Supplemental Digital Content is Available in the Text.
Keywords: care continuum, HIV/AIDS, retention, surveillance, validation
Abstract
Context:
Improving retention in care is a key element of the National HIV/AIDS Strategy (NHAS). However, definitions for measuring retention in care are not standardized.
Objective:
To compare measures of retention based on both clinic visit data and HIV laboratory surveillance data.
Design:
Retrospective cohort study.
Setting:
New York City (NYC), New York.
Participants:
We matched adult patients with HIV infection seen at the Spencer Cox Center for Health (SCC) in 2010 or 2011 with the NYC HIV Surveillance Registry.
Main Outcome Measures:
Retention in care was measured on the basis of SCC electronic medical record (EMR) data (≥1 medical visits in 2012) and Surveillance Registry data (≥2 CD4/viral load [VL] tests ≥90 days apart in 2012).
Results:
There were 5746 adult HIV-infected patients seen at SCC between 2010 and 2011 who matched with the Surveillance Registry. Seventy-eight percent (n = 4469) had 1 or more medical visits at SCC in 2012 and were considered retained on the basis of the EMR definition, among which 3831 (86%) met the surveillance definition for retention in care. Patients who did not have a medical visit at SCC in 2012 (n = 1277) were lost to care in NYC (n = 485; 36%), engaged in care at an alternate provider (n = 622; 49%), or died after their last SCC visit (n = 197; 15%).
Implications:
This study is an important comparison of laboratory surveillance versus clinic visit-based measures of retention in care in an urban setting with the largest HIV epidemic in the country. Collaborative projects between local health departments and clinical care providers can help validate the care status of patients and inform the allocation of resources to reengage patients who are lost to care.
Conclusion:
The combined use of laboratory and clinic visit–based data to measure retention in care provides a more accurate representation of the care status of HIV-infected patients than use of a single data source alone. Routine sharing of data by public health institutions and clinical care providers would help target resources toward reengaging patients who are lost to care in jurisdictions with universal HIV-related laboratory reporting.
Medical advancements in antiretroviral therapy regimens enable HIV-infected individuals to lead long, healthy, and productive lives.1,2 However, despite the availability of effective therapy for people living with HIV/AIDS (PLWHA), and the availability of biomedical and other HIV/AIDS prevention methods, there are still a substantial number of new HIV infections reported in the United States each year.3 Gaps in retention in care alone, a key metric along the HIV care continuum, may be responsible for as many as 60% of new HIV transmissions.4
The 2010 National HIV/AIDS Strategy (NHAS) presented target rates for retention in care to reduce new infections, limit disparities in access to HIV care and treatment, and improve overall care and viral suppression outcomes.5 Measuring outcomes along the continuum of care, including retention in care, is a key element of monitoring progress toward the targets laid out in the NHAS.
The proportion of patients considered to be retained in HIV clinical care can vary depending upon the data sources used and the specific definition of retention that is applied. For example, clinical or surveillance data sources can be used, and several definitions have been proposed and evaluated. One commonly used measure recommended by the Health Resources and Services Administration HIV/AIDS Bureau (HRSA HAB) defines retention in HIV clinical care in a given 12-month period as 2 or more HIV care visits 90 or more days apart.6 Several studies have shown that using CD4 T-cell counts or HIV viral load (VL) test results as proxies for the care visits can accurately estimate the proportion of patients retained in clinical care over a given time period.7–9 For jurisdictions with universal HIV-related laboratory reporting, surveillance registries offer a unique, population-based view of the care that patients are accessing at multiple providers and can be used to evaluate whether patients are lost to care in the jurisdiction.
