Abstract
There is an urgent need for novel antibiotics as the current antibiotics are losing their value due to increased resistance among clinically important bacteria. Sertraline, an on-marked anti-depressive drug, has been shown to modify bacterial activity in vitro, including increasing the susceptibility of Escherichia coli to antibiotics. The aim of the present study was to investigate if the antimicrobial activity of sertraline could be documented under clinical settings, hereunder if sertraline could potentiate the effect of tetracycline in treatment of an experimentally induced ascending infection in poultry. A total of 40 chickens were divided in four groups of 10 chickens each. All chickens were challenged with 4x103 colony forming units (CFU) of a tetracycline resistant E. coli strain using a surgical infection model, and subsequently treated with either high-dose sertraline, tetracycline, a combination hereof or received no treatment. Seven days post challenge all birds were submitted to necropsy and scored pathologically for lesions. The average lesion scores were significantly higher (P<0.05) in the groups that were treated with high-dose sertraline or high-dose sertraline combined with tetracycline. In conclusion high-dose treatments (four times the maximum therapeutic dose for treating human depression) with sertraline as an adjuvant for treatment of antibiotic resistant E. coli infections exacerbate the pathological outcome of infection in chickens.
Introduction
The progressive incline in antimicrobial resistance of clinical important bacteria has led to an increased focus of so-called non-antibiotics, which may be defined as medical compounds whose primary indication for use is non-infectious diseases, but also processes antimicrobial or antimicrobial helper compound effects [1]. Neurotropic medical compounds, such as phenothiazine and selective serotonin reuptake inhibitors (SSRIs) have been largely recognized for their non-antibiotic properties [2–8]. Phenothiazine and derivatives hereof have shown promising results for reversal of antimicrobial properties of multi-resistant Mycobacterium strains [8–13], the causative agent of tuberculosis, which is a serious threat to human health and highly prevalent in developing countries. In industrialized countries the emergence of multi-resistant, clinically important Gram positive and Gram negative bacteria are likely to constitute a health threat to humans and domestic animals [14–16]. Tetracycline is a valued antibiotic in animal husbandry due to its broad-spectrum activities, low toxicity and several oral-based formulations [17,18]. Tetracycline is also used in humane medicine, although the use has decreased in the last decade, likely due to development of better antibiotics and due to a dramatic increase in tetracycline resistant bacteria [17]. The increase in the prevalence of tetracycline resistance in clinically important bacteria in animals is a major problem as it forces the use of other antibiotics for treatment of infectious disease in livestock production. Substituting tetracycline with other antibiotics primarily used for human infections, e.g. cephalosporines would favour selection of cephalosporin-resistant bacteria, which may be directly or indirectly transferred to humans [19,20].
In vitro, sertraline, an SSRI compound, has shown synergy with tetracycline; hence increased the sensitivity of tetracycline-resistant Escherichia coli strains to tetracycline [2,3,21]. If so, the combined treatment with sertraline and tetracycline may allow for the current use of tetracycline even in infections caused by tetracycline resistant organisms in livestock production, and even in human infections in which the infective organism has faced resistance to the last line of antibiotics, such as colistin [22].
Therefore, the aim of the present study was to assess the effect of a combinational treatment of sertraline and tetracycline in an experimentally induced infection caused by high-tetracycline resistant E. coli in a chicken model of ascending bacterial infection.
Material and methods
Bacterial model organism
The strain of E. coli APEC_O2 (NCBI association nr. GCA_001620375.1) was used as challenge strain. The strain originates from a joint of a diseased chicken with arthritis [23]. The strain carries two large plasmids, one being a virulence-associated plasmid [24] and the other being an antimicrobial resistance plasmid, encoding resistance towards a number of antibiotics and heavy metals, including tetracycline [25]. The virulence plasmid has been shown to increase virulence in an avian air sac model [26] as well as contributing to enhanced killing of embryos, growth in human urine and colonization of the murine kidney [27]. The present study is the first to evaluate virulence of E. coli APEC_O2 in an avian ascending model.
Minimal inhibitory concentration (MIC) determinations and synergy assessment
The minimum inhibitory concentration (MIC) of APEC_O2 for sertraline (Sigma-aldrich, Denmark) and tetracycline (Sigma-aldrich, Denmark), respectively, were determined by serial broth dilutions method [28].
Effect of sertraline on the MIC of tetracycline
Synergistic effect of sertraline on tetracycline activity against E. coli APEC O2 was evaluated by checkerboard method with 96-well microtiter plates using MH broth, as described elsewhere [29]. For each combination, the fractional inhibitory concentration (FIC) was calculated as the MIC of the tetracycline in combination with sertraline divided by the MIC of the tetracycline alone and likewise for sertraline. The FIC indexes were derived from summation of individual FICs. The efficacy of the combination effect was interpreted from the FIC index as follows: synergism was defined as an FIC index ≤ 0.5; indifference as 0.5 < FIC index ≤ 4; and antagonism as FIC index > 4 [28].
Experimental groups and housing
Forty brown layers (Bowan brown) were purchased from a commercial layer farm. At 42 weeks of age the layers were transferred from the farmer to the experimental housing unit at University of Copenhagen. They were allowed three weeks of acclimatisation before the experimental infections. During the acclimatisation period the birds were monitored for wellbeing, normal avian behaviour and appetite. Birds were kept free range on deep litter in separate floor pens (2.4 × 2.4 m) with unlimited access to nests, perches and dust baths. Water and commercially layer feed was provided ad libitum in all groups throughout the experiment. All procedures performed on the birds were approved and licensed by the Danish Animal Experiments Inspectorate (license no. 2013-15-2934-00923).
