Supplemental Digital Content is available in the text
Keywords: meta-analysis, mindfulness, substance use disorder, systematic review
Abstract
Objectives:
Substance use disorder (SUD) is a prevalent health issue with serious personal and societal consequences. This review aims to estimate the effects and safety of Mindfulness-based Relapse Prevention (MBRP) for SUDs.
Methods:
We searched electronic databases for randomized controlled trials evaluating MBRP for adult patients diagnosed with SUDs. Two reviewers independently assessed citations, extracted trial data, and assessed risks of bias. We conducted random-effects meta-analyses and assessed quality of the body of evidence (QoE) using the Grading of Recommendations Assessment, Development, and Evaluation approach.
Results:
We identified 9 randomized controlled trials comprising 901 participants. We did not detect statistically significant differences between MBRP and comparators on relapse (odds ratio [OR] 0.72, 95% confidence interval [CI] 0.46–1.13, low QoE), frequency of use (standardized mean difference [SMD] 0.02, 95% CI −0.40 to 0.44, low QoE), treatment dropout (OR 0.81, 95% CI 0.40 to 1.62, very low QoE), depressive symptoms (SMD −0.09, 95% CI −0.39 to 0.21, low QoE), anxiety symptoms (SMD −0.32, 95% CI −1.16 to 0.52, very low QoE), and mindfulness (SMD −0.28, 95% CI −0.72 to 0.16, very low QoE). We identified significant differences in favor of MBRP on withdrawal/craving symptoms (SMD −0.13, 95% CI −0.19 to −0.08, I2 = 0%, low QoE) and negative consequences of substance use (SMD −0.23, 95% CI −0.39 to −0.07, I2 = 0%, low QoE). We found negligible evidence of adverse events.
Conclusions:
We have limited confidence in estimates suggesting MBRP yields small effects on withdrawal/craving and negative consequences versus comparator interventions. We did not detect differences for any other outcome. Future trials should aim to minimize participant attrition to improve confidence in effect estimates.
Substance use disorder (SUD) is a prevalent health issue with serious personal and societal consequences. SUDs are often associated with various physical health problems (Cargiulo, 2007; Rehm, 2011; Degenhardt and Hall, 2012), comorbid with other psychiatric disorders (Grant et al., 2015a, 2016), and implicated in significant social and economic consequences (National Institute on Drug Abuse, 2008; Rehm et al., 2009; National Drug Intelligence Center, 2011; World Health Organization, 2014). Recent substantial increases in access to care for SUDs have led to greater emphasis on evaluating interventions to identify best practices for SUD treatment in healthcare systems (Institute of Medicine, 2015). However, few adults with SUDs actually seek and obtain treatment (Substance Abuse and Mental Health Services Administration, 2008), and the majority of these adults experience relapse within 12 months (McLellan et al., 2000). Consequently, improving access to, engagement with, and retention in interventions that specifically address the chronic relapsing nature of SUDs are policy priorities (Connors et al., 1996).
Relapse to Substance Use
Several mechanisms may lead to relapse to substance use, including stress (Hodgins et al., 1995; Brewer et al., 1998) and a failure to cope with urges or temptations to use (Ramo and Brown, 2008). Traditional relapse prevention is based on the theory that certain interactions between the individual and environment (eg, social influences, greater access to substances), along with the inability of the individual to cope with craving caused by these interactions, can increase the risk of relapse (Witkiewitz and Marlatt, 2004). Practitioners delivering relapse prevention therapy therefore aim to help the client in identifying situations that trigger relapse, and also learning cognitive and behavioral skills to cope with these situations (Marlatt and Gordon, 1985).
Mindfulness-based Relapse Prevention
Mindfulness-based interventions are an increasingly utilized approach for addressing behavioral health issues like SUDs (Teasdale et al., 2000; Morone et al., 2008; Chiesa and Serretti, 2009; Hofmann et al., 2010; McCown and Reibel, 2010), either as standalone interventions or integrated into existing treatments (Ramel et al., 2004; Walsh and Shapiro, 2006). Primarily derived from Buddhist theory, “mindfulness” involves a purposeful attention to the present moment, with an openness to accepting things as they are (Segal et al., 2007). Within the context of medical treatment, for example, patients may foster mindfulness to identify, acknowledge, and ultimately disengage from dysfunctional cognitions (Brown and Ryan, 2003).
Mindfulness-based Relapse Prevention (MBRP) is a recently developed mindfulness intervention specifically for substance use that integrates traditional psychotherapeutic relapse prevention techniques (Marlatt and Gordon, 1985; Carroll, 1996; Irvin et al., 1999; Lancaster et al., 2006; Brandon et al., 2007) with mindfulness-based meditation practices (Bowen et al., 2011). The addition of these mindfulness meditation practices to traditional relapse prevention techniques is intended to further reduce the risk of relapse by helping patients with psychological discomfort that often precipitates relapse. Neurologically, mindfulness is hypothesized to reduce activity in circuitry related to craving (Way et al., 2010), and stimulate activity in circuitry related to cognitive self-regulation of behavior (Seeley et al., 2007; Craig, 2009; Xue et al., 2011; Hasenkamp and Barsalou, 2012; Hasenkamp et al., 2012). The core components of MBRP are typically delivered in weekly 2-hour group sessions for 8 weeks (16 hours total contact time) (Bowen et al., 2011, 2014a). During these sessions, MBRP providers teach patients meditation practices related to a central theme for the session (Table 1), to facilitate patients’ awareness of and healthier responses to challenging emotional, cognitive, and physical states they may experience due to craving or withdrawal from substance use (Bowen et al., 2011, 2014a).
TABLE 1.