In 2012, the New York City Department of Health and Mental Hygiene (NYC DOHMH) partnered with the Spencer Cox Center for Health (SCC), then one of the largest HIV care providers in NYC, to conduct an analysis of HIV clinic and surveillance data. Our first objective was to validate surveillance-based methods used to calculate patient retention rates via a match of HIV-infected patients in care at the SCC against HIV-related laboratory test records in the NYC DOHMH HIV Surveillance Registry. Our second objective was to apply the surveillance definition to characterize the prospective care status of SCC patients who were not retained in care at the SCC using citywide surveillance data. We report on characteristics of the lost-to-care patients versus those who have died or reengaged in care elsewhere.
Methods
Data sources and study population
The NYC DOHMH HIV Surveillance Registry contains named reports of all HIV and AIDS diagnoses and HIV-related laboratory results (including CD4 and VL tests) for persons newly and previously diagnosed with HIV/AIDS. The population-based Surveillance Registry is routinely updated with vital status data from local and national death records (quarterly and yearly, respectively) and continuously updated with HIV laboratory test data of persons seeking HIV-related medical care at an NYC provider.
In 2012, the SCC (formerly the Center for Comprehensive Care) provided care to more than 6000 HIV-positive children and adults, representing approximately 5% of all PLWHA in NYC.10 Patients at the SCC represent a diverse range of underserved communities, including women of color, lesbian, gay, bisexual, and transgender people, youth, people with low income, the formerly incarcerated, immigrants, and the homeless from the 5 boroughs of NYC. Patients were seen at any one of the 3 clinics located along the west side of Manhattan. Each clinic uses an electronic medical record (EMR) and offers a comprehensive model of care, including mental health care services, dentistry, case management, and medical subspecialties such as dermatology, neurology, and cardiology. Patient clinical data are stored in the EMR and can be extracted for review and analysis. The SCC was integrated into the Mount Sinai Institute for Advanced Medicine in 2013 after completion of the work described.
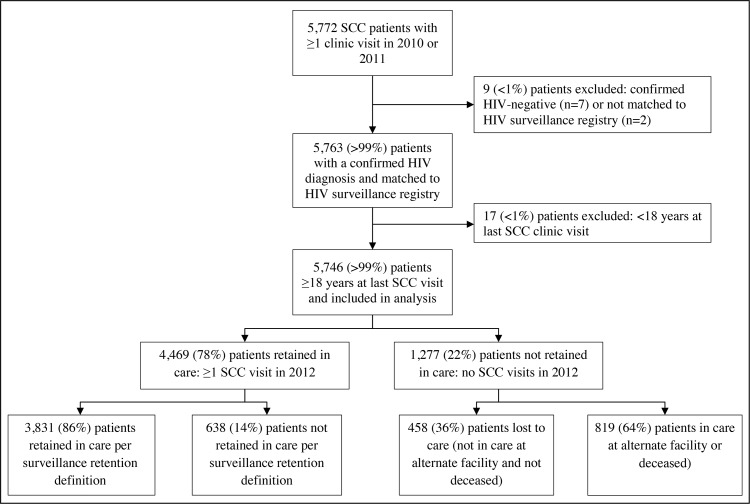
We selected all adult (≥18 years) HIV-infected patients who were seen for at least 1 medical visit at the SCC between January 1, 2010, and December 31, 2011. We extracted race/ethnicity, age, residential zip code, and date of most recent medical visit between January 1, 2010, and December 31, 2012, from the SCC EMR system. For matching purposes alone, we also extracted patient name and date of birth. On the basis of a deterministic, internally developed algorithm,11 we matched the SCC patients with persons in the NYC HIV Surveillance Registry. Patients initially considered a nonmatch via the matching algorithm were reviewed manually to ascertain whether they could be matched with a record in the Surveillance Registry data. From the Surveillance Registry, we extracted date of HIV/AIDS diagnosis; sex at birth; age at time of HIV/AIDS diagnosis; HIV transmission risk factors; poverty level of zip code of residence (based on the proportion of residents living below the Federal Poverty Level) at most recent SCC visit between 2010 and 2012; and clinical indicators (VL and CD4 cell counts). Patients eligible for analysis had a confirmed HIV/AIDS diagnosis via a match with the Surveillance Registry and were aged 18 years or older at their most recent SCC medical visit (Figure).