Preparation of challenge inoculum
E. coli APEC_O2 was stored at—80°C in Brain and Heart Infusion (BHI) broth (Oxoid, Basingstoke, UK) in 15% (v/v) glycerol until needed. The day before inoculation the strain was grown overnight in BHI broth. The overnight culture of the strain was diluted to 4×104 CFU, and 0.1 ml of the diluted inoculum were aspirated in 1 ml syringes and kept on ice until infection.
Oviduct infections, post challenge treatments and post mortem assessment
Initially all birds were weighed, before the experiments were performed as described by Pors et al. [30]. Briefly, each hen underwent laparotomy under general anaesthesia and received a dose of 0.1 ml of approximately 4×104 CFU injected into the salpinx lumen. To avoid post-surgical pain a semisynthetic opioid, buprenorphine (0.1 mg/kg), was administered to all hens in all experimental groups during surgery, and repeated after 8 and 16 hours.
After completion of the oviduct infections, the forty layers were divided into four treatment groups as shown in Table 1. The individual treatment dose was based on the body weight of the chicken the day of experimental infection. Group one did not receive any further treatment, while all chickens in group 2–4 were each treated once a day in four days, starting 48 hours post challenge. All treatments were given orally. Powder-form of either tetracycline (Doxylin Vet.) (group 2), sertraline (Sertrone) (group 3) or both tetracycline and sertraline (group 4) were dissolved in 1 ml 0.9% NaCl and administered orally to each hen with a dispensable 3 ml plastic transfer pipette.
Table 1. Overview of experimental groups.
Each group consisted of 10 commercially brown layers, 45 weeks of age.
| Group | Treatment | Daily dose/ kg bodyweight (Day 2–5 after challenge) |
|---|---|---|
| 1 | None (infection control) | None |
| 2 | Tetracycline | 25 mg1 |
| 3 | Sertraline | 8 mg2 |
| 4 | Tetracycline +sertraline | 25 mg1 + 8 mg2 |
1 Corresponding to 50 mg Doxylin vet/ kg bodyweight,
2Corresponding to 16 mg Sertrone /kg bodyweight
All layers were weighed and euthanized by cervical dislocation seven days post challenge (d.p.i.) and directly submitted to post mortem analysis, where lesions were scored according to the scorings system outlined by Pors et al. [30]. Briefly, each organ system (ovary, peritoneum and salpinx) was evaluated based on pre-defined criteria and assigned a score between 0 (no macroscopically pathology) and 4 (extensive macroscopic pathology) for each criteria assessed. The total cumulative score is the sum of scores for all evaluated organ systems in addition to scores on spleen proliferation and lymphatic reaction. Two pathologists scored all lesions of all chickens during the post mortem examination, and if any incongruence in scoring occurred, a third pathologist also participated in the scoring process.
From each chicken, sampling from the bone marrow and the cranial and caudal salpinx was done using cotton swabs and plated directly on agar plates (Oxoid, Basingstoke, UK) with 5% bovine blood. Re-isolation was considered positive if abundant growth in pure culture of E. coli was obtained.
For determination of CFU/ gram salpinx tissue, approximately five centimetres of the middle part of salpinx (magnum) was removed aseptically, weighed off and added to 20 ml 0.9% NaCl solution in a sterile Stomacher filterbag. The contents were homogenised in a stomacher machine for 5 min, and 100 μl of the homogenate was used to make serial 10-fold dilutions in 0.9% NaCl. From each dilution 100 μl was placed on a Müller-Hinton (MH) plate supplemented with 10 mg/ml tetracycline, and distributed equally on the plate using sterile plastic beads. All CFU dilutions were done in duplicate to ensure reproducibility.
Confirmation of re-isolated bacteria as being E. coli APEC O2 was done by PCR using primers designed in the present study and described below.
E. coli APEC_O2 specific primer design
A draft genome sequence of E. coli APEC_O2 strain was used in this study for E. coli APEC_O2 strain-specific primer design. pAPEC-O2-ColV and pAPEC-O2-R plasmids were removed from the genome assembly, and the remaining contigs were aligned to 28 genetically-related strains of E. coli representing sequence types ST135, ST452, ST-15, ST131, ST95, ST140, ST127 and ST73 using progressive MAUVE [31]. Based on the alignment there were 168 regions found to be present specifically in E. coli APEC_O2. These regions were further checked for similarity to other E. coli strains by BLAST in non-redundant nucleotide collection of National Centre of Biotechnology Information (NCBI). Hits (n = 46) that were found to be strictly located on a chromosome and/or representing phage sequences were further checked for their similarity to other species in non-redundant nucleotide database, and for the presence of their duplicates in APEC O2. This final step revealed 11 APEC_O2 strain-specific hits, one of which of 217 bp representing phage-related antirepressor was used for primer design using Primer3Plus [32] with the settings for qPCR within the program. Based on this five primer pairs have been selected, and tested against 12 strains of genetically diverse collection of APEC strains described by Ronco et al. [33]. Finally, one primer pair (Forward primer ACCGTTTAGTGCTTCCCAAG; reverse primer ATTGCGACTTCTGTCATGC) with PCR condition including an initial denaturation of 5 min at 95°C followed by 25 cycles of 30 sec at 95°C, 30 sec annealing at 60°C and 30 sec extension at 72°C, and hereafter a finally 10 min of extension at 72°C, yielding a 141 bp PCR product. The primers and PCR condition was proved to be E. coli APEC_O2 strain-specific in the tested strain collection and was used in this study to confirm re-isolated strains of E. coli as E. coli APEC_O2 in the present study.
