Abstract
Ischemic stroke occurs more often among the elderly, and within this demographic, women are at an increased risk for stroke and have poorer functional recovery than men. This is also well replicated in animal studies where aging females are shown to have more extensive brain tissue loss as compared to adult females. Astrocytes provide nutrients for neurons, regulate glutamate levels, and release neurotrophins and thus play a key role in the events that occur following ischemia. In addition, astrocytes express receptors for gonadal hormones and synthesize several neurosteroids suggesting that the sex differences in stroke outcome may be mediated through astrocytes. This review discusses key astrocytic responses to ischemia including, reactive gliosis, excitotoxicity, and neuroinflammation. In light of the age and sex differences in stroke outcomes, this review highlights how aging and gonadal hormones influence these responses. Lastly, astrocyte specific changes in gene expression and epigenetic modifications during aging and following ischemia are discussed as possible molecular mechanisms for impaired astrocytic functioning.
Keywords: Astrocyte, Stroke, Hormones, Estrogen, Progesterone, Glutamate, Excitotoxicity, Neuroinflammation, IGF-1, VEGF, MicroRNA, Epigenetics, HDAC, H3K4me3, Methylation, Oxidative stress, GFAP, Sex differences, Blood-brain barrier
Background
By 2030, it is estimated that 19% of the population will be greater than 65 years of age. As the world’s population ages, the prevalence of age-related neurological diseases will increase. Specifically, the prevalence of stroke increases during aging. The average age of ischemic stroke patients is 71 and 17.8% of the population over 45 years of age showed at least one stroke-related symptom (Ovbiagele et al., 2013; Fonarow et al., 2010). The increased risk for stroke during aging is accompanied with poorer stroke outcomes and the cost associated with treating stroke totals more than 36 billion dollars annually in the United States (Go et al., 2014). In addition, elderly patients are less likely to be discharged home and more likely to die in the hospital (Copen et al., 2001; Fonarow et al., 2010).
Given the effects of normal aging on the brain (reviewed in Juraska and Lowry, 2012) it is not surprising that the aged brain responds differently to stroke than the adult brain. Ischemia leads to a series of events including increased intracellular calcium levels and increased glutamate release resulting in excitotoxicity, upregulation of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and loss of normal protein structure and function (Yenari and Han, 2012). Excessive calcium levels can trigger activation of signaling pathways that cause an overproduction of free radicals and dysfunction of mitochondria, which leads to oxidative stress and cell death (Starkov et al., 2004). In astrocytes, higher levels of mitochondrial calcium can also enhance neuroprotection due to increased in ATP production (Zheng et al, 2010; Zheng et al., 2013). Furthermore, ischemic injury induces a cascade of events that lead to disruption of the blood–brain barrier (Yang and Rosenberg, 2011). The blood–brain barrier is composed of specialized brain microvascular endothelial cells interconnected by tight junction proteins, surrounded by pericytes, and a defined basement membrane. Astrocytic end feet located on the outer side of the basement membrane further regulate blood–brain barrier function and deliver nutrients to nearby neurons (Figley and Stroman, 2011; Abbott et al., 2006). The blood–brain barrier is designed to maintain homeostasis of the brain microenvironment and the known disruption after ischemia may make changes during aging particularly important. In humans, normal aging results in increased blood–brain barrier permeability (Farrall and Wardlaw, 2009; Montagne et al., 2015) and decreased microvessel density (Brown and Thore, 2011). Furthermore, studies in a senescence-accelerated mouse model have shown that the passage of cytokines through the blood–brain barrier is altered (Banks et al., 2001; McLay et al., 2000), the expression of the glucose transporter GLUT-1 is reduced (Vorbrodt et al., 1999) and there is increased permeability of the blood–brain barrier (Ueno et al., 1993; Pelegri et al., 2007). This increased permeability in the blood–brain barrier may exacerbate cell loss following ischemia. Although the blood–brain barrier consists of several different cell types that are susceptible to age-related changes, this review will highlight the importance of astrocytes in stroke outcomes during aging with an emphasis on the influence of sex since both are risk factors for stroke.