FIGURE.
Analytic Population for Assessing Surveillance-Based Retention in Care Status: SCC Patients With 1 or More Clinic Visits in 2010 or 2011
Abbreviation: SCC, Spencer Cox Center for Health.
Outcome measures
Retention in care
We used SCC EMR data and Surveillance Registry laboratory data to determine the proportion of patients who were retained in HIV care in 2012. Specifically, we calculated the proportion of patients who were retained in care as per the SCC EMR (defined as ≥1 medical visits in 2012) and who were also retained in care as per the HIV surveillance database (defined as having ≥2 CD4/VL tests ≥90 days apart in 2012 at the SCC or at SCC-affiliated St Luke's or Roosevelt Hospital).
Care status
We used the Surveillance Registry to determine the outcomes of patients who did not have a medical visit at the SCC in 2012 (ie, not retained in care as per the SCC EMR) as lost to care, in care at an alternate care provider, or died after their last medical visit. Patients categorized as lost to care in NYC had no laboratory evidence of being in care at an alternate NYC HIV medical provider in 2012. For those categorized as in care at an alternate provider, we defined being in care as having 2 or more CD4/VL tests 90 or more days apart at the same facility in 2012.
Analysis
We conducted a descriptive analysis of demographic and clinical characteristics of SCC patients. We compared demographic and clinical characteristics of patients lost to care with characteristics of those in care at an alternate provider to determine factors associated with loss to care. We also assessed the demographic and clinical characteristics of patients who died in the time period between their last medical visit at the SCC and the end of 2012. Chi-square tests were used to test the significance of differences in categorical variables. Wilcoxon rank sum tests were used to assess the significance of differences in continuous variables. P values less than .05 were considered statistically significant. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc, Cary, North Carolina).
The study was approved by the institutional review board of St Luke's Roosevelt Hospital Center.
Results
Study population
There were 5763 HIV-infected patients seen at the SCC between January 1, 2010, and December 31, 2011, who matched with the NYC HIV Surveillance Registry. Seventeen (<1%) patients were younger than 18 years as of their most recent medical visit and were excluded, resulting in a study population of 5746 patients. The majority of patients were male (n = 4472; 78%); black or Hispanic (n = 4604; 80%); 40 years or older (n = 4285; 75%), and resided in a zip code classified as having a high or very high poverty level as of their most recent SCC visit in 2010-2012 (n = 3434; 60%). More than 30% (n = 1749) of the patients were diagnosed with HIV/AIDS before 1997, and 45% (n = 2606) were known to have men who have sex with men (MSM) transmission risk. The median CD4 count at or before the last documented SCC visit was 496 cells/μL (interquartile range [IQR] = 304-713), and the proportion with suppressed HIV VL was 71% (n = 4070) (Table).
TABLE. Characteristics of SCC Patients Who Had 1 or More Clinic Visits in 2010 or 2011 Based on EMR Dataa.
