Abstract
Study Design.
A pooled patient-level analysis of two multicenter randomized controlled trials and one multicenter single-arm prospective trial.
Objective.
The aim of this study was to identify predictors of outcome of conservative and minimally invasive surgical management of pain originating from the sacroiliac joint (SIJ).
Summary of Background Data.
Three recently published prospective trials have shown that minimally invasive SIJ fusion (SIJF) using triangular titanium implants produces better outcomes than conservative management for patients with pain originating from the SIJ. Due to limitations in individual trial sample size, analyses of predictors of treatment outcome were not conducted.
Methods.
We pooled individual patient data from the three trials and used random effects models with multivariate regression analysis to identify predictors for treatment outcome separately for conservative and minimally invasive surgical treatment. Outcome was measured using visual analogue scale (VAS), Oswestry Disability Index (ODI), and EuroQOL-5D (EQ-5D).
Results.
We included 423 patients assigned to either nonsurgical management (NSM, n = 97) or SIJF (n = 326) between 2013 and 2015. The reduction in SIJ pain was 37.9 points larger [95% confidence interval (95% CI) 32.5–43.4, P < 0.0001] in the SIJF group than in the NSM group. Similarly, the improvement in ODI was 18.3 points larger (95% CI 14.3–22.4), P < 0.0001). In NSM, we found no predictors of outcome. In SIJF, a reduced improvement in outcome was predicted by smoking (P = 0.030), opioid use (P = 0.017), lower patient age (P = 0.008), and lower duration of SIJ pain (P = 0.028).
Conclusions.
Our results support the view that SIJF leads to better treatment outcome than conservative management of SIJ pain and that a higher margin of improvement can be predicted in nonsmokers, nonopioid users, and patients of increased age and with longer pain duration.
Level of Evidence: 1
Keywords: disability, fusion of the sacroiliac joint, low back pain, opioid use, sacroiliac joint pain
The sacroiliac joint (SIJ) contributes to 15% to 30%1–5 of all chronic low back pain (LBP) with an even higher contribution (35–43%6–8) after lumbar fusion. Patients with SIJ pain have decreased quality of life9 with levels similar to other common surgically treated spine conditions.10 Nonsurgical treatments for SIJ pain, including physical therapy, chiropractic, intraarticular SIJ steroid injections, and radiofrequency neurotomy of sacral nerve root branches, have some literature support,11–15 but high-quality evidence supporting long-term improvements and describing potential predictors of favorable outcomes is lacking.
Surgical treatments for SIJ dysfunction include open and minimally invasive SIJ fusion (SIJF). Most published evidence on minimally invasive SIJF reports use of triangular titanium implants (TTIs), including retrospective case series,16–27 a combined multicenter case series,28 comparative case series against open SIJF,29–31 systematic reviews,32–34 and three prospective multicenter clinical trials.35–37 Even though previously published results from the three prospective trials have shown concordant improvements in pain, disability and quality of life after SIJF compared with nonsurgical management (NSM), the number of patients included in each of those trials was too low to identify potential predictors of clinical outcomes both for conservative management and SIJF. We therefore conducted a patient-level pooled analysis using the data from all three prospective multicenter TTI clinical trials to determine whether patient characteristics predicted clinical outcomes after either surgical or nonsurgical treatment.
MATERIALS AND METHODS
Data Sources
The three pooled trials are prospective clinical trials of SIJF with TTI. Trial characteristics are presented in Table 1. Literature searches using Medline, Embase, and ClinicalTrials.gov [primary search terms: sacroiliac joint AND (arthrodesis OR fusion)] revealed no other ongoing prospective TTI trials.
TABLE 1.
Trial Characteristics of Studies Included In Pooled Analysis
| Characteristic | Study | ||
| INSITE | iMIA | SIFI | |
| NCT number | NCT01681004 | NCT01741025 | NCT01640353 |
| Number of study centers | 19 | 9 | 26 |
| Number enrolled/treated | 148 | 103 | 172 |
| Geography | US | EU | US |
| Enrollment period | 2013–2014 | 2013–2015 | 2012–2013 |
| Design | RCT | RCT | SAT |
| Randomization ratio (surgery:nonsurgery) | 2:1 | 1:1 | NR |
| Control group | NSM | CM | — |
| Data availability, mo | 24 | 12 | 24 |
| Percent of subjects with available data at long-term follow-up* | 85% | 92% | 87% |
| Inclusion criteria | |||
| Age 21–70 yrs | ✓ | ✓ | ✓ |
| SIJ pain for >6 mo | ✓ | ✓ | ✓ |
| Diagnosis of SIJ dysfunction based on Fortin Finger Test, 3/5 positive exam and block | ✓ | ✓ | ✓ |
| ODI at least 30% | ✓ | ✓ | ✓ |
| SIJ pain at least 50 points | ✓ | ✓ | ✓ |
| Exclusion criteria | |||
| Severe back/hip pain due to something else | ✓ | ✓ | ✓ |
| Other known sacroiliac pathology | ✓ | ✓ | ✓ |
| History of recent (<1 yr) major pelvic trauma | ✓ | ✓ | ✓ |
| Previously diagnosed osteoporosis | ✓ | ✓ | ✓ |
| Osteomalacia or other metabolic bone disease | ✓ | ✓ | ✓ |
| Chronic rheumatologic condition | ✓ | ✓ | ✓ |
| Condition or anatomy making iFuse treatment infeasible | ✓ | ✓ | ✓ |
| Chondropathy | ✓ | ✓ | ✓ |
| Known allergy to titanium or titanium alloys | ✓ | ✓ | ✓ |
| Use of medication known to have detrimental effects on bone | ✓ | ✓ | ✓ |
| Neuropathy that would interfere with physical therapy | ✓ | ✓ | ✓ |
| Current local or systemic infection | ✓ | ✓ | ✓ |
| Currently receiving long-term worker's compensation, disability, involved in injury litigation | ✓ | ✓ | ✓ |
| Pregnant or planning pregnancy in next 2 years | ✓ | ✓ | ✓ |
| Prisoner | ✓ | ✓ | ✓ |
| Known or suspected alcohol or drug abuse | ✓ | ✓ | ✓ |
| Uncontrolled psychiatric disease | ✓ | ✓ | ✓ |
| Participating in another study | ✓ | ✓ | ✓ |
| Fibromyalgia | ✓ | ||
| Spine surgery in the past 12 months | ✓ | ||
CM indicates conservative management; mo, months; NR, not relevant; NSM, nonsurgical management; RCT, randomized controlled trial; SAT, single-arm trial.
