenuated Shigella dysenteriae strain, elevated MAIT cell frequencies and MAIT cell activation markers were associated with a significant Shigella LPS-specific antibody–secreting cell response. Finally, activated MAIT cells have been shown to produce sCD40L [16], a key factor involved in T cell effects on B cells. However, there remains a lack of investigation into the ability of MAIT cells to provide help to B cells.
In this study, we examine the ability of human MAIT cells to stimulate B cell antibody production ex vivo. We activated MAIT cells with microbial, direct TCR, and cytokine stimulation, and the resulting supernatant was applied to purified autologous B cells and assayed for B cell stimulatory cytokines. This study provided the first direct evidence that MAIT cells induce B cell plasmablast differentiation and antibody production, a potentially important function of MAIT cells in the defense against microbial invasion.
MATERIALS AND METHODS
Primary cells for ex vivo studies
We obtained blood for this study from same-day discarded leukocyte filtration packs obtained from healthy anonymous blood donors, and isolated PBMCs by density gradient centrifugation using Lymphoprep (StemCell Technologies, Vancouver, BC, Canada). We isolated TCR Vα 7.2+ cells from PBMC via positive selection of Vα 7.2 PE-labeled (clone 3C10; BioLegend, San Diego, CA, USA) PBMCs, using anti-PE microbeads and MACS columns (Miltenyi, Bergisch Gladbach, Germany), and isolated primary B cells by subjecting the flow-through from Vα 7.2+ selection to a human B cell−-selection kit (eBioscience, San Diego CA, USA). We also isolated primary human monocytes by using a human CD14+-selection kit (eBioscience). Where indicated, we obtained highly purified populations of CD3−CD19+ B cells and CD3+CD4− Vα 7.2+CD161++ or CD161− cells by flow sorting of previously magnetically purified populations on a FACS Aria II (BD, Franklin Lakes, NJ, USA), with a postsorting purity greater than 99.5%. We defined MAITs as CD3+CD4− Vα 7.2+CD161++ cells. Media used for all studies was RPMI 1640 with 10% FBS with 1% penicillin/streptomycin.
E. coli for MAIT cell stimulation
We used the E. coli strains BL21, BSV18 (a RibA deficient strain), and 1100-2 (the parental strain of BSV18 that has an intact RibA gene) for bacterial stimulations of MAIT cells. Strain BSV18 required 20 µg/ml supplemental riboflavin to allow for growth. We based bacterial counts on OD600 absorption, and live bacterial cultures were frozen at −80 for later use. For bacterial stimulations, we spun down thawed aliquots of Escherichia coli, fixed them in 1% paraformaldehyde in PBS for 10 min at room temperature, and washed them twice in PBS immediately before addition to cells. Strains 1100-2 and BSV18 were provided by the Coli Genetics Stock Center (Yale University, New Haven, CT, USA).
MAIT and B cell stimulation
For activation of purified MAIT cell populations with E. coli, we incubated 50,000 MAIT cells overnight with 100,000 THP-1 cells (a monocyte cell line; ATCC, Manassas, VA, USA), or 100,000 primary human CD14+ monocytes, in a total volume of 300 µl in 96-well flat bottom plates, with formaldehyde-fixed E. coli added at the indicated MOI per THP-1 cell and 1.25 µg/ml anti-CD28 [17] (clone CD28.2; BioLegend). We added anti-MR1 blocking antibody (BioLegend) at 10 µg/ml in MAIT/THP-1/BL21 cultures for selected experiments. For TCR MAIT cell stimulation, we coated flat-bottom tissue culture wells for 5 h at 37°C with 1 µg/ml anti-CD3 (clone OKT3; BioLegend) and 2 µg/ml anti-CD28 in PBS, followed by washing 2 times with PBS 2% FBS before addition of MAIT cells. For cytokine stimulation, we added various combinations of recombinant IL-12 at 10 ng/ml (PeproTech, Rocky Hill, NJ, USA), IL-15 at 50 ng/ml (PeproTech), and IL-18 at 50 ng/ml (MBL Biotech, Arlington, VA, USA). When supernatant from IL-12/IL-18–stimulated MAIT cells was added to B cells, blocking antibodies to IL-12/23 p40 (5 µg/ml; eBioscience) and IL-18 (MBL, 5 µg/ml) were added to neutralize the exogenous cytokines. For other experiments, blocking antibody to CD154 (CD40L; BioLegend) was added to MAIT supernatant at 20 µg/ml. Supernatant (200 µl) from MAIT cells activated overnight was added to 250,000 B cells (100 µl) in 96-well flat-bottom plates, followed by 7 d incubation at 37°C.
ELISA for whole-molecule IgA, IgG, and IgM
We measured IgA, IgG, and IgM antibody levels in B cell cultures by sandwich ELISA. We coated Nunclon 96-well Maxisorp plates (Thermo Fisher Scientific) with goat Fab2 anti-human IgA, IgG, or IgM (The Jackson Laboratory, Bar Harbor, ME, USA) overnight in PBS, followed by washing; blocking with PBS 1% BSA; washing; addition of samples and standards; washing; addition of HRP-conjugated anti-human IgA, IgG, or IgM antibodies; washing; and addition of OPD sodium citrate solution, with addition of 2 M HCl as a stop solution after 10 min.
