Abstract
The hypothalamic hormone oxytocin (OXT) plays an important role in a range of physiological processes and social-emotional functioning in both sexes. In women, physiological stimuli, such as suckling and parturition, result in pulsatile release of OXT into the peripheral circulation via the posterior pituitary gland. However, data regarding OXT secretory patterns in men during a state of rest are limited. Further, the relationship between secretory dynamics of OXT and emotional measures has never been evaluated. We hypothesized a pulsatile pattern of OXT secretion in men, and explored the relationship between OXT secretory patterns and social-emotional functioning.
Methods
Deconvolution analysis was performed on serum OXT levels obtained every 5 minutes over a period of 10 hours in 5 healthy normal weight men. Area under the curve (AUC), average OXT values, and pulse characteristics [pulse number, inter-pulse interval, pulse height and mass (area under each pulse)] were calculated. State Adult Attachment Measure (SAAM) assessed types of human attachment. Interpersonal Support Evaluation List (ISEL) assessed perception of social support. Toronto Alexithymia Scale (TAS-20) measured the ability to express and identify one's own emotions.
Results
Mean age was 22.8±1.2 years, and BMI was 21.7±0.4 kg/m2 (mean±SEM). Assuming a basal secretion of zero and a half life of five to seven minutes, we demonstrated the following: OXT AUC: 5422 ± 595 pg/ml/10hr, mean OXT level: 9.1 ± 1.0 pg/ml, mean pulse number: 22 ± 1/10hr, mean pulse height: 1.81 ± 0.21 pg/ml, mean pulse mass: 30.34 ± 4.6 pg/ml and mean inter-pulse interval: 27 ± 2 min. The SAAM Avoidant scale correlated negatively with mean OXT pulse height (r=-0.90, p=0.04) and pulse mass (r=-0.95, p=0.01). The ISEL Belonging score correlated positively with OXT AUC (r=0.89, p=0.04) and average OXT (r=0.93, p= 0.02). ISEL Appraisal score also had a positive association with mean OXT pulse height (r=0.99, p=0.0006) and pulse mass (r=0.98, p=0.003). Finally, ISEL total score had a significant correlation with average OXT values (r=0.90, p=0.04). While none of the subjects had a score in the alexithymia range, TAS-20 Difficulty describing feelings score had an inverse correlation with OXT pulse height (r=-0.96, p=0.01) and pulse mass (r=-0.99, p=0.001). TAS-20 total score also had an inverse correlation with OXT pulse height (r=-0.94, p=0.02) and pulse mass (r=-0.96, p=0.009).
Conclusion
We demonstrate a pulsatile pattern of peripheral OXT secretion in healthy men at rest. Subjects with lower OXT pulse height and pulse mass had a more avoidant style of attachment, felt less supported, and expressed greater difficulty in describing their feelings. Our findings support the concept that OXT is a key mediator of social-emotional functioning. Future studies to determine causality are warranted.
Keywords: Oxytocin, Secretory Dynamics, Social-Emotional functioning
Introduction
The neurohypophyseal hormone oxytocin (OXT), long known for its role in lactation and parturition, is increasingly recognized as a key mediator in a range of physiologic processes in both sexes1-4. Parvocellular neurons of the hypothalamic paraventricular nucleus (PVN) release OXT within the brain, while action potentials in magnocellular neurons of the PVN and supraoptic nucleus (SON) result in the release of OXT into the peripheral circulation via the posterior pituitary gland5. Physiological stimuli such as suckling and parturition are associated with an increase in excitatory bursts in these neurons and result in pulsatile release of OXT into the peripheral circulation6. Studies in chronically catheterized female sheep show an episodic OXT release not only during parturition, but also during non-pregnant states7.
In humans, peripheral OXT levels increase in response to physiological factors such as lactation8 and parturition9 in women, and sexual stimulation10 in men, suggesting pulsatile secretion. However, data regarding the secretory dynamics of OXT in men and women at rest, during which the effect of external stimuli are minimized, and thus variation can be attributed to mostly intrinsic sources, are limited. Further, prior studies utilized the fluctuations in peak peripheral levels over the baseline levels to detect the pulsatile nature of secretion. The “gold standard” for detection of hormone secretory patterns, deconvolution, is a mathematical technique that identifies secretory events based on serial peripheral hormone levels11. Deconvolution-based estimates of secretory bursts largely rely on the rate of elimination of hormones from the circulation and therefore on the sampling frequency. Due to its short half-life12, 13, OXT must be measured from blood samples taken at a frequency of at least every five minutes in order to determine secretory patterns. To date, deconvolution studies for OXT have not been performed in humans. Understanding the pattern of OXT secretion may help provide mechanistic insights into the actions of this important hormone.