Histopathology and immunohistochemistry
For histopathological examination, pieces of the liver and kidney were fixed in 4% neutral buffered formaldehyde solution for 48 h, subsequently trimmed, dehydrated and embedded in paraffin wax prior to preparation of 3 to 5 μm thick sections, which were mounted on adhesive slides (Super Frost/Plus; Menzel-Gläser, Braunschweig, Germany).
Haematoxylin and eosin staining was performed according to a standard protocol [34].
An indirect immunostaining technique based on a specific E. coli rabbit monoclonal antibody (ab137967; Abcam plc, Cambridge, UK) according to the protocol described by Rossez et al [35] was used for detections of E. coli in the tissue sections.
Statistical analysis
For the scoring of pathology, Kruskal–Wallis followed by Dunn’s Multiple Comparison test was used for comparisons of lesion scores. CFU/per gram salpinx and the body weight changes p.i., respectively, one-way ANOVA was used to compare group levels. Subsequently, Tukey's multiple comparison test compared group levels two-by-two for each of the two parameters. All the statistical analysis was done with Graphpad Prism (Graphpad Software, Inc., La Jolla, USA). P<0.05 was considered statistically significant.
Results
In vitro synergy assessment
For E. coli APEC_O2, MICs for tetracycline and sertraline were determined to 64 mg/L and 32 mg/L, respectively (Table 2). Synergy between tetracycline and sertraline as defined by FIC ≤ 0.50, could be obtained for E. coli APEC_O2 at an exposure of 8 mg/L tetracycline combined with 16 mg/L sertraline.
Table 2. Minimal inhibitory concentration (MIC) of sertraline and tetracycline alone or combined.
For each combination exposure on sertraline and tetracycline, the fractional inhibitory concentration index (FICI) is stated.
| MIC | FICI | |
|---|---|---|
| Tetracycline (μg/ml) | Sertraline (μg/ml) | |
| 0 | 32 | - |
| 64 | 0 | - |
| 32 | 0.25 | 0.96 |
| 16 | 8 | 0.50 |
| 4 | 16 | 0.56 |
Pathological outcome of infection
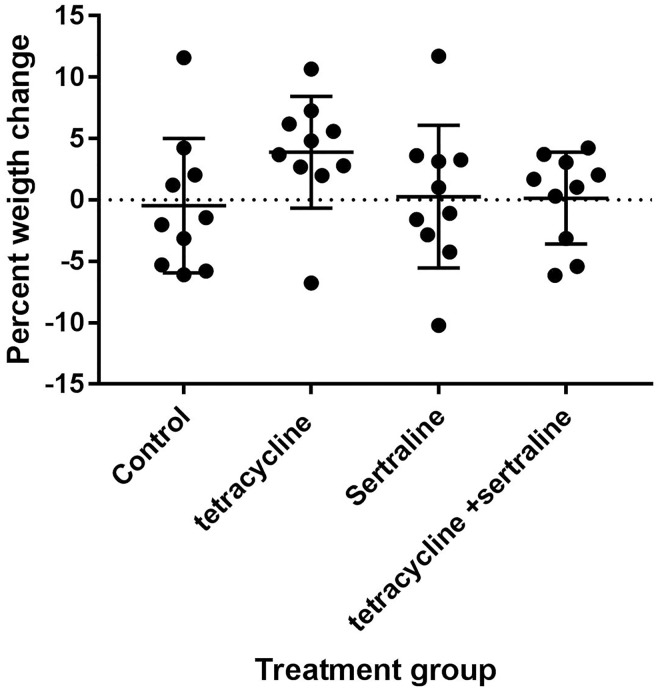
The surgical procedure of infection was conducted in an uncomplicated manner for all chickens. There were no mortality in any of the groups at day seven following challenge, and besides a slight depression observed in all groups 12–24 hours after challenge, all chickens displayed normal avian behaviour, and normal drinking and feeding activities, and the average weight change per chicken did not differ significantly between groups (Fig 1).
Fig 1. Weight changes during infection.
Percent weight change seven days after infection compared to before infection per chicken in the different treatment groups.
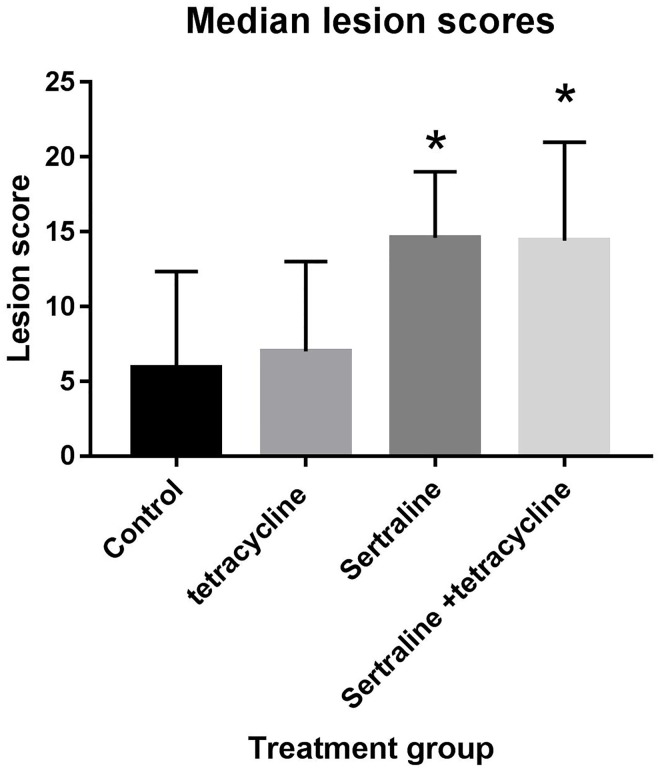
At post mortem examination seven days post challenge, large variations in the pathological outcome was observed (Fig 2). Overall, irrespective of post infection treatment gross macroscopic observation consisted of varying degrees of peritonitis, salpingitis and oophoritis. The least severe pathology as defined by lowest lesions score were observed among chickens in the untreated control group, although the median score did not differ significantly from the median lesion score of chickens in the tetracycline-treated group. For the untreated group of chickens, 6/10 did not demonstrate any pathology at all (lesion score 0), while 5/10 chickens in the tetracycline-treated group all had lesion score of 5 or lower.