There are well documented sex differences in stroke risk and stroke outcomes. According to the Framingham Heart Study, women had an overall lower risk of stroke than men (Petrea et al., 2009), however although men and women had a similar prevalence of stroke at age 35 to 44 years, between the ages of 45 and 54 women were more than twice as likely to have had a stroke than men (Towfighi et al., 2007). In addition, partially due to longer life-spans and age of stroke, women are discharged to long term care facilities more often, have poorer functional outcomes, and a lower probability of achieving independence in activities of daily living after discharge (Appelros et al., 2009; Fukuda et al., 2009; Kapral et al., 2005; Gargano et al., 2011). A recent study found that even though acute stroke care was similar between males and females, women had poorer functional outcomes and increased dependence on nursing care three months after ischemia (Gattringer et al., 2014).
Preclinical studies have historically examined stroke severity and recovery in young animals; however, in agreement with the human data, more recent animal studies have shown that the aged brain responds differently to experimental stroke than the young brain. For example, following cerebral ischemia adult females (3–7 months) have smaller infarcts than middle-aged females (rats: 9–12 months, mice: 12–15 months) (Selvamani and Sohrabji, 2010b; Manwani et al., 2011; Selvamani et al., 2014) and aged females (>16 months) (Rosen et al., 2005; DiNapoli et al., 2008; Liu et al., 2009). However, studies examining the outcome of ischemia and the effects of aging in male animals have produced varying results. Similar to the results observed in females, several studies have shown that aged male rodents exhibit larger infarcts, increased edema, and worse neurological functional deficits compared to adult males (Dong et al., 2014; Miao et al., 2013; Tennant et al., 2014). The accelerated development of degenerating neurons and apoptotic cells following ischemia in males may contribute to the poorer outcomes (Popa-Wagner et al., 2007). There is also evidence that chronic infection resulting in pre-existing inflammation, a condition known to influence stroke outcome, results in increased infarct size in aged male mice but not in young mice 24 h post stroke (Dhungana et al., 2013). Importantly, some studies have found opposite effects of aging or no effects of aging in male mice following ischemia. For example, 16 month old male mice have smaller infarcts and less atrophy than adult male mice following transient focal ischemia (Liu et al., 2009; Manwani et al., 2011), and adult rats had more extensive neurological damage than aged rats following occlusion of the external carotid artery (Shapira et al., 2002). In contrast, other studies have shown no differences in infarct volumes following ischemia between adult and middle-aged males (Selvamani et al., 2014). These differences suggest that several factors may influence the outcome of ischemia during aging, including the type of occlusion, inclusion of middle-aged versus aged animals, and the time point examined following stroke. Irrespective of infarct size, several studies have found more severe behavioral deficits and poorer functional recovery in aging animals following stroke than adult animals. Whereas adult males began to recover sensorimotor functioning 1–2 days after stroke and fully recovered within 15 days, this was delayed in aged rats and functionality only returned to 70% of pre-stroke levels at day 15 (Popa-Wagner et al., 2007). Although rehabilitative training on a novel task following ischemia resulted in behavioral improvement in adult males, improvement was only observed in aged males with a previously learned task (Tennant et al., 2014).
Sex has been identified as an important variable for stroke outcome in animal models of stroke as well. In particular, adult females have smaller infarcts and better cerebral blood flow than adult males (Hall et al., 1991; Alkayed et al., 1998; Selvamani et al., 2014), however the sex difference is reversed in aging and middle-aged females have larger infarct volumes than middle-aged males (Manwani et al., 2013). In a direct comparison of adult and middle-aged male and female rats, adult females displayed smaller infarct volumes as compared to middle-aged females and young males, while young and middle-aged males did not differ (Selvamani et al., 2014). These studies indicate that ischemia may be more detrimental to aging females than aging males. Despite the increased burden of stroke on aging females, very little is known about how ischemia differentially affects males and females.