Central Themes of Mindfulness-based Relapse Prevention Sessions (Witkiewitz et al., 2014b)
| Week | Theme | Content |
| 1 | Automatic pilot and relapse | Discuss tendency to behave mechanically or unconsciously without full awareness of what one is doing, specifically in relation to substance use (acting upon cravings and urges without awareness) |
| Explore mindfulness through guided experience | ||
| Body scan to practice paying attention to the body | ||
| 2 | Awareness of thoughts and emotions related to triggers and craving | Introduce ways of experiencing triggers, cravings and thoughts of using without “automatically” reacting |
| Notice how triggers are experienced in thoughts, emotions, and sensations | ||
| Discuss how the automatic tendency to interpret and judge experience prevents being “fully present” and aware of helpful options | ||
| 3 | Mindfulness practices in daily life | Introduce practices that encourage present-moment awareness of thoughts, emotions, and sensations, to be used in informal, everyday challenging situations |
| Practice recognizing what is needed and possible options for getting needs met in healthy ways | ||
| 4 | Mindfulness practices in high-risk situations | Identify past triggering situations and factors associated with relapse, and personal high-risk situations |
| Practice ways of using mindfulness in triggering situations to stay present and “be with” versus reacting to the sensations, thoughts, and feelings that emerged | ||
| 5 | Balancing acceptance and skillful action | Discuss the meaning and importance of acceptance as a means of supporting skillful action |
| Discussed skillful action versus automatic reactions | ||
| Explored relating differently to unwanted experiences (eg, craving, difficult emotions, negative thoughts) | ||
| 6 | The role of thoughts in relapse (seeing thoughts as thoughts) | Introduce the idea of recognizing thoughts as just thoughts versus facts that must be believed or acted upon |
| Discuss and explore the connection between thoughts and relapse | ||
| Complete diagram showing how triggers can lead to a chain of events leading to relapse or skillful action | ||
| Practice distancing oneself from thoughts and taking a more neutral observer stance | ||
| 7 | Balancing self-care and one's lifestyle | Discuss the importance of lifestyle balance and taking care of oneself to reduce vulnerability to relapse |
| Identify personal warning signs for relapse, and how to best respond when these warning signs arise | ||
| Complete a list of typical daily activities, identifying ones that were draining, nurturing, or both and | ||
| Discuss ways to increase nurturing and modify draining activities wherever possible | ||
| Complete reminder cards listing helpful people to call and alternative activities to using substances | ||
| 8 | Building social support and continuing mindfulness practices | Participate in the body scan exercise |
| Discuss the importance of building a support system | ||
| Reflect on what they’ve learned about themselves through meditation and daily mindfulness practice |
Notes: Facilitators and clients reviewed home practice efforts at every session weeks 2 to 8.
Objectives
Rigorous studies that estimate the clinical effects and safety of interventions are critical before recommendations for widespread dissemination, such as the use of mindfulness-based interventions by healthcare professionals to treat SUDs (Institute of Medicine, 2005, 2015). Meta-analytic estimates of specific effects of specific interventions are particularly importance for efforts to improve evidence-based practice such as the development of clinical practice guidelines (Institute of Medicine, 2011). Reviews of the overall literature on mindfulness treatments for substance use and addiction suggest such interventions may be an effective tool, yet these have not involved meta-analyses of intervention effects (Zgierska et al., 2009; Brewer et al., 2013; Garland and Froeliger, 2013; Witkiewitz et al., 2013; Black, 2014; Chiesa and Serretti, 2014; Witkiewitz et al., 2014a) or include the SUDs of interest to this review (de Lisle et al., 2011; Oikonomou et al., 2016). MBRP specifically has been evaluated in several randomized controlled trials (RCTs) (Bowen et al., 2009; Witkiewitz and Bowen, 2010; Lee et al., 2011). An up-to-date systematic review is needed to synthesize these findings to provide comprehensive estimates of the effects of MBRP on specific patient-important clinical outcomes to subsequently inform guidelines about whether to recommend its use in everyday practice.
METHODS
We conducted a systematic review to identify RCTs evaluating the effects and safety of MBRP for adults with SUDs. This manuscript updates a previous review that we registered on an international prospective register of systematic reviews, PROSPERO (CRD42015016380), before completing formal screening of search results against eligibility criteria (Grant et al., 2015b); we identified 3 additional completed RCTs in our update search. The specific efficacy outcomes of interest included relapse to substance use (primary outcome), frequency and quantity of substance use, withdrawal/craving symptoms, treatment dropout, depressive and anxiety symptoms, negative consequences from substance use, and health-related quality of life. We evaluated safety via reported adverse events.
Search Strategy
We searched 2 trial registries (ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform) and the following databases from January 2000 through August 2016: Allied and Complementary Medicine Database, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central, PsycINFO, PubMed, and Web of Science. Search strings involved variants of terms related to “mindfulness-based relapse prevention” and “substance use disorder” (the reproducible search strings are available in Online Supplement 1). We conducted database searches from 2000 onward because MBRP was developed and the first papers by its developers were published after the start of the 21st century (Zgierska et al., 2009; Bowen et al., 2010). In addition, we examined reference lists from included studies and previous reviews of mindfulness meditation for SUDs. We also contacted authors of included studies about any RCTs we may have missed, and also data not reported in manuscripts.
Eligibility Criteria
We included parallel group, individually, or cluster-randomized controlled trials with adult patients (male and female) who were 18 years of age or older. Participants must have been diagnosed with alcohol, opioid, stimulant, and/or cannabis use disorder; diagnoses included abuse or dependence using criteria from the Fourth and Fifth Editions of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV and DSM-V, respectively), or harmful use or dependence syndrome using the International Classification of Diseases (ICD) criteria. We included RCTs that evaluated MBRP as either a monotherapy or adjunctive therapy; we excluded RCTs evaluating other mindfulness-based interventions, such as mindfulness-based cognitive therapy or mindfulness-based stress reduction. We did not limit RCTs by comparator. We did not restrict eligibility by treatment duration, outcome follow-up period, clinical setting, or geographic location. Due to project team capabilities, we included studies published in English language only.
Eligibility Screening
Two independent reviewers screened titles and abstracts of retrieved citations. We obtained full texts for citations judged as potentially eligible by at least 1 reviewer. The reviewers then assessed full texts against the specified eligibility criteria; we resolved any disagreements regarding eligibility through discussion within the review author team.
Data Extraction
Two reviewers independently extracted study-level data using a form designed by the project team (the full data extraction form is available in Online Supplement 2). They also independently assessed risks of bias of included studies using the Cochrane Risk of Bias tool, Cochrane's recommended approach for assessing risks of bias in RCTs included in systematic reviews of interventions (Higgins et al., 2011), and also involvement of the developers of the program (Bowen et al., 2010) in the RCT to indicate whether each RCT was an independent replication (Gottfredson et al., 2015). The project lead (S.G.) extracted all outcome data.
