Abstract
Use of organophosphate flame retardants (PFRs) has increased over the past decade with the phase out of polybrominated diphenyl ethers. Urinary metabolites of PFRs are used as biomarkers of exposure in epidemiologic research, which typically uses samples collected and stored in polypropylene plastic cryovials. However, a small study suggested that the storage vial material may influence reported concentrations. Therefore, we aimed to examine the influence of the storage vial material on analytical measurement of PFR urinary metabolites. Using urine samples collected from participants in the Environment and Reproductive Health (EARTH) Study, we analyzed the PFR metabolites in duplicate aliquots that were stored in glass and plastic vials (n=31 pairs). Bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), diphenyl phosphate (DPHP) and isopropyl-phenyl phenyl phosphate (ip-PPP) were detected in 97%, 97% and 78% of duplicates. We observed high correlations between glass-plastic duplicates for BDCIPP (rs=0.95), DPHP (rs =0.79) and ip-PPP (rs =0.82) (p<0.0001). Urinary ip-PPP was an average of 0.04 ng/ml (p=0.04) higher among samples stored in glass, with a mean relative difference of 14%. While this difference is statistically significant, it is small in magnitude. No differences were observed for BDCIPP or DPHP, however future research should seek to reduce the potential for type II error (false negatives). We conclude that storing urine samples in polypropylene plastic cryovials may result in slightly reduced concentrations of urinary ip-PPP relative to storage in glass vials and future research should seek to increase the sample size, reduce background variability and consider the material of the urine collection cup.
Keywords: urinary biomarkers, organophosphate flame retardants, human, sample storage, quality control
Graphical Abstract
Introduction
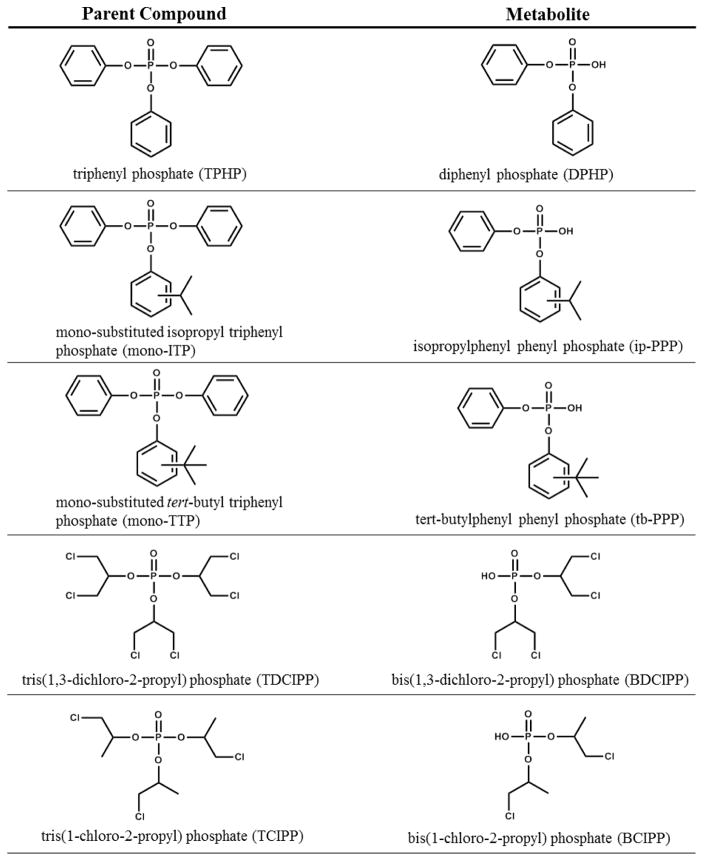
Organophosphate flame retardants (PFRs) have been used in the polyurethane foam of upholstered furniture [1] with increasing prevalence over the past decade following the phase out polybrominated diphenyl ethers (PBDEs) [2]. Flame retardants used in polyurethane foam are additives that are not chemically bound, and therefore migrate into the air and dust of indoor environments [3] and lead to human exposure. Two commonly used PFRs are triphenyl phosphate (TPHP) and tris(1,3-dichloro-2-propyl)phosphate (TDCIPP), which after intake are excreted within hours primarily as the urinary metabolites diphenyl phosphate (DPHP) and bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), respectively [4–6]. TPHP is often used in the Firemaster® 550 mixture in combination with two brominated flame retardants as well as various mono-substituted triphenyl phosphate isomers (Figure 1). In addition to being used as a flame retardant, TPHP is also used as a plasticizer and is found in nail polish, hydraulic fluids and polyvinyl chloride (PVC) [7, 3]. Additionally, the primary metabolite of TPHP, diphenyl phosphate (DPHP), is also sold and used as a plasticizer, although production volumes of DPHP are significantly lower than TPHP. TCIPP in contrast, is primarily used as a flame retardant, primarily in rigid polyurethane foam for insulation and construction (80% of use) as well in flexible polyurethane foam (e.g., furniture cushions) [8].