*SIJF group only.
INSITE, a prospective 2-year multicenter randomized controlled trial (RCT) conducted at 19 centers in the US,28 included 148 patients with diagnosed SIJ dysfunction unresponsive to at least 6 months of conservative care. Patients were included between January 2013 and May 2014. Diagnosis was based on history, physical examination tests,38 and a ≥50% decrease in SIJ pain after image-guided joint block with local anesthetic.39–42 Subjects were randomized in a 2:1 fashion to either SIJF as previously described35 or NSM. NSM included anti-inflammatory and opioid pain medications, physical therapy, intra-articular SIJ steroid injections, and radiofrequency neurotomy, delivered serially as needed to manage pain and disability. Assessments included SIJ pain using a visual analog scale, Oswestry Disability Index (ODI),43 EuroQoL-5D (EQ-5D),44 and Short Form-36 (SF-36).45 In the NSM group, crossover to surgical care was allowed only after the 6-month visit was complete.
iMIA, a prospective multicenter randomized controlled clinical trial (n = 103), was conducted at nine European centers.36 Patients were included between June 2013 and May 2015. Key differences between iMIA and INSITE include 1) iMIA used 1:1 randomization, 2) nonsurgical treatment in iMIA included only physical therapy per European guidelines,46 3) iMIA included Zung Depression Scale47 but not SF-36, and 4) iMIA included a functional test48 and self-reported walking distance.
SIFI is a prospective, multicenter, single-arm clinical trial (n = 172) conducted at 26 centers in the US.37 Patients were included between August 2012 and December 2013. All subjects underwent SIJF. SIFI subjects underwent computed tomography (CT) scan at 1 year; otherwise, study parameters were identical to INSITE.
Surgical Revisions and Wound Infections
Adverse events, defined broadly using an international clinical trial standard, were collected continuously during the trials. Events of interest included wound-related problems and early and late surgical revisions of the target SIJ.
Statistical Analysis
We applied random effects models, performed using the nlme49 and lme450 R51 packages, that used appropriate covariance structures to take into account individual patient characteristics (fixed effects) as well as repeated measures and site-level factors (random effects). Both univariate and multivariate regression techniques were used, including interaction terms. Outcomes assessed in a single trial only were not evaluated. As both RCTs allowed crossover from NSM to SIJF after month 6, the treatment effect in the NSM cohorts was estimated using only 1, 3, and 6-month data. Models regarding patient age and pain duration used values grouped by quartiles. Opioid use was defined as continuous daily opioid use, including oral medication and/or transdermal application.
RESULTS
Four hundred twenty-three patients in three trials were analyzed, including 326 who underwent SIJF and 97 who underwent NSM. Two-year follow-up data were available from the two completed US studies; 1-year data are currently available from the European RCT.
Baseline Characteristics
In the three pooled trials, mean (SD) age was 50.4 (11.2) years, most (70.4%) subjects were women, and pain duration averaged 5.4 years (SD 6.7, Table 2). Mean baseline SIJ pain (80 points, SD 12.5) and ODI scores (56 points, SD 12.7) were high. Quality of life was diminished (mean EQ-5D Time Trade-off Index (TTO) of 0.43, SD 0.20 and mean SF-36 Physical Component Summary of 31, SD 5.9). Body mass index, baseline pain scores, the proportion using opioids, and the proportion with prior SIJ steroid injections were higher in the two US studies; smoking was less common in US patients. In the two RCTs, baseline characteristics (age, body mass index, pain duration, baseline pain, and ODI and QOL scores) were distributed equally across groups. Current smoking and a history of prior RF ablation were more common in the SIJF group (P = 0.0100 and 0.0197, respectively). Operative characteristics were similar across studies: Operating time averaged 48 minutes and three implants were used in most cases, with no significant variation in the mean number of implants used across studies (P = 0.970). Mean hospital length of stay was longer in the European RCT (3.6 days) versus US studies (0.8 days, P < 0.0001).
TABLE 2.