Data Synthesis
Random-effects Meta-analyses
We conducted random-effects meta-analyses on the longest outcome using the restricted maximum-likelihood estimator method for the amount of heterogeneity and the Hartung-Knapp-Sidik-Jonkman adjustment for standard errors (Hartung, 1999; Hartung and Knapp, 2001; Sidik and Jonkman, 2006), using the “metafor” package in R (Version 3.2.3) (Viechtbauer and Viechtbauer, 2015). Effect estimates are expressed either as odds ratios (ORs) or Hedges g—a small study bias-adjusted estimate of the standardized mean difference (SMD)—along with 95% confidence intervals (CIs). For consistency, we coded outcome data such that an SMDs <0 and ORs < 1 favor MBRP, and we used common indices for interpreting clinical effect sizes: SMD ≤ −0.2 or OR ≤ 0.60 for a small clinical effect, SMD ≤ −0.5 or OR ≤ 0.29 for a medium clinical effect, and SMD ≤ −0.8 or OR ≤ 0.15 for a large clinical effect (Chen et al., 2010). We used the I2 statistic to assess the degree of heterogeneity in each analysis (Higgins et al., 2003).
Additional Analyses
We examined publication bias using Begg rank-correlation test for funnel plot asymmetry (Begg and Mazumdar, 1994) and Egger test for funnel plot asymmetry (Egger et al., 1997), and applied Duval trim and fill method (Duval and Tweedie, 2000) in the presence of publication bias. To explore sources of heterogeneity, we conducted meta-regressions when possible to examine whether there were differences in effect sizes by substance targeted, co-intervention status, and comparison group (Viechtbauer et al., 2015). To explore the robustness of our meta-analyses, we conducted sensitivity analyses using earlier time-points than longest follow-up when reported, and we followed recommendations to calculate a prediction interval for considering the whole distribution of effects, and also to examine whether effects exist and are consistent across individual studies (Higgins et al., 2009).
Quality of the Body of Evidence
We assessed the quality of the body of evidence (QoE) for each outcome using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (Balshem et al., 2011), which rates on a 4-item scale (very low, low, moderate, and high) the confidence that an effect estimate is close to the population parameter. We specifically assessed the following aspects of the body of evidence underpinning each effect estimate, as recommend by the GRADE approach: study limitations via our risk of bias assessments; directness via how well studies addressed our questions of interest; and consistency via the magnitude of heterogeneity; precision via the width of confidence intervals; and publication bias (see below).
RESULTS
We identified 97 citations through our search strategy (Fig. 1). Of 50 full texts identified as potentially eligible, we excluded 21, including 2 terminated trials and 9 ongoing trials that would likely meet eligibility criteria for this review once completed (see Online Supplement 3). Overall, we identified nine studies (see Online Supplement 5) meeting inclusion criteria (Bowen et al., 2009, 2014b; Brewer et al., 2009; Uhlig, 2009; Lee et al., 2011; Zgierska, 2014; Imani et al., 2015; Glasner et al., 2017).
FIGURE 1.
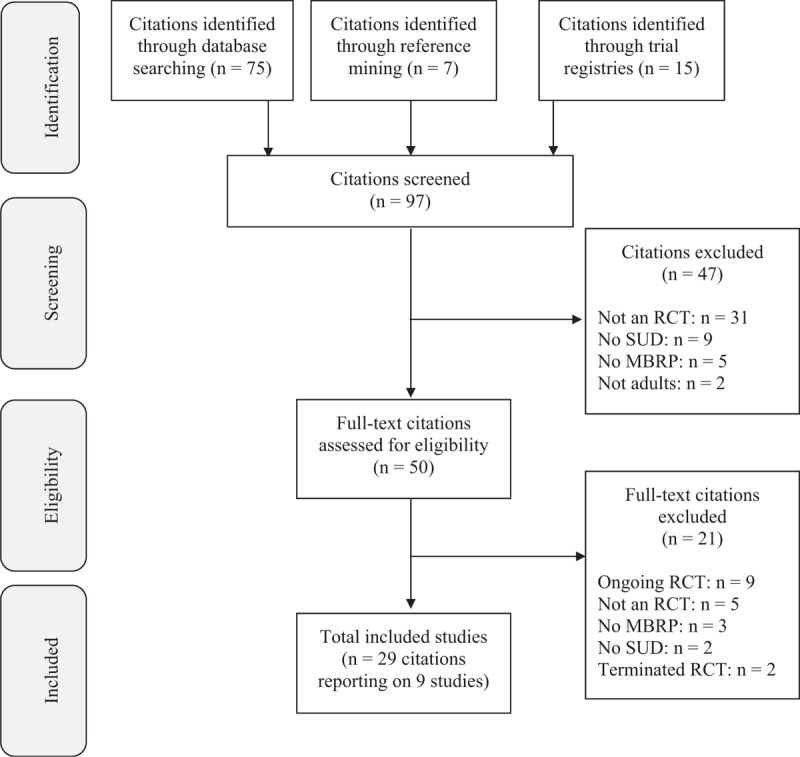
Flow diagram of search results.
Description of Included Studies
Methods and Setting
All studies took place in SUD specialty care settings; participants typically were in outpatient care, though 1 study took place in prison (Lee et al., 2011) and another in a residential treatment center (Bowen et al., 2014b). Most RCTs took place in the United States, 1 took place in Iran (Imani et al., 2015), and another in Taiwan (see Table 1) (Lee et al., 2011). All RCTs randomized participants individually (as opposed to cluster randomization); 1 RCT randomized participants to either MBRP or 1 of 2 other comparators (Bowen et al., 2014b), whereas all other RCTs evaluated MBRP against a single comparator. In all, 901 participants were randomized to receive either MBRP (425 participants) or a comparator intervention (476 participants), such as treatment as usual (TAU; 291 participants), relapse prevention (138 participants), health education (32 participants), or cognitive behavioral therapy (CBT) (15 participants).
Participants
Average age of participants ranged from 34 to 45 years old (median 39), and percentage of male participants ranged from 0% to 100% (median 72%). The majority of studies did not restrict participants by primary substance of misuse, with participants reporting use of various substances including alcohol, cocaine, marijuana, methamphetamines, and opioids. One study recruited patients meeting DSM criteria specifically for either alcohol or cocaine (Brewer et al., 2009), whereas 3 other studies only recruited participants dependent on stimulants (Glasner et al., 2017), opioids (Imani et al., 2015), or alcohol (Zgierska, 2014). Many RCTs excluded patients with concurrent psychotic disorder, significant suicide risk, or cognitive impairments, though notably 43% (n = 27) of participants in 1 study had an axis I mood or anxiety disorder (Glasner et al., 2017).