| 2010 and 2011 SCC Patients | ||
|---|---|---|
| n | % | |
| Total | 5 746 | 100.0 |
| Sex | ||
| Male | 4 472 | 77.8 |
| Female | 1 274 | 22.2 |
| Race/ethnicity | ||
| Black | 2 460 | 42.8 |
| Hispanic | 2 144 | 37.3 |
| White | 1 053 | 18.3 |
| Asian/Pacific Islander | 61 | 1.1 |
| Other/unknownb | 28 | 0.5 |
| Age group as of most recent SCC clinic visit | ||
| 18-29 y | 562 | 9.8 |
| 30-39 y | 899 | 15.6 |
| 40-49 y | 1 917 | 33.4 |
| 50-59 y | 1 733 | 30.2 |
| 60+ y | 635 | 11.1 |
| Year of HIV diagnosis | ||
| ≤1996 | 1 749 | 30.4 |
| 1997-2000 | 1 479 | 25.7 |
| 2001-2005 | 1 217 | 21.2 |
| 2006-2010 | 1 119 | 19.5 |
| 2011-2012 | 182 | 3.2 |
| Area-based poverty levelc | ||
| Low poverty (<10% below FPL) | 539 | 9.4 |
| Medium poverty (10% to <20% below FPL) | 1 548 | 26.9 |
| High poverty (20% to <30% below FPL) | 1 716 | 29.9 |
| Very high poverty (≥30% below FPL) | 1 718 | 29.9 |
| Area-based poverty level not available | 225 | 3.9 |
| Transmission risk | ||
| Men who have sex with men | 2 606 | 45.4 |
| Injection drug use history | 1 010 | 17.6 |
| Heterosexuald | 873 | 15.2 |
| Perinatal | 58 | 1.0 |
| Other | 5 | 0.1 |
| Unknown | 1 194 | 20.8 |
| Virally suppressed (≤200 copies/mL) at most recent SCC clinic visite | ||
| Yes | 4 070 | 70.8 |
| No | 1 651 | 28.7 |
| Unknown—no VL on or before clinic visit date | 25 | 0.4 |
| Median VL (copies/mL) among unsuppressed at most recent SCC clinic visit (IQR)e | 13 776 (1 628-61 366) | |
| CD4 (cells/μL) at most recent SCC clinic visite | ||
| ≤200 | 821 | 14.3 |
| 201-349 | 930 | 16.2 |
| 350-500 | 1 137 | 19.8 |
| ≥501 | 2 834 | 49.3 |
| Unknown—no CD4 on or before clinic visit date | 24 | 0.4 |
| Median CD4 cell count (cells/μL) at most recent SCC clinic visit (IQR)e | 496 (304-713) | |
| SCC care status as of December 31, 2012 | ||
| Retained in care | ||
| ≥1 SCC clinic visits January 1-December 31, 2012 | 4 469 | 77.8 |
| Lost to clinic | ||
| No SCC clinic visits January 1-December 31, 2012 | 1 277 | 22.2 |
Abbreviations: EMR, electronic medical record; FPL, Federal Poverty Level; IQR, interquartile range; NYC, New York City; SCC, Spencer Cox Center for Health; VL, viral load.
aPrepared September 2014 by the Department of Health and Mental Hygiene Bureau of HIV Prevention and Control with data reported as of March 31, 2014.
bIncludes Native American and Multiracial.
cPoverty level based on most recent NYC zip code of residence at most recent SCC visit 2010-2012. Not available for persons missing zip code information or living outside NYC.
dIncludes persons who had heterosexual sex with a person they know to be HIV-infected, an injection drug user, or a person who has received blood products. For females-only, also includes history of prostitution, multiple sex partners, sexually transmitted disease, crack/cocaine use, sex with a bisexual male, probable heterosexual transmission as noted in medical chart, or sex with a male and negative history of injection drug use.
eViral suppression, median VL, CD4 category, and median CD4 count are determined by a patient's most recent CD4/VL test on or before his or her most recent SCC visit 2010-2012.
Validation of surveillance-based retention in care measures
A total of 4469 (78%) patients had at least 1 medical visit at the SCC in 2012 and therefore were considered retained in care as per the SCC EMR. HIV surveillance data confirmed that 3831 (86%) of these patients were retained in care at the SCC based on having 2 or more CD4/VL tests 90 or more days apart at an SCC facility in 2012. Of the remainder (n = 638), 65% (n = 415) did not meet the surveillance-based definition because they had only 1 HIV-related laboratory result in 2012; 23% (n = 148) had 2 HIV-related laboratory reports in 2012 but the laboratory reports were drawn less than 90 days apart; and 12% (n = 75) had no results reported to the Surveillance Registry in 2012 from SCC-affiliated laboratories (see Table, Supplemental Digital Content 1, available at http://links.lww.com/JPHMP/A275, which shows characteristics of SCC patients who were retained in care).