Fig 2. Lesion scores.
Median lesion score per chicken in the different treatment groups. Vertical bars indicate standard deviation, and asterisk indicated statistical difference in lesion score compared to the non-treated control group.
When compared to the untreated and tetracycline-treated groups, the pathology observed in the remaining groups (sertraline and sertraline/tetracycline) was considerably more severe and productive/suppurative, and only a single chicken had a lesion score below 10 in the sertraline-treated group, and for the sertraline/tetracycline treatment group, 3/10 chickens had a lesion score greater than 20. The median lesion score in both the sertraline and sertraline/ tetracycline treatment group were significantly higher than the control group (P< 0.011 and P<0.014, respectively).
Histopathology and immunohistochemistry
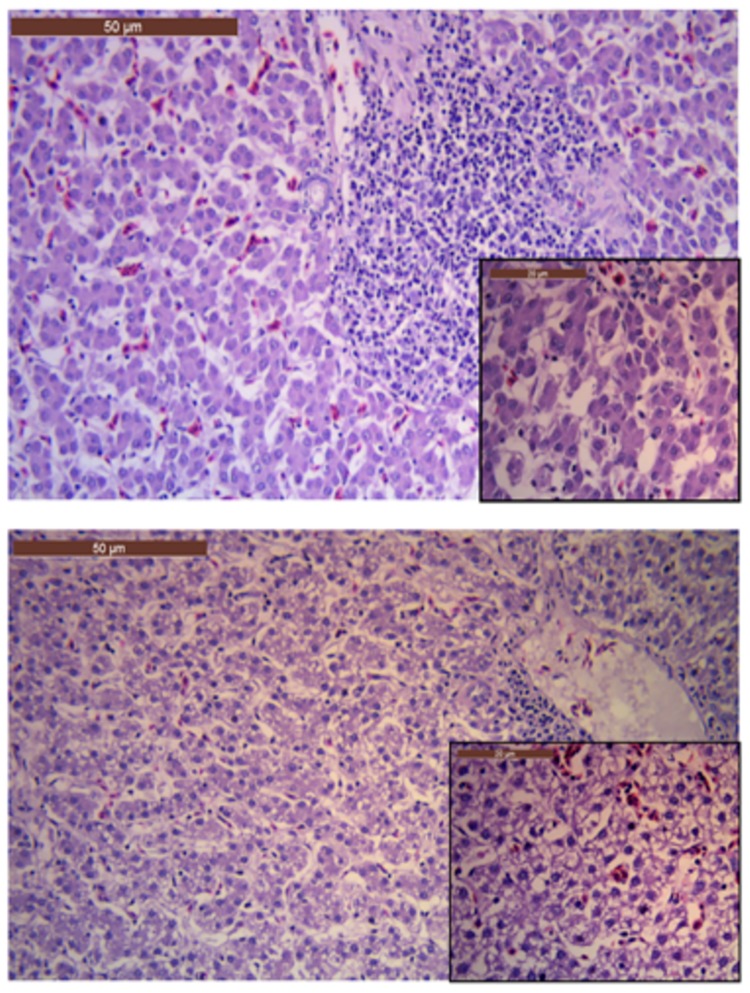
Similar to the macroscopic variations observed, the histopathological observations also ranged from no histopathological findings to severe vacuolisation of the hepatic cells in the different groups (Fig 3). Severe vacuolisation of the hepatic cells were solely found in liver sections from chickens in the sertraline- or sertraline/tetracycline groups. No histopathological findings were observed in the kidney sections, irrespectively of treatment groups. Positive staining for E. coli was not observed in any of the tissue samples.
Fig 3. Liver histology.
a) Liver section from a chicken in the control group (not receiving any treatment after challenge with E. coli APEC_O2). Notice the normal and regular hepatocytes; b) Liver section from a chicken in the sertraline-treated group. Notice the major vacuolization of the hepatocytes. Each of the large images is at 20X magnification, insert in the corners are from the same image, but at 40X magnification.
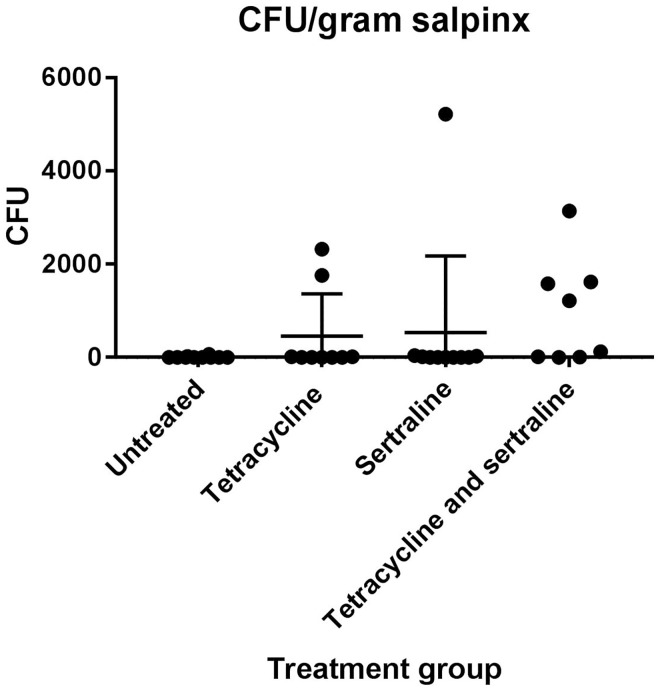
CFU of the salpinx
For all chickens, CFU of E. coli APEC_O2 in approximately 20 gram of the salpinx was determined. There was no difference between the average CFU/g salpinx per chicken between groups (p = 0.171) (Fig 4).