Interventions
Several RCTs evaluated MBRP according to the original manual (Bowen et al., 2009, 2014b; Zgierska, 2014). As such, sessions in these RCTs likely included 20 to 30 minutes of guided meditations, experiential skills-based practices, and discussion of practical applications, with participants also receiving handouts, audio-recorded mindfulness homework exercises, and daily craving and mood tracking sheets, as per the MBRP manual (Bowen et al., 2011, 2014a). One RCT evaluated the manual translated into Farsi (Imani et al., 2015), and the remaining RCTs shortened the MBRP manual to be delivered in 9 to 15 hours of total contact time (Brewer et al., 2009; Uhlig, 2009; Lee et al., 2011; Glasner et al., 2017). MBRP providers ranged from trained graduate-level therapists with experience in CBT and mindfulness meditation, to certified meditation instructors, to “trained instructors.” We confirmed involvement of the MBRP developers on the study team of 5 RCTs (Bowen et al., 2009, 2014b; Lee et al., 2011; Zgierska, 2014; Imani et al., 2015), and consultation with the MBRP developers in the development and implementation phases of another RCT (Glasner et al., 2017).
Five RCTs reported additional interventions or services received by MBRP participants, including TAU services the participants in the comparator group received (Uhlig, 2009; Zgierska, 2014; Imani et al., 2015), contingency management (which participants in the health education comparison group also received as a co-intervention) (Glasner et al., 2017), and “multiple other treatment programs” (which participants in the relapse prevention comparison group also received as a co-intervention) (Bowen et al., 2014b). Comparator interventions included relapse prevention, health education, cognitive behavioral therapy, and TAU (ie, substance use education, the Matrix Model, a predominantly 12-step process-orientated group, or medical management including pharmacotherapy and weekly individual counseling sessions).
Risks of Bias
Regarding selection bias, the majority of studies reported adequate random sequence generation methods, though only 4 reported an adequate concealment of the allocation sequence. All studies were de facto high risk of performance bias due to knowledge of the allocated interventions by participants and providers, as blinding participants and providers to assigned interventions is generally not possible for behavioral interventions. We rated 4 RCTs as low risk of detection bias due to use of blinded outcome assessors, 1 RCT as high risk of detection bias due to lack of blinding outcome assessors, and the remaining 4 RCTs as unclear risk of detection bias due to insufficient information. Attrition bias is a significant concern for this body of evidence, as we rated 4 RCTs as high risk of attrition bias at all follow-up points due to substantial attrition rates and 1 RCT as low risk at 1 follow-up point and high at all others due to varying attrition rates. Lastly, we rated 4 RCTs as low risk of reporting bias due to complete reporting of all outcomes contained in a trial registration or providing all outcome data in response to e-mails asking for study data (our justifications for all risk of bias assessments can be found in Online Supplement 5).
Effects of MBRP
The below analyses are summarized in Table 2 (outputs for all analyses and underlying data can be found in Online Supplements 4 and 5).
TABLE 2.
Evidence Table for Included Studies
| Study | Country | Participants | Substance Use Issue | MBRP Program | MBRP Provider | Co-intervention | Comparator | Longest Follow-up | Level of Care |
| Bowen et al., 2009 | United States | 168 randomized; 41 yrs; 64% male | Alcohol and drug use disorders | Standard manual (16 h) | Experienced masters-level therapists | NR | TAU | 4 mos | Outpatient |
| Bowen et al., 2014b | United States | 286 randomized; 39 yrs; 72% male | Substance use disorders | Standard manual (16 h) | Experienced masters/doctoral-level therapists | NR | TAU; RP | 12 mos | Outpatient |
| Brewer et al., 2009 | United States | 36 randomized; 38 yrs; 72% male | DSM-IV criteria for alcohol/cocaine abuse/dependence | Shortened version (9 h) | Experienced doctoral-level therapists | NR | CBT | Postintervention | Outpatient |
| Glasner et al., 2017 | United States | 63 randomized; 45 yrs; 71% male | DSM-IV diagnosis of stimulant dependence | Shortened version (10 h) | Experienced masters-level therapist | CM (both MBRP and comparator) | Health education | 1 mo | Outpatient |
| Imani et al., 2015 | Iran | 30 randomized; 37 yrs; 100% male | Opioid dependence according to DSM-IV-TR criteria | Translated manual (16 h) | NR | TAU (ie, comparator) | TAU | Postintervention | Outpatient |
| Lee et al., 2011 | Taiwan | 24 randomized; 41 yrs; 100% male | Illicit drug user | Shortened version (15 h) | Certified clinical psychologists | NR | TAU | Residential (Prison) | |
| Uhlig, 2009 | United States | 66 randomized; 39 yrs; 73% male | Substance dependence | Shortened version (13 h) | Certified meditation instructor | TAU (ie, comparator) | TAU | Postintervention | Outpatient |
| Witkiewitz et al., 2014b | United States | 105 randomized; 34 yrs; 0% male | Requiring residential addiction treatment | Shortened Version (13 h) | Experienced masters-level clinicians | Other programs (both MBRP and comparator) | RP | 3.5 mos | Residential |
| Zgierska, 2014 | United States | 123 randomized; 41 yrs; 57% male | Alcohol dependence diagnosis | Standard Manual (16 h) | Trained instructors | TAU (ie, comparator) | TAU | 4 mos | Outpatient |
Abbreviations: CM, contingency management; NR, not reported; RP, relapse prevention.
MBRP Versus Any Comparator
Relapse was operationalized across included studies as either any substance use or proportion of substance-free urine samples in the 30 or 90 days before outcome assessments. Random-effects meta-analysis of the pooled RCTs yielded no significant difference on average between MBRP and any comparator (relapse prevention, health education, CBT, and TAU) for relapse to substance use (OR 0.72, 95% CI 0.46 to 1.13, 7 RCTs, I2 = 0%, low QoE; see Fig. 2). We downgraded the QoE for this outcome due to a high risk of attrition bias and a wide CI.
FIGURE 2.