Studies suggest that OXT may play a key role in mediating social-emotional functioning in animal models as well as in humans. In rodents, chronic OXT infusion into the lateral ventricles of the brain significantly promotes adult social interactions such as physical contact and auto grooming behaviors14. In humans, the presence of specific single nucleotide polymorphisms in the OXT receptor has been linked to pair bonding behavior in adults, suggesting a role for OXT in social attachment15. Children and adults with autism are characterized by difficulties with social interaction, and a single-dose of intranasal OXT improves social-emotional functioning in this population16, reinforcing its link with social-emotional functioning. Furthermore, in healthy adults, intranasal administration of OXT is associated with improved processing of social signals (e.g., decoding facial expressions)17 and an increase in social trust18. Confirmatory evidence implicating OXT in social-emotional functioning comes from neuroimaging studies, which demonstrate reduced amygdala activation to fearful faces with OXT administration in healthy males19.
In addition to its critical role in mediating many aspects of social support and attachment, OXT may be involved in interoception20, which refers to the ability to understand one's own emotions. It has been proposed that a dysregulation of the OXT system, which plays a major part in encoding interoceptive signals, could be responsible for the verbal and language deficits in autism20. However, the relationship between social-emotional functioning and OXT secretory dynamics has not been examined in humans. Deconvolution analysis would allow for in depth examination of the relationship between pulsatile and integrated OXT secretory parameters and social-emotional measures and may improve our understanding of how OXT levels relate to social behavior. Our objective was to determine resting state secretory dynamics of OXT in men, who are not subject to cyclical changes in hormone levels across the menstrual cycle that are known to impact OXT secretion in women21. Further, OXT's socio-emotional effects may be sexually dimorphic19, 22-24. In order to reduce the variability of our results from gender specific effects, we limited our recruitment to healthy men only for this preliminary study. We hypothesized a pulsatile pattern of resting state OXT secretion. As a secondary exploratory aim of the study, we examined the relationship between OXT secretory pulse parameters and measures of social-emotional functioning.
Methods
Subjects
The study was approved by the Partners Human Research Committee. Informed consent was obtained from all subjects who participated in the study. We recruited five healthy men with normal body weight (BMI between 18.5 and 24.9) from the community between May and September 2013. We excluded subjects with serious medical conditions (e.g., diabetes mellitus, cardiovascular disease, untreated thyroid disease); anemia; a history of gastrointestinal surgery; psychiatric disease or history of taking psychotropic medications; history of an eating disorder; active substance abuse; tobacco use; or excessive exercise within three months of the study (running more than 25 miles in any one week or having exercised more than 10 hours in any one week).
Study Procedures
Eligibility was determined at a screening visit that included a comprehensive medical history, complete physical exam with height, weight, and vital signs, and blood draw for laboratory evaluations. Paffenbarger activity assessment was used to evaluate exercise patterns. The Eating Disorder Module of the Structured Clinical Interview for DSM Disorders-IV (SCID) was administered in person by trained study personnel to evaluate for disordered eating.
All subjects were hospitalized overnight for the main study visit. An intravenous catheter was inserted by 7pm, and subjects began fasting at 8 pm. Frequent serum sampling for OXT was performed every 5 minutes from 10 pm until 8 am to obtain a total of 120 samples from each subject. The frequency of sampling was determined based on the requirement for deconvolution analysis of intervals less than or equal to the half-life of OXT12, 13. The following questionnaires were completed before blood sampling began: State Adult Attachment Measure (SAAM), Interpersonal Support Evaluation List (ISEL), and the Toronto Alexithymia Scale 20 (TAS-20). Subjects were allowed to sleep through the night in a dark and quiet environment, and any interaction and conversation with subjects during this time was minimized to avoid effects of social stimulation on OXT levels. For the same reasons, subjects were restricted from using television, phones or reading after 9 pm.
Questionnaires
State Adult Attachment Measure (SAAM)25
This is a measure of attachment of an individual toward others, designed to capture an individual's situation-dependent sense of attachment security, anxiety, and avoidance. The measure consists of 21 Likert-like questions. Each scale runs from 1 to 7 with a midpoint of 4. The SAAM measures three different aspects of adult attachment, namely: a. Security; b. Anxiety; and, c. Avoidance. High scores on the Security Scale indicate a current presence of security-related strategies. Higher scores on the Anxiety Scale indicate a higher current anxiety about attachment, while higher scores on the Avoidance Scale indicate a stronger current tendency toward avoidance of attachment.
Interpersonal Support Evaluation List (ISEL)26
This is a 40-item self-report questionnaire that measures perceived availability of four types of social support: a. Tangible support (availability of material help); b. Belonging support (perception of doing things with others); c. Self esteem (perception of one's own positive comparison with others); and d. Appraisal support (a measure of perception of having someone to talk to). Subjects rate each on a 4-point scale ranging from “Definitely true” to “Definitely false”. Summative scores are calculated for each type of social support with higher scores indicating better support.