Cytometric bead array
We measured the cytokine levels (IL-2, -4, -5, -6, -9, -10, -13, -17A, -17F, -21, and -22; TNF-α; and IFN-γ) in activated MAIT cell supernatant with the Legendplex Human Th Cytokine Panel (BioLegend), according to the manufacturer’s instructions and analyzed the data with Legendplex software (BioLegend). Where cytokine levels were too low to compute values, 1 pg/ml was used.
Flow cytometry
We ran flow cytometry of MAIT and B cell cocultures after 7 d of coculture on a BD Fortessa, using a panel consisting of CD3 APC-e780, CD19 PerCP-Cy5.5, HLA-DR BV421, CD27 FITC, CD38 PE-Cy7, CD24 APC, CD20 A700, IgD PE, CD138 PE-Dazzle 594, and GV510 viability dye [antibodies obtained from BioLegend, eBioscience, or Tonbo (San Diego, CA, USA)]. We sorted cells on a FACS Aria II (BD). We defined plasmablasts as CD3−CD19+CD38++CD24−CD138− HLA-DR+ cells. For intracellular staining, we added Brefeldin A and monensin 4 h before staining the cells and used the BD Cytofix/Cytoperm kit according to the manufacturer’s instructions, with a panel consisting of CD3 PerCIp-Cy5.5 (BioLegend), Vα7.2 PE (BioLegend), CD161 APC (BioLegend), TNF-α e450 (eBioscience), and IFN-γ FITC (BioLegend). We analyzed flow cytometry data using FlowJo software ver. 10.0.8 (FlowJo, LLC, Ashland, OR, USA).
Statistical analysis
We performed statistics and generated figures with Prism ver. 6.07 (GraphPad Software, La Jolla, CA, USA), comparing differences between groups using the Mann-Whitney U test, 1-way ANOVA followed by Tukey’s multiple-comparisons test, or 2-way ANOVA followed by Fisher’s LSD test, as indicated.
RESULTS
Activation of MAIT cells by APC/E. coli ex vivo induces IFN-γ and TNF-α
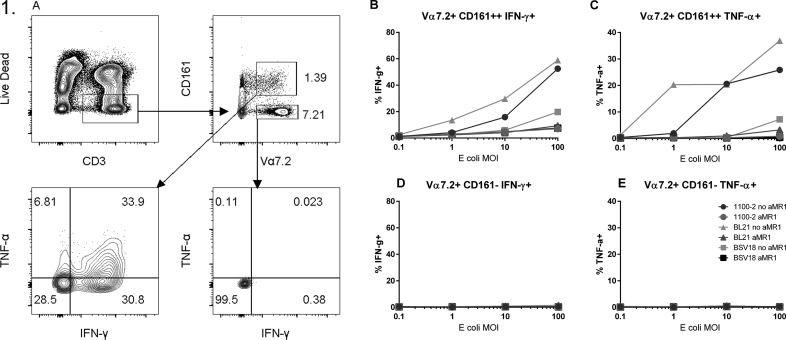
MAIT cells are known to be activated by an intermediate of the riboflavin synthesis pathway that is present in many bacterial and fungal species [3]. For E. coli stimulation of MAIT cells, we used a strain of E. coli deficient in the RibA gene (BSV18), which prevents downstream synthesis of the MAIT ligand [18]. As a positive control, we also used strain 1100-2 (the RibA nonmutant strain upon which BSV18 is based) and BL21, a wild-type E. coli strain which has been shown to result in high levels of MAIT-cell activation [19].
As expected, bacterial stimulation of PBMCs by strains BL21 and 1100-2 resulted in significant upregulation of IFN-γ and TNF-α in Vα7.2+CD161++ MAIT cells, but not in conventional Vα7.2+CD161− T cells (Fig. 1). Blockade of MR1, which prevents TCR-mediated activation of MAIT cells, almost completely eliminated TNF-α expression; however, some residual IFN-γ activity remained. In contrast, MAIT cells stimulated with RibA-deficient BSV18 failed to express similarly high levels of IFN-γ and TNF-α, although the levels of IFN-γ expression were still higher than in Vα7.2+ CD161− cells (Fig. 1B–E), suggesting that MAIT cells are also activated by non-TCR–mediated mechanisms, such as cytokines released from APC reactive to bacterial ligands present in the culture.
Figure 1. MR1-dependent activation of MAIT cells by E. coli.
(A) Gating strategy of PBMCs stimulated overnight with formaldehyde-fixed BL21, 1100-2, or BSV18 E. coli, with or without anti-MR1 antibody (10 µg/ml) at 0.1, 1, 10, and 100 MOI, with 1.25 µg/ml anti-CD28. Levels of TNF-α and IFN-γ in (B and C) MAIT (Vα7.2+CD161++) and (D and E) non-MAIT (Vα7.2+CD161−) T cell populations were measured by flow cytometry. Data are from a single donor and are representative of 2 independent experiments.