Prospective care status of patients not retained at SCC
There were 1277 (22%) patients who did not have a medical visit at the SCC in 2012 and therefore were not considered retained in care based on the SCC EMR definition. Four hundred fifty-eight (36%) of these did not have laboratory evidence of being retained in care at any HIV medical provider in NYC and were classified as lost to care in NYC. Eight hundred nineteen (64%) patients not retained in care per the SCC EMR were not lost to care in NYC according to the HIV surveillance database: 622 (49%) patients were retained in care at an alternate provider in 2012, and 197 (15%) patients died after their last SCC care visit (see Table, Supplemental Digital Content 2, available at http://links.lww.com/JPHMP/A276, which shows characteristics of SCC patients lost to care from SCC).
The majority (n = 420; 68%) of patients retained in care at an alternate provider had HIV-related laboratory results within 9 months of their last SCC medical visit (median = 214 days; IQR = 94-941 days). Based on the latest laboratory results in 2012, patients' median CD4 count was 348 cells/μL (IQR = 209-600) and 56% (n = 345) of patients had a suppressed VL. Most often, the alternate provider was classified by surveillance as either an outpatient facility (53%) or a hospital (36%). Approximately 6% of patients were in care at a correctional facility (data not shown).
There were a total of 252 patients who died by the end of the study period, 22% (n = 55) of whom died while considered retained in care at the SCC. When compared with patients who were not retained in care at the SCC at the time of their death (n = 197), a higher proportion of these retained patients had a medical visit at the SCC in the month prior to their death (47% vs 26%, P < .001) and were virally suppressed (69% vs 39%, P < .001) at that time. The majority (n = 152; 60%) of the 197 patients who died and were not retained in care had a medical visit at the SCC within the 3 months prior to their death. Forty-two percent (n = 82) of these patients had a history of injection drug use, and 52% (n = 102) were diagnosed with HIV/AIDS before 1997. The median CD4 count prior to death in this group was 198 cells/μL (IQR = 69-403). More than two-thirds (n = 39; 71%) of retained patients who died had a non–HIV-related cause of death, such as cardiovascular diseases (n = 10; 18%) or non–HIV-related malignant neoplasms (n = 12; 22%), whereas those who were not retained in care were more likely to die of HIV-related causes (n = 87; 44%; P = .045 vs retained patients; see Table, Supplemental Digital Content 3, available at http://links.lww.com/JPHMP/A277, which shows characteristics of SCC patients who died).
Characteristics of lost-to-care patients
When compared with the group of patients in care at an alternate provider, the group of patients lost to care in NYC had a higher proportion of patients who were male (80% vs 75%, P = 0.04), white (19% vs 13%, P = 0.002), MSM (46% vs 34%, P < .001), and in the younger age group of 18- to 29-year-olds (17% vs 8%, P < .001). More than 29% of the patients lost to care were diagnosed with HIV/AIDS between 2006 and 2012 compared with only 16% of patients retained at an alternate NYC provider (P < .001). In addition, compared with patients reengaged in care elsewhere, patients lost to care in NYC had a higher median CD4 count (412 vs 338 cells/μL) but a similar proportion of VL suppression (44% vs 41%, P = 0.15; see Supplemental Digital Content 2, available at http://links.lww.com/JPHMP/A276, which shows characteristics of SCC patients lost to care from SCC).
Similarly, when compared with the group of patients who died, the group of patients lost to care in NYC were more likely to be male (80% vs 70%, P = 0.005), white (19% vs 14%, P = 0.04), MSM (46% vs 21%, P < .001), and in the younger age group of 18- to 29-year-olds (17% vs 4%, P < .001). A higher proportion of those lost to care were diagnosed in the recent years of 2006-2012 (30% vs 12%, P < .001). Patients lost to care had similar rates of VL suppression as those who died (44% vs 38%, P = 0.07) and had a higher median CD4 count at the last visit (412 vs 198 cells/μL; see Supplemental Digital Content 2, available at http://links.lww.com/JPHMP/A276, which shows characteristics of SCC patients lost to care from SCC).