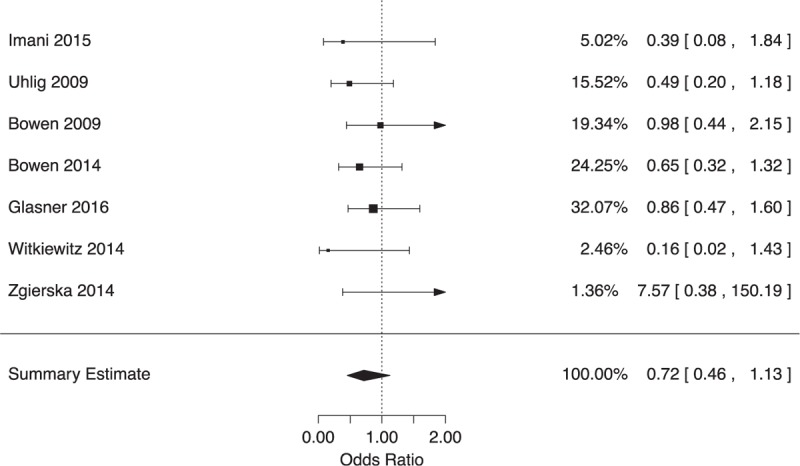
Forest plot of effects on relapse at longest follow-up.
Random-effects meta-analysis of the pooled RCTs yielded no significant difference on average for the secondary outcomes frequency of use (SMD 0.02, 95% CI −0.40 to 0.44, I2 = 42%, 5 RCTs, low QoE), quantity of use (SMD 0.26, 95% CI −0.13 to 0.64, 1 RCT, very low QoE), treatment dropout (OR 0.81, 95% CI 0.40 to 1.62, 5 RCTs, I2 = 44%, very low QoE), depressive symptoms (SMD −0.09, 95% CI −0.39 to 0.21, 4 RCTs, I2 = 0%, low QoE), anxiety symptoms (SMD −0.32, 95% CI −1.16 to 0.52, 4 RCTs, I2 = 78%, very low QoE), and mindfulness (SMD −0.28, 95% CI −0.72 to 0.16, 6 RCTs, I2 = 58%, very low QoE). We identified a small clinical effect in favor of MBRP on withdrawal/craving symptoms (SMD −0.13, 95% CI −0.19 to −0.08, 5 RCTs, I2 = 0%, low QoE), with QoE downgraded due to high risks of attrition and publication bias; and on negative consequences from substance use (SMD −0.23, 95% CI −0.39 to −0.07, 4 RCTs, I2 = 0%, low QoE), with QoE downgraded due to high risk of attrition bias and a wide confidence interval. Lastly, we identified a medium clinical effect on health-related quality of life in favor of MBRP versus an active comparison group (relapse prevention) that shared the same co-intervention as MBRP recipients (SMD −0.64, 95% CI −1.19 to −0.09, 1 RCT, very low QoE). However, we significantly downgraded the QoE for this outcome due to high risks of selection, detection, and attrition bias; only 1 study providing data for this outcome; evaluation of an adapted version of MBRP at a different stage of the clinical pathway than intended; and a wide CI.
Publication Bias
We did not detect evidence of publication bias (see Table 2) for any outcomes using the Begg rank correlation test for funnel plot asymmetry (Begg and Mazumdar, 1994), and Egger test for funnel plot asymmetry (Egger et al., 1997). Model results including estimated missing studies did not substantially change results for relapse (OR 0.74, 95% CI 0.53 to 1.05, I2 = 0%), depressive symptoms (SMD −0.00, 95% CI −0.16 to 0.16, I2 = 0%), anxiety symptoms (SMD −0.20, 95% CI −0.70 to 0.31, I2 = 79%), negative consequences (SMD −0.21, 95% CI −0.37 to −0.05, I2 = 0%), and mindfulness (SMD −0.18, 95% CI −0.51 to 0.15, I2 = 51%), but results for withdrawal/craving symptoms (SMD −0.13, 95% CI −0.30 to 0.04, I2 = 0%) were no longer statistically significant when including an estimated missing study.
Meta-regressions
Indirect evidence suggests that MBRP may lead to significantly greater reductions in depressive symptoms when targeting patients specifically with a stimulant use disorder rather than any SUD (SMD −0.46, 95% CI −0.81 to −0.11), and also greater reductions in withdrawal/craving when targeting patients specifically with an alcohol use disorder rather than any SUD (SMD −0.09, 95% CI −0.18 to −0.01). We did not detect differences in results by type of substance targeted for other outcomes. We did not detect differences in results by co-intervention status. Meta-regressions did not indicate that the type of comparator systematically affected the results for any outcome (see Table 2).
Additional Analyses
Results were not sensitive to using earlier time-points from individual studies than longest follow-up when reported for relapse to substance use, frequency of use, negative consequences, withdrawal/craving, anxiety symptoms, and mindfulness. However, results were not statistically significant in 2 of 7 sensitivity analyses for withdrawal/craving, whereas results were statistically significant (and in favor of MBRP) in 1 of 5 sensitivity analyses for depressive symptoms and 2 in 4 sensitivity analyses for mindfulness. The full range of the prediction interval for the true effect in a new study favors MBRP for withdrawal/craving symptoms (SMD −0.19 to −0.07) and negative consequences (SMD −0.45 to −0.01), whereas the prediction intervals range from clinical benefit to clinical harm for all other outcomes (see Table 2).
Adverse Events
Three RCTs indicated that no adverse events were reported (Bowen et al., 2009, 2014b; Brewer et al., 2009). Another RCT listed death as 1 reason for exclusion from analyses in follow-up assessments for standard relapse prevention, and one participant receiving MBRP was admitted to inpatient care at six-month follow-up for reasons unknown (Bowen et al., 2014b). Authors from another RCT indicated in correspondence that no serious adverse events were reported; 1 participant receiving MBRP reported nightmares, increased anxiety, and trauma memories at a follow-up visit (symptoms resolved after medications were changed via psychiatrist consultation) (Zgierska, 2014). The other 4 RCTs did not provide data on adverse events (Uhlig, 2009; Lee et al., 2011; Imani et al., 2015; Glasner et al., 2017).