Supernatant from APC/E. coli– and TCR/cytokine–activated MAIT cells induces B cell plasmablast expansion
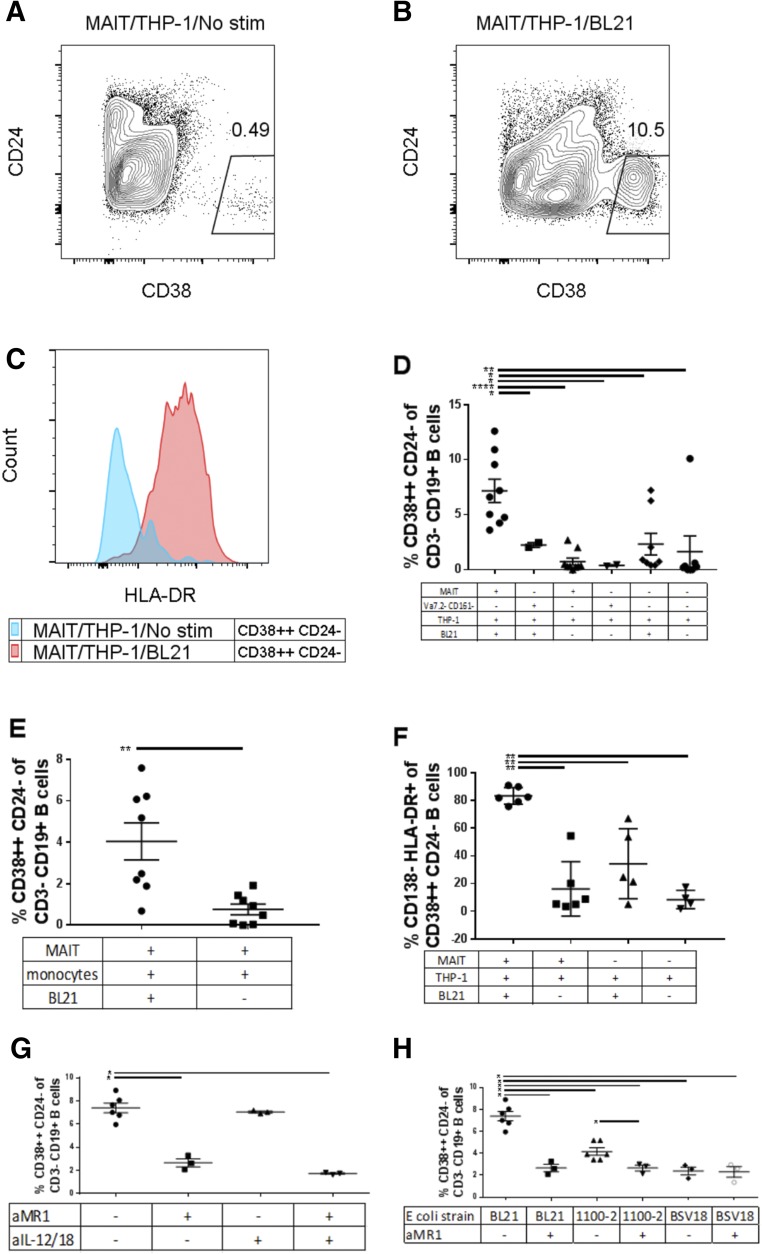
MAIT cells have been shown to be potently stimulated by THP-1 cells (monocyte cell line) exposed to formaldehyde-fixed E. coli [16]. We wanted to know whether MAIT cells are capable of secreting factors that may influence B cell differentiation and antibody production. We coincubated several combinations of TCR Vα7.2+ T cells (MAIT cells), sort purified Vα 7.2− CD161− T cells and THP-1 cells for 24 h in the presence or absence of fixed E. coli strain BL21 (MAIT/THP-1/BL21 or MAIT/THP-1), and then applied the resulting supernatant to purified B cells for 7 d. B cells that had been exposed to MAIT/THP-1/BL21 supernatant had a significantly higher proportion of CD38++CD24− B cells compared to that induced by MAIT/THP-1 supernatant (Fig. 2A, B, and D). In addition, supernatant from MAIT/THP-1/BL21 induced significantly higher levels of CD38++CD24− B cells than that from non-MAIT (Vα 7.2− CD161−) T cells with THP-1 and BL21, indicating a MAIT-cell–specific effect, rather than a generalized T cell effect. A similar effect was seen when primary human monocytes were used in place of THP-1 cells as APCs (Fig. 2E). A significantly higher proportion of CD38++CD24− cells induced by MAIT/THP-1/BL21 supernatant were HLA-DR+ (Fig. 2C and F), a phenotype characteristic of B cell plasmablasts [20]. This phenotype is in contrast to B cells exposed to supernatant from unstimulated MAIT cells, where CD38++CD24− cells were predominantly CD138++ and HLA-DR−, a phenotype that corresponds to plasma cells [20]. Supernatant from THP-1 cells stimulated with BL21 in the absence of MAIT cells (THP-1/BL21) did have a modest effect on B cell plasmablast frequency (∼1% CD38++CD24−) (Fig. 2D), but this was still significantly lower than when MAIT cells were present. Thus, factors released by E. coli-stimulated MAIT cells appear to be responsible for the increased B cell plasmablast frequency.
Figure 2. B cell phenotypic changes after addition of E. coli-stimulated MAIT cell supernatant.
We cultured B cells from different subjects in the presence of supernatant from subject-matched MAIT cells and THP-1 cells (A) or MAIT/THP-1/BL21 (B). Of CD38++CD24− cells, we measured HLA-DR (C) in B cells stimulated with MAIT/THP-1 (blue) or MAIT/THP-1/BL21 supernatant. Frequency of CD38++CD24− cells after addition of MAIT supernatant with THP-1 (D) and primary monocytes (E) as APCs, frequency of CD138−HLA-DR+ of CD38++CD24− B cells (F), the effect of addition of anti-MR1 (10 µg/ml) and/or anti-IL-12 (5 µg/ml) and anti-IL-18 (5 µg/ml) during MAIT/THP-1/BL21 culture on the ability of resulting supernatant to differentiate B cells (G), the effect of different E. coli strains, with or without added anti-MR1 in MAIT/THP-1/E. coli supernatant (H). Data are means ± sem, and significance was determined by Mann-Whitney U tests. Data are representative of 3 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.00001.
