Abstract
Objective:
Hormone receptor-positive breast cancers respond favorably to subcutaneous testosterone combined with an aromatase inhibitor. However, the effect of testosterone combined with an aromatase inhibitor on tumor response to chemotherapy was unknown. This study investigated the effect of testosterone-letrozole implants on breast cancer tumor response before and during neoadjuvant chemotherapy.
Methods:
A 51-year-old woman on testosterone replacement therapy was diagnosed with hormone receptor-positive invasive breast cancer. Six weeks before starting neoadjuvant chemotherapy, the patient was treated with subcutaneous testosterone-letrozole implants and instructed to follow a low-glycemic diet. Clinical status was followed. Tumor response to “testosterone-letrozole” and subsequently, “testosterone-letrozole with chemotherapy” was monitored using serial ultrasounds and calculating tumor volume. Response to therapy was determined by change in tumor volume. Cost of therapy was evaluated.
Results:
There was a 43% reduction in tumor volume 41 days after the insertion of testosterone-letrozole implants, before starting chemotherapy. After the initiation of concurrent chemotherapy, the tumor responded at an increased rate, resulting in a complete pathologic response. Chemotherapy was tolerated. Blood counts and weight remained stable. There were no neurologic or cardiac complications from the chemotherapy. Cost of therapy is reported.
Conclusions:
Subcutaneous testosterone-letrozole was an effective treatment for this patient's breast cancer and did not interfere with chemotherapy. This novel combination implant has the potential to prevent side effects from chemotherapy, improve quality of life, and warrants further investigation.
Keywords: Breast cancer, Chemotherapy, Implant, Letrozole, Testosterone
One out of eight women will develop breast cancer over their lifetime, which is of great concern to both premenopausal and postmenopausal women, and a significant economic burden on healthcare systems. Breast cancer treatments can initiate sterility, menopause, and worsen menopausal symptoms. In addition, long-term toxicities from chemotherapy can be devastating in this patient population. Novel treatments that can reduce the occurrence of breast cancer, increase tumor response to therapy, and prevent side effects from cancer therapies should be investigated.
Testosterone (T) implants have been used for over 70 years in both men and women to treat symptoms and various diseases including breast cancer. Since July 2009, T has been combined with an aromatase inhibitor (AI) in the implant to prevent excess aromatization to estradiol (E2). Implants release continuous therapeutic doses of active ingredients for approximately 3 months in women. Subcutaneous T and T combined with an AI (T + AI) have been shown to reduce the observed occurrence of breast cancer.1,2 Hormone receptor-positive breast cancers respond to androgen therapy, including subcutaneous T and intramammary T + AI implant therapy.3-5 In addition, subcutaneous T + AI has been shown to significantly improve psychological, somatic, and urogenital symptoms in breast cancer survivors without adverse effects or disease recurrence in up to 8 years of therapy.6 The lack of recurrent disease on T + AI therapy is consistent with preclinical and clinical data, which support T direct inhibitory (antiproliferative) effect via the androgen receptor.7
Chemotherapy is associated with known toxicities including neurologic, cardiac, and gastrointestinal toxicities, immunosuppression, anemia, granulocytopenia, and pain, which can lead to reduction of chemotherapy dose, cessation of therapy, or death. Host toxicities can also have an adverse effect on quality of life during and after therapy.
Androgen receptors are located throughout the body including the brain, peripheral nervous system, heart, gastrointestinal tract (GI), lungs, bone, bone marrow, spleen, and other immunological tissue. T is neuroprotective and cardioprotective.8-11 Androgens, including T, stimulate immune function, prevent inflammation, and have been used as treatment for anemia and bone marrow failure.12,13
Preclinical evidence supports that T may protect against chemotherapy-induced toxicities without interfering with antitumor activity.14-16 However, the effect of subcutaneous T + AI on chemotherapeutic response had not been previously studied in humans.