In view of the effects of age and sex on stroke outcomes, it is not surprising that these two factors influence the effectiveness of potential therapeutics. Although treatment with apocynin, a NOX2 inhibitor, prior to middle cerebral artery occlusion (MCAO) increased mortality and failed to reduce infarct volume in aging female rats, treatment in adult females resulted in decreased infarct volumes and improved functional recovery (Kelly et al., 2009). Co-administration of EEIIMD, a plasminogen activator inhibitor type 1 derived peptide, and tPA resulted in significant improvement in cortical and total infarction volumes, edema formation, and functional outcome in adult females, whereas infarct volume was decreased in aged females without a reduction in edema or functional improvement (Tan et al., 2009). Several studies in males have also shown age differences in the effectiveness of various treatments. The recombinant T-cell receptor ligand, RTL1000, was recently shown to decrease infarct volume in both adult and aged male mice following ischemia albeit through different mechanisms of protection (Dotson et al., 2014). In aged animals this protection was due to a reduction in the number of activated microglia, T cells, and dendritic cells in the ischemic hemisphere following MCAO, however, treatment in adult mice reduced the percentage of T cells and macrophages in the spleen (Dotson et al., 2014). Furthermore, post-conditioning with sevoflurane significantly reduced infarct size and edema formation, and improved the neurological outcome in adult male rats but failed to alter these in aged males (Dong et al., 2014). Interestingly, overexpression of adiponectin, an adipose-specific plasma protein, reduced ischemic brain injury and promoted neurobehavioral outcomes in aged male mice more efficiently than in adult animals (Miao et al., 2013). Sex has been shown to alter the effects of hypothermia on brain injury with males displaying greater response to therapy than females. For example, hypothermia reduces markers of oxidative damage in cerebral spinal fluid after brain injury to a greater degree in males than females (Wagner et al., 2004) and after traumatic brain injury protects against neuronal loss in males, but not in females (Suzuki et al., 2003).
The mechanism underlying the ‘young female advantage’ is not well understood, although the ovarian hormones, estrogen and progesterone, are believed to play a role in mediating neuroprotection (Simpkins et al., 1997). The sex difference in infarct volume observed between intact adult males and females is abolished with removal of the ovaries which results in larger infarcts in adult females (Alkayed et al., 1998) and this is prevented by administering estrogen to ovariectomized adult females (Dubal et al., 1998; Rusa et al., 1999; Selvamani and Sohrabji, 2010b). In addition, estrogen treatment improved sensorimotor deficits and spatial memory deficits that were observed after ischemia in ovariectomized females (Li et al., 2004; Gulinello et al., 2006). A recent meta-analysis of preclinical studies concluded that progesterone administration results in reduced lesion size following experimental stroke (Wong et al., 2013). Additionally, deletion of the gene that codes for aromatase P450, an enzyme involved in the biosynthesis of estrogen, results in greater ischemic damage as compared to wild type animals (McCullough et al., 2003).
Menopause, which usually occurs between 45 and 55 years of age, results from a depletion of ovarian follicles that leads to increased follicle-stimulating hormone and luteinizing hormone levels and a dramatic decrease in estrogen and progesterone levels. The sex difference in risk for stroke in humans reverses around the time of menopause in females and because of this gonadal hormones may also play a role in stroke risk and outcome during aging. However, the effects of ovarian hormones on stroke outcome in aging animals are complex. Although middle-aged constant diestrus females have larger lesions than adult females, treatment with estradiol increases infarction in aging animals rather than providing neuroprotection (Selvamani and Sohrabji, 2010b; Leon et al., 2012; De Butte-Smith et al., 2007; however see Inagaki and Etgen, 2013). Progesterone administration following ischemia decreased lesion size in aging females but had no effect on neurological outcome (Gibson et al., 2011).
In adult males, several studies have shown that castration reduces infarct size and androgen replacement increases infarct to levels similar to that observed in intact males (Cheng et al., 2007, 2009; Vagnerova et al., 2010). In agreement with this, circulating levels of androgens decrease during aging in males, and aging males have smaller infarct volumes and less atrophy than adult males (Liu et al., 2009; Manwani et al., 2011). However, some studies have shown dose-dependent effects of androgen treatment and not all studies have shown that low levels of androgens result in reduced infarcts (Cheng et al., 2009; Uchida et al., 2009). There is evidence that the effects of gonadal hormones may be mediated in part though the actions of astrocytes (Wang et al., 2014).