Toronto Alexithymia Scale 20 (TAS-20)27, 28
This is a 20-item self-report scale that measures the degree of alexithymia, an individual's difficulty in understanding his/her own emotions, using a global score and three subscales: a. Difficulty describing feelings; b. Difficulty identifying feelings; and, c. Externally-oriented thinking (the tendency to focus attention on external stimuli rather than internal sensations). A global score of ≤51 indicates non-alexithymia, scores between 52 and 60 indicate possible alexithymia, and scores ≥61 indicate alexithymia.
Biochemical Analysis
Samples were frozen and stored at -80° C until analysis. Plasma OXT was measured following extraction using an ELISA (Assay Designs, Ann Arbor, MI). The extraction procedure involves acidification (which precipitates very large proteins) and solid phase binding of hydrophobic peptides and proteins to remove hydrophilic molecules that likely interfere with the assay. It may also remove large molecular weight proteins that may bind or cross-link OXT29, 30. Extraction was performed according to the protocol recommended by the ELISA manufacturer. Briefly, specimens were acidified with an equal volume of 0.01% trifluoroacetic acid (TFA) and then subjected to solid phase extraction using C-18 Sep-Pak® columns eluted with TFA/acetonitrile. The eluted protein fraction was lyophilized. Lyophilized extracts were reconstituted with a minimal volume of assay buffer to concentrate the oxytocin prior to assay. Assay results were corrected for concentration factor and are reported in the range of 3-30 pg/ml for the original specimen. Pooled serum quality control (QC) specimens were processed in the same manner for each batch of specimens tested. For unextracted OXT run in duplicate, the inter-assay and intra-assay coefficients of variation (CV) for the low range (51pg/ml) were 9.5% and 4.5%, and for the high range (371 pg/ml) were 9.5% and 3.7%, respectively. The inter-assay CV for extracted QCs containing 32 to 411pg/mL OXT was 11.8 to 18.7%, respectively.
We analyzed the secretory dynamics of OXT using deconvolution analysis software31. A basal rate of zero and a half-life of five to seven minutes was assumed for the deconvolution analysis based on the reported half life of OXT12, 13. The software calculated the OXT area under the curve, average oxytocin levels, the number of pulses, frequency of pulse interval, mean pulse height (the height of a pulse over the determined basal value) and mean pulse mass (area under the curve for each pulse). The magnitude of pulsatile secretion is determined by the mean pulse height and pulse mass. These variables (mean pulse height and mass) along with the number of pulses secreted in a given period contribute to the variation in the peripheral levels of OXT over a given period of time.
Approximate Entropy (ApEn) was used to measure the repetitiveness of patterns in this time series data set. The more repetitive the patterns are, the smaller the ApEn and the less random the data. The ApEn program of the Decon software provides the ability to shuffle the data more than 1000 times and provides a p value for randomness. A low p value indicates that there is a low probability that these data are random.
Statistical Analysis
We used JMP12 PRO for analysis (SAS Institute, NC). CV of OXT levels between subjects and within each subject were calculated using the mean and standard deviation values of OXT with the formula: CV=(standard deviation/mean)×100. Pearson's correlations were used to assess the relationships between secretory characteristics of OXT and social-emotional functioning measures. A p-value of <0.05 was considered statistically significant.
Results
The mean (±SEM) age of the subjects was 22.8±1.2 years, and mean BMI was 21.7±0.4 kg/m2. Subjects reported no alcohol consumption or participation in physical activity in the 24 hours leading up to the study visit, as instructed.
Variability of OXT Between Subjects and Within Each Subject
The inter-subject CV, defined as the variability of OXT levels between subjects, was 24%. Within each individual, the CV for OXT levels ranged from 33-51%.
OXT Deconvolution Analysis
Figure 1 depicts pulsatile secretory patterns in the subjects. OXT area under the curve was 5422±595 pg/mL/10h and the mean OXT level was 9.1±1.0 pg/mL. Assuming a basal secretion of zero and a half-life of five-seven minutes, we demonstrated a mean of 22±1 significant pulses over the ten-hour sampling period. The mean pulse height was 1.81±0.21 pg/mL with a mean pulse mass of 30.3±4.6 pg/mL and mean inter pulse interval of 27±2 minutes. Despite having 120 samples per subject, deconvolution analysis of the data resulted in high residuals, indicating a residual difference between the actual data points and the fitted curve (Figure 1). The p values for randomness obtained from the ApEn program for our subjects were all less than 0.1, indicating the non-randomness of our data.
Figure 1. Deconvolution analysis.