METHODS
Testosterone-letrozole combination implant
The combination testosterone-letrozole (T + L) implants were compounded at Millennium Wellness Center. Nonmicronized T, letrozole, and stearic acid powders were mixed by mechanical means in a geometric ratio of 15:1:1. A manual pellet press was used to compress the triturate into 3.1 mm diameter cylindrical pellets containing 60 mg of T and 4 mg of letrozole (60 mg T + 4 mg L). The pellets were then placed in sealed glass vials and terminally sterilized via autoclave for 40 minutes at 121°C.
Case
JR is a 51-year-old postmenopausal patient, with a 30-year smoking history (1978-2008) followed on a prospective IRB trial examining the incidence of breast cancer in women treated with subcutaneous T implants. She began treatment with T implants in October of 2008 for symptoms of hormone deficiency. Since February of 2013, she occasionally received T in combination with a low dose (ie, 4 mg) of anastrozole combined in the implant for symptoms of excess estrogen including irritability, fluid retention, and weight gain.
The patient received a dose of 180 mg T + 4 mg anastrozole in July of 2015 before diagnosis. In October of 2015, her primary care physician palpated a suspicious right breast mass on clinical examination, which was highly suspicious on mammography. Ultrasound revealed a 3.3 × 3.0 cm right breast mass at the 1 o’clock position. The patient underwent an ultrasound-guided core biopsy of the breast mass and a 1.5 cm right axillary lymph node. The right breast mass revealed a (stage 2) grade 3, Estrogen receptor-positive, Progesterone receptor-positive, Human epidermal growth factor receptor 2-positive, KI67 60%, infiltrating ductal carcinoma. The axillary node was negative. The consensus recommendation was that the patient be treated with neoadjuvant chemotherapy.
Consideration was given to discontinuing androgen therapy. The patient was reluctant to stop therapy because of the significant beneficial effects she experienced on T therapy including improvement in sleep, hot flashes, mood, memory, physical energy, strength, joint and muscular pain, bladder control, and sex drive. The patient made an informed decision to continue T + AI therapy before and during chemotherapy, and was enrolled in an IRB-approved study following women with a diagnosis or history of breast cancer treated with T or T + AI implants. Before each insertion procedure, the patient signed a separate consent, which included a description of the benefits, risks, and “off-label” use of the T + AI implants.
In October (after her diagnosis), the patient was treated with 12 mg of letrozole in combination with 180 mg of T. Three, 60 mg T + 4 mg letrozole implants (T + L) were placed in the subcutaneous tissue of the gluteal area under local anesthesia using a disposable trocar kit. Once chemotherapy was initiated, the dose of subcutaneous letrozole on subsequent insertions was reduced from 12 to 8 mg for a total dose of 180 mg T with 8 mg of letrozole (180 mg T + 8 mg L). The patient was very compliant with the whole food, low-glycemic diet she was instructed to follow.
Between mid-October and early December (day 0-42), the patient was treated with subcutaneous T + L alone, before initiation of chemotherapy. December 2015 through March 2016, the patient was treated with six cycles of chemotherapy including docetaxel (Taxotere); carboplatin, trastuzumab (Herceptin); pertuzumab (Perjeta); palonosetron (for nausea); dexamethasone, fosaprepitant (for nausea); and diphenhydramine. The day after chemotherapy infusion, the patient returned to the oncologist's office and was treated with pegfilgrastim (Neulasta) to stimulate granulocytosis. The patient did refuse her sixth and final dose of docetaxel due to anorexia and malaise, but did agree to take the other three chemotherapeutic agents. Definitive surgery was performed 4 weeks after the final dose of chemotherapy. In addition, the patient continued intravenous trastuzumab injections at 3-week intervals for a total of 12 cycles.
Tumor response to therapy was monitored before and during neoadjuvant chemotherapy using serial transverse and longitudinal 2D ultrasound measurements. Tumor volume was calculated using the ellipsoid formula, 4/3π (a/2 × b/2 × c/2), and reported in cubic centimeters (cc). A model of the logarithm of tumor volume on number of days after the initial T + L implant (day 0) was fitted to the data. To account for the possibility that the rate of decline in tumor size changed after the addition of chemotherapy at day 43, a two-stage model with a known change point was employed.17
Clinical status, weight, and serial blood counts were monitored. Serum E2 and T levels were measured. Cost of therapy was analyzed by obtaining the “Explanation of benefits-Claim for services” from the insurance company.