Given that MAIT cells are stimulated via their TCR through interaction with MR1 expressed on APCs, we wanted to know whether blockade of MR1 would decrease MAIT-mediated stimulation of B cells. When the MR1-blocking antibody was added to the MAIT/THP-1/E. coli stimulation, addition of the supernatant to B cells resulted in significantly lower B cell plasmablast frequency (Fig. 2G). MAIT cells are also thought to be stimulated by IL-12 and IL-18 secreted by APCs [12]; however, addition of IL-12 and -18 blocking antibodies to the MAIT/THP-1 E. coli coculture did not result in reduced plasmablast frequency. Combinations of MR1 and IL-12/18 blockade did not seem to have an additive effect on decreasing plasmablast frequency. Thus, MAIT TCR stimulation seems to be critical for the ability of MAIT cells to provide help to B cells.
To confirm the need for TCR stimulation of MAIT cells, we also exposed MAIT/THP-1 cocultures to BSV18, a strain of E. coli that is defective for the RibA gene and thus lacks the ability to synthesize the MAIT ligand [18], as well as strain 1100-2, the congenic strain for BSV18 that is not defective in RibA (Fig. 2H). Addition of MR1 blocking antibodies reduced the B cell plasmablast frequency induced by MAIT/THP-1/1100-2 supernatant. Addition of MAIT/THP-1/BSV18 supernatant, however, resulted in no changes in plasmablast frequency between MR1 blockade and no-blockade conditions. Thus, B cell differentiation induced by activated MAIT cells is dependent on TCR stimulation.
Supernatant from MAIT cells and APC incubated with E. coli stimulates B cell antibody production
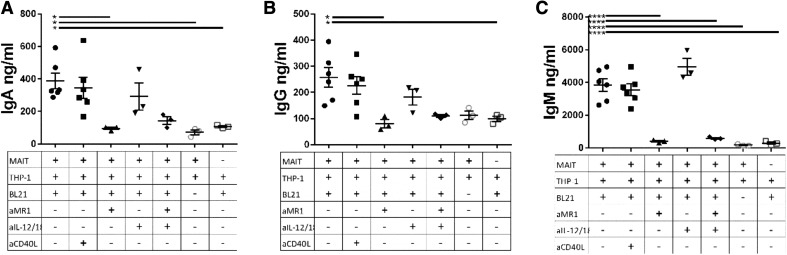
Next, we measured IgA, IgG, and IgM levels in supernatant from B cells stimulated with MAIT/THP-1/BL21 supernatant by ELISA. As MAIT cells stimulated with strain BL21 resulted in more potent B cell stimulatory responses than strain 1100-2 for undetermined reasons, BL21 was used for the remainder of MAIT/B cell experiments. Supernatant from MAIT/THP-1/BL21 coculture stimulated B cells to produce significantly higher levels of IgA, IgG, and IgM, compared to either MAIT/THP-1 supernatant, without BL21, or THP-1 alone, with BL21 (Fig. 3). The antibodies produced were not, however, LPS-specific, as seen by LPS ELISA (data not shown). As seen with B cell plasmablast expansion, blockade of MR1 during the MAIT/THP-1/BL21 stimulation resulted in decreased antibody levels, similar to those seen for the MAIT/THP-1 no stimulation or THP-1 only with BL21 conditions. Also similar to the B cell phenotype results, IL-12 and -18 blockade during MAIT/THP-1/BL21 stimulation failed to result in significant decreases in B cell antibody production. When we added anti-CD40L blocking antibody to MAIT/THP-1/BL21 supernatant, no significant decrease in IgA, IgG, or IgM levels were seen, thus CD40L produced by MAIT cells does not appear to be required for MAIT-mediated help for B cells.
Figure 3. MR1 is essential for induction of B cell antibody production by MAIT/THP-1/BL21 supernatant.
We cultured B cells from different subjects for 7 d in supernatant from subject-matched MAIT cells and THP-1 cells incubated overnight with BL21. (A–C) IgA, IgG, and IgM levels for the different conditions, with anti-MR1 (10 µg/ml), anti-IL-12/18, or both at 5 µg/ml each during initial MAIT/THP-1/BL21 stimulation or anti-CD40L (20 µg/ml) added to supernatant after MAIT culture and before addition to B cells. Data are means ± sem, and significance was determined by 1-way ANOVA using Tukey’s multiple-comparisons test. Data are representative of 2 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.00001.
TCR- and cytokine- stimulated MAIT cells promote increased B cell plasmablast frequency and antibody production
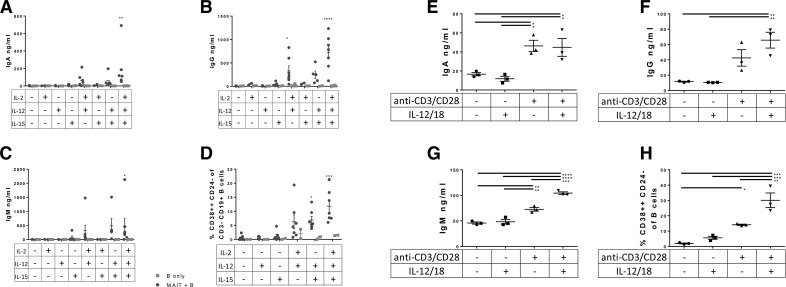
We tested various combinations of cytokines for their ability to stimulate MAIT cells to induce B cell differentiation and antibody production in coculture of purified Vα7.2+CD161++ MAIT cells and sorted B cells (Fig. 4A–D). Data from experiments performed with MAIT cells in direct coculture with B cells and E. coli are not shown because of extremely low B cell viability at the end of the 7 d coculture. The combinations of IL-12 and either IL-2 or IL-15 were found to induce IgA, IgG, and IgM antibody production (Fig. 4A–C) and result in significant expansion of CD38++CD24− plasmablasts (Fig. 4D). These same cytokines, in the absence of MAIT cells, failed to stimulate B cell differentiation or antibody production to the same extent. IL-2, -12, or -15 alone also failed to stimulate MAIT cells sufficiently.