Astrocytes produce and respond to gonadal hormones
No longer considered merely a brain support cell, astrocytes are known to regulate the levels of extracellular glutamate and thereby influence synaptic activity, release several gliotransmitters and play a role in the elimination of useless synapses (Pfrieger, 2010; Stipursky et al., 2011; Jourdain et al., 2007; Araque and Navarrete, 2010). Astrocytes from both males and females express estrogen receptor (ER) α, ERβ, the transmembrane ER, GPR30, and progesterone and androgen receptors (Azcoitia et al., 1999; Pawlak et al., 2005b; Arnold et al., 2008; Duncan et al., 2013; Kuo et al., 2010b) and estradiol facilitates the synthesis of progesterone in astrocytes (Micevych et al., 2007; Chaban et al., 2004). In addition, astrocytes were found to be the most steroidogenic brain cells producing several neurosteroids, such as progesterone, testosterone, and estradiol, and expressing enzymes involved in hormone synthesis, including aromatase (Zwain and Yen, 1999). PPT, a selective ERα agonist, induced a similar calcium response as estradiol in astrocytes and was able to facilitate progesterone synthesis by astrocytes. The estradiol induced response was attenuated in ERαKO mouse astrocytes, suggesting that estradiol signaling occurs through the membrane receptor ERα (Kuo et al., 2010b). Interestingly, the neuroprotective effects of an ERα ligand in experimental autoimmune encephalomyelitis, a model of brain inflammation, were completely prevented by deletion of ERα from astrocytes (Spence et al., 2011). Not surprisingly, some of the effects of sex hormones on astrocytes are sexually dimorphic. Estradiol treatment increased insertion of ERα into the cell membrane, and enhanced progesterone synthesis in female astrocytes, but failed to alter these measures in male astrocytes (Kuo et al., 2010a). Astrocyte estrogen receptors appear to be altered by the hormone loss that occurs during aging. Cortical astrocytes from regularly cycling middle-aged females retained normal ERα levels whereas acyclic middle-aged females had increased expression of ERα (Arimoto et al., 2013) (Fig. 1).
Fig. 1.
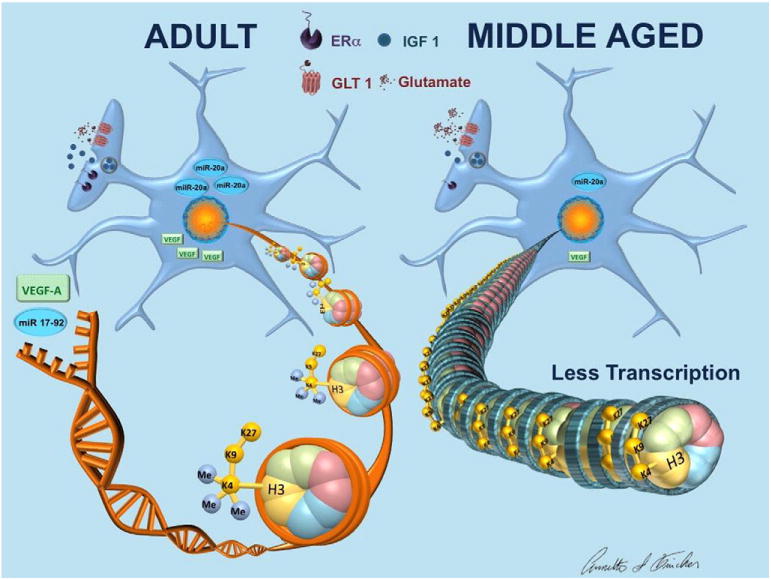
Age differences in cortical astrocytes. Cortical astrocytes from acyclic middle-aged females have increased ERα expression (Arimoto et al., 2013). Following ischemia, astrocytes from middle-aged females secrete less IGF-1 and clear less glutamate as compared to adult astrocytes and this is not explained by a difference in GLT-1 expression between groups (Lewis et al., 2012; Selvamani and Sohrabji, 2010a). Astrocytes isolated from adult females had more H3K4me3 enriched genes, including the mir17-92 cluster and VEGFa, and this was accompanied with greater mir20a mRNA expression and protein expression of VEGF in astrocytes from adult females relative to aging females following ischemia (Chisholm et al., 2015). Decreased H3K4me3 in astrocytes from middle-aged females would result in heterochromatin and reduced gene transcription.
Brain injury and cerebral ischemia induce aromatase activity and expression in astrocytes (Garcia-Segura et al., 1999; Carswell et al., 2005) and inhibition of aromatase exacerbates neuronal death following kainic acid treatment indicating that this response is neuroprotective (Azcoitia et al., 2003). Furthermore, an increase in astrocyte derived estradiol was accompanied with neuroprotective and anti-inflammatory effects following global ischemia, an effect that was blocked by aromatase antisense oligonucleotides (Zhang et al., 2014). Sex differences in aromatase activity and expression following brain injury provide an alternative mechanism for the sex differences in stroke severity. Female astrocytes were more resistant to oxygen glucose deprivation and resilient to H2O2 induced oxidant stress and this was mediated in part by an increased expression of P450 and aromatase activity in female astrocytes as compared to males (Liu et al., 2007). Deletion of the P450 aromatase gene significantly increased cell death after oxygen glucose deprivation in female astrocytes and abolished this sex difference (Liu et al., 2008).