Baseline Characteristics of Patients Included in Pooled Analysis
| Characteristic | Study | Total | P Across Studies* | P Across Treatment† | ||
| INSITE (n = 148) | iMIA (n = 103) | SIFI (n = 172) | ||||
| Age, yrs, mean [range] | 51.3 [26–72] | 48.1 [23–70] | 50.9 [23–72] | 50.4 [23–72] | 0.2073 | 0.7480 |
| Women, n (% female) | 103 (69.6%) | 75 (72.8%) | 120 (69.8%) | 298 (70.4%) | 0.8322 | 0.2425 |
| Race, n (%) | ||||||
| White | 141 (95.3%) | ND | 166 (96.5%) | 307 (95.9%) | 0.3370 | 0.8681 |
| Black | 5 (3.4%) | ND | 2 (1.2%) | 7 (2.2%) | ||
| Ethnicity | ||||||
| Hispanic or Latino, n (%) | 8 (5.4%) | ND | 7 (4.1%) | 15 (4.7%) | 0.7654 | 0.1817 |
| Body mass index, mean [range] | 30.4 [17–50] | 27.1 [16–44] | 29.4 [17–51] | 29.2 [16–51] | 0.0085 | 0.7567 |
| Smoking status, n (%) | ||||||
| Current smoker | 29 (19.6%) | 39 (37.9%) | 44 (25.6%) | 112 (26.5%) | 0.0287 | 0.0100 |
| Former smoker | 43 (29.1%) | 22 (21.4%) | 49 (28.5%) | 114 (27.0%) | ||
| Never smoker | 76 (51.4%) | 42 (40.8%) | 79 (45.9%) | 197 (46.6%) | ||
| Prior lumbar fusion (n, %) | 58 (39.2%) | 37 (35.9%) | 76 (44.2%) | 171 (40.4%) | 0.3732 | 0.8458 |
| Years of pain, mean [range] | 6.4 [0.48–41] | 4.7 [0.45–44] | 5.1 [0.43–41] | 5.4 [0.43–44] | 0.2515 | 0.1052 |
| Prior treatments | ||||||
| Physical therapy | 107 (72.3%) | 59 (57.3%) | 111 (64.5%) | 277 (65.5%) | 0.0456 | 0.9074 |
| Steroid SI joint injection | 127 (85.8%) | 75 (72.8%) | 162 (94.2%) | 364 (86.1%) | <0.0001 | 0.2677 |
| RF ablation | 25 (16.9%) | 17 (16.5%) | 27 (15.7%) | 69 (16.3%) | 0.9575 | 0.0197 |
| Taking opioids (n, %) | 99 (66.9%) | 53 (51.5%) | 131 (76.2%) | 283 (66.9%) | <0.0001 | 0.1654 |
| Questionnaire scores, mean (SD) | ||||||
| VAS | 82.3 (11.3) | 75.3 (12.8) | 79.8 (12.8) | 79.6 (12.5) | 0.0056 | 0.0631 |
| ODI | 56.8 (13.2) | 56.6 (14.0) | 55.2 (11.5) | 56.1 (12.7) | 0.4531 | 0.3670 |
| EQ-5D | 0.45 (0.18) | 0.36 (0.25) | 0.43 (0.18) | 0.4 (0.2) | 0.1837 | 0.5518 |
| PCS | 30.4 (6.2) | ND | 31.7 (5.6) | 31.1 (5.9) | 0.0476 | 0.5709 |
| MCS | 43.1 (11.6) | ND | 38.5 (11.3) | 40.6 (11.7) | 0.0029 | 0.8356 |
EQ-5D indicates EuroQOL-5D Time Trade-off Index; MCS, SF-36 Mental Component Summary; ND, not done; ODI, Oswestry Disability Index; PCS, SF-36 Physical Component Summary; SI, sacroiliac; VAS, visual analogue scale.
*Mixed model across studies.
†Mixed model across treatment groups (SIJF vs. NSM, RCTs only).
Treatment Effect
Taking into account all assessments before month 6, the adjusted reduction in SIJ pain was 37.9 points larger [95% confidence interval (95% CI) 32.5–43.4, P < 0.0001] in the SIJF groups versus the NSM groups. Similarly, the improvement in ODI was 18.3 points larger (95% CI 14.3–22.4), P < 0.0001) and the improvement in EQ-5D TTO index was 0.24 points larger (95% CI 0.17–0.30, P < 0.0001). Extensive modeling was used to evaluate for effect modifiers (i.e., interaction terms), but none were found.
Predictors of Treatment Outcome
Table 3 and Figure 1 show associations of clinical characteristics with treatment outcomes for NSM and SIJF. In the NSM cohort (n = 97), none of the examined variables showed a significant association with pain, disability (ODI), or quality of life (EQ-5D) at 6 months of follow-up. For the SIJF group (n = 326), predictors of treatment outcome were assessed over the 24-month follow-up period. For SIJ pain, we found that older age [effect size (ES) 9.1 points; P = 0.0080] and longer pain duration (ES 7.7 points; P = 0.0282) were associated with larger improvements after SIJF, while current smokers (ES 5.9 points; P = 0.0299) and patients using opioids at baseline (ES 6.4 points; P = 0.0166) had smaller responses. For disability (ODI), improvements after SIJ were smaller among current smokers (ES 4.4 points; P = 0.0292) and those using opioids at baseline (ES 6.1 points; P = 0.0029). For EQ-5D, only longest pain duration was predictive of statistically significantly greater improvements after SIJF (ES 0.105 points; P = 0.0035).
TABLE 3.
Associations Between Baseline Patient Characteristics and Treatment Outcome
| SIJ Fusion | Nonsurgical Management | |||||
| VAS SIJ | ODI | EQ-TTO | VAS SIJ | ODI | EQ-TTO | |
| Age quartile | ||||||
| 1 (<24 yrs) | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 (42–50) | −2.3 (0.5135) | 1.92 (0.4643) | −0.0294 (0.4219) | 5.5 (0.2976) | 5.3 (0.1374) | −0.001 (0.9905) |
| 3 (50–59) | −4.7 (0.1750) | 0.17 (0.9482) | −0.0231 (0.5210) | 5.8 (0.3026) | −0.6 (0.8725) | −0.041 (0.6536) |
| 4 (>59) | −9.1 (0.0080) | −1.39 (0.5921) | 0.0013 (0.9707) | 2.4 (0.6745) | 1.4 (0.6940) | 0.026 (0.7664) |
| Pain duration quartile | ||||||
| 1 (<1.5 yrs) | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 (1.5–3) | −4.9 (0.1677) | 0.88 (0.7417) | 0.057 (0.1147) | 0.74 (0.8831) | 1.2 (0.7237) | −0.103 (0.2037) |
| 3 (3–6) | −7.7 (0.0282) | −0.19 (0.9431) | 0.067 (0.0644) | 3.42 (0.5048) | −1.5 (0.6693) | −0.082 (0.3234) |
| 4 (>6) | −5.2 (0.1384) | −0.26 (0.9235) | 0.105 (0.0035) | 1.50 (0.7872) | −3.9 (0.2969) | −0.101 (0.2619) |
| Current smoker | 5.9 (0.0299) | 4.4 (0.0292) | 0.0027 (0.9232) | −4.9 (0.3028) | −1.2 (0.7189) | 0.112 (0.1423) |
| Male gender | −1.3 (0.6324) | −2.4 (0.2453) | −0.029 (0.3111) | 3.1 (0.4344) | −0.52 (0.8493) | −0.030 (0.6418) |
| Bilateral SIJF | 2.1 (0.5172) | 1.5 (0.5484) | 0.027 (0.4155) | — | — | — |
| History of lumbar fusion | 3.0 (0.2236) | 1.6 (0.3868) | −0.027 (0.2841) | 1.9 (0.6403) | 0.17 (0.9501) | 0.044 (0.4959) |
| Opioids at baseline | 6.4 (0.0166) | 6.1 (0.0029) | −0.025 (0.3656) | 5.1 (0.1922) | 2.2 (0.3970) | −0.042 (0.5001) |
Each entry shows the regression coefficient for the subgroup level for changes in SIJ pain, ODI, or EQ-5D TTO index for the SIJF group and NSM group separately. Negative values indicate a decrease. Associated P values are given in parentheses. Significant values (P < 0.05) are bolded.