Figure 4. Induction of antibody production and plasmablast differentiation by TCR- and cytokine-stimulated MAIT cells.
(A–D) We cocultured Vα7.2+CD161++ MAIT cells and B cells from different subjects in the presence of several combinations of IL-2, -12, and -15 for 7 d and measured antibody levels and B cell phenotype. Data are means ± sem, and were analyzed by 2-way ANOVA, with Fisher’s LSD test, comparing MAIT+B cells with B cells only. We also cultured Vα7.2+CD161++ T cells for 24 h with plate-bound anti-CD3/CD28 stimulation and/or recombinant IL-12/18, and supernatant with IL-12/18 blocking antibodies was applied to B cells for 7 d, followed by ELISA (E–G) and flow cytometry (H) for B cell phenotype. Data shown are mean ± sem, and significance was determined by 1-way ANOVA using Tukey’s multiple comparisons test. Data are representative of 2 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.00001.
We next tested whether MAIT cells, independent of THP-1 APCs or E. coli, would stimulate B cells, via activation by TCR, cytokines, or both. Vα7.2+CD161++ sort purified cells were incubated for 24 h with medium alone, wells coated with anti-CD3/anti-CD28 antibodies (TCR stimulation), IL-12/IL-18, or both TCR and IL-12/18 stimulation (Fig. 4E–H). Supernatant tested for antibodies by ELISA revealed that supernatant from MAIT cells stimulated with anti-CD3/CD28 or anti-CD3/CD28 and IL-12/18 induced IgA, IgG, and IgM secretion (Fig. 4E–G). In addition, supernatant from MAIT cells stimulated with anti-CD3/CD28, and particularly anti-CD3/CD28 stimulation with exogenous IL-12/18, induced greatly increased plasmablast frequencies (Fig. 4H). This is in agreement with findings from Slichter et al. [21] in which TCR and inflammatory signals synergize to elicit potent effector function in MAIT cells. Thus, secreted factors from TCR- and cytokine-activated MAIT cells, in the absence of other cell types such as THP-1, are capable of providing help to B cells.
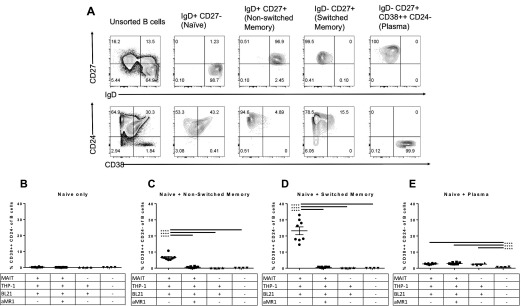
Memory B cells differentiate into plasmablasts after stimulation with MAIT/THP-1/BL21 supernatant
To determine whether activated MAIT cell supernatant drives B cell differentiation into plasmablasts, or whether it merely induces expansion and/or survival of preexisting plasma cell or plasmablast populations, we sorted B cells into 4 different populations: IgD+CD27− (naive), IgD+CD27+ with CD38++ − cells excluded (nonswitched memory nonplasma), IgD−D27+ with CD38++CD24− cells excluded (switched memory nonplasma) and IgD− CD27+CD38++CD24− (plasma cells). Sort purity of each of the defined populations was >99% (Fig. 5A). Either naive B cells alone or naive B cells plus the different B cell populations were cultured with activated MAIT cell supernatant. Sorted naive B cells were included in each condition to ensure a sufficient number of cells present per well to facilitate survival and staining efficacy for flow cytometry. Purified naive B cells exposed to MAIT/THP-1/BL21 supernatant did not generate plasmablasts (Fig. 5B). However, when both switched and nonswitched CD27+ memory CD38− B cells were exposed to MAIT/THP-1/BL21 supernatant, there was a marked expansion of CD38++CD24− plasmablasts (Fig. 5C and D). These cells were not present at the beginning of culture, nor did they expand with either THP-1/BL21 supernatant or medium alone, indicating that MAIT-specific factors induced plasmablast differentiation. Addition of anti-MR1 during the MAIT/THP-1/BL21 coculture resulted in a significant decrease in the ability of supernatant to induce plasmablast differentiation. IgD−CD27+CD38++ plasma cells did not undergo expansion in response to MAIT/THP-1/BL21 supernatant (Fig. 5E), as compared to MAIT/THP-1/BL21/aMR1 or THP-1/BL21 supernatant, though there was an unexplained decrease in plasma cell population with the medium-alone condition, perhaps reflecting survival signals provided by some cytokines produced by either E. coli-exposed THP-1 cells, or by bacterial ligands present in the supernatant. Thus, preexisting plasma cells are likely not the source of expanded CD38++CD24− plasmablasts after stimulation by E. coli-activated MAIT supernatant, and they are rather a result of differentiation from nonplasma IgD+/−CD27+ memory B cells. It is possible that activated MAIT cells engage in crosstalk with THP-1 cells, which in turn secretes factors needed for B cell differentiation. Together, these data clearly indicate the soluble factors secreted after the interaction of TCR-activated MAIT cells with THP-1 cells act preferentially on memory B cells to induce plasmablast differentiation.