DISCUSSION
Across studies, our analyses did not indicate that MBRP has beneficial clinical effects beyond comparator interventions (such as relapse prevention, health education, CBT, and TAU) on substance use relapse. We also did not identify significant differences between MBRP and comparator interventions at longest follow-up for other substance use outcomes, including frequency and quantity of substance use. We also did not detect systematic differences in several other patient-important outcomes, including treatment dropout, depressive symptoms, and anxiety symptoms, and a purported mediator of MBRP (ie, mindfulness). Although we have limited confidence in results indicating that MBRP yields decreases in withdrawal/craving and negative consequences, the clinical effects were small. Although we also found clinical effects in favor of MBRP on health-related quality of life, we have very limited to no confidence in this effect estimate due to inadequacies of the body of evidence underlying this analysis. The majority of meta-regression analyses did not detect moderators of effect estimates. Whereas the available evidence on adverse events is also very limited, very few adverse events were reported, indicating that MBRP appears relatively safe from direct harm (Table 3).
TABLE 3.
Summary of Findings Table
| Outcome | Studies | Summary Estimate (95% CI) | QoE | Publication Bias | Meta-regressions | Prediction Interval | Sensitivity to Additional Analyses |
| Relapse to substance use | k = 7; n = 841 | OR 0.72 (0.46 to 1.13) | Low1,2 | τ = −0.14, P = 0.77; t (5) = 0.11, P = 0.92; OR 0.74 (0.53 to 1.05) | Substance: P = 0.44; Co-int: P = 0.99; Comparator: P = 0.42 | OR 0.44 to 1.15 | No significant differences across sensitivity analyses |
| Frequency of use | k = 5; n = 718 | SMD 0.02 (−0.40 to 0.44) | Low1,2 | τ = 0.20, P = 0.82; t (3) = 1.30, P = 0.28; SMD 0.02 (−0.40 to 0.44) | Substance: P = 0.07; Co-int: P = 0.46; Comparator: P = 0.20 | SMD −0.74 to 0.77 | No significant differences across sensitivity analyses |
| Quantity of use | k = 1; n = 123 | SMD 0.26 (−0.13 to 0.64) | Very low1,2,7 | Insufficient evidence | Insufficient evidence | Insufficient evidence | No significant differences across sensitivity analyses |
| Withdrawal | |||||||
| Craving symptoms | k = 5; n = 718 | SMD −0.13 (−0.19 to −0.08) | Low1,11 | τ = −0.40, P = 0.48; t (3) = −1.09, P = 0.35; SMD −0.13 (−0.30 to 0.04) | Substance: P = 0.04; Co-int: P = 0.39; Comparator: P = 0.21 | SMD −0.19 to −0.07 | Results not statistically significant in 2 of 7 analyses |
| Treatment dropout | k = 5; n = 556 | OR 0.81 (0.40 to 1.62) | Very low1,2,4 | τ = −0.40, P = 0.48; t (3) = −0.65, P = 0.56; OR 0.81 (0.40 to 1.62) | Substance: P = 0.97; Co-int: P = 0.23; Comparator: P = 0.28 | OR 0.19 to 3.42 | N/A |
| Health-related quality of life | k = 1; n = 105 | SMD −0.64 (−1.19 to −0.09) | Very low1,4–9 | Insufficient evidence | Insufficient evidence | Insufficient evidence | N/A |
| Negative consequences | k = 4; n = 682 | SMD −0.23 (−0.39 to −0.07) | Low1,9 | τ = −0.67, P = 0.33; t (2) = −1.78, P = 0.22; SMD −0.21 (−0.37 to −0.05) | Substance: P = 0.53; Co-int: P = 0.21; Comparator: P = 0.79 | SMD −0.45 to −0.01 | No significant differences across sensitivity analyses |
| Depressive symptoms | k = 4; n = 622 | SMD −0.09 (−0.39 to 0.21) | Low1,2 | τ = −0.67, P = 0.33; t (2) = −1.98, P = 0.19; SMD −0.00 (−0.16 to 0.16) | Substance: P = 0.03; Co-int: P = 0.51; Comparator: P = 0.21 | SMD −0.49 to 0.32 | Results statistically significantly favor MBRP in 1 of 5 analyses |
| Anxiety symptoms | k = 4; n = 553 | SMD −0.32 (−1.16 to 0.52) | Very low1,2,10 | τ = −0.67, P = 0.33; t (2) = −2.18, P = 0.16; SMD −0.20 (−0.70 to 0.31) | Substance: P = 0.32; Co-int: P = 0.60; Comparator: N/A | SMD −2.37 to 1.74 | No significant differences across sensitivity analyses |
| Mindfulness | k = 6; n = 525 | SMD −0.28 (−0.72 to 0.16) | Very low1,2,10 | τ = −0.20, P = 0.72; t (4) = −1.43, P = 0.23; SMD −0.18 (−0.51 to 0.15) | Substance: P = 0.65; Co-int: P = 0.12; Comparator: P = 0.93 | SMD −1.35 to 0.78 | Results statistically significantly favor MBRP in 2 of 4 analyses |
Reasons for downgrading QoE: 1, high risk of attrition bias; 2, CI consistent with benefit/harm; 3, substantial statistical heterogeneity; 4, adapted version of MBRP; 5, high risk of selection bias; 6, high risk of detection bias; 7, only 1 study (no replications to assess consistency); 8, not outpatient aftercare; 9, wide CI; 10, considerable statistical heterogeneity; 11, evidence of publication bias.
OR < 1 favors MBRP; SMD < 0 favors MBRP.
k, Number of studies; τ, Kendall tau for Begg rank-correlation test for funnel plot asymmetry; t, Egger regression test for funnel plot asymmetry.
We decided to update a previous systematic review on MBRP for SUDs (Grant et al., 2015b), commissioned by the Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury to assist with clinical decision-making, to share the results with a wider audience. In addition, since that review of the evidence, more studies had been published in this small research area, so we updated our review in accordance with guidance on when to update reviews (Garner et al., 2016), which indicates the emergence of new studies that were likely to influence the direction, magnitude, and credibility of our previous reviews findings as important factors for embarking on a systematic review update. As a result of identifying 3 new trials, and also obtaining additional information for 5 trials through author correspondence, this manuscript updates our previous review in several important ways. First, we had sufficient power to detect a statistically significant result for 1 outcome included in our previous review (withdrawal/craving symptoms). Second, we included 5 outcomes not included in our previous review (quantity of substance use, negative consequences of substance use, depressive symptoms, anxiety symptoms, and mindfulness), 1 of which also demonstrated a statistically significant result (negative consequences of substance use). Lastly, we increased our GRADE ratings (ie, we had higher confidence in effect estimates) for our primary outcome (relapse to substance use) and 2 key secondary outcomes (frequency of use and withdrawal/craving symptoms). This updated review therefore provides more current and accurate effect estimates of MBRP to guide policy and practice decision-making and recommendations in addiction medicine (Institute of Medicine, 2011).