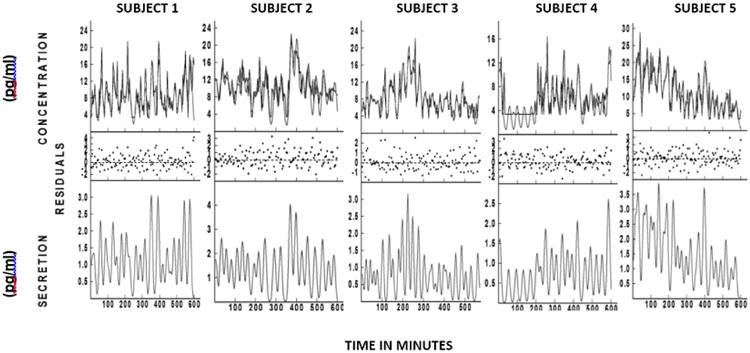
The top panel depicts the absolute concentration of OXT over time for each of the subjects. The middle panel represents the residuals. The bottom panel depicts secretory bursts of OXT for the same period of time. The Y axis represents the magnitude of the secretory bursts (pg/ml) and the X axis represents time in minutes.
OXT Secretory Parameters and Social-Emotional Functioning
Table 1 reports scores of social-emotional functioning measures. The relationships between pulsatile characteristics of OXT and social-emotional functioning are presented in Table 2.
Table 1. Measures of social-emotional functioning in study participants.
| Assessment | Scores |
|---|---|
| State Adult Attachment Measure | |
| Secure | 45.6±0.93 |
| Anxious | 23.8±2.39 |
| Avoidant | 14.4±2.16 |
| Interpersonal Support Evaluation List | |
| Appraisal | 25.8±1.59 |
| Belonging | 27.2±1.24 |
| Self esteem | 23.2±0.73 |
| Tangible | 26.8±1.83 |
| Total score | 103.0±4.73 |
| Toronto Alexithymia Scale-20 | |
| Difficulty describing feelings | 12±1.73 |
| Difficulty identifying feelings | 10.4±2.04 |
| Externally oriented thinking | 15.6±1.47 |
| Total score | 38.0±3.29 |
Values are presented as Mean±SEM.
Table 2. Correlations of oxytocin secretory characteristics with socio-emotional measures.
| Assessment | Mean Pulse Mass | Mean Pulse Height | AUC | Average Value |
|---|---|---|---|---|
| State Adult Attachment Measure | R (p value) | R (p value) | R (p value) | R (p value) |
| Secure | 0.77 (0.12) | 0.80 (0.10) | 0.87 (0.06) | 0.85 (0.07) |
| Anxious | 0.63 (0.25) | 0.60 (0.29) | 0.50 (0.39) | 0.47 (0.43) |
| Avoidant | -0.95 (0.01) | -0.90 (0.04) | -0.82 (0.09) | -0.77 (0.13) |
| Interpersonal Support Evaluation List | ||||
| Appraisal | 0.98 (0.003) | 0.99 (0.0006) | 0.88 (0.05) | 0.84 (0.07) |
| Belonging | 0.54 (0.35) | 0.72 (0.17) | 0.89 (0.04) | 0.93 (0.02) |
| Self esteem | 0.35 (0.57) | 0.53 (0.36) | 0.53 (0.36) | 0.56 (0.32) |
| Tangible | 0.29 (0.63) | 0.52 (0.37) | 0.69 (0.20) | 0.74 (0.15) |
| Total score | 0.65 (0.24) | 0.81 (0.09) | 0.88 (0.05) | 0.90 (0.04) |
| Toronto Alexithymia Scale-20 | ||||
| Difficulty describing feelings | -0.99 (0.001) | -0.96 (0.01) | -0.84 (0.07) | -0.79 (0.11) |
| Difficulty identifying feelings | -0.85 (0.07) | -0.72 (0.17) | -0.45 (0.44) | -0.38 (0.52) |
| Externally oriented thinking | 0.20 (0.75) | 0.02 (0.97) | -0.07 (0.91) | -0.12 (0.85) |
| Total score | -0.96 (0.009) | -0.94 (0.02) | -0.75 (0.14) | -0.71 (0.18) |
AUC: Area under the curve, R represents the Pearsons' correlation coefficient. Significant p values are bolded.
SAAM
The SAAM Avoidant scale, on which higher scores indicate a more avoidant style of attachment, correlated negatively with mean OXT pulse height and pulse mass (r=-0.90, p=0.04 and r=-0.95, p=0.01, respectively).
ISEL
The ISEL Belonging score, for which higher scores indicate subjective perception of engaging in more activities with others, correlated positively with OXT AUC (r=0.89, p=0.04) and average OXT value (r=0.93, p= 0.02). The ISEL Appraisal score, for which higher scores indicate subjective perception of having someone to talk to, also had a positive association with mean OXT pulse height and pulse mass (r=0.99, p=0.0006 and r=0.98, p=0.003, respectively). Finally, ISEL total score had a significant direct correlation with OXT average values (r=0.90, p=0.04).