Figure 5. Activated MAIT supernatant induces plasmablast differentiation from memory B cells.
(A) B cells from multiple subjects were sorted and then pooled into CD3−CD19+ B cell populations based on IgD, CD27, CD38, and CD24 expression. (B–E) Supernatant from cocultures of different combinations of MAIT cells from multiple subjects, THP-1 cells, and BL21 (100 MOI per THP-1 cell) were added to different populations of sort-purified B cells, each population containing 150,000 IgD+CD27− cells per well plus either no extra cells (B), 150,000 added IgD+CD27+CD38− cells (C), 150,000 added IgD-CD27+CD38− cells (D), or 150,000 IgD−CD27+CD38++ cells (E), and cultured for 7 d. After 7 d, B cells were assayed for the frequency of CD38++CD24− plasmablasts. Data are s ± sem, and representative of 2 independent experiments. Significance was determined by 1-way ANOVA using Tukey’s multiple comparisons test. ****P ≤ 0.00001.
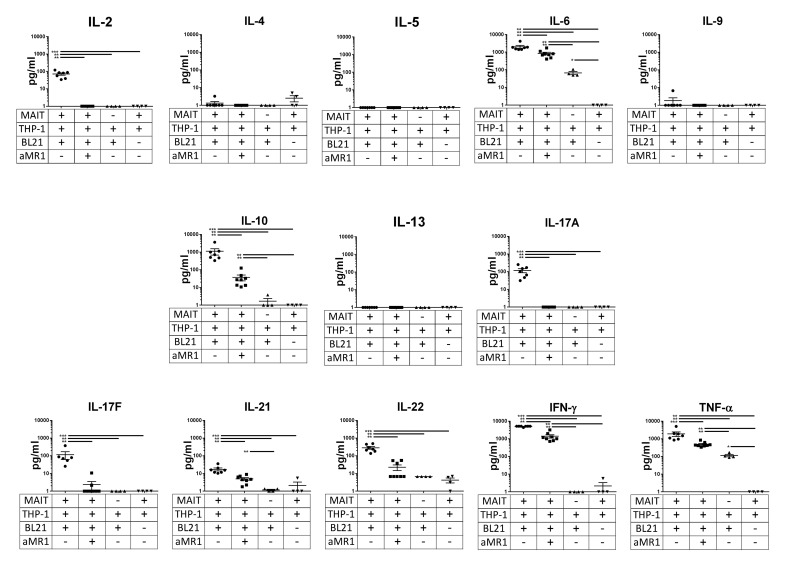
APC/E. coli-stimulated MAIT cells produce multiple B cell stimulatory factors
As supernatant from MAIT cells cocultured with THP-1 cells and stimulated with fixed E. coli was found to stimulate B cell differentiation and antibody production, we wanted to know which specific soluble factors contribute to this effect. We measured 13 different cytokine concentrations in primary MAIT cell supernatant by multiplex cytokine array using a T-helper-specific panel (Fig. 6). MAIT/THP-1/BL21 supernatant contained increased amounts of IL-2, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, TNF-α, and IFN-γ, relative to MAIT/THP-1/BL21 with added anti-MR1, MAIT/THP-1 or THP-1/BL21 supernatant. No significant upregulation compared to controls was seen for IL-4, -5, -9, and -13. The effect of anti-MR1 blockade suggests that MAIT signaling via TCR is necessary for expression of these cytokines, either from the MAIT cells themselves or from THP-1 cells via MAIT-mediated activation.
Figure 6. Cytokine profile of ex vivo-stimulated MAIT cells.
Vα7.2+ primary T cells from multiple subjects were cultured overnight with THP-1 cells in the presence or absence of fixed E. coli (strain BL21) at an MOI of 100 per THP-1 cell and with or without anti-MR1 (10 µg/ml) and cytokine levels were measured with a T-helper-specific multiplex cytokine array. Data shown are means ± sem, significance was determined by Mann-Whitney U tests and are representative of 2 independent experiments. **P ≤ 0.01; ***P ≤ 0.001.
DISCUSSION
Recent studies have revealed that subsets of innate lymphocytes, such as iNKT cells [22] and γδ T cells [23], have the capacity to become B cell helpers. In this report, we provide evidence to suggest that MAIT cells have the capacity for B cell help, through increasing plasmablasts and promoting Ig production in an MR1-dependent manner, likely by increasing differentiation from memory B cells.
Previous studies have shown that B cells are capable of modulating MAIT cell responses [13], and B cells have been shown to be essential to the formation of MAIT cells in mice [8], with their accumulation in the gut being dependent on MR-1-expressing B cells [24], although B cells were not found to be essential for MAIT cell development in humans [25]. Because of the ex vivo nature of our experiments, we are unable to determine the in vivo location and context in which MAIT and B cells potentially interact to provide the necessary help to B cells. We failed to detect CXCR5 expression in either activated or nonactivated MAIT cells (data not shown), and it is unclear whether MAIT cells provide help to B cells within germinal centers, as is the case with follicular T-helper cells, or at extrafollicular sites. Given that MAIT cells represent up to 40% of liver lymphocytes [26], that the liver receives the majority of its blood supply from the gastrointestinal tract (thus acting as a sentinel site for gut flora), and in light of recent work identifying the liver as a site of IgA production by B cells [27], it is possible that MAIT cell stimulation of B cells may occur in the liver.