EQ-TTO indicates EQ-5D Time Trade-off Index; ODI, Oswestry Disability Index; Ref, reference level; VAS SIJ, visual analog scale sacroiliac joint pain.
Figure 1.
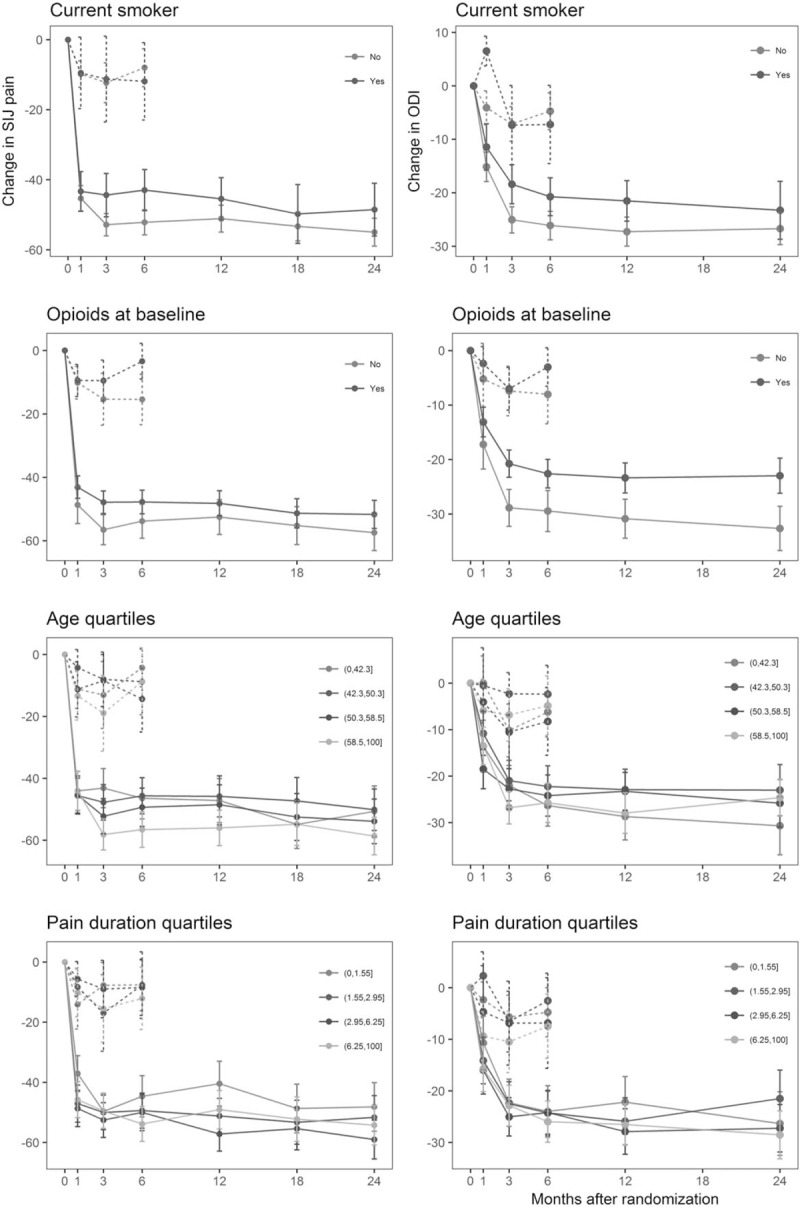
Changes in pain (by VAS, left) and disability (by ODI, right) over time for NSM (dotted lines) and SIJF (solid lines) in relation to baseline smoking, opioid use, patient age, and pain duration.
Surgical Revisions and Wound Infections
Of the 326 patients undergoing SIJF, 1.2% (n = 4) underwent early surgical revision (<1 month). In each of those patients, one of the implants had been inadvertently placed into a sacral neuroforamen, causing postoperative neuropathic symptoms and requiring surgical repositioning of the implant. Late revision surgery (>1 month), performed in 2.8% (n = 9), was typically done to address pain, sometimes associated with poor implant position, with placement of additional implants in most cases. Signs of wound infection occurred in eight subjects overall, including deep wound infection requiring surgical washout (n = 1), drainage from wound treated with antibiotics (n = 3), redness treated with antibiotics (n = 3), and slow healing treated with antibiotics (n = 1). No subject had bony infection or implant removal for infection.
DISCUSSION
Combining data from three separate prospective studies allowed us to assess in more detail which patient groups may have a better chance of benefitting from conservative or minimally invasive surgical treatment of chronic SIJ pain. Our principal findings are that, within the patient cohort undergoing SIJF, two factors (current smoking and opioid use at baseline) predicted lower and two factors (higher patient age and longer duration of SIJ pain) predicted higher degrees of improvement in SIJ pain and pain-related disability. Older age also predicted higher improvements in quality of life (EQ-5D TTO). Even though one may argue that each of these differences may be of relatively modest clinical significance, it is important to note that they all reached statistical significance. Moreover, subgroups with smaller improvements after SIJF, such as smokers or opioid users, still displayed larger and clinically important improvements compared with patients in the NSM cohort. Another important difference between SIJF and NSM was that within NSM, we found no predictors of treatment outcome at all.