Reactive astrocytes: a response to aging and injury
Astrocyte morphology changes and GFAP expression increases progressively during aging in humans and rodents (Middeldorp and Hol, 2011). Astrocyte proliferation is an indication of reactive gliosis, a process which may enhance neuronal damage (Arevalo et al., 2013). Importantly, an increase in reactive astrocytes has been shown to occur in response to a variety of brain diseases and brain traumas (Sofroniew and Vinters, 2010; Eng and Ghirnikar, 1994) and this response appears to aid in recovery following brain injury (Liu et al., 2015). For example, GFAP/vimentin double knockout mice subjected to photothrombotic stroke had attenuated astrocytic reactivity in response to injury and this resulted in reduced neurological recovery (Liu et al., 2015). However, aging alters the proliferation of reactive astrocytes after injury and this results in accelerated glial scar formation in aged animals which may not benefit stroke recovery (Badan et al., 2003). Along with an accelerated astrocytic response, the expression of GFAP positive cells was increased in the aged brain versus adult brain and this increase was still present 30 days following stroke (Manwani et al., 2011). Gene expression studies have shown that reactive astrocytes display a unique gene expression profile relative to quiescent astrocytes (Zamanian et al., 2012) and that aged reactive astrocytes have an altered response to stroke including an upregulation of genes associated with inflammation and scar formation as compared to adult astrocytes (Buga et al., 2008).
Reactive astrocytes: influence of ovarian hormones
Several studies have provided evidence that estrogen and progesterone treatment decrease astrocyte proliferation following injury (Perez-Alvarez et al., 2012; Garcia-Estrada et al., 1993, 1999; Djebaili et al., 2005). Sex differences have been observed in GFAP expression in the hippocampus of adult animals and the number of GFAP positive cells varied with the estrous cycle (Arias et al., 2009). Live imaging of the ischemic brain found significantly greater GFAP induction in adult female mice in diestrus, as compared with males and this upregulation of GFAP in response to ischemia was dependent on the estrus cycle and serum estrogen levels (Cordeau et al., 2008). Similarly, following kainic acid induced injury, astrocyte proliferation is enhanced in aging females as compared to aging males (Zhang et al., 2008). There is evidence that estrogen may influence astrocyte proliferation by increasing N-myc downstream-regulated gene 2 expression, a protein known to inhibit cell proliferation (Ma et al., 2014).
Release of neurotrophic factors: impact of aging
Reactive astrocytes release a variety of trophic factors that promote neuronal survival (Ridet et al., 1997). Astrocytes synthesize and release IGF-1 and therefore may play an important role in neuroprotection, in view of the beneficial effects reported for this growth factor in stroke (Sohrabji, 2015). Recently it was shown that astrocytes protect neurons from oxidative stress induced by H2O2 and this neuroprotection is abrogated with blockade of the IGF-1 receptor in astrocytes (Genis et al., 2014). In addition, IGF-1 protected astrocytes, but not neurons against H2O2-induced death (Genis et al., 2014). IGF-1 plasma levels are decreased in middle-aged females, and astrocytes from middle-aged females secrete significantly less IGF-1 as compared to adult astrocytes (Lewis et al., 2012; Selvamani and Sohrabji, 2010a) (Fig. 1). The age-related decreases in secreted IGF-1 observed in aging astrocytes may result in more severe stroke outcomes observed in this group.
Astrocytes also produce vascular endothelial growth factor (VEGF), a potent angiogenic factor that induces endothelial cell proliferation, inhibits apoptosis, and promotes cell migration (Neufeld et al., 1994). Coexpression of GFAP and VEGF in the entorhinal cortex decreases during normal aging and there is decreased gene expression of VEGF in the dentate gyrus in aged female rats (Bernal and Peterson, 2011). Furthermore, VEGF plays a major role in cell protection and recovery after cerebral ischemia (Sun et al., 2003; Wang et al., 2012). There is an enhancement of VEGF expression in astrocytes after ischemia that promotes astrocyte activation (Liu et al., 2014a).