Human MAIT cells have been shown to upregulate factors known to have B cell stimulatory ability in response to bacterial stimulation, including IL-4, -5, -10, and -13 and CD40L [16, 28]. In our experiments, we observed production of IL-2, -6, -10, and -21 in cocultures of MAIT cells with E. coli and THP-1 cells. Several of these upregulated cytokines are known to promote B cell differentiation and antibody production. IL-2 is known to induce B cell differentiation and proliferation [29]. IL-6 is known to induce greatly elevated levels of IgG [30]. IL-10 can enhance B cell survival, proliferation, and antibody production [31]. IL-21 is well recognized for its ability to promote B cell proliferation, plasma cell differentiation, and antibody production [32]. IFN-γ enhances antibody production and entry into the S phase in human B cells [33], and TNF-α may provide costimulation in mitogen-activated B cells [34]. Clearly, there are many factors in activated MAIT cell supernatant that may be responsible for MAIT-mediated B cell differentiation and antibody production. Further studies are needed to determine the specific mechanism(s) responsible for these effects.
The generation of antibodies by B cells is critical for the development of protective immunity against pathogens. A large body of literature has implicated T cells, in particular Tfh cells, in mediating the process of affinity maturation and isotyping switching [1]. More recent work on iNKT cells has also described their role in providing both cognate and noncognate help to B cells [2]. In this report, we demonstrate a newly-described ability of MAIT cells to provide help to B cells ex vivo. Given their relative abundance in humans (∼5% of circulating T cells) compared to that of iNKT and Tfh cells, and although further studies are needed to demonstrate the in vivo relevance of these findings, our data suggest that MAIT cells may be a potential target for improving B cell responses in humans and may be useful for reactivation and differentiation of memory B cells.
AUTHORSHIP
M.S.B., J.S.H., and D.T.L. designed the research; M.S.B. analyzed the data; M.S.B., S.T., and A.S.I. performed the research; and M.S.B. and D.T.L. wrote the manuscript.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the U.S. National Institutes of Health Grants K08AI100923 and R01AI130378 (to D.T.L.) We thank the University of Utah Flow Cytometry Core for the use of the BD FACS Canto, BD Fortessa, and BD FACS Aria II flow cytometers.
Glossary
- iNKT
invariant natural killer T (cell)
- LSD
least significant difference
- MAIT
mucosal associated variant T
- MOI
multiplicity of infection
- MR
MHC class-related protein
- OPD
o-phenylenediamine dihydrochloride
- Tfh
T follicular helper (cell)
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Crotty S. (2015) A brief history of T cell help to B cells. Nat. Rev. Immunol. 15, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellabona P., Abrignani S., Casorati G. (2014) iNKT-cell help to B cells: a cooperative job between innate and adaptive immune responses. Eur. J. Immunol. 44, 2230–2237. [DOI] [PubMed] [Google Scholar]
- 3.Kjer-Nielsen L., Patel O., Corbett A. J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., Williamson N. A., Purcell A. W., Dudek N. L., McConville M. J., O’Hair R. A., Khairallah G. N., Godfrey D. I., Fairlie D. P., Rossjohn J., McCluskey J. (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723. [DOI] [PubMed] [Google Scholar]
- 4.Walker L. J., Kang Y. H., Smith M. O., Tharmalingham H., Ramamurthy N., Fleming V. M., Sahgal N., Leslie A., Oo Y., Geremia A., Scriba T. J., Hanekom W. A., Lauer G. M., Lantz O., Adams D. H., Powrie F., Barnes E., Klenerman P. (2012) Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 119, 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckle S. B., Birkinshaw R. W., Kostenko L., Corbett A. J., McWilliam H. E., Reantragoon R., Chen Z., Gherardin N. A., Beddoe T., Liu L., Patel O., Meehan B., Fairlie D. P., Villadangos J. A., Godfrey D. I., Kjer-Nielsen L., McCluskey J., Rossjohn J. (2014) A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J. Exp. Med. 211, 1585–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold M. C., McLaren J. E., Reistetter J. A., Smyk-Pearson S., Ladell K., Swarbrick G. M., Yu Y. Y., Hansen T. H., Lund O., Nielsen M., Gerritsen B., Kesmir C., Miles J. J., Lewinsohn D. A., Price D. A., Lewinsohn D. M. (2014) MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J. Exp. Med. 211, 1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reantragoon R., Corbett A. J., Sakala I. G., Gherardin N. A., Furness J. B., Chen Z., Eckle S. B., Uldrich A. P., Birkinshaw R. W., Patel O., Kostenko L., Meehan B., Kedzierska K., Liu L., Fairlie D. P., Hansen T. H., Godfrey D. I., Rossjohn J., McCluskey J., Kjer-Nielsen L. (2013) Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 210, 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bourhis L., Martin E., Péguillet I., Guihot A., Froux N., Coré M., Lévy E., Dusseaux M., Meyssonnier V., Premel V., Ngo C., Riteau B., Duban L., Robert D., Huang S., Rottman M., Soudais C., Lantz O. (2010) Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708. [DOI] [PubMed] [Google Scholar]
- 9.Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259. [DOI] [PubMed] [Google Scholar]