RESULTS
Ultrasound measurements demonstrated a 43% reduction in tumor volume (12.28 vs 6.96 cc) 41 days after the patient's initial 180 mg T + 12 mg L subcutaneous implant therapy and dietary changes (Fig. 1). This significant reduction in tumor volume occurred before the initiation of chemotherapy. The tumor continued to respond to T + L with concurrent chemotherapy (0.784 cc, day 107; 0.12 cc, day 126). Based on the two-stage model, tumor volume decreased at 1.4% (SE = 0.09%) per day during days 1 to 43, and at a significantly greater rate (P = 0.02) of 3.6% (SE = 0.5%) afterwards, after the addition of chemotherapy (Fig. 2). It should be noted that as the tumor responded to therapy, it became more irregular in shape, making tumor volume calculated by the ellipsoid formula more difficult and possibly less accurate. Regardless, after five cycles of chemotherapy, the tumor was no longer palpable on clinical examination and unable to be identified on ultrasound, that is, complete clinical response. Most significant, there was no residual invasive cancer at the time of definitive surgery, that is, complete pathologic response.
FIG. 1.
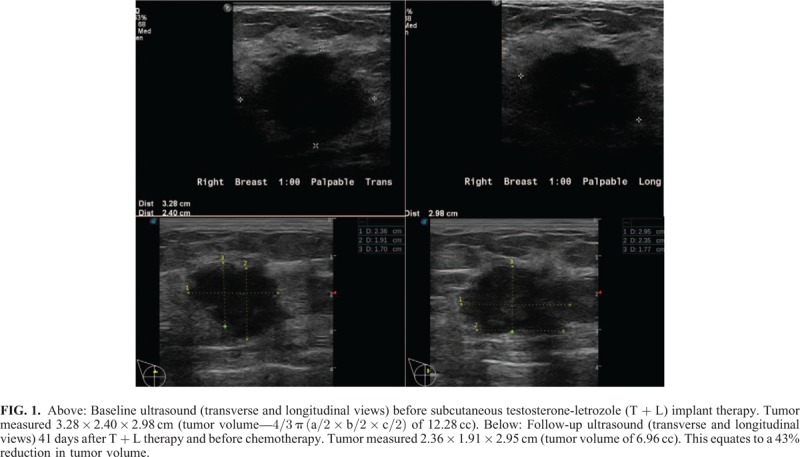
FIG. 2.
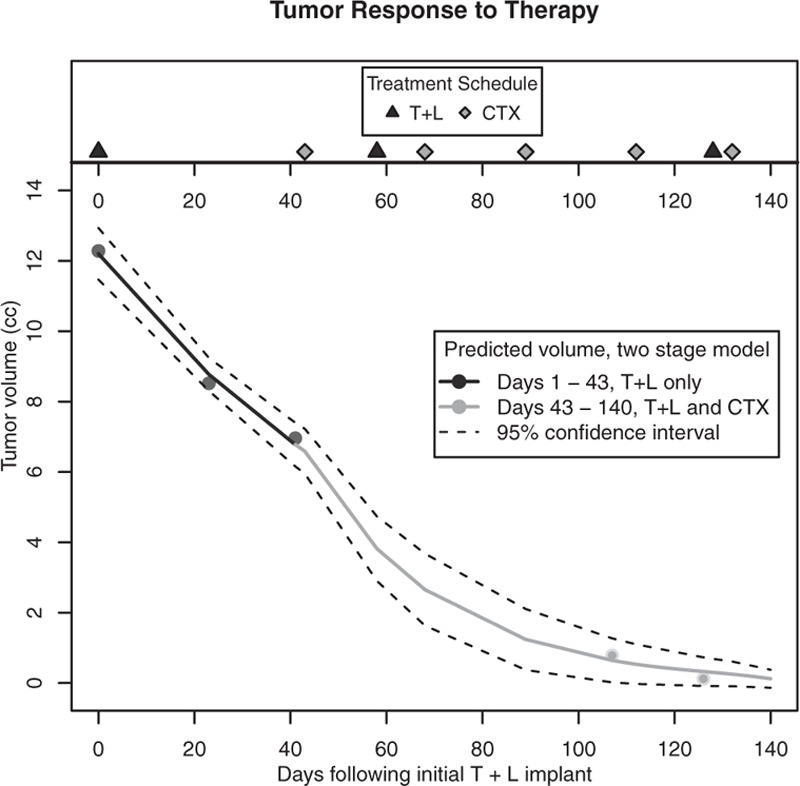
Biphasic tumor response to testosterone-letrozole (T + L) therapy and T + L therapy with concurrent chemotherapy (CTX) showing the predicted value and its 95% confidence interval (CI). The confidence interval describes the level of uncertainty about the estimated size of the tumor. In this case, it means that if the measurement process were repeated on the tumor using the same procedure, it would be expected that the CI would contain the new measurement 95% of the time. There was a 43% reduction in tumor volume (12.3-6.96 cc) 41 days after initial T + L implant, before initiation of CTX on day 43. Two-stage model demonstrates the rate of response to therapy increased after the addition of CTX to T + L.