The conclusions from this review may surprise some, as individual trials on MBRP for SUDs have reported positive conclusions for substance use outcomes. However, some positive conclusions within trial reports were based on analyses comparing combined data from MBRP and relapse prevention with TAU (Bowen et al., 2014b), or focused on select positive results (Bowen et al., 2009), rather than the totality of findings within a trial (Boutron et al., 2010). Those considering MBRP should weigh our reported effect estimates and our confidence in them with other factors, such as resource requirements, impact on health equity, acceptability to patients, feasibility to implement, and opportunity costs, before deciding whether to recommend it as a treatment in lieu of or in combination with other available interventions for patients with SUDs (Alonso-Coello et al., 2016). Furthermore, it is worth noting that we only examined 1 specific mindfulness intervention amongst others that would benefit from focused systematic reviews to inform recommendations for practice (Zgierska et al., 2008).
Limitations in the current body of evidence indicate how future trials can provide data for firmer conclusions about the effects of MBRP and more reliably inform clinical decision-making. First, most RCTs resembled pilot efficacy trials rather than pragmatic effectiveness trials, with more than half of RCTs randomizing less than 40 participants to each trial group; larger samples are needed to reach the optimal information size for detecting robust results (Guyatt et al., 2011). For most subgroup comparisons in our review, there was insufficient power to statistically detect whether MBRP is efficacious for specific substances, more efficacious when offered either adjunctively or as a monotherapy, or more efficacious when compared with certain interventions than others. Second, attrition bias is a critically high-risk for this evidence base. Future researchers should invest more study resources into ensuring adequate follow-up rates. Given that much outcome data were not reported, we implore future researchers to pre-register trial protocols and subsequently report all outcomes measured in trial manuscripts to have greater statistical power to detect effects amongst all outcomes of interest (Chan et al., 2013). Lastly, researchers should write RCT reports that are in compliance with reporting guidelines for RCTs to allow full critical appraisal of all potential risks of bias, understand the settings and populations to which results are most applicable, and facilitate replication of the intervention (Moher et al., 2010; Grant et al., 2013).
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Patricia Smith and Whitney Dudley for their assistance on this study. We also would like to thank Dr Kristie Gore for her support and guidance, our project officers and points of contact at DCoE, Dr Marina Khusid, and Dr Paul Shekelle and Dr Tracy Simpson for their time and helpful suggestions. Lastly, we thank the authors of included trials who took the time and effort to respond to our queries about trial data. Any errors of fact or interpretation in this report remain the responsibility of the review authors.
Footnotes
Authors’ contributions: S.G., S.H., and M.S. designed the study and wrote the protocol. R.S. designed and conducted the literature searches. S.G. and A.M. designed the data collection forms. S.G. and B.C. screened studies for inclusion and extracted the data. S.G. and M.B. conducted statistical analyses. S.G. and B.C. wrote the first draft of the manuscript, and all authors contributed to and have approved the final manuscript. S.G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This research updates a previous systematic review that was funded through a contract from the US Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury (DCoE) to the RAND Corporation to conduct evidence synthesis reviews to determine the efficacy and comparative effectiveness of integrative medicine approaches for psychological health conditions. A DCoE representative provided assistance during the project and commented on drafts of project reports, but the funder did not directly participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of specific manuscripts for publication. The authors are solely responsible for the content and the decision to submit this manuscript for publication. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Department of Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury.
Conflicts of interest: S.G.'s spouse is a salaried-employee of Eli Lilly and Company, and owns stock. S.G. has accompanied his spouse on company-sponsored travel. All other authors declare no conflicts of interest.
REFERENCES
- Alonso-Coello P, Schünemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: introduction. BMJ 2016; 353:i2016. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–406. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- Black DS. Mindfulness-based interventions: an antidote to suffering in the context of substance use, misuse, and addiction. Subst Use Misuse 2014; 49:487–491. [DOI] [PubMed] [Google Scholar]
- Boutron I, Dutton S, Ravaud P, et al. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA 2010; 303:2058–2064. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins S, et al. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abuse 2009; 30:205–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Marlatt GA. Mindfulness-based Relapse Prevention for Addictive Behaviors: A Clinician's Guide. New York, NY: Guilford Press; 2010. [Google Scholar]
- Bowen S, Chawla N, Marlatt GA. Mindfulness-based Relapse Prevention for Addictive Behaviors: A Clinician's Guide. New York, NY: Guilford Press; 2011. [Google Scholar]
- Bowen S, Chawla N, Witkiewitz K. Baer R. Mindfulness-based relapse prevention for addictive behaviors. Mindfulness-based Treatment Approaches: A Clinician's Guide Elsevier Academic Press, 2nd ed.San Diego, CA: 2014. [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, et al. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry 2014; 71:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol 2007; 3:257–284. [DOI] [PubMed] [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, et al. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction 1998; 93:73–92. [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abuse 2009; 30:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Elwafi HM, Davis JH. Craving to quit: psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychol Addict Behav 2013; 27:366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol 2003; 84:822. [DOI] [PubMed] [Google Scholar]
- Cargiulo T. Understanding the health impact of alcohol dependence. Am J Health Syst Pharm 2007; 64:S5–S11. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: a review of controlled clinical trials. Exp Clin Psychopharmacol 1996; 4:46–54. [Google Scholar]
- Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013; 346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput 2010; 39:860–864. [Google Scholar]
- Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med 2009; 15:593–600. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Subst Use Misuse 2014; 49:492–512. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA, Donovan DM. Conceptualizations of relapse: a summary of psychological and psychobiological models. Addiction 1996; 91 Suppl 1 to Issue 12:5–14. [PubMed] [Google Scholar]
- Craig AD. How do you feel: now? the anterior insula and human awareness. Nat Rev Neurosci 2009; 10:59–70. [DOI] [PubMed] [Google Scholar]