Fig 4. Colony-forming units (CFU).
CFU per gram salpinx in the different treatment groups. Vertical bars indicate the standard deviation.
The weight of each chicken was measured before and seven days after challenge. There were no statistical differences in the average weight changes per chicken between groups (Fig 1).
Discussion
In humans, urinary tract infections (UTI) due to E. coli are one of the most common reasons for antibiotic treatment in clinical practise [36]. Emergence of multi-resistant E. coli will consequently have major impact of treatment success of UTI in human medicine. Similar, in poultry E. coli is the most frequent cause of ascending infections, and accumulating evidence suggest that the clones of E. coli causing ascending infections in poultry are highly similar to clones of E. coli causing UTI in human [23,37]. In the present study we have assessed the potential of sertraline as antimicrobial or as an antimicrobial helper compound in the treatment of experimental ascending E. coli infections in poultry.
In the study, the strain E. coli APEC_O2 was used as challenge strain. The strain was chosen because it is a tetracycline resistant avian pathogenic E. coli and because it has been shown to possess a plasmid enhancing survival in human urine and increasing colonization of the murine kidney [27]. The strain has, however, never been used to experimentally infect the salpinx of poultry, therefore the virulence potential in this aspect was unknown prior to the present study. In the infection control group (Group 1) only 4/10 chickens had recognisable macroscopic pathology, while the remaining six chickens in the group showed no signs of gross pathology. Out of these six macroscopically unaffected chickens, E. coli APEC_O2 could not be re-isolated from the tissue either (Fig 3). The host-related factors resulting in varying susceptibility is well-documented, although it could be argued if the infection dose used was too low [30]. Ideally, 10/10 chickens in the untreated group would had shown gross pathology consistent with an ascending E. coli infection. However, previous studies have documented that host-related factors, the infection dose and strain-related characteristics are important parameters for infection outcome [38]. In a recent study, applying the same method, but using another strain of E. coli as challenge, a 100% mortality two days post challenge was observed when applying a dose of 5x105 CFU [39]. To optimize the chances for full survival of all chickens in all groups, while still ensuring an infection to be established in at least part of the chickens, the dose was set two log units lower than in the previously mentioned study.
As E. coli APEC_O2 is tetracycline resistant, it was not surprising that the pathology in the tetracycline-treated group was highly similar to the observations done in the un-treated control group. The CFU per gram salpinx were also similar with the counts in the control group, although two chickens had CFU counts significantly higher than for any of the chickens in the control group (Fig 4), indicating clearly that E. coli APEC_O2 is not inhibited by tetracycline, unless administered in concentrations eight times higher than the cut-off value of tetracycline sensitivity of E. coli as defined by EUCAST[40] (Table 2).
In contrast to all other groups, all but one chicken in the tetracycline-treated group, actually had gained weight in the week following infection (Fig 1). If this is due to ease of infection, and consequently greater appetite, or a better feed conversion rate when tetracycline is administered as suggested by several studies [41,42] is unknown as all groups were feed ad libitum with no records on actual feed intake, but anorexia/decrease in appetite was not observed in any of the groups during the trial.
At seven days post challenge, the bone-marrow of all chickens from all groups was culture negative, indicative of the lack of a septic/bacteraemic condition. In agreement E. coli was not detected in any of the histopathological slides. The severity of the macroscopic pathology observed for chickens in sertraline and sertraline/tetracycline treated groups was highly similar, and more pronounced than in the un- or tetracycline- treated groups (Fig 1). The infections were considerably more productive and more extensive cases of peritonitis involving the entire peritoneal cavity were observed. The histopathology revealed the chickens in these groups also had marked vacuolisation of the hepatic cells (Fig 3), a finding previously associated with infectious diseases in avian species [43]. In contrast, the level of CFU in salpinx was statistically indifferent from counts in the control group although there was a tendency to higher counts among chickens in the sertraline/tetracycline treated group (Fig 4). Based on the in vitro synergy assessment assay, it was evident that a relative high concentration of sertraline is needed to reduce the tetracycline resistance of E. coli APEC_O2 to 8 mg/L tetracycline, the epidemiological cut-off for resistance [40]. As sertraline is known to have a low toxicity [44], and due to the in vitro observations (Table 2), a daily dose of 8 mg sertraline/kg body mass chicken was considered as a relevant experimental dose, which is approximately 2.5 times the concentration used to treat severe clinical depression in human medicine [45].