The patient's weight remained stable throughout chemotherapy (67.7 ± 0.72 kg). White blood count (7.7 ± 2.5 k/mm3) and platelet count (228.0 ± 45.3 k/mm3) also remained stable during chemotherapy. Baseline hemoglobin was 12.3 g/dL. Average hemoglobin during chemotherapy was 11.0 ± 0.5 (range 10.8-12.2 g/dL). T level was therapeutic/pharmacologic, 315 ng/dL,18 and E2 remained <0.5 pg/mL. The patient's main symptoms during chemotherapy were nausea, lack of appetite, and myalgia. There were no reported febrile episodes.
Although the patient reported significant pain after each chemotherapy treatment, she did not have any long-term neurologic side effects from therapy. Memory was intact. There was no evidence or complaints of neuropathy, asthenia, or persistent pain. The patient had no signs or symptoms of cardiac toxicity or compromise. There was no significant change on echocardiogram after six cycles of chemotherapy. Cost of therapy in US dollars is listed in Table 1.
TABLE 1.
Cost of treatment in US dollars
| Amount billed | Amount covered by insurance | |
| T + AI implant | 230a | 0b |
| Chemotherapy (six cycles) | 125,000 | 60,600 |
| Six additional trastuzumab | 46,500 (7,750 × 6) | 22,590 (3,765 × 6) |
| Pegfilgrastim | 46,200 (7,700 × 6) | 23,400 (3,900 × 6) |
| Two-day hospital charge | 71,000 | 61,420 |
| Additional expensesc (estimate) | 45,000 | 25,000 |
Amount billed and amount covered by patient's insurance. Total amount billed for chemotherapy $217,700 ($106,590 covered). T + AI, testosterone-aromatase inhibitor.
aThere was no charge for this patient. Listed is the average total fee per insertion.
bTestosterone implants for women are not covered by insurance in the United States.
c“Additional expenses” include surgeon's fee, anesthesia, plastic surgeon, pathology, radiology, scans, labs, biopsy, port, and other expenses.
DISCUSSION
Testosterone and T + AI therapy have been shown to reduce the observed occurrence of breast cancer.1,2 This patient had been treated with subcutaneous T since 2008. Beginning February of 2013, she had received T in combination with a low-dose AI and was on T + 4 mg anastrozole at the time of diagnosis. Consideration was given to discontinuing T therapy. However, because of the significant impact on this patient's quality of life, she was reluctant to stop therapy. Preclinical and clinical evidence support that androgens, including T, inhibit proliferation of both benign and malignant breast tissue, and would not be directly causative.4,5 However, caution must be observed when treating breast cancer patients with T therapy where adequate aromatase inhibition is critical. T is the major substrate for E2 in postmenopausal patients and has a stimulatory effect via the estrogen receptor. This is particularly important in the neoadjuvant setting where malignant epithelial cells and surrounding fibroblasts overexpress aromatase, increasing local estrogen production, which stimulates cancer cell growth.19 Lack of direct causation via the androgen receptor is supported by the significant reduction in tumor volume after the insertion of T + L implants, before initiation of chemotherapy. Although the patient was treated with the combination T with lower-dose anastrozole (ie, 4 mg) before diagnosis, she received a higher dose of AI after her diagnosis, which may have been more effective in preventing local (intratumoral and peritumoral) aromatization at the tumor site, thus shifting the balance from the mitogenic effect of even low levels of E2 to the inhibitory effect of T. Also, some studies have shown that letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels.20 Because chemotherapy was added at day 43, it remains unknown if the tumor would have eventually become resistant to hormonal therapy.