- de Lisle SM, Dowling NA, Allen JS. Mindfulness and problem gambling: a review of the literature. J Gambling Studies 2011; 28:719–739. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012; 379:55–70. [DOI] [PubMed] [Google Scholar]
- Duval SJ, Tweedie RL. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front Psychiatry 2013; 4: (173). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner P, Hopewell S, Chandler J, et al. When and how to update systematic reviews: consensus and checklist. BMJ 2016; 354:i3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner S, Mooney LJ, Ang A, et al. Mindfulness-based relapse prevention for stimulant dependent adults: a pilot randomized clinical trial. Mindfulness 2017; 8:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson DC, Cook TD, Gardner FE, et al. Standards of evidence for efficacy, effectiveness, and scale-up research in prevention science: next generation. Prev Sci 2015; 16:893–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Montgomery P, Hopewell S, et al. Letter to the Editor: new guidelines are needed to improve the reporting of trials in addiction sciences. Addiction 2013; 108:1687–1688. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015; 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Hempel S, Colaiaco B, et al. Mindfulness-based Relapse Prevention for Substance Use Disorders: A Systematic Review; RR-1031. RAND Corporation, Santa Monica; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions: III. JAMA Psychiatry 2016; 73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence: imprecision. J Clin Epidemiol 2011; 64:1283–1293. [DOI] [PubMed] [Google Scholar]
- Hartung J. An alternative method for meta-analysis. Biom J 1999; 41:901–916. [Google Scholar]
- Hartung J, Knapp G. A refined method for the meta analysis of controlled clinical trials with binary outcome. Stat Med 2001; 20:3875–3889. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front Hum Neurosci 2012; 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, et al. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 2012; 59:750–760. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009; 172:137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Altman D, Gøtzsche P, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. J Consult Clin Psychol 1995; 63:400. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol 2010; 78:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imani S, Vahid MKA, Gharraee B, et al. Effectiveness of mindfulness-based group therapy compared to the usual opioid dependence treatment. Iran J Psychiatry 2015; 10:175–184. [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Complementary and Alternative Medicine in the United States. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Institute of Medicine. Psychosocial Interventions for Mental and Substance Use Disorders: A Framework for Establishing Evidence-based Standards. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- Irvin JE, Bowers CA, Dunn ME, et al. Efficacy of relapse prevention: a meta-analytic review. J Consult Clin Psychol 1999; 67:563–570. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead LF, et al. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med 2006; 166:828–835. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Bowen S, An-Fu B. Psychosocial outcomes of mindfulness-based relapse prevention in incarcerated substance abusers in Taiwan: a preliminary study. J Subst Use 2011; 16:476–483. [Google Scholar]
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 1985. [Google Scholar]
- McCown D, Reibel D. Monti DA, Beitman BD. Mindfulness and mindfulness-based stress reduction. Integrative Psychiatry. New York, NY: Oxford University Press; 2010. 289–338. 494, xvii. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000; 284:1689–1695. [DOI] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain 2008; 134:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Drug Intelligence Center. The Economic Impact of Illicit Drug Use on American Society. Washington, DC: US Department of Justice; 2011. [Google Scholar]
- National Institute on Drug Abuse. Addiction science: from molecules to managed care. Bethesda, MD: National Institute on Drug Abuse; 2008. [Google Scholar]
- Oikonomou MT, Arvanitis M, Sokolove RL. Mindfulness training for smoking cessation: a meta-analysis of randomized-controlled trials. J Health Psychol 2016; doi:10.1177/135910531663766. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Carmona PE, et al. The effects of mindfulness meditation on cognitive processes and affect in patients with past depression. Cognit Ther Res 2004; 28:433–455. [Google Scholar]
- Ramo DE, Brown SA. Classes of substance abuse relapse situations: a comparison of adolescents and adults. Psychol Addict Behav 2008; 22:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health 2011; 34:135–143. [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet 2009; 373:2223–2233. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal Z, Williams J, Teasdale J, et al. The mindful way through depression. New York: The Guilford Press; 2007. [Google Scholar]
- Sidik K, Jonkman JN. Robust variance estimation for random effects meta-analysis. Comput Stat Data Anal 2006; 50:3681–3701. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS): 2005: Discharges From Substance Abuse Treatment Services: DASIS Series: S-41. Dept of Health & Human Services publication No. (SMA) 08-4314, in: Services, U.D., o.H.H., (Ed.). Rockville, MD; 2008. [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000; 68:615. [DOI] [PubMed] [Google Scholar]
- Uhlig DJ. Mindfulness based relapse prevention and the matrix model in substance abuse relapse prevention. Dissertation Abstracts Int B Sci Eng 2009; 70:2596. [Google Scholar]
- Viechtbauer W, Viechtbauer MW. Package ‘metafor’; 2015. Available at: http://cran.r-project.org/web/packages/metafor/metafor.pdf Accessed August 7, 2016. [Google Scholar]
- Viechtbauer W, López-López JA, Sánchez-Meca J, et al. A comparison of procedures to test for moderators in mixed-effects meta-regression models. Psychol Methods 2015; 20:360–374. [DOI] [PubMed] [Google Scholar]
- Walsh R, Shapiro SL. The meeting of meditative disciplines and Western psychology: a mutually enriching dialogue. Am Psychol 2006; 61:227. [DOI] [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, et al. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion 2010; 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. J Consult Clin Psychol 2010; 78:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol 2004; 59:224–235. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Lustyk MK, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav 2013; 27:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Harrop EN, et al. Mindfulness-based treatment to prevent addictive behavior relapse: theoretical models and hypothesized mechanisms of change. Subst Use Misuse 2014; 49:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Warner K, Sully B, et al. Randomized trial comparing mindfulness-based relapse prevention with relapse prevention for women offenders at a residential addiction treatment center. Subst Use Misuse 2014; 49:536–546. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- Xue S, Tang Y-Y, Posner MI. Short-term meditation increases network efficiency of the anterior cingulate cortex. Neuroreport 2011; 22:570–574. [DOI] [PubMed] [Google Scholar]
- Zgierska A. Mindfulness Meditation for Health. ClinicalTrials.gov Identifier: NCT01056484; 2014. [Google Scholar]
- Zgierska A, Rabago D, Zuelsdorff M, et al. Mindfulness meditation for alcohol relapse prevention: a feasibility pilot study. J Addict Med 2008; 2:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abuse 2009; 30:266–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