Discussion
The comparison of NYC HIV surveillance data with SCC EMR data found that HIV-related laboratory test results from the NYC DOHMH Surveillance Registry are a valid source for estimating retention rates of patients engaged in HIV clinical care in NYC. Using laboratory data from the Surveillance Registry and the HRSA HAB definition of retention in care,6 86% of the 4469 patients who had at least 1 medical visit at the SCC in 2012 were classified correctly as retained in care at the SCC. Of the 638 (14%) patients not retained in care, 415 (65%) patients fell short of meeting the surveillance definition of retention because they had only 1 laboratory result in 2012.
In addition, we found that the majority of patients classified as “lost to care” from the SCC were not in need of follow-up by the clinic. Laboratory data from Surveillance Registry suggest that the majority of these patients had reengaged in care with an alternate provider and most had done so within 9 months of their last visit at the SCC. This reflects the high degree of “churn” of HIV-infected patients in cities such as NYC that have a large and diverse group of HIV care providers.12,13 These findings suggest that the combined use of clinical and surveillance data can help providers identify patients who are lost to care in NYC and direct resources toward reengaging them as opposed to patients who are reengaged in care with alternate providers or who have died.
A closer examination of the data showed that SCC patients who were lost to care in NYC were more likely to be young, white, and have an MSM transmission risk category when compared with those who have either reengaged in care with an alternate provider or died. Targeted interventions to retain patients who match the profile of those lost to care may be warranted to help reduce risks of attrition.
Patients lost to care in NYC were more likely to have higher CD4 counts than those who reengaged in care with an alternate provider or those who died. Reports in the literature support these findings, and the suggestion that patients who feel healthy are more likely to fall out of care.14 In fact, a significant proportion of patients who died during the study period were engaged in care at the time of death and had a medical visit within 3 months of their death. This may explain the relatively high rate of VL suppression among this group of patients and the fact that many of the deaths were due to non–HIV-related causes.
This study has several limitations. The validation of surveillance-based care measures depends on the definitions being used for retention. In this study, only 1 care visit in a calendar year was required to be considered retained in the clinical data, yet we validated this care status against a surveillance-based definition of a minimum of 2 laboratory results (as a proxy for 2 visits) at least 90 days apart. If the surveillance definition had required just 1 laboratory result at an SCC-affiliated facility, more than 98% of patients would have been considered retained. The growing recognition that clinically stable patients may not need as frequent laboratory monitoring suggests that alternate surveillance-based definitions of detection may be more appropriate. While adjusting the surveillance definition of retention to 1 laboratory result per year may capture a higher percentage of clinically stable patients, the metric loses specificity if not combined with the use of clinic-based visit data. This is another illustration of the advantages of combining data sources to validate the care status of patients. In addition, our analysis evaluated care patterns within a calendar year versus 12-month intervals between care visits. This may have resulted in misclassification of certain patients as not retained if they had a laboratory test late in 2011 but then just 1 laboratory report in 2012.
The study's findings have limited generalizability outside NYC, as NYC's HIV epidemic and service accessibility are unique. In addition, our findings are based on a study population of patients who were known to have engaged in care at an HIV care provider. These patients may be different from other NYC patients who have not accessed or engaged in care, as well as from patients living with HIV/AIDS elsewhere. Furthermore, our study population was known to have had a medical visit with an HIV care provider during the study period and thus surveillance-based retention was expectedly higher in this group than in the general NYC HIV population.15
Implications for Policy & Practice
This study is an important comparison of laboratory surveillance versus clinic visit–based measures of retention in care in an urban setting with the largest HIV/AIDS epidemic in the country.