TAS-20
None of the subjects had a score in range for alexithymia, which measures the individual's difficulty in understanding his/her own emotions. The TAS-20 Difficulty describing feelings subscale score, where higher scores indicate more difficulties in describing one's own feelings, had an inverse correlation with OXT pulse height (r=-0.96, p=0.01) and pulse mass (r=-0.99, p=0.001). The TAS-20 total score, for which higher levels reflect higher degrees of alexithymia, also had an inverse correlation with OXT pulse height (r=-0.94, p=0.02) and pulse mass (r=-0.96, p=0.009).
The rest of the measures of socio-emotional functioning had no significant correlations with OXT pulse characteristics.
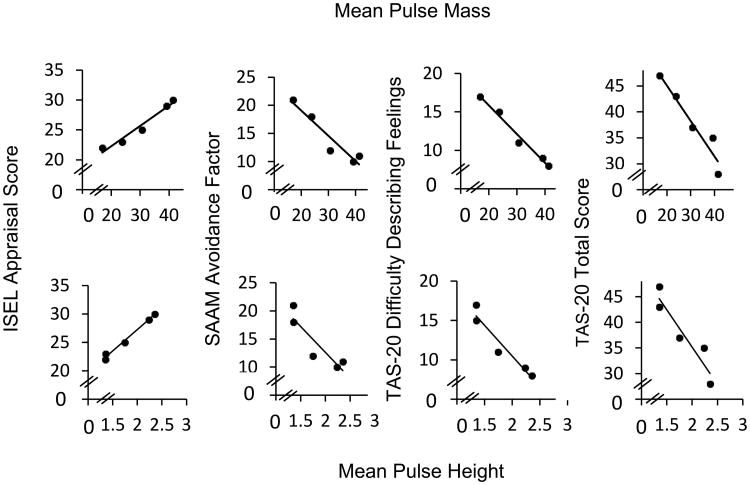
Figure 2 shows scatter plots of the relationship between OXT pulse characteristics and social-emotional functioning measures.
Figure 2. Scatter plots of mean pulse height and mean pulse mass with ISEL Appraisal, SAAM Avoidance Factor and TAS-20 Difficulty describing feelings and total TAS-20 scores.
The figure shows a positive correlation of ISEL Appraisal scores with oxytocin mean pulse height (bottom row) and pulse mass (top row), and negative correlations of SAAM Avoidance scores and TAS-20 Difficulty describing feelings and total score with oxytocin mean pulse height and mass. ISEL: Interpersonal Support Evaluation List, SAAM: State Adult Attachment Measure, TAS-20: Toronto Alexithymia Scale-20.
Discussion
To our knowledge this is the first description of OXT secretory dynamics using sophisticated deconvolution analysis in humans. In this novel physiologic study, we performed deconvolution analysis on serum OXT levels sampled every 5 minutes overnight in healthy men at rest, and demonstrate a pulsatile pattern of OXT secretion. In addition, we show robust correlations between OXT secretory parameters and measures of social-emotional functioning.
OXT was one of the first hormones found to be released in pulses, leading researchers to propose a pulsatile release of other hypothalamic hormones32. Animal studies have identified a pulsatile pattern of OXT secretion during parturition, lactation6, 7 as well as in non-pregnant states in females. In anesthetized male rats, stimuli such as touch and pain elicit an increase in plasma OXT levels33. Similarly in humans, multiple physiological stimuli impact OXT secretion in both sexes. In females, lactation and parturition are the main physiological states during which OXT is secreted in pulses9, 34. In men, peripheral levels have been shown to increase during sexual arousal10. Some authors have utilized peripheral OXT levels during physiological stimuli to estimate OXT pulse characteristics in humans9, 34, 35. Ueda et al defined an OXT pulse as an increase in peripheral OXT of more than 2 standard deviations above the basal OXT concentration34. In this study conducted in lactating women, blood samples were collected every two minutes for a 30 minute period and the authors observed 2.25±0.71 pulses over 20 minutes of suckling34 .
Although these data support a pulsatile peripheral OXT pattern during physiological stimuli in both sexes, studies in humans have not yet shown a pulsatile pattern of ‘resting state’ OXT release. In contrast to data in female animals that demonstrate pulsatile OXT secretion in the non-pregnant resting state7, Amico et al found no OXT pulses in six men and women under basal conditions in peripheral blood samples obtained every 15 minutes over four hours 21. Limitations in this study included the use of variation in peripheral OXT levels to evaluate for the presence of pulses and low frequency of blood sampling, which may have reduced the ability to detect pulses.