- 10.Leeansyah E., Svärd J., Dias J., Buggert M., Nyström J., Quigley M. F., Moll M., Sönnerborg A., Nowak P., Sandberg J. K. (2015) Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 infection. PLoS Pathog. 11, e1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sattler A., Dang-Heine C., Reinke P., Babel N. (2015) IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur. J. Immunol. 45, 2286–2298. [DOI] [PubMed] [Google Scholar]
- 12.Ussher J. E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T. H., Klenerman P., Willberg C. B. (2014) CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salerno-Goncalves R., Rezwan T., Sztein M. B. (2014) B cells modulate mucosal associated invariant T cell immune responses. Front. Immunol. 4, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung D. T., Bhuiyan T. R., Nishat N. S., Hoq M. R., Aktar A., Rahman M. A., Uddin T., Khan A. I., Chowdhury F., Charles R. C., Harris J. B., Calderwood S. B., Qadri F., Ryan E. T. (2014) Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl. Trop. Dis. 8, e3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bourhis L., Dusseaux M., Bohineust A., Bessoles S., Martin E., Premel V., Coré M., Sleurs D., Serriari N. E., Treiner E., Hivroz C., Sansonetti P., Gougeon M. L., Soudais C., Lantz O. (2013) MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 9, e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepore M., Kalinichenko A., Colone A., Paleja B., Singhal A., Tschumi A., Lee B., Poidinger M., Zolezzi F., Quagliata L., Sander P., Newell E., Bertoletti A., Terracciano L., De Libero G., Mori L. (2014) Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat. Commun. 5, 3866. [DOI] [PubMed] [Google Scholar]
- 17.Leeansyah E., Ganesh A., Quigley M. F., Sönnerborg A., Andersson J., Hunt P. W., Somsouk M., Deeks S. G., Martin J. N., Moll M., Shacklett B. L., Sandberg J. K. (2013) Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121, 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett A. J., Eckle S. B., Birkinshaw R. W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H., Williamson N. A., Strugnell R. A., Van Sinderen D., Mak J. Y., Fairlie D. P., Kjer-Nielsen L., Rossjohn J., McCluskey J. (2014) T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365. [DOI] [PubMed] [Google Scholar]
- 19.López-Sagaseta J., Dulberger C. L., McFedries A., Cushman M., Saghatelian A., Adams E. J. (2013) MAIT recognition of a stimulatory bacterial antigen bound to MR1. J. Immunol. 191, 5268–5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminski D. A., Wei C., Qian Y., Rosenberg A. F., Sanz I. (2012) Advances in human B cell phenotypic profiling. Front. Immunol. 3, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slichter C. K., McDavid A., Miller H. W., Finak G., Seymour B. J., McNevin J. P., Diaz G., Czartoski J. L., McElrath M. J., Gottardo R., Prlic M. (2016) Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 1, e86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vomhof-DeKrey E. E., Yates J., Leadbetter E. A. (2014) Invariant NKT cells provide innate and adaptive help for B cells. Curr. Opin. Immunol. 28, 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal R. R., Mackay C. R., Moser B., Eberl M. (2012) IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur. J. Immunol. 42, 110–119. [DOI] [PubMed] [Google Scholar]
- 24.Martin E., Treiner E., Duban L., Guerri L., Laude H., Toly C., Premel V., Devys A., Moura I. C., Tilloy F., Cherif S., Vera G., Latour S., Soudais C., Lantz O. (2009) Stepwise development of MAIT cells in mouse and human. PLoS Biol. 7, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeansyah E., Loh L., Nixon D. F., Sandberg J. K. (2014) Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat. Commun. 5, 3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X. Z., Jo J., Tan A. T., Sandalova E., Chia A., Tan K. C., Lee K. H., Gehring A. J., De Libero G., Bertoletti A. (2013) IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J. Immunol. 190, 3142–3152. [DOI] [PubMed] [Google Scholar]
- 27.Moro-Sibilot L., Blanc P., Taillardet M., Bardel E., Couillault C., Boschetti G., Traverse-Glehen A., Defrance T., Kaiserlian D., Dubois B. (2016) Mouse and human liver contain immunoglobulin A-secreting cells originating from Peyer’s patches and directed against intestinal antigens. Gastroenterology 151, 311–323. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery H. C., van Wilgenburg B., Kurioka A., Parekh K., Stirling K., Roberts S., Dutton E. E., Hunter S., Geh D., Braitch M. K., Rajanayagam J., Iqbal T., Pinkney T., Brown R., Withers D. R., Adams D. H., Klenerman P., Oo Y. H. (2016) Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1 J. Hepatol. 64, 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. (1984) Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature 312, 641–643. [DOI] [PubMed] [Google Scholar]
- 30.Maeda K., Mehta H., Drevets D. A., Coggeshall K. M. (2010) IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood 115, 4699–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh K., Hirohata S. (1995) The role of IL-10 in human B cell activation, proliferation, and differentiation. J. Immunol. 154, 4341–4350. [PubMed] [Google Scholar]
- 32.Spolski R., Leonard W. J. (2014) Interleukin-21: a double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 13, 379–395. [DOI] [PubMed] [Google Scholar]
- 33.Snapper C. M., Paul W. E. (1987) Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236, 944–947. [DOI] [PubMed] [Google Scholar]
- 34.Kehrl J. H., Miller A., Fauci A. S. (1987) Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J. Exp. Med. 166, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]