In the SIJF cohort, smokers showed reduced pain response (by 5.9 VAS points) and higher disability levels (by 4.4 ODI points) than nonsmokers. These results are consistent with previously published data describing a significant negative association between smoking and spine surgery outcomes.52
Patients using opioids at baseline also benefitted less from SIJF (by 6.4 VAS points and by 6.1 ODI points) when compared with opioid-naive patients. These findings add to the somewhat controversial discussion regarding opioids as part of LBP treatment overall, as current evidence suggests an absence of long-term superiority of opioids over placebo in the treatment of LBP, which has led some authors to call for avoiding any opioid use in LBP treatment.53,54 Opioid use may even increase the risk of recurrence of already existing depression as well as the risk of developing new onset depression.55
In the SIJF cohort, patients younger than 45 years displayed a reduced pain response (by 9.1 VAS points) compared with patients in the oldest age quartile. Whether young age reflects a true biologic effect or is a marker for more severe disability is not known, but our results suggest that SIJF should be discussed with greater caution in younger patients. However, our findings are in line with previously published reports on patients undergoing lumbar fusion surgery, which found that older patients were not at a higher risk of poor treatment outcomes.56
Within the SIJF cohort, we observed that patients in the third quartile of pain duration (3–6 years) had a larger improvement in pain. Also, patients in the fourth pain duration quartile (>6 years) had larger improvements in quality of life (EQ-5D). The significance of this finding is uncertain and is therefore the most difficult to integrate into decision-making during patient selection. However, as increased pain duration has been described to be a risk factor for poor treatment outcome in LBF,57 our contrasting results provide reassurance that in patients with long-standing pain originating from the SIJ, SIJF is a reasonable option.
Procedure-related safety was reasonable in our analysis, with a low rate of wound problems and a low surgical revision rate consistent with a previous report in the commercial setting.58
Combined with retrospective case series,16–31 our findings provide high-level evidence for the safety and effectiveness of SIJF with TTI and support its use as a relevant treatment choice in patients with SIJ dysfunction unresponsive to NSM.
Minimally invasive SIJF is gaining increasing attention in spine surgery. Two different surgical approaches to SIJF have been reported. In the dorsal approach, which was not used in the trials evaluated in our analysis, a midline dorsal incision is made with dissection to the dorsal ligamentous recess followed by device placement. Stabilization is achieved through ligamentotaxis. Published outcomes from this approach are scant.59 In the lateral-to-medial approach, which was used in the trials analyzed by us, the implants transfix the SIJ. Published TTI studies include the three trials we summarized as well as retrospective case series,16–28 including some with 3-,26 4-,22 and 5-year24 follow-up, and comparative case series versus open SIJF.29–31 Three additional case series report good outcomes with hollow modular anchor screws60–62 and a recent small case series suggests good outcomes with an additional transfixing device.63 Minimally invasive SIJF using TTI was shown to not only improve the LBP component of SIJ pain but also the referred leg pain component.64 Because of differences in approaches, device design, acute impact on the joint, and long-term fusion strategies, it is unclear whether results from our analysis apply to other laterally transfixing devices or to devices placed via a dorsal approach.
The main strength of our analysis is that all three pooled studies were of high quality, used standardized enrollment and diagnostic criteria, and were rigorously monitored. The two RCTs were designed to directly estimate the clinical value of surgery compared with a nonsurgical treatment control group. However, certain limitations should be mentioned. First, because the study protocols of iMIA and INSITE allowed crossover from nonsurgical to surgical treatment after 6 months and the majority of patients made use of this option, long-term information for NSM (beyond 6 months) was not evaluated in our analysis. Nevertheless, while crossover prevented calculation of treatment ES after month 6, it allowed us to completely avoid early crossover, which has complicated interpretation of other surgery versus nonsurgery trials.65,66 Another limitation of our analysis is that all trials included were not blinded and therefore patient-specific expectations cannot be ruled out as potential confounders to overall outcome results. Nevertheless, the large observed ES suggest a true underlying effect. Finally, the fact that all three trials included in our analysis were industry-sponsored may be viewed by some as a limitation. However, industry-sponsorship is the norm in spine surgery device trials.67
CONCLUSION
Our pooled analysis suggests that the success of conservative management of SIJ pain is limited and difficult to predict. In contrast, improvements in pain, disability, and quality of life with minimally invasive SIJF were large; moreover, the extent of improvement was modestly associated with smoking, opioid use, patient age, and duration of pain. Procedure-related safety of SIJF was reasonable.
Key Points
Recent evidence suggests that minimally invasive surgical treatment of pain originating from the sacroiliac joint (SIJ) may be a relevant alternative to frequently unsuccessful conservative management.
We pooled data from the only existing prospective trials using triangular titanium implants to treat SIJ pain to identify predictors of treatment outcome.
Minimally invasive surgical management produced significantly better outcome than conservative management.
We found no predictors of outcome for conservative management of SIJ pain.
For minimally invasive surgical management, we found that smoking and opioid use predicted poorer outcome, while higher patient age and longer duration of pain were associated with better outcome.
Acknowledgments
Study Groups
iMIA: D. Kools, G. Lesage, F. Martens, H. Keymeulen (Department of Neurosurgery, Onze-Lieve-Vrouw Hospital Aalst, Belgium); Y. Lecomte (Montegnée, Belgium); J. Dengler, S. Bayerl, J. Kopetzki (Department of Neurosurgery, Charité - Berlin, Germany); R. Pflugmacher, M. Webler, R. Bornemann, Tom Jansen (Department of Orthopedics and Traumatology, University Hospital Bonn, Germany); A. Mues (Hilden, Germany); A. Gasbarrini, C. Griffoni, S. Colangeli, R. Ghermandi (Instituto Ortopedico Rizzoli di Bologna, Bologna, Italy); D. Prestamburgo, F. Valli (Department of Orthopedics and Traumatology, Ospedale di Legnano, Italy); P. Gaetani, V. Silvani, M. Minelli, D. Adinolfi, M. Verlotta, A. Cattalani (Pavia, Italy); B. Sturesson, I. Dahlberg (Department of Orthopedics, Aleris, Ängelholm Hospital, Ängelholm, Sweden).