The more severe outcome of infection in the sertraline/tetracycline group was unexpected, as it was hypothesized to significantly improve/combat the infection. An explanation for the unexpected discrepancy of the pathology and CFU counts when compared to control group was sought for in the literature. As to the authors knowledge this is the first time sertraline treatment in chickens in an infectious model has been described. In behavioural (non-infectious) avian studies, exposure of SSRI compounds in concentrations from 5.0 mg/L [46] to 25 mg/L [47] have been published with no reports on observed pathology/toxically when SSRIs are used in poultry. Neither did the histopathological examination in the present study show any signs of increased toxicities. There were, however, signs of exacerbated infection (liver vacuolisation), indicating the bacterial infection rather than the compound of sertraline per see worsened the outcome. These observations may lead to the tentative conclusion that the sertraline compromise the innate immune system of the chickens. In humans, SSRIs has been documented to suppress lymphocyte proliferation, cytokine secretion and viability in vitro, although the mechanism behind SSRI-induced immunological effects remains to be elucidated [48]. The finding that SSRIs suppress unwanted immune reactions has been demonstrated in animal models of autoimmune disorders, such as rheumatoid arthritis [49] and multiple sclerosis [50]. Furthermore, a beneficial effect of SSRIs in the treatment of lymphoma has been proposed, presumably attributed to both a direct suppressive effect on the malignant cells and a stimulatory effect on anti-cancer immunity [51,52]. Finally, recent evidence showed an effect of fluoxetine, another SSRI compound, on neutrophil adhesion and recruitment to inflammatory sites, demonstrating that not only cellular but also innate immunity is impacted by SSRIs [53].
Only few studies have investigated the interaction of SSRI and bacterial infection, experimentally induced [54] or clinical observations [55]. For the latter, a recent study documented that there is an increased risk of acquiring a Clostridium difficile infection when taking daily doses of SSRI [56,57], despite that it is generally assumed that the required doses for immunosuppression has been suggested to be higher than those used to treat depression [48]. In contrast, relatively low concentrations (0.1–1 μM) of a SSRI compound, fluoxetine, have been found to stimulate T cell proliferation [58].
The avian immune system resembles that of mammals since both evolved from a common reptilian ancestor and have inherited many commonalities [59]. They have also developed a number of different strategies that are unique to birds. The lymphoid organs plays a major role in avian immunity, with the bursa of Fabricius (site of B-cell origin) and the thymus (site of T-cell origin) are considered primary lymphoid organs [60]. Since T-cells are suggested to be particular sensitive to high-doses sertraline [61], it may be speculated that avian species are particular susceptible to SSRI-induced immunosuppression.
In conclusion, although sertraline has a synergic effect with tetracycline in vitro, combinational treatment with sertraline and tetracycline significantly worsen the pathological outcome of infection in an experimental bacterial infection model. These findings may be explained by an immunosuppressive effect of sertraline when applied in doses four times that of clinical doses recommended for treatment of central nervous system disorders in man.
A possible beneficial effect of lower doses of sertraline in combination with tetracycline treatment of tetracycline resistant E. coli infections requires future studies to answer.
Supporting information
(XLSX)
Acknowledgments
Dr. Lisa Nolan, Iowa State University, USA, is thanked for providing the strain E. coli APEC O2.
Data Availability
All relevant data are included in the paper and it Supporting Information files.
Funding Statement
This project has received funding from the Danish Council of Independent Research, grant agreement no. 4184-00512 to RHO.
References
- 1.Vandevelde NM, Tulkens PM, Van BF (2016) Modulating antibiotic activity towards respiratory bacterial pathogens by co-medications: a multi-target approach. Drug Discov Today 21: 1114–1129. doi: 10.1016/j.drudis.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Ayaz M, Subhan F, Ahmed J, Khan AU, Ullah F, Ullah I et al. (2015) Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J Biol Res (Thessalon) 22: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amantaa A, Chattopadhyayb D, Sinhaa C, Janaa A, Ghosha S, Banerjeea A et al. (2012) Evaluation of In Vivo and In Vitro Antimicrobial Activities of a Selective Serotonin Reuptake Inhibitor Sertraline Hydrochloride. Anti-Infective Agents 10. [Google Scholar]
- 4.Hendricks O, Molnar A, Butterworth TS, Butaye P, Kolmos HJ, Christensen JB et al. (2005) In vitro activity of phenothiazine derivatives in Enterococcus faecalis and Enterococcus faecium. Basic Clin Pharmacol Toxicol 96: 33–36. doi: 10.1111/j.1742-7843.2005.pto960105.x [DOI] [PubMed] [Google Scholar]
- 5.Molnar J, Schneider B, Mandi Y, Farkas S, Holland IB (1980) New mechanism of plasmid curing by psychotropic drugs. Acta Microbiol Acad Sci Hung 27: 309–315. [PubMed] [Google Scholar]
- 6.Amaral L, Lorian V (1991) Effects of chlorpromazine on the cell envelope proteins of Escherichia coli. Antimicrob Agents Chemother 35: 1923–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viveiros M, Jesus A, Brito M, Leandro C, Martins M, Ordway D et al. (2005) Inducement and reversal of tetracycline resistance in Escherichia coli K-12 and expression of proton gradient-dependent multidrug efflux pump genes. Antimicrob Agents Chemother 49: 3578–3582. doi: 10.1128/AAC.49.8.3578-3582.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaral L, Viveiros M, Kristiansen JE (2006) "Non-Antibiotics": alternative therapy for the management of MDRTB and MRSA in economically disadvantaged countries. Curr Drug Targets 7: 887–891. [DOI] [PubMed] [Google Scholar]
- 9.Molnar J, Beladi I, Foldes I (1977) Studies on antituberculotic action of some phenothiazine derivatives in vitro. Zentralbl Bakteriol Orig A 239: 521–526. [PubMed] [Google Scholar]
- 10.Ordway D, Viveiros M, Leandro C, Bettencourt R, Almeida J, Martins M et al. (2003) Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 47: 917–922. doi: 10.1128/AAC.47.3.917-922.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justice SS, Hunstad DA, Cegelski L, Hultgren SJ (2008) Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol 6: 162–168. doi: 10.1038/nrmicro1820 [DOI] [PubMed] [Google Scholar]
- 12.Stoute ST, Bickford AA, Walker RL, Charlton BR (2009) Mycotic pododermatitis and mycotic pneumonia in commercial turkey poults in northern California. J Vet Diagn Invest 21: 554–557. doi: 10.1177/104063870902100424 [DOI] [PubMed] [Google Scholar]
- 13.Deshpande D, Srivastava S, Musuka S, Gumbo T (2016) Thioridazine as Chemotherapy for Mycobacterium avium Complex Diseases. Antimicrob Agents Chemother 60: 4652–4658. doi: 10.1128/AAC.02985-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO (2014) Antimicrobial resistance—Global report on surveillance 2014.