Thus far very few studies have described detailed secretory characteristics of OXT in humans and state of the art methodology, deconvolution analysis, has never been used to determine OXT dynamics. To our knowledge, one study conducted in mares has used sophisticated methodology (cluster analysis) to determine OXT secretory dynamics36. Another study conducted in humans utilized a pulse detection program called “Pulsar”, in which original serial hormone concentrations are superimposed on smoothed series of hormonal values to detect pulses. In this study, Marchini et al demonstrated one pulse in peripheral blood samples obtained every 20 seconds for 4 min in 42% of newborn babies37. When used in lactating women, “Pulsar” technique demonstrated an average of one OXT pulse during 10 minutes of breast feeding35. In women in labor, this technique yielded 1.2 ± 0.54 pulses per 30 minutes before labor, which increased to 4.2 and 6.7 pulses per 30 minutes during the first stage, and second/third stages of labor, respectively9. Challinar at al assessed the effect of menstrual cycle and fasting on peripheral OXT levels in healthy women and men, respectively, using a “Detect program”, a pulse detection software similar to “Pulsar”, and demonstrated no OXT pulses under basal conditions38. Of note, these techniques do not provide details of the secretory events and do not account for the rate of elimination from the circulation39.
In our study of men, we demonstrate approximately 22 pulses (range: 19-27) over 10 hours occurring about every 27 minutes. The mean pulse height in our study was 1.81±0.21 pg/ml. The pulse frequency noted in our study is similar to that reported in pregnant women prior to labor and less frequent than the pulse frequency reported in women during active labor or lactation9, 34, 35. The pulse amplitude we found in men (1.81±0.21 pg/ml) is lower than that reported in lactating women (9.1±1.3 pg/ml)34, which may be due to physiological differences (e.g., influence of lactation; or higher levels of estrogen, a hormone known to stimulate oxytocin secretion, in the women21), or assay differences. The behavioral and metabolic effects of OXT are sexually dimorphic19, 22-24; whether this is partly related to differences in the timing, amplitude and/or burst mass of OXT pulses between sexes is an important area for future investigation. Another interesting finding we elucidate is the biological variability of OXT levels overnight within an individual as well as between subjects. We show here that, within the same individual at rest, levels of OXT vary as much as 33-51%. Further, we demonstrate that OXT levels vary by 24% between subjects. The magnitude of variation within and between individuals is much larger than the CV of the assay. In a study of tamarins by Snowdon et al where basal OXT levels were assessed by measuring urinary OXT for 3 days per week for 3 weeks, there was a tenfold variation in urinary OXT values between individuals of the same sex40. Similarly, in humans, a wide range of OXT values were observed between subjects during the same phases of pregnancy41. Consistent with these reports of biological variability of OXT, we show that resting state OXT levels are highly variable within and between men.
Affiliative behaviors have been found to be associated with peripheral OXT levels in animals as well as in humans, suggesting an association of OXT with social-emotional functioning40, 42. Several studies also attest to the crucial role of OXT in mediating maternal fetal bonding43, 44 and indicate that post partum OXT levels influence bonding between mother and infant45. In a study of healthy males with insecure attachment, a single dose of intranasal OXT increased the feeling of secure attachment46. Further, it has been postulated that low OXT levels mediate the disorganized attachment representations in patients with borderline personality disorders47. In our study, scores on the SAAM Avoidant scale, a measure of aversion to intimacy and closeness, had a negative association with OXT pulse height and pulse mass, reinforcing this link between OXT and attachment. While we found an inverse association between avoidant attachment and OXT levels, others have shown a positive relationship between secure attachment style and OXT45. In this study, mothers with secure attachment had higher peripheral OXT levels during physical interaction with infants associated with fMRI activation in reward areas of the brain45. Together, these studies indicate that OXT may modulate attachment types in humans45. Our data suggest that pulsatile secretion of OXT may be of relevance with regard to the types of attachment.
The perception of having friends or family for support is crucial to psychological well-being. Men who received intranasal OXT felt supported during stressful situations in a manner similar to having close friends around, indicating that OXT may act to enhance social support in humans48. Consistent with these findings, a prior study showed that men and women who reported better social support also had higher OXT levels before and after warm contact with their partners49. Our study found that men who feel more supported have greater pulsatile OXT secretion, consistent with OXT's established role in social support.
OXT has been implicated in better decoding of facial signals with improved performance in ‘reading the mind test’17. While these data attest for the role of OXT in inferring others' mental states, studies of individuals with autism shed light on the importance of OXT in interoception20. The TAS-20 Describing feelings and total scores had significant negative correlations with OXT pulse height and mass in our study, suggesting that larger OXT pulses are associated with a better ability to understand and express one's own emotions. The findings of Luminet et al also support this observation in that subjects with higher alexithymia scale scores assessed by TAS-20 performed better at complex emotion recognition when administered OXT compared to placebo50.
A limitation of our study is the inability to completely resolve the secretory patterns of OXT using deconvolution analysis despite having about 120 samples for each individual. Our study indicates that more than one data point in a 5-minute period is necessary to optimally capture pulse characteristics of OXT using deconvolution analysis. Our analyses with samples every 5 minutes resulted in high residuals; a higher frequency of sampling would circumvent this issue. This first study included five healthy men at rest. Future studies aimed at sampling at 2-3 minute intervals in a larger population in both sexes are warranted in order to provide further insight into the secretory patterns of OXT.