INSITE: John Swofford MD, John Cummings MD, James Cole MD, Elizabeth Pertile, Ellen Looney, Patti Hunker, Mary Anne Gfell (Indiana Interventional Pain and Community Hospital, Indianapolis, IN); Clay Frank MD, Jamie Edwards MD, Gordon Mortensen MD, Tracy Mente RN (Wheaton Franciscan Healthcare, Wauwatosa, WI); Scott Kitchel MD, Christopher Miller MD, Gregory Moore MD, Shawn Potts, Brett Barnes (Neurospine Institute, Eugene, OR); Robert Limoni MD, Nilesh Patel MD, Taylor Romdenne, Denise Barnes RN, Nicholas Peterson (Aurora BayCare Medical Center & Advanced Pain Management, Green Bay, WI); Harry Lockstadt MD, Elaine Wilhite MS, James Farris PA-C (Bluegrass Orthopaedics & Hand Care, Lexington, KY); Don Kovalsky MD, Laura Pestka RN, Cristy Newman (Orthopaedic Center of Southern Illinois, Mount Vernon, IL); Peter Whang MD, Donna Ann Thomas MD, Bethany Samperi, Stacey Lombardi (Yale University, New Haven, CT); Emily A. Darr MD, John A. Glaser MD, Laura Fields, Jennifer Philp, Monica Baczko (Medical University of South Carolina, Charleston, SC); Charles Harvey, MD, Jason Peterman PA-C, Karim Bouferrache MPAS PA-C, Lori Latham (Riverside Medical Center, Kankakee, IL); Pierce Nunley MD, Andrew Utter MD, Marcus Stone PhD, Norma Rivera, Monicah Jepkemboi, Anthony Juarez (Spine Institute of Louisiana, Shreveport, LA); Jonathan Sembrano MD, Ed Santos MD, David Polly MD, Charles Ledonio MD, Sharon Yson MD (University of Minnesota, Minneapolis, MN); Philip Ploska MD, Terry Price PA (Regenerative Orthopaedics and Spine Institute, Stockbridge, GA); Michael Oh MD, Gary Schmidt MD, Matthew Yeager (Allegheny General Hospital, Pittsburgh, PA); Merle Stringer MD, Douglas Stringer MD, Carolyn Henderson (Brain & Spine Center, Panama City, FL); Farshad Ahadian MD, Yu-Po Lee MD, Katie Lam (University of California, San Diego, CA); Gowriharan Thaiyananthan MD, Bryan Oh MD, Navid Farahmand MD, Tungie Williams (Basic Spine, Newport Beach, CA); William Rosenberg MD, Amy Akins RN BSN CCRC, Pamela McCann RN BSN, Jennifer Feeback CCRP (Midwest Division-RMC, LLC,-Research Medical Center, Kansas City, MO); Vikas Patel MD, Scott Laker MD, Venu Akuthota MD, Christopher Cain MD, Evalina Burger MD, Christopher Kleck MD, Claire Cofer, David Calabrese (University of Colorado, Aurora, CO); Mark C. Gillespy MD, Sherri Zicker RN (Orthopaedic Clinic of Daytona Beach, Daytona Beach, FL).
SIFI: Harry Lockstadt, MD, Elaine Wilhite, MS, James Farris, PA-C (Bluegrass Orthopaedics and Hand Care, Lexington, Kentucky); Don Kovalsky, MD, Cristy Newman, Laura Pestka, RN (Orthopaedic Center of Southern Illinois, Mount Vernon, Illinois); Cheng Tao, MD, Jackie Makowski, Toni Kelly (Spine and Neuro Center, Huntsville, Alabama); S. Craig Meyer, MD, Vicki Jones, Michelle Vogt (Columbia Orthopaedic Group, Columbia, Missouri); Scott Kutz, MD, Linda Thompson, RN, BSN, FNP (Mercy Medical Research Center, Springfield, Missouri); Dimitriy Kondrashov, MD, Irina Kondrashov (SF Spine Group, San Francisco, California); Andy J. Redmond, MD, Jennifer Piazza, MS, Laurie Doredant, Beth Short, BSN, MS, Jessica Mayfield, RN (Precision Spine Care, Tyler, Texas); CL Soo, MD, Julie White, MBA, Kallena Haynes (Medical Research International, Oklahoma City, Oklahoma); Bradley Duhon, MD, Amber Pfister (Neurosurgical and Spine Specialists, Parker, Colorado); Ali Mesiwala, MD, Stephanie Bose, RN (Southern California Center for Neuroscience and Spine, Pomona, California); Leonard Rudolf, MD, John Thibodeau Jr RN (Alice Peck Day Memorial Hospital, Lebanon, New Hampshire); Kevin Stevenson, MD, Logan Mahoney, LPN (Piedmont Orthopaedic Complex, Macon, Georgia); Fabien Bitan, MD, Stephanie Gomez (Manhattan Orthopaedics, New York City, New York); John Stevenson, MD, Ana Marichal (The Orthopaedic Institute, Gainesville, Florida); Donald Sachs, MD, Robin Cambron, MSN, MBA, RN, Missy White, Ana Colburn, RN, Sally Raiden, RN, MSN (Center for Spinal Stenosis and Neurologic Care, Lakeland, Florida); Abhineet Chowdhary, MD, Tina Fortney, RN, BSN (Overlake Hospital Medical Center, Bellevue, Washington); Gowriharan Thaiyananthan, MD, Tungie Williams (BASIC Spine, Orange, California); Michael Oh, MD, Gary Schmidt, MD, Matthew Yeager (Allegheny General Hospital, Pittsburgh, Pennsylvania); David Wiles, MD, Susan Maye, RN, MS (East Tennessee Brain & Spine, Johnson City, Tennessee); Michael Hasz, MD, Carrie Califano (Virginia Spine Institute, Reston, Virginia); William Rosenberg, MD, Pamela McCann, RN, BSN (Midwest Division-RMC, LLC,-Research Medical Center, Kansas City, Missouri); Jeffrey D. Coe, MD, Julia Coe, Marlene Coe (Silicon Valley Spine, Campbell, California); Jed Vanichkachorn, MD, Jessica Lynch (Tuckahoe Orthopaedics Associates, Richmond, Virginia); Mark C. Gillespy, MD, Sherri Zicker, RN (Orthopaedic Clinic of Daytona Beach, Daytona Beach, Florida); Ralph Rashbaum, MD, Shannon Rusch, BA, CCRC (Texas Back Institute, Plano, Texas); Emily A. Darr, MD, John A. Glaser, MD, Laura Fields, Monica Baczko (Medical University of South Carolina, Charleston, South Carolina).