Figure 1.
Organophosphate flame retardant parent compound and primary urinary metabolite.
While population-wide data are limited, studies suggest that exposure to these PFRs is likely widespread in the U.S. as DPHP and BDCIPP have been detected in over 90% of adult urine samples [9–12]. This is of concern because TDCIPP and TPHP are suspected endocrine disrupting chemicals that have been shown to disrupt thyroid hormone and estrogen signaling as well as to reduce reproductive performance in zebrafish and chickens [13–15]. TPHP is a suspected obesogen that can initiate adipocyte differentiation and antagonize osteogenesis [16, 17]. TCIPP is considered a carcinogen under Proposition 65 regulated by the State of California [18] and is a potential developmental neurotoxicant [19]. TCIPP can also disrupt the endocrine system, with in vitro evidence of antiandrogenic and antiestrogenic activity [20]. In vivo studies report morphological changes in the thyroid and adverse effects on reproduction including changes to the estrous cycle, increased uterine weights, low birth weight, and delayed hatching [21, 22, 13, 23]. Little is known regarding toxicity of the mono-substituted triphenyl phosphate isomers. Few epidemiologic studies have investigated PFRs, however an exploratory analysis of 33 men found that urinary BDCIPP and DPHP were associated with reductions in sperm motility and increased total T3 [24].
Epidemiologic studies investigating PFRs are needed and may utilize urinary metabolites as biomarkers of exposure. Typically, urine samples are collected in plastic (polypropylene) specimen cups and frozen in plastic cryovials. However, preliminary results have suggested that PFR metabolites may adhere to these collection and storage containers [25]. Therefore, our objective was to determine whether the material of the storage vial material biases analytical determination of PFR urinary metabolites using a subset of aliquots from a U.S. preconception cohort that were stored both in glass and plastic vials.
Materials and Methods
Participants
Study participants were women recruited into the Environment and Reproductive Health (EARTH) study between November 2005 and October 2015 from patients undergoing assisted reproductive technologies at the Massachusetts General Hospital Fertility Center. Female participants must be between the ages of 18 and 46 to enroll in the study. The EARTH study was approved by the Human Studies Institutional Review Boards of Massachusetts General Hospital and Harvard T.H. Chan School of Public Health. Participants signed an informed consent after the study procedures were explained by a research nurse and any questions were answered.
Urine Samples
Urine was collected in a sterile polypropylene cup and specific gravity (SG) was measured using a handheld refractometer (National Instrument Company, Inc.). Each sample was divided into aliquots (2.5 to 5 mL) and stored at −80°C. We randomly selected duplicate samples collected between 2008–2009 that were stored in glass vials (Shorty Vials®, Borosilicate Glass, PTFE lined Screw Cap, Wheaton) and plastic cryovials (Nalgene® Cryogenic Vials, Polypropylene, Sterile, External Thread with Screw Cap, Thermo Scientific) (glass-plastic duplicates). While the main objective of our analysis was to compare analytical results from duplicates stored in glass and plastic storage vials, we also evaluated analytical variability from duplicates stored only in plastic storage vials. To do so, we selected duplicate samples collected between 2005–2015 that were stored in plastic cryovials (plastic-plastic duplicates). The glass-plastic (n=31 pairs) and plastic-plastic (n=30 pairs) duplicates were shipped on dry ice overnight to Duke University (Durham, NC, USA) for quantification of urinary metabolites used as biomarkers of exposure to PFRs.
Extraction and Instrumental Analysis
Extraction and analysis methods for BCIPP, BDCIPP, DPHP, ip-PPP and tb-PPP followed methods previously developed by our laboratory [9]. Briefly, urine samples were thawed and a 2.5 to 5 ml aliquot was transferred to a clean glass test tube and spiked with mass-labeled internal standards (d10-BDCIPP = 80 ng, d10-DPHP = 60 ng). After acidifying to pH <6.5 with formic acid, samples were diluted 1:1 with water and, concentrated and cleaned using solid-phase extraction techniques (SPE). The SPE eluent was blown to dryness under a gentle nitrogen stream, reconstituted in 500 μl of 1:1 H2O:MeOH and spiked with the recovery standard (13C2-DPHP = 81.5 ng). Extracts were analyzed by negative electrospray ionization liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described [9]. Chromatography was achieved under gradient conditions using a Luna C18(2) column (50 x 2.0 mm, 2.5 μm particle size, Phenomenex, Torrance, CA) preceded by a SecurityGuard Polar-RP (4 x 2.0 mm) guard cartridge. The mobile phases were methanol and water (modified with 0.8 mM ammonium acetate), flow rate was 300 μl/min, the injection volume was 5 μl and the column oven was 45°C. Data were acquired under multiple reaction monitoring conditions using optimized parameters. Analyte responses were normalized to internal standard responses. BCIPP and BDCIPP were normalized using d10-BDCIPP, while DPHP, ip-PPP and tb-PPP were normalized using d10-DPHP. Urinary specific gravity ranged from 1.002 to 1.028 with a mean of 1.016.
Quality assurance/quality control
In the urine samples, the mean recovery of the mass-labeled standards was 103% (standard error = 1.7%) for d10-DPHP and 146% (5.7%) for d10-BDCIPP. The apparent high recovery for d10-BDCIPP was presumably due to matrix effects in the urine samples since the blanks (clean water only) showed d10-BDCIPP mean recovery of 112%. This issue has been observed in our previous studies [9] and is related to the fact that 13C2-DPHP was used as the recovery standard. One laboratory blank (5 ml Milli-Q water only) sample was extracted with every batch (n=5). Two of the individual sub-samples were analyzed in duplicate to assess method precision and were generally within 10% with the exception of BDCIPP which was within 30%. Very low levels of DPHP (mean = 0.71 ng) and ip-PPP (mean = 0.28 ng) were routinely detected in the laboratory blanks and analyte values were blank corrected using the mean laboratory blank values. Method detection limits (MDLs) were calculated as three times the standard deviation of laboratory blanks normalized to the volume of water extracted (5 ml). MDLs were 68 pg/ml for BCIPP, 56 pg/ml for BDCIPP, 183 pg/ml for DPHP, 59 pg/ml for ip-PPP, 154 pg/ml for tb-PPP, respectively.
Data Analysis
Results below the method detection limit (MDL) were imputed using MDL/v2. Because aliquots from the same sample would have the same specific gravity, all statistical analyses were performed on urinary metabolite data that was not normalized to urinary specific gravity. As urinary metabolite concentrations were log-normally distributed, geometric mean (GM) urinary metabolite concentrations were calculated for each duplicate pair. Spearman correlation coefficients between duplicates were calculated separately for glass-plastic and plastic-plastic duplicates, and agreement was presented graphically via scatterplots. Agreement between duplicate samples was assessed using Bland-Altman plots. Pairwise differences were calculated as glass minus plastic. As pairwise differences appeared to follow an approximate normal distribution, a one-sample t-test was conducted to test whether the mean difference was zero. Detection frequencies between glass and plastic duplicates were compared using Fisher’s exact test. To compare urinary metabolite concentrations to those measured in other populations, we accounted for urinary dilution by normalizing to urinary specific gravity (SG) using the approach described by Pearson et al. [26]: CSG=(SGm-1)/(SGi-1) where CSG=SG normalized urinary metabolite concentration, SGm=population mean SG and SGi= SG for an individual sample. Correlations and Bland-Altman plots were visualized using JMP® Pro (version 12.0.1, SAS Institute Inc., Carey, NC). Relative difference was calculated as the concentration of urinary metabolite measured in the sample stored in a plastic vial divided by the concentration measured in the sample stored in a glass vial, minus 1 and multiplied by 100. Statistical analyses were performed using SAS® (version 9.3; SAS Institute Inc., Cary, NC) with statistical significance defined as α = 0.05.
Results and Discussion
Among all samples, detection frequencies were high for BDCIPP (98%), DPHP (97%) and ip-PPP (87%) but low for BCIPP (0%) and tb-PPP (13%) (Table 1).
Table 1.
Distribution of urinary metabolites (ng/ml) from all 61 duplicate pairs.
| N > MDL (%)a | GM (95% CI) | Minimum | 25th Pctl | 50th Pctl | 75th Pctl | Maximum | |
|---|---|---|---|---|---|---|---|
| BCIPP | 0 (0) | <MDL | <MDL | <MDL | <MDL | <MDL | <MDL |
| BDCIPP | 60 (98) | 0.76 (0.55, 1.05) | <MDL | 0.30 | 0.76 | 2.14 | 10.38 |
| DPHP | 59 (97) | 0.92 (0.66, 1.28) | <MDL | 0.48 | 0.90 | 1.41 | 340.36 |
| ip-PPP | 53 (87) | 0.26 (0.20, 0.35) | <MDL | 0.18 | 0.28 | 0.56 | 4.00 |
| tb-PPP | 8 (13) | <MDL | <MDL | <MDL | <MDL | <MDL | 0.47 |
Each pair of duplicates was considered >MDL when one or more duplicate was >MDL.
Summary statistics calculated using geometric mean concentrations from each pair of duplicates.
Similar detection frequencies and GM concentrations were observed between subsamples of glass-plastic and plastic-plastic duplicate pairs (Supplemental Table S2).
BDCIPP, DPHP and ip-PPP were detected among all of the 31 (100%) glass aliquots but only 29 (94%) of the plastic duplicates; this difference was not statistically significant (p=0.49, Fisher’s exact test). Detection frequencies and GM concentrations were similar to other adult populations in the U.S. and Norway [9, 10, 27, 28] and lower than measured in pooled samples from an Australian study [29] (Supplemental Table S2).
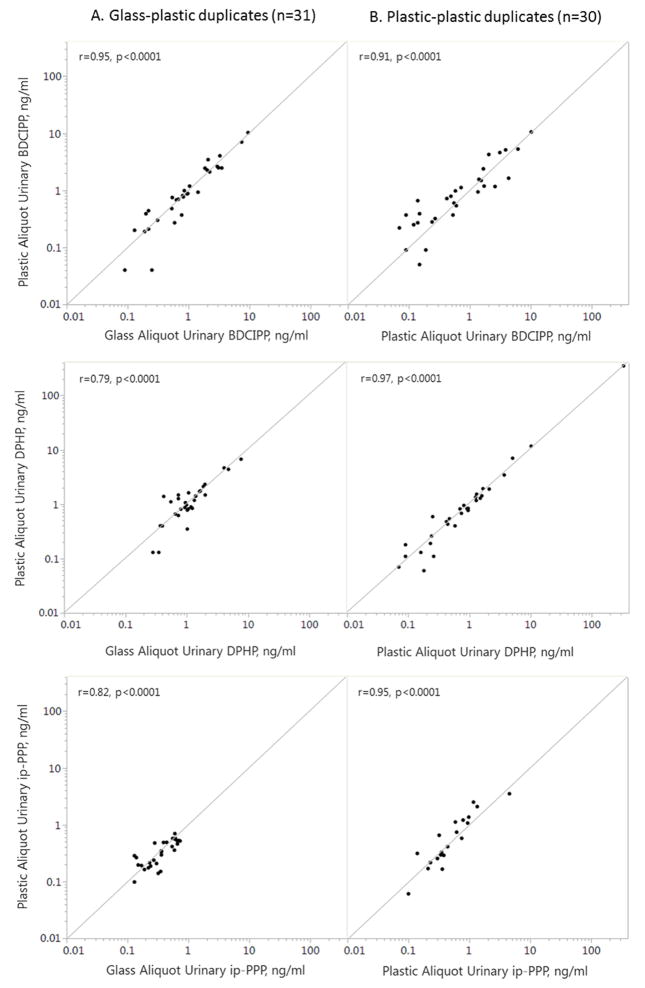
For BDCIPP and ip-PPP, duplicate samples were highly correlated for both the glass-plastic (rs > 0.8) and plastic-plastic (rs > 0.9) duplicates (Figure 2). For DPHP, the plastic-plastic duplicates were more highly correlated (rs > 0.97) than the glass-plastic duplicates (rs > 0.79). Pearson correlations using log transformed data were similar.
Figure 2.
Comparison between duplicate glass-plastic (n=31) and duplicate plastic-plastic (n=30) aliquots with infinity line and Spearman correlation coefficients (r).
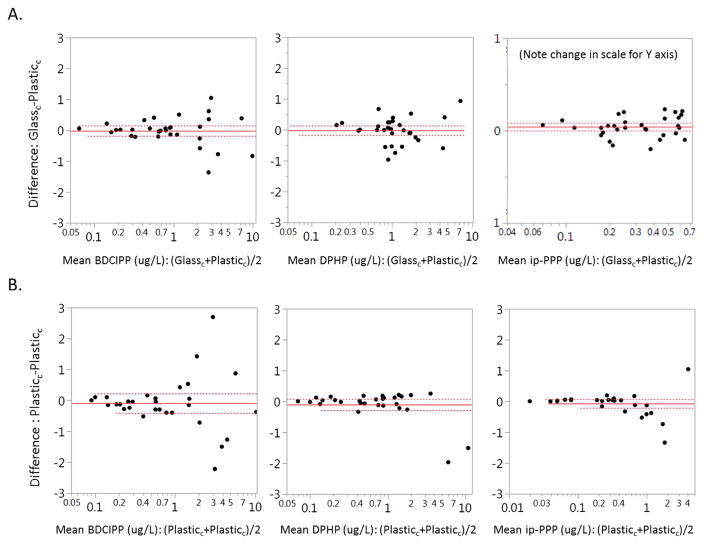
We used a one-sample t-test to determine whether the difference between glass-plastic duplicates differed from zero and found that urinary ip-PPP was an average of 0.04 ng/ml (p=0.04) higher in glass duplicates, with a mean relative difference of 14% (Figure 3). There was no significant difference for BDCIPP (−0.02 ng/ml, p=0.80) or DPHP (−0.02 ng/ml, p=0.80) between glass plastic duplicates. Sample volume, the length of storage time, and urine SG were not associated with absolute or relative differences between duplicate samples (data not shown).
Figure 3.
Bland-Altman plot of relative difference between duplicate aliquots: A) Glass-plastic, B) Plastic-plastic. Solid line indicates the average for all samples and dotted lines delineate the 1.96 standard deviation.
This analysis suggests that storage of urine samples in plastic cryovials as compared to glass vials may result in a systematic bias of reduced ip-PPP. The finding of lower measured concentrations of ip-PPP among urine samples stored in plastic cryovials is consistent with our hypothesis that the concentrations in aliquots stored in plastic cryovials would be lower than duplicates stored in glass, which was based on a very limited study that reported lower concentrations of urinary DPHP and BDCIPP in 2 out of 3 samples collected and stored both in glass and polypropylene containers [25]. Note that while the observed difference is statistically significant, it is very small in magnitude.
We hypothesize two possible explanations for the absence of a difference between glass-plastic aliquots for BDCIPP and DPHP. First, this may be related to the higher hydrophobicity (Log Kow) of ip-PPP (4.79) compared to BDCIPP (2.18) and DPHP (2.88), which could lead to increased absorption to polypropylene [30]. Second, this may be related to potential type II error (false negative) for BDCIPP (27%) and DPHP (56%) due to the higher variability in the difference between duplicates for these PFRs. Some of this variability may be from analytical noise or from sorption to the polypropylene specimen cup during urine sample collection. While collection using a polypropylene specimen cup is common for collection of biological samples, including in our study, samples used by Cooper et al. [25] were collected using glass. Therefore, future work should seek to control for potential differences introduced by the material of the specimen collection cup as well as other variables that may influence the degree of sorption such as sample volume, urine properties, storage time and storage temperature.
Conclusion
We conclude that storing urine samples in polypropylene plastic cryovials may result in reduced concentrations of urinary ip-PPP relative to storage in glass vials, although this difference is small compared to other sources of variability. Future research should seek to increase the sample size, reduce background variability and consider the material of the urine collection cup.
Supplementary Material
Highlights.
We measured PFR metabolites in duplicate urine samples stored in glass and plastic vials
Concentrations were highly correlated between duplicates
ip-PPP was slightly but significantly higher using glass compared to plastic vials for storage
DPHP and BDCIPP showed no storage difference between the glass and plastic vials
Acknowledgments
The authors gratefully acknowledge all members of the EARTH study team, specifically the Harvard T.H. Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd and Patricia Morey, physicians and staff at Massachusetts General Hospital fertility center and a special thanks to all the study participants.
Funding: This study was funded by grants ES009718, ES022955, ES000002, and T32ES007069 from the National Institute of Environmental Health Sciences (NIEHS).
Abbreviations
- BDCIPP
Bis(1,3-dichloro-2-propyl) phosphate
- BCIPP
bis(1-chloro-2-propyl) phosphate
- DPHP
diphenyl phosphate
- EARTH
Environment and Reproductive Health
- GM
Geometric mean
- ip-PPP
isopropyl-phenyl phenyl phosphate
- MDL
Method detection limit
- PFRs
Organophosphate flame retardants
- SG
Specific gravity
- tb-PPP
tert-butyl-phenyl phenyl phosphate
Footnotes
Conflict of Interest: The authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ Sci Technol. 2012;46(24):13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 4.Nomeir AA, Kato S, Matthews HB. The metabolism and disposition of tris(1,3-dichloro-2- propyl) phsophate (Fyrol FR-2) in the rat. Toxicol Appl Pharm. 1981;57(3):401–13. doi: 10.1016/0041-008x(81)90238-6. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki K, Suzuki T, Takeda M, Uchiyama M. Metabolism of phosphoric-acid triesters by rat- liver homogenate. Bull Environ Contam Toxicol. 1984;33(3):281–8. doi: 10.1007/BF01625544. [DOI] [PubMed] [Google Scholar]
- 6.Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicology letters. 2013;223(1):1879–3169. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, et al. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 2016;86:45–51. doi: 10.1016/j.envint.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Union Risk Assessment Report. Tris(2-chloro-1-methylethyl) phosphate (TCPP) 2008 May; CAS No: 13674-84-5, EINECS No: 237-158-7. [Google Scholar]
- 9.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol. 2014;48(17):10432–8. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- 10.Carignan CC, McClean MD, Cooper E, Watkins D, Fraser AJ, Heiger-Bernays W, et al. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman K, Daniels JL, Stapleton HM. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int. 2014;63:169–72. doi: 10.1016/j.envint.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect. 2013;121(5):580–5. doi: 10.1289/ehp.1205907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhat A, Crump D, Chiu S, Williams KL, Letcher RJ, Gauthier LT, et al. In Ovo effects of two organophosphate flame retardants--TCPP and TDCPP--on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. 2013;134(1):92–102. doi: 10.1093/toxsci/kft100. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Ji K, Jo A, Moon HB, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio) Aquatic Toxicol. 2013;134–135:104–11. doi: 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Lam JC, Han J, Wang X, Guo Y, Lam PK, et al. Developmental exposure to the organophosphorus flame retardant tris(1,3-dichloro-2-propyl) phosphate: estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquatic Toxicol. 2015;160:163–71. doi: 10.1016/j.aquatox.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol Letters. 2014;228(2):93–102. doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, et al. Ligand binding and activation of PPARgamma by Firemaster® 550: Effects on adipogenesis and osteogenesis. Environ Health Perspect. 2014 doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evidence on the Carcinogenicity of Tris(1,3-dichloro-2-propyl) phosphate. Reproductive and Cancer Hazard Assessment Branch, Office of Environmental Health Hazard Assessment, California Environmental Protection Agency; Jul, 2011. [accessed October 12, 2012]. http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/TDCPP070811.pdf. [Google Scholar]
- 19.Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (Danio rerio) Toxicol Sci. 2014;142(2):445–54. doi: 10.1093/toxsci/kfu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohyama K, Nagata S, Hosogoe N. Hormonal effects of organic phosphate triesters study by the reporter gene assay. Tokyo-to Kenko Anzen Kenkyu Senta Kenkyu Nenpo. 2006;56:6. [Google Scholar]
- 21.TNO Quality of Life. Oral two-generation reproduction toxicity study (including a dose range finding study) with Tris(2-chloro-1-methylethyl)-phosphate in rats. 2007 (Unpublished report) [Google Scholar]
- 22.Freudenthal R, Henrich R. A subchronic toxicity study of Fyrol PCF in Sprague-Dawley rats. Int J Toxicol. 1999;18(3):173. doi: 10.1080/109158101753253009. [DOI] [PubMed] [Google Scholar]
- 23.U.S. EPA. Flame retardants used in flexible polyurethane foam: An alternatives assessment update. U.S. Environmental Protection Agency, Design for the Environment; 2014. Jun, [Accessed August 31, 2015]. Draft Report. EPA 744-D-14-001. https://www.epa.gov/sites/production/files/2015-08/documents/ffr_final.pdf. [Google Scholar]
- 24.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocrine disrupt. 2013;1(1):e26306. doi: 10.4161/endo.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper EM, Covaci A, van Nuijs ALN, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(7):2123–32. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J Expos Sci Environ Epidemiol. 2008;19(3):336–42. doi: 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ Health Perspect. 2015;123(2):160–5. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol. 2014;48(23):13625–33. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, et al. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int. 2015;74:1–8. doi: 10.1016/j.envint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 30.US EPA. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11. United States Environmental Protection Agency; Washington, DC, USA: 2016. p. 777. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.