- 15.Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109: 309–318. doi: 10.1179/2047773215Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover FC (1995) Emerging problems in antimicrobial resistance. J Intraven Nurs 18: 297–300. [PubMed] [Google Scholar]
- 17.Chopra I, Roberts M (2001) Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65: 232–260. doi: 10.1128/MMBR.65.2.232-260.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnappinger D, Hillen W (1996) Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch Microbiol 165: 359–369. [DOI] [PubMed] [Google Scholar]
- 19.Depoorter P, Persoons D, Uyttendaele M, Butaye P, De ZL, Dierick K et al. (2012) Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium. Int J Food Microbiol 159: 30–38. doi: 10.1016/j.ijfoodmicro.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 20.Chambers L, Yang Y, Littier H, Ray P, Zhang T, Pruden A et al. (2015) Metagenomic Analysis of Antibiotic Resistance Genes in Dairy Cow Feces following Therapeutic Administration of Third Generation Cephalosporin. PLoS One 10: e0133764 doi: 10.1371/journal.pone.0133764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Kromann S, Olsen JE, Svenningsen SW, Olsen RH (2017) Insight into synergetic mechanisms of tetracycline and the selective serotonin reuptake inhibitor, sertraline, in a tetracycline-resistant strain of Escherichia coli. J Antibiot (Tokyo) 70: 944–953. doi: 10.1038/ja.2017.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paveenkittiporn W, Kerdsin A, Chokngam S, Bunthi C, Sangkitporn S, Gregory CJ (2017) Emergence of plasmid-mediated colistin resistance and New Delhi metallo-beta-lactamase genes in extensively drug-resistant Escherichia coli isolated from a patient in Thailand. Diagn Microbiol Infect Dis 87: 157–159. doi: 10.1016/j.diagmicrobio.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 23.Zhu G, X, Jiang J, Pan Z, Hu L, Wang S, Wang H et al. (2014) Comparative genomic analysis shows that avian pathogenic Escherichia coli isolate IMT5155 (O2:K1:H5; ST complex 95, ST140) shares close relationship with ST95 APEC O1:K1 and human ExPEC O18:K1 strains. PLoS One 9: e112048 doi: 10.1371/journal.pone.0112048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson TJ, Siek KE, Johnson SJ, Nolan LK (2006) DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J Bacteriol 188: 745–758. doi: 10.1128/JB.188.2.745-758.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson TJ, Siek KE, Johnson SJ, Nolan LK (2005) DNA sequence and comparative genomics of pAPEC-O2-R, an avian pathogenic Escherichia coli transmissible R plasmid. Antimicrob Agents Chemother 49: 4681–4688. doi: 10.1128/AAC.49.11.4681-4688.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skyberg JA, Johnson TJ, Nolan LK (2008) Mutational and transcriptional analyses of an avian pathogenic Escherichia coli ColV plasmid. BMC Microbiol 8: 24 doi: 10.1186/1471-2180-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skyberg JA, Johnson TJ, Johnson JR, Clabots C, Logue CM, Nolan LK (2006) Acquisition of avian pathogenic Escherichia coli plasmids by a commensal E. coli isolate enhances its abilities to kill chicken embryos, grow in human urine, and colonize the murine kidney. Infect Immun 74: 6287–6292. doi: 10.1128/IAI.00363-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52: 1 doi: 10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- 29.Pillai SK, Moellering R, Eliopoulos G (2005) Antimicrobial combinations In: Lorian V, editors. Antibiotics in Laboratory Medicine. Baltimore: Wlliams & Wilkins; pp. 365–441. [Google Scholar]
- 30.Pors SE, Olsen RH, Christensen JP (2014) Variations in virulence of avian pathogenic Escherichia coli demonstrated by the use of a new in vivo infection model. Vet Microbiol 170: 368–374. doi: 10.1016/j.vetmic.2014.02.043 [DOI] [PubMed] [Google Scholar]
- 31.Darling AC, Mau B, Blattner FR, Perna NT (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14: 1394–1403. doi: 10.1101/gr.2289704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35: W71–W74. doi: 10.1093/nar/gkm306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronco T, Stegger M, Olsen RH, Sekse C, Nordstoga AB, Pohjanvirta T et al. (2017) Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genomics 18: 13 doi: 10.1186/s12864-016-3415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens A, Wilson IG (1996) The haematoxylins and eosin In: Theory and practice of histological techniques. New York: Churchill Livingstone; pp. 99–112. [Google Scholar]
- 35.Rossez Y, Holmes A, Lodberg-Pedersen H, Birse L, Marshall J, Willats WG et al. (2014) Escherichia coli common pilus (ECP) targets arabinosyl residues in plant cell walls to mediate adhesion to fresh produce plants. J Biol Chem 289: 34349–34365. doi: 10.1074/jbc.M114.587717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goossens H, Ferech M, Vander SR, Elseviers M (2005) Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365: 579–587. doi: 10.1016/S0140-6736(05)17907-0 [DOI] [PubMed] [Google Scholar]
- 37.Ewers C, Li GW, Wilking H, Kiessling S, Alt K, Antao EM et al. (2007) Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int J Med Microbiol 297: 163–176. doi: 10.1016/j.ijmm.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 38.Olsen RH, Thofner IC, Pors SE, Pires Dos ST, Christensen JP (2016) Experimental induced avian E. coli salpingitis: Significant impact of strain and host factors on the clinical and pathological outcome. Vet Microbiol 188: 59–66. doi: 10.1016/j.vetmic.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 39.Li L, Thofner I, Christensen JP, Ronco T, Pedersen K, Olsen RH (2016) Evaluation of the efficacy of an autogenous Escherichia coli vaccine in broiler breeders. Avian Pathol 1–26. doi: 10.1080/03079457.2016.1267857 [DOI] [PubMed] [Google Scholar]
- 40.EUCAST (2015) European committe on antimicrobial susceptibility testing. Antimicrobial wild type distributions of microorganisms.