Footnotes
The device(s)/drug(s) is/are FDA-cleared or approved by corresponding national agency for this indication.
Si-Bone Inc. is funding the iMIA trial in support of this work.
Relevant financial activities outside the submitted work: board membership, consultancy, grants, stocks, royalties, employment, travel/accommodations/meeting expenses.
Contributor Information
Collaborators: On behalf of the INSITE, iMIA, SIFI study groups
References
- 1.Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res 1987; 217:266–280. [PubMed] [Google Scholar]
- 2.Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976) 1995; 20:31–37. [DOI] [PubMed] [Google Scholar]
- 3.Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976) 1996; 21:1889–1892. [DOI] [PubMed] [Google Scholar]
- 4.Irwin RW, Watson T, Minick RP, Ambrosius WT. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil 2007; 86:37–44. [DOI] [PubMed] [Google Scholar]
- 5.Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine (Phila Pa 1976) 2009; 34:E27–E32. [DOI] [PubMed] [Google Scholar]
- 6.Maigne JY, Planchon CA. Sacroiliac joint pain after lumbar fusion. A study with anesthetic blocks. Eur Spine J 2005; 14:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DePalma MJ, Ketchum JM, Saullo TR. Etiology of chronic low back pain in patients having undergone lumbar fusion. Pain Med 2011; 12:732–739. [DOI] [PubMed] [Google Scholar]
- 8.Liliang P-C, Lu K, Liang C-L, et al. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med 2011; 12:565–570. [DOI] [PubMed] [Google Scholar]
- 9.Cher D, Polly D, Berven S. Sacroiliac joint pain: burden of disease. Med Devices Evid Res 2014; 7:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cher DJ, Reckling WC. Quality of life in preoperative patients with sacroiliac joint dysfunction is at least as depressed as in other lumbar spinal conditions. Med Devices Evid Res 2015; 8:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luukkainen R, Nissilä M, Asikainen E, et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol 1999; 17:88–90. [PubMed] [Google Scholar]
- 12.Luukkainen RK, Wennerstrand PV, Kautiainen HH, et al. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondylarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin Exp Rheumatol 2002; 20:52–54. [PubMed] [Google Scholar]
- 13.Maugars Y, Mathis C, Berthelot JM, et al. Assessment of the efficacy of sacroiliac corticosteroid injections in spondylarthropathies: a double-blind study. Br J Rheumatol 1996; 35:767–770. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SP, Hurley RW, Buckenmaier CC, et al. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology 2008; 109:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel N, Gross A, Brown L, et al. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med 2012; 13:383–398. [DOI] [PubMed] [Google Scholar]
- 16.Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J 2012; 6:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolf L. MIS fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J 2013; 7:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res 2012; 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop 2013; 2013:536128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings J, Jr, Capobianco RA. Minimally invasive sacroiliac joint fusion: one-year outcomes in 18 patients. Ann Surg Innov Res 2013; 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder JE, Cunningham ME, Ross T, et al. Early results of sacro-iliac joint fixation following long fusion to the sacrum in adult spine deformity. Hosp Spec Surg J 2013; 10:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanaclocha-Vanaclocha V, Verdú-López F, Sánchez-Pardo M, et al. Minimally invasive sacroiliac joint arthrodesis: experience in a prospective series with 24 patients. J Spine 2014; 3:185. [Google Scholar]
- 23.Gaetani P, Miotti D, Risso A, et al. Percutaneous arthrodesis of sacro-iliac joint: a pilot study. J Neurosurg Sci 2013; 57:297–301. [PubMed] [Google Scholar]
- 24.Rudolf L, Capobianco R. Five-year clinical and radiographic outcomes after minimally invasive sacroiliac joint fusion using triangular implants. Open Orthop J 2014; 8:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JT, Rudolf LM, Glaser JA. Outcome of percutaneous sacroiliac joint fixation with porous plasma-coated triangular titanium implants: an independent review. Open Orthop J 2013; 7:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachs D, Kovalsky D, Redmond A, et al. Durable intermediate- to long-term outcomes after minimally invasive transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices Evid Res 2016; 9:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornemann R, Roessler PP, Strauss A, et al. Two-year clinical results of patients with sacroiliac joint syndrome treated by arthrodesis using a triangular implant system. Technol Health Care 2017; 25:319–325. [DOI] [PubMed] [Google Scholar]
- 28.Sachs D, Capobianco R, Cher D, et al. One-year outcomes after minimally invasive sacroiliac joint fusion with a series of triangular implants: a multicenter, patient-level analysis. Med Devices Evid Res 2014; 7:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res 2013; 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledonio CGT, Polly DW, Swiontkowski MF. Minimally invasive versus open sacroiliac joint fusion: are they similarly safe and effective? Clin Orthop Relat Res 2014; 472:1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledonio C, Polly D, Swiontkowski MF, et al. Comparative effectiveness of open versus minimally invasive sacroiliac joint fusion. Med Devices Evid Res 2014; 2014:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiney J, Capobianco R, Cher D. A systematic review of minimally invasive sacroiliac joint fusion utilizing a lateral transarticular technique. Int J Spine Surg 2015; 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lingutla KK, Pollock R, Ahuja S. Sacroiliac joint fusion for low back pain: a systematic review and meta-analysis. Eur Spine J 2016; 25:1924–1931. [DOI] [PubMed] [Google Scholar]