The study's strengths lie in the robust capacity of the NYC Surveillance Registry that documents the trajectory of care for patients, from reengaging with any provider in NYC to reporting on vital status.
As illustrated with a large sample of diverse patients who accessed care from a single provider, collaborative projects between local health departments and clinical care providers can help validate the care status of patients and inform the allocation of resources to reengage patients who are lost to care in jurisdictions with universal HIV-related laboratory reporting. Recently, the DOHMH launched the HIV Care Status Reports Web-based application for providers to ascertain the current care status of lost-to-care HIV-infected patients according to surveillance data.16
Departments of health and clinical care providers should adopt practices to routinely share data in a combined effort to improve patient retention in HIV care.
Supplementary Material
Footnotes
The authors acknowledge Christopher Beattie and Joyce Park from the Spencer Cox Center for Health for their contribution in preparing the clinical data set.
The authors have no conflicts of interest to declare.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (http://www.JPHMP.com).
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26(3):335–343. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV Surveillance Report, 2013. http://www.cdc.gov/hiv/library/reports/surveillance. Published 2015. Accessed June 1, 2015.
- 4.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–596. [DOI] [PubMed] [Google Scholar]
- 5.The White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. https://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf. Published 2010. Accessed June 1, 2015.
- 6.Health Resources and Services Administration. The HIV/AIDS Program: HAB Performance Measures Group 1. http://hab.hrsa.gov/deliverhivaidscare/files/habgrp1pms08.pdf. Published 2009. Accessed March 12, 2013.
- 7.Sabharwal CJ, Braunstein SL, Robbins RS, Shepard CW. Optimizing the use of surveillance data for monitoring the care status of persons recently diagnosed with HIV in NYC. J Acquir Immune Defic Syndr. 2014;65(5):571–578. [DOI] [PubMed] [Google Scholar]
- 8.Lubelchek RJ, Finnegan KJ, Hotton AL, et al. Assessing the use of HIV surveillance data to help gauge patient retention-in-care. J Acquir Immune Defic Syndr. 2015;69(suppl 1):S25–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean BB, Hart RL, Buchacz K, Bozzette SA, Wood K, Brooks JT. HIV laboratory monitoring reliably identifies persons engaged in care. J Acquir Immune Defic Syndr. 2014;68(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HIV Epidemiology and Field Services Program. HIV Surveillance Annual Report, 2012. New York, NY: New York City Department of Health and Mental Hygiene; 2013. [Google Scholar]
- 11.Drobnik A, Pinchoff J, Bushnell G, et al. Matching HIV, tuberculosis, viral hepatitis, and sexually transmitted diseases surveillance data, 2000-2010: identification of infectious disease syndemics in New York City. J Public Health Manag Pract. 2014;20(5):506–512. [DOI] [PubMed] [Google Scholar]
- 12.Krentz HB, Gill MJ. The effect of churn on “community viral load” in a well-defined regional population. J Acquir Immune Defic Syndr. 2013;64(2):190–196. [DOI] [PubMed] [Google Scholar]
- 13.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62(3):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castel AD, Tang W, Peterson J, et al. Sorting through the lost and found: are patient perceptions of engagement in care consistent with standard continuum of care measures? J Acquir Immune Defic Syndr. 2015;69(suppl 1):S44–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HIV Epidemiology and Field Services Program. Care and clinical status of persons with HIV/AIDS in NYC in 2013 as based on HIV surveillance data. http://www.nyc.gov/html/doh/downloads/pdf/dires/hiv-related-medical-care-2013.pdf. Published 2015. Accessed December 2, 2015.
- 16.New York City Department of Health and Mental Hygiene. HIV care status report system. http://www1.nyc.gov/site/doh/health/health-topics/aids-hiv-care-status-reports-system.page. Published 2015. Accessed December 2, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.