There was also concern that T might interfere with, or adversely affect the response to chemotherapy. Although the data on nolvadex are conflicting,21 preclinical evidence does not support this concern for T. A recent study demonstrated that androgen receptor agonists inhibited proliferation additively with chemotherapy in breast cancer cell lines.14 Even more compelling is Stolfi et al's data demonstrating that coadministration of T with chemotherapy did not reduce the antitumor activity of chemotherapy in a murine breast tumor model. In addition, coadministration of T resulted in the amelioration of chemotherapy host toxicities including intestinal toxicity, leukopenia and mortality, and increased tolerance to higher doses of chemotherapy.15 In a clinical study of 31 breast cancer patients treated with Thiotepa, T was shown to improve tumor response rate, prevent side effects from therapy, and increase tolerance to chemotherapy by preventing bone marrow depression.16 Our patient's tumor response rate did increase after the addition of chemotherapy (Fig. 2). Although possible, it is not known if T + L and chemotherapy acted synergistically. The complete pathological response supports that T + L did not interfere with chemotherapy. For reference, the expected complete pathologic response rate is less than 40%.22
Neurotoxicity and cardiac toxicity are possible side effects of this patient's chemotherapy regimen. Both taxanes and platin compounds are listed as frequent causes of chemotherapy-induced peripheral neuropathy, which can be a major dose-limiting factor.23 T is both neuroprotective and cardioprotective, and therefore has the potential to reduce these major side effects of chemotherapy.8-11 T improves memory and has been used to treat peripheral nerve pain and numbness.9,24 In addition, T has been used to treat congestive heart failure (cardiac toxicity), an uncommon but serious side effect of docetaxel and both trastuzumab and pertuzumab.11,25,26 Even though this patient had smoked for 30 years, there were no adverse effects to her heart as documented by serial echocardiograms. In addition, there were no persistent neurological changes or complications including memory loss or peripheral neuropathy.
Testosterone reduces inflammation.12 Inflammation is a key factor in the etiology and progression of breast cancer, resistance to endocrine therapy, and also cardiac and neurologic side effects of chemotherapy including “chemo brain.”12,27-31 T protects and stimulates the bone marrow. Its therapeutic effects in various hematologic diseases including aplastic anemia, anemia of renal failure, cyclic neutropenia, and chemotherapy-induced depression of bone marrow have been reported.13,15,16 Although this patient received pegfilgrastim after each chemotherapy treatment, T may have also helped maintain her blood counts. Novel cancer therapeutic strategies focus on supporting the immune system. Androgens, including T, are candidates for such treatments since they modulate immune function.30,32-34
Breast cancer cells rely on aerobic metabolism and require glucose for metabolism.35,36 High glucose and insulin levels trigger multiple direct and indirect mechanisms that promote cancer growth. Evidence supports the beneficial role of the whole food, low-glycemic diet in this patient's (prechemotherapy) tumor response.36 T also improves insulin resistance, which may be an indirect mechanism through which T protects against cancers and other disease states including cardiovascular disease and metabolic syndrome.37 Diet changes were made simultaneously with the T + L therapy, so it is not known how much each individual variable contributed to the reduction in tumor volume. However, previous case reports and two of the authors’ (RG, CD) clinical experience (unpublished data) have demonstrated similar tumor responses to T + AI combination implant therapy in the neoadjuvant setting.3
Five years of adjuvant oral AI therapy has been recommended for this postmenopausal patient. There are no trials comparing oral AI therapy to subcutaneous T + AI, which the patient is continuing. We know that this patient's tumor did respond favorably to T + L therapy. It is not known how the patient's tumor would have responded to an oral AI alone. Nevertheless, subcutaneous delivery of an AI is not “standard of care.” However, the 8 mg dose of subcutaneous letrozole maintained serum E2 levels below 5 pg/mL, which is the target level of E2 used in comparative (efficacy) studies on oral AIs.20