The measurement of OXT in peripheral blood is controversial29, 51, 52. For our study, we used an ELISA following extraction, a method considered to be among the best available options29, 51, 53. It is also important to note that central and peripheral OXT secretion may be coordinated or independent of one another; therefore plasma levels may not reflect central functioning54, 55, 56. Furthermore, some authors have questioned the behavioral effects of intranasal OXT due to the lack of dose response data and methodological drawbacks of earlier studies30, 57.
Both emotional functioning and OXT levels may be affected by stress and anxiety,58 indicating that there could be common mediators underlying the associations we demonstrate in this study. Further, variations in the state of wakefulness during study procedures might contribute to the variability in OXT levels. Some authors have proposed that effects of OXT on social cognition and functioning may be selective and depend the individual's social proficiency59 and that these effects may not be generalizable to all populations. Our exploratory analysis suggests that OXT may have an important role in socio-emotional functioning in healthy men. Whether the associations that we identified indicate causality and/or are applicable to different populations and both sexes needs to be investigated in detail in future studies. Further, the association of measures of attachment and interoception with only OXT pulse characteristics and not with average OXT levels highlights the importance of assessing OXT secretory dynamics, and the need to examine the interactions between OXT pulse characteristics and social-emotional functioning measures in a larger number of subjects of both genders.
Conclusion
In this first study using deconvolution analysis to examine resting state secretory dynamics of OXT in healthy men, we have demonstrated that OXT is released in pulsatile manner. Men with lower OXT pulse height and pulse mass displayed a more avoidant style of attachment, felt less supported, and expressed greater difficulty in describing their feelings. The strong associations of OXT secretory parameters with attachment style, perceptions of social support, and measures of interoception are consistent with OXT's important role in social emotional functioning in humans.
Highlights.
Oxytocin is secreted in a pulsatile manner in healthy men during a resting state.
Oxytocin pulse characteristics (pulse height and pulse mass) correlate positively with measures of social support and negatively with avoidant attachment style and with measures of interoception.
Our findings support the hypothesis that oxytocin plays a major role in mediating socio-emotional functioning in humans.
Acknowledgments
Funding Sources: This study was supported by funding from the following sources:
National Institutes of Health Grant K23 MH092560, Bethesda, MD
Harvard Catalyst, the Harvard Clinical and Translational Science Center UL1 RR025758, Boston, MA
National Institutes of Health Grant Training grant T32 DK007028
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caldwell HK, Aulino EA, Freeman AR, Miller TV, Witchey SK. Oxytocin and behavior: Lessons from knockout mice. Dev Neurobiol. 2016 doi: 10.1002/dneu.22431. [DOI] [PubMed] [Google Scholar]
- 2.Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 3.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 4.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 5.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 6.Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: recruitment and synchronization. J Physiol. 1986;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell MD, Kraemer DL, Brennecke SP, Webb R. Pulsatile release of oxytocin during the estrous cycle, pregnancy and parturition in sheep. Biol Reprod. 1982;27:1169–1173. doi: 10.1095/biolreprod27.5.1169. [DOI] [PubMed] [Google Scholar]
- 8.McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed) 1983;286:257–259. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515–1523. doi: 10.1016/0002-9378(91)90399-c. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- 11.Johnson ML, Virostko A, Veldhuis JD, Evans WS. Deconvolution analysis as a hormone pulse-detection algorithm. Methods Enzymol. 2004;384:40–54. doi: 10.1016/S0076-6879(04)84004-7. [DOI] [PubMed] [Google Scholar]
- 12.Chard T, Boyd NR, Forsling ML, McNeilly AS, Landon J. The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J Endocrinol. 1970;48:223–234. doi: 10.1677/joe.0.0480223. [DOI] [PubMed] [Google Scholar]
- 13.Fabian M, Forsling ML, Jones JJ, Pryor JS. The clearance and antidiuretic potency of neurohypophysial hormones in man, and their plasma binding and stability. J Physiol. 1969;204:653–668. doi: 10.1113/jphysiol.1969.sp008937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]
- 15.Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsater H, Larsson H, Westberg L. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71:419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, Iwashiro N, Natsubori T, Inoue H, Suga M, Takao H, Sasaki H, Gonoi W, Kunimatsu A, Kasai K, Yamasue H. Oxytocin improves behavioural and neural deficits in inferring others' social emotions in autism. Brain. 2014;137:3073–3086. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- 17.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 19.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–430. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amico JA, Seif SM, Robinson AG. Oxytocin in human plasma: correlation with neurophysin and stimulation with estrogen. J Clin Endocrinol Metab. 1981;52:988–993. doi: 10.1210/jcem-52-5-988. [DOI] [PubMed] [Google Scholar]