Astrocyte regulation of glutamate homeostasis: impact of aging and ischemia
Under basal conditions, astrocyte specific glutamate transporters, GLT-1 and GLAST, respond to neuronal activity by clearing glutamate from the extracellular space (Anderson and Swanson, 2000). Glutamate in astrocytes is converted to glutamine and released by astrocytes to be taken up by neurons (Cotrina and Nedergaard, 2002). Disruption in the ability of astrocytes to remove glutamate can result in excitotoxicity and several studies have shown that aging astrocytes have an impaired glutamatergic response. Normal aging resulted in decreased amounts of vesicular glutamate transporters and reduced astroglial glutamate uptake capacity in male rats (Latour et al., 2013). A recent publication suggests that not only is the effect of aging on astrocytes region-specific but it is also dependent on the astroglial marker used (Rodriguez et al., 2014). A significant increase in GFAP surface was detected during aging in hippocampus but a reduction was observed in the entorhinal cortex of male mice (Rodriguez et al., 2014). However, the same study found a decrease in cellular service area positive for glutamine synthetase in the hippocampus, whereas no changes were observed in the entorhinal cortex (Rodriguez et al., 2014). Importantly, glutamine synthetase converts glutamate to glutamine, which is then released by astrocytes and used by neurons for the synthesis of glutamate and GABA (Verkhratsky and Kirchhoff, 2007). Using D-galactose to induce aging in male astrocytes resulted in a down-regulated expression of glutamine synthetase mRNA and decreased cell viability after glutamate exposure (Shen et al., 2014). Ischemia induces a neurotoxic cascade that results in increased glutamate release resulting in excitotoxicity which is likely a determining factor in the extent of the ischemic lesion. The baseline differences in the aging astrocytes’ ability to maintain glutamate homeostasis provide a possible mechanism for larger infarcts observed in aging animals.
In addition to changes observed during normal aging, aging astrocytes are also impaired following ischemia. Cortical astrocytes collected from the ischemic hemisphere of aging females cleared less glutamate than astrocytes from adult females and this was not explained by a difference in GLT-1 expression between groups (Lewis et al., 2012) (Fig. 1). These age-related changes in the glutamate/glutamine cycle may alter glutamate balance and synaptic transmission as well lead to poorer outcomes following ischemia.
Astrocyte regulation of glutamate homeostasis: influence of estrogen
To our knowledge only two studies have directly compared the ability of male and female astrocytes to maintain normal glutamate levels. Spinal astrocytes from female neonates cleared glutamate at a faster rate than male astrocytes (Morizawa et al., 2012) whereas there were no differences in the clearance of glutamate between adult male and female astrocytes following ischemia (Lewis et al., 2012). Although few studies have directly compared astrocyte functioning between males and females, it is known that estrogen regulates the expression of astrocytic components involved in removing excess extracellular glutamate. Estrogen treatment increased the expression of GLT-1 and GLAST mRNA and protein (Pawlak et al., 2005a) and increased glutamate transporter function (Lee et al., 2009). These results indicate that estrogen enhances glutamate uptake in astrocytes which could explain the age effect observed in Lewis et al. (2012), since the middle-aged females in that study were in constant diestrus, a state characterized by low estrogen levels.
Astrocytes and neuroinflammation following injury: impact of aging
Neuroinflammatory responses play a critical role in stroke outcomes and early inflammatory events are mediated in part by astrocytes (Xia et al., 2010), which are both targets for and producers of cytokines and proinflammatory mediators (Sofroniew, 2014; Pineau et al., 2010). The astrocytic response to neuroinflammation is altered by age and sex. NFκB is a transcription factor that is sequestered in the cytosol under basal conditions and then translocates to the nucleus upon stimulation where it induces genes involved in inflammation. Astrocytes from adult males showed moderate activation of cytosolic NFκB after stimulation with IL-1β, whereas astrocytes from aging males showed a much stronger response and had a larger increase in nuclear translocation of NFκB (Jiang and Cadenas, 2014). H2O2 production promotes activation of NFκB and the rate of H2O2 release was increased 50% from primary astrocytes isolated from aged males as compared to adult astrocytes (Jiang and Cadenas, 2014). Similarly, in vivo, normal aging is associated with increased release of inflammatory cytokines including IL1β and TNFα and the expression of these cytokines was mostly attributed to astrocytes (Campuzano et al., 2009). These studies suggest increased inflammatory responses in aging astrocytes which could result in detrimental stroke outcomes.