Because of the lack of adverse drug events from T + L therapy and the beneficial effect on quality of life, this therapy may be an option for patients who are not candidates for cytotoxic chemotherapy, possibly in combination with trastuzumab. Unfortunately, subcutaneous T implants are not new and therefore unlikely to warrant commercial development. The necessity for an invasive procedure, no matter how simple, may be another obstacle to further investigation of this therapy. However, the clinical implications for prevention and therapy of cancer, along with reduction of side effects from cancer therapies, could conceivably make an impact on the staggering cost of health care in the United States.38
This is the first case report of the concurrent use of T combined with an AI during neoadjuvant chemotherapy. Although the concepts presented in this study are thought to be provoking and biologically plausible, this report is limited by being a single case study. Nevertheless, the objective findings do suggest a testable hypothesis and likely valid conclusions, which may eventually affect 12.4% of the female population diagnosed with breast cancer. This case report also supports previous data on the safety and efficacy of the combination T + AI on quality of life in breast cancer survivors.6 These preliminary findings may stimulate interest in further research on the prevention of chemotherapy-induced toxicities, and also the treatment of menopausal symptoms in millions of breast cancer survivors worldwide. Although currently prescribed “off-label,” the combination T + AI may also be an option for hormone therapy in perimenopausal and menopausal women at significant increased risk for breast cancer where excess estrogen is contraindicated.
CONCLUSIONS
Subcutaneous T + L therapy in conjunction with a whole food, low (processed)-carbohydrate diet was beneficial in the neoadjuvant therapy of breast cancer. In addition, T + L did not interfere with chemotherapy, supporting preclinical and clinical data. The T + AI combination implant seems to be a promising therapy that has the potential to simultaneously treat breast cancer, prevent side effects of chemotherapy, and improve health and quality of life in breast cancer survivors.
Footnotes
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1.Glaser RL, Dimitrakakis C. Reduced breast cancer incidence in women treated with subcutaneous testosterone, or testosterone with anastrozole: a prospective, observational study. Maturitas 2013; 76:342–349. [DOI] [PubMed] [Google Scholar]
- 2.Glaser R, Dimitrakakis C. Reduced incidence of breast cancer in women adherent to testosterone or testosterone-anastrozole hormone therapy: updated interim analysis. Maturitas 2015; 81:189. [Google Scholar]
- 3.Glaser RL, Dimitrakakis C. Rapid response of breast cancer to neoadjuvant intramammary testosterone-anastrozole therapy: neoadjuvant hormone therapy in breast cancer. Menopause 2014; 21:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaser RL. Testosterone and breast cancer prevention. Maturitas 2015; 81:104. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrakakis C. Androgens and breast cancer in men and women. Endocrinol Metab Clin N Am 2011; 40:533–547. [DOI] [PubMed] [Google Scholar]
- 6.Glaser RL, York AE, Dimitrakakis C. Efficacy of subcutaneous testosterone on menopausal symptoms in breast cancer survivors. J Clin Oncol 2014; 32 Suppl 2:109. [Google Scholar]
- 7.McNamara KM, Sasano H. Androgen and breast cancer: an update. Curr Opin Endocrinol Diab Obesity 2016; 23:249–256. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui AN, Siddiqui N, Khan RA, et al. Neuroprotective role of steroidal sex hormones: an overview. CNS Neurosci Therapeut 2016; 22:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giatti S, Romano S, Pesaresi M, et al. Neuroactive steroids and the peripheral nervous system: an update. Steroids 2015; 103:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iellamo F, Volterrani M, Caminiti G, et al. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol 2010; 55:1310–1316. [DOI] [PubMed] [Google Scholar]