- 41.Singer RS, Hofacre CL (2006) Potential impacts of antibiotic use in poultry production. Avian Dis 50: 161–172. doi: 10.1637/7569-033106R.1 [DOI] [PubMed] [Google Scholar]
- 42.Graham JP, Boland JJ, Silbergeld E (2007) Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep 122: 79–87. doi: 10.1177/003335490712200111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howell J, MacDonald DW, Christian RG (1970) Inclusion body hepatitis in chickens. Can Vet J 11: 99–101. [PMC free article] [PubMed] [Google Scholar]
- 44.Peretti S, Judge R, Hindmarch I (2000) Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl 403: 17–25. [DOI] [PubMed] [Google Scholar]
- 45.Taragano F, Lyketsos CG, Paz J, Schapira M, Comesana DE, Klimovsky S (1999) An open-label trial of sertraline for the treatment of major depression in primary care. Ann Clin Psychiatry 11: 67–71. [DOI] [PubMed] [Google Scholar]
- 46.Feltenstein MW, Sufka KJ (2005) Screening antidepressants in the chick separation-stress paradigm. Psychopharmacology (Berl) 181: 153–159. doi: 10.1007/s00213-005-2227-1 [DOI] [PubMed] [Google Scholar]
- 47.Amita H, Matsunami S, Matsushima T (2008) Impulsive choice in chicks: Effects of competitive foraging and SSRI (fluvoxamine). Neurosci Res 61: 112. [Google Scholar]
- 48.Gobin V, Van SK, Denys D, Deforce D (2014) Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. Int Immunopharmacol 20: 148–156. doi: 10.1016/j.intimp.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 49.Baharav E, Bar M, Taler M, Gil-Ad I, Karp L, Weinberger A et al. (2012) Immunomodulatory effect of sertraline in a rat model of rheumatoid arthritis. Neuroimmunomodulation 19: 309–318. doi: 10.1159/000339109 [DOI] [PubMed] [Google Scholar]
- 50.Taler M, Gil-Ad I, Korob I, Weizman A (2011) The immunomodulatory effect of the antidepressant sertraline in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neuroimmunomodulation 18: 117–122. doi: 10.1159/000321634 [DOI] [PubMed] [Google Scholar]
- 51.Frick LR, Rapanelli M, Arcos ML, Cremaschi GA, Genaro AM (2011) Oral administration of fluoxetine alters the proliferation/apoptosis balance of lymphoma cells and up-regulates T cell immunity in tumor-bearing mice. Eur J Pharmacol 659: 265–272. doi: 10.1016/j.ejphar.2011.03.037 [DOI] [PubMed] [Google Scholar]
- 52.Frick LR, Palumbo ML, Zappia MP, Brocco MA, Cremaschi GA, Genaro AM (2008) Inhibitory effect of fluoxetine on lymphoma growth through the modulation of antitumor T-cell response by serotonin-dependent and independent mechanisms. Biochem Pharmacol 75: 1817–1826. doi: 10.1016/j.bcp.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 53.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A et al. (2013) Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121: 1008–1015. doi: 10.1182/blood-2012-06-437392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C et al. (2007) Anti-inflammatory properties of desipramine and fluoxetine. Respir Res 8: 35 doi: 10.1186/1465-9921-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purslow C (2013) Linking Clostridium difficile infection with depression. Expert Rev Anti Infect Ther 11: 763 [DOI] [PubMed] [Google Scholar]
- 56.Rogers MA, Greene MT, Young VB, Saint S, Langa KM, Kao JY et al. (2013) Depression, antidepressant medications, and risk of Clostridium difficile infection. BMC Med 11: 121 doi: 10.1186/1741-7015-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers MA, Aronoff DM (2013) Depression and clostridium difficile infection. J Psychosoc Nurs Ment Health Serv 51: 5 doi: 10.3928/02793695-20130903-01 [DOI] [PubMed] [Google Scholar]
- 58.Edgar VA, Genaro AM, Cremaschi G, Sterin-Borda L (1998) Fluoxetine action on murine T-lymphocyte proliferation: participation of PKC activation and calcium mobilisation. Cell Signal 10: 721–726. [DOI] [PubMed] [Google Scholar]
- 59.Seto F (1981) Early development of the avian immune system. Poult Sci 60: 1981–1995. [DOI] [PubMed] [Google Scholar]
- 60.Lillehoj HS (1991) Cell-mediated immunity in parasitic and bacterial diseases In: Sharma JM, editors. Avian cellular immunology. Boca Raton, Fla.: CRC Press; pp. 151–181. [Google Scholar]
- 61.Taler M, Gil-Ad I, Lomnitski L, Korov I, Baharav E, Bar M et al. (2007) Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur Neuropsychopharmacol 17: 774–780. doi: 10.1016/j.euroneuro.2007.03.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are included in the paper and it Supporting Information files.