Astrocytes and neuroinflammation following injury: influence of estrogen
In addition, sex differences have been observed in the inflammatory response and there is evidence that this may be mediated through the estrogen’s actions on astrocytes (Azcoitia et al., 2010). Neonatal astrocytes treated with lipopolysaccharide (LPS) showed significant increases in levels of inflammatory markers including, IL-1β, IL6, and TNFα in both males and females; however, this increase was significantly greater in astrocytes from males as compared to females (Loram et al., 2012; Santos-Galindo et al., 2011). Pre-treatment with estradiol prevented the LPS induced increase in IL-1β, TNFα, and MMP-9 in astrocyte media derived from young and middle-aged females supporting the involvement of estrogen in this sex difference (Lewis et al., 2008). Furthermore, the LPS induced increase in the expression of IL-6 and interferon gamma-induced protein (IP-10) mRNA levels in astrocytes was attenuated by selective estrogen receptor modulators (Cerciat et al., 2010) and estradiol repressed NFκB-dependent transcription in astrocytes (Giraud et al., 2010). Reactive oxygen species production, a trigger for inflammatory responses, is increased in astrocytes after oxygen–glucose deprivation and this is prevented with estradiol treatment (Guo et al., 2012). Therefore, the effects of estradiol on astrocytic inflammatory responses may be one mechanism involved in the sex differences documented previously.
Gene expression and epigenetic modifications: impact of aging and ischemia
More recently, research has focused on the molecular mechanisms that may underlie changes in cellular function during aging. Gene expression is altered with aging; however, it is not clear how aging alters gene expression (Xu et al., 2007; Berchtold et al., 2008; Lu et al., 2004). Epigenetic processes, such as histone acetylation and DNA methylation, are critical regulators of transcriptional activity and studies have shown that aging influences acetylation levels (Peleg et al., 2010; Castellano et al., 2012). Levels of acetylation are maintained by a balance between histone acetyltransferases (HATs) and histone deacetylases (HDACs) (reviewed in Yang and Seto, 2007) and changes in both the activity and cellular localization of HDAC’s have been identified during aging (Dos Santos Sant’Anna et al., 2013; Baltan, 2012). Understanding how these enzymes are influenced by aging and sex may provide a target for blunting some of the negative aspects of normal aging.
Furthermore, disruption of epigenetic measures may be involved in the aging brain’s inability to mount effective cellular and molecular responses to ischemia. At the molecular level, ischemia results in changes in transcriptional activity of several genes (Kim et al., 2002, 2004; Kury et al., 2004). Age differences have been observed in gene expression following stroke in male rats, with adult male rats upregulating genes involved with the oxidative stress whereas aged rats displayed increased expression of pro-apoptotic and phagocytosis-promoting genes (Buga et al., 2012). A second study examined transcriptional responses across the lifespan following ischemia in males and found that genes involved in the inflammatory and immune response are regulated in an age-dependent manner (Sieber et al., 2014). It is notable that the previously mentioned literature on gene expression and epigenetic modifications in normal aging and following stroke examined males only. However, a recent profiling study of microRNAs, post-transcriptional gene regulators, found that five days following stroke, there is a unique expression pattern of microRNAs in adult females, as compared to adult males and middle-age males and females, including age differences in several microRNAs that are members of the mir17-92 cluster (Selvamani et al., 2014).
Astrocyte specific changes in gene expression and epigenetic modifications
In addition to the whole brain changes that have been observed during aging and following ischemia in gene expression and epigenetic modifications, astrocyte specific alterations have been identified. Astrocytes isolated from adult mice showed higher expression of genes involved in hemoglobin synthesis and neuronal differentiation than aging astrocytes, while aged astrocytes showed higher expression of genes implicated in zinc ion binding and an increased inflammatory phenotype indicating that that normal aging alters gene expression profiles in astrocytes (Orre et al., 2014). Our lab has shown recently that astrocytes from adult females had greater H3K4 specific methyltransferase activity than middle-aged females following ischemia and consistent with this finding, astrocytes from adult females displayed more H3K4me3 enriched peaks than middle-aged females (Chisholm et al., 2015). This study also identified astrocytes as a potential mediator in the increased expression of circulating microRNAs previously observed in adult females following ischemia (Selvamani et al., 2014). Astrocytes isolated from adult females had more H3K4me3 enriched peaks at the mir17-92 cluster and greater mir20a mRNA expression as compared to astrocytes from middle-aged females