- 11.Herring MJ, Oskui PM, Hale SL, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the basic science literature. J Am Heart Assoc 2013; 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 2004; 89:3313–3318. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Schmidt PJ, Fleming MD, Bhasin S. Effects of testosterone on erythropoiesis in a female mouse model of anemia of inflammation. Endocrinology 2016; 157:2937–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakkar A, Wang B, Picon-Ruiz M, Buchwald P, Ince TA. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res Treatment 2016; 157:77–90. and supplemental Figure 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolfi RL, Sawyer RC, Nayak R, Spiegelman S, Martin DS. Protection by testosterone from fluorouracil-induced toxicity without loss of anticancer activity against autochthonous murine breast tumors. Cancer Res 1980; 40:2730–2735. [PubMed] [Google Scholar]
- 16.Watson GW, Turner RL. Breast cancer. Br Med J 1959; 1:1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julious SA. Inference and estimation in a change-point regression problem. J Roy Stat Soc Ser D (The Statistician) 2001; 50:51–61. [Google Scholar]
- 18.Glaser R, Kalantaridou S, Dimitrakakis C. Testosterone implants in women: pharmacological dosing for a physiologic effect. Maturitas 2013; 74:179–184. [DOI] [PubMed] [Google Scholar]
- 19.Bulun SE, Lin Z, Zhao H, et al. Regulation of aromatase expression in breast cancer tissue. Ann N Y Acad Sci 2009; 1155:121–131. [DOI] [PubMed] [Google Scholar]
- 20.Geisler JÃ, Helle H, Ekse D, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res 2008; 14:6330–6335. [DOI] [PubMed] [Google Scholar]
- 21.Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009; 374:2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valachis A, Mauri D, Polyzos NP, Chlouverakis G, Mavroudis D, Georgoulias V. Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast 2011; 20:485–490. [DOI] [PubMed] [Google Scholar]
- 23.Grisold W, Cavaletti G, Windebank A. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-Oncology 2012; 14:iv45–iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davison SL, Bell RJ, Gavrilescu M, et al. Testosterone improves verbal learning and memory in postmenopausal women: results from a pilot study. Maturitas 2011; 70:307–311. [DOI] [PubMed] [Google Scholar]
- 25.Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure a meta-analysis. Circ Heart Fail 2012; 5:315–321. [DOI] [PubMed] [Google Scholar]
- 26.Valachis A, Nilsson C. Cardiac risk in the treatment of breast cancer: assessment and management. Breast Cancer Targets Ther 2015; 7:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol 2015; 26:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyengar N, Hudis C, Danneberg A. Obesity and inflammation: new insights into breast cancer development and progression. American Society of Clinical Oncology educational book/ASCO. American Society of Clinical Oncology. Meeting 33; 2013; NIH Public Access; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011; 4:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol 2010; 120:105–115. [DOI] [PubMed] [Google Scholar]
- 31.Dieci MV, Griguolo G, Miglietta F, Guarneri V. The immune system and hormone-receptor positive breast cancer: is it really a dead end. Cancer Treat Rev 2016; 46:9–19. [DOI] [PubMed] [Google Scholar]
- 32.Walecki M, Eisel F, Klug J, et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell 2015; 26:2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giltay EJ, Fonk JC, von Blomberg BM, Drexhage HA, Schalkwijk C, Gooren LJ. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocrinol Metab 2000; 85:1648–1657. [DOI] [PubMed] [Google Scholar]
- 34.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol 2013; 9:56–62. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 36.Klement RJ. Calorie or carbohydrate restriction? The ketogenic diet as another option for supportive cancer treatment. Oncologist 2013; 18:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol 2013; 217:R25–R45. [DOI] [PubMed] [Google Scholar]
- 38.Giordano SH, Niu J, Chavez-MacGregor M, et al. Estimating regimen-specific costs of chemotherapy for breast cancer: observational cohort study. Cancer 2016; 122:3447–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]