- 22.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Murai Y, Nakashima T, Miyata S, Kiyohara T. Different effect of oxytocin on membrane potential of supraoptic oxytocin neurons in virgin female and male rats in vitro. Neurosci Res. 1998;30:35–41. doi: 10.1016/s0168-0102(97)00117-x. [DOI] [PubMed] [Google Scholar]
- 25.Omri Gillath JH, Noftle Erik E, Stockdale Gary D. Development and validation of a state adult attachment measure (SAAM) Journal of Research in Personality. 2009;43:362–373. [Google Scholar]
- 26.Sheldon Cohen RM, Kamarck Tom, Hoberman Harry M. Social Support: Theory, Research and Applications. Ed IGSe al; 1985. Measuring the Functional Components of Social Support; pp. 73–94. [Google Scholar]
- 27.Bagby RM, Taylor GJ, Parker JD. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 28.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 29.Leng G, Sabatier N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng G, Ludwig M. Intranasal Oxytocin: Myths and Delusions. Biol Psychiatry. 2016;79:243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Johnson ML. Deconvolution analysis of hormone data. Methods Enzymol. 1992;210:539–575. doi: 10.1016/0076-6879(92)10028-c. [DOI] [PubMed] [Google Scholar]
- 32.Lincoln DW. Dynamics of Oxytocin Secretion. 1974 [Google Scholar]
- 33.Stock S, Uvnas-Moberg K. Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiol Scand. 1988;132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x. [DOI] [PubMed] [Google Scholar]
- 34.Ueda T, Yokoyama Y, Irahara M, Aono T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obstet Gynecol. 1994;84:259–262. [PubMed] [Google Scholar]
- 35.Nissen E, Uvnas-Moberg K, Svensson K, Stock S, Widstrom AM, Winberg J. Different patterns of oxytocin, prolactin but not cortisol release during breastfeeding in women delivered by caesarean section or by the vaginal route. Early Hum Dev. 1996;45:103–118. doi: 10.1016/0378-3782(96)01725-2. [DOI] [PubMed] [Google Scholar]
- 36.Alexander SL, Irvine CH. The effect of endotoxin administration on the secretory dynamics of oxytocin in follicular phase mares: relationship to stress axis hormones. J Neuroendocrinol. 2002;14:540–548. doi: 10.1046/j.1365-2826.2002.00815.x. [DOI] [PubMed] [Google Scholar]
- 37.Marchini G, Stock S. Pulsatile release of oxytocin in newborn infants. Reprod Fertil Dev. 1996;8:163–165. doi: 10.1071/rd9960163. [DOI] [PubMed] [Google Scholar]
- 38.Challinor SM, Winters SJ, Amico JA. Pattern of oxytocin concentrations in the peripheral blood of healthy women and men: effect of the menstrual cycle and short-term fasting. Endocr Res. 1994;20:117–125. doi: 10.3109/07435809409030403. [DOI] [PubMed] [Google Scholar]
- 39.Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Boyd DG, Evans WS. AutoDecon, a deconvolution algorithm for identification and characterization of luteinizing hormone secretory bursts: description and validation using synthetic data. Anal Biochem. 2008;381:8–17. doi: 10.1016/j.ab.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58:614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 44.Kendrick KM. Oxytocin, motherhood and bonding. Exp Physiol. 2000;85 Spec No:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 45.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, O'Connor MF, Gundel H. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 2009;34:1417–1422. doi: 10.1016/j.psyneuen.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jobst A, Padberg F, Mauer MC, Daltrozzo T, Bauriedl-Schmidt C, Sabass L, Sarubin N, Falkai P, Renneberg B, Zill P, Gander M, Buchheim A. Lower Oxytocin Plasma Levels in Borderline Patients with Unresolved Attachment Representations. Front Hum Neurosci. 2016;10:125. doi: 10.3389/fnhum.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 49.Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- 50.Luminet O, Grynberg D, Ruzette N, Mikolajczak M. Personality-dependent effects of oxytocin: greater social benefits for high alexithymia scorers. Biol Psychol. 2011;87:401–406. doi: 10.1016/j.biopsycho.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 51.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 52.Rutigliano G, Rocchetti M, Paloyelis Y, Gilleen J, Sardella A, Cappucciati M, Palombini E, Dell'Osso L, Caverzasi E, Politi P, McGuire P, Fusar-Poli P. Peripheral oxytocin and vasopressin: Biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res. 2016;241:207–220. doi: 10.1016/j.psychres.2016.04.117. [DOI] [PubMed] [Google Scholar]
- 53.Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. J Clin Endocrinol Metab. 1990;71:1531–1535. doi: 10.1210/jcem-71-6-1531. [DOI] [PubMed] [Google Scholar]
- 55.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 56.Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol. 2013;25:668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- 57.Conlisk. Professor Zak's empirical studies on trust and oxytocin. J Econ Behav Organ. 2011;78:160–166. http://doi.org/10.1016/j.jebo.2011.01.002. [Google Scholar]
- 58.Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]