- 34.Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine 2015; 23:59–66. [DOI] [PubMed] [Google Scholar]
- 35.Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg 2016; 10: Article 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturesson B, Kools D, Pflugmacher R, et al. Six-month outcomes from a randomized controlled trial of minimally invasive SI joint fusion with triangular titanium implants vs. conservative management. Eur Spine J 2017; 26:708–719. [DOI] [PubMed] [Google Scholar]
- 37.Duhon BS, Cher DJ, Wine KD, et al. Triangular titanium implants for minimally invasive sacroiliac joint fusion: a prospective study. Glob Spine J 2016; 6:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szadek KM, van der Wurff P, van Tulder MW, et al. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain 2009; 10:354–368. [DOI] [PubMed] [Google Scholar]
- 39.Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: Guidance and recommendations. Pain Physician 2013; 16 2 Suppl:S49–S283. [PubMed] [Google Scholar]
- 40.Bogduk N. Sacroiliac Joint Access. Practice Guidelines for Spinal Diagnostic and Treatment Procedures 2nd ed.San Francisco: International Spine Intervention Society; 2013. 523–555. [Google Scholar]
- 41.Manchikanti L, Boswell MV, Singh V, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009; 12:699–802. [PubMed] [Google Scholar]
- 42.American Society of Anesthesiologists Task Force on Chronic Pain Management, American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology 2010; 112:810–833. [DOI] [PubMed] [Google Scholar]
- 43.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000; 25:2940–2952. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 44.The EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Healthy Policy 1990; 16:199–208. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473–483. [PubMed] [Google Scholar]
- 46.Vleeming A, Albert HB, Östgaard HC, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J 2008; 17:794–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry 1965; 13:508–515. [DOI] [PubMed] [Google Scholar]
- 48.Mens JMA, Vleeming A, Snijders CJ, et al. Validity of the active straight leg raise test for measuring disease severity in patients with posterior pelvic pain after pregnancy. Spine (Phila Pa 1976) 2002; 27:196–200. [DOI] [PubMed] [Google Scholar]
- 49.Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and Nonlinear Mixed Effects Models [Internet]. 2016. Available at: http://CRAN.R-project.org/package=nlme Accessed February 12, 2016. [Google Scholar]
- 50.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67:1–48. [Google Scholar]
- 51.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available at: http://www.R-project.org/ Accessed August 1, 2012.
- 52.Jackson KL, Devine JG. The effects of smoking and smoking cessation on spine surgery: a systematic review of the literature. Glob Spine J 2016; 6:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel Shaheed C, Maher CG, Williams KA, et al. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med 2016; 176:958–968. [DOI] [PubMed] [Google Scholar]
- 54.Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976) 2014; 39:556–563. [DOI] [PubMed] [Google Scholar]
- 55.Scherrer JF, Salas J, Lustman PJ, et al. Change in opioid dose and change in depression in a longitudinal primary care patient cohort. Pain 2015; 156:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marbacher S, Mannion AF, Burkhardt J-K, et al. Patient-rated outcomes of lumbar fusion in patients with degenerative disease of the lumbar spine: does age matter? Spine (Phila Pa 1976) 2016; 41:893–900. [DOI] [PubMed] [Google Scholar]
- 57.Viniol A, Jegan N, Brugger M, et al. Even worse - risk factors and protective factors for transition from chronic localized low back pain to chronic widespread pain in general practice: a cohort study. Spine (Phila Pa 1976) 2015; 40:E890–E899. [DOI] [PubMed] [Google Scholar]
- 58.Cher DJ, Reckling WC, Capobianco RA. Implant survivorship analysis after minimally invasive sacroiliac joint fusion using the iFuse Implant System. Med Devices Evid Res 2015; 8:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Endres S, Ludwig E. Outcome of distraction interference arthrodesis of the sacroiliac joint for sacroiliac arthritis. Indian J Orthop 2013; 47:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khurana A, Guha AR, Mohanty K, et al. Percutaneous fusion of the sacroiliac joint with hollow modular anchorage screws: clinical and radiological outcome. J Bone Joint Surg Br 2009; 91:627–631. [DOI] [PubMed] [Google Scholar]
- 61.Mason LW, Chopra I, Mohanty K. The percutaneous stabilisation of the sacroiliac joint with hollow modular anchorage screws: a prospective outcome study. Eur Spine J 2013; 22:2325–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Khayer A, Hegarty J, Hahn D, et al. Percutaneous sacroiliac joint arthrodesis: a novel technique. J Spinal Disord Tech 2008; 21:359–363. [DOI] [PubMed] [Google Scholar]
- 63.Kube RA, Muir JM. Sacroiliac joint fusion: one year clinical and radiographic results following minimally invasive sacroiliac joint fusion surgery. Open Orthop J 2016; 10:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dengler J, Sturesson B, Kools D, et al. Referred leg pain originating from the sacroiliac joint: 6-month outcomes from the prospective randomized controlled iMIA trial. Acta Neurochir (Wien) 2016; 158:2219–2224. [DOI] [PubMed] [Google Scholar]
- 65.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 2007; 356:2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delitto A, Piva SR, Moore CG, et al. Surgery versus nonsurgical treatment of lumbar spinal stenosis: a randomized trial. Ann Intern Med 2015; 162:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cher D, Capobianco R. Spine device clinical trials: design and sponsorship. Spine J 2015; 15:1133–1140. [DOI] [PubMed] [Google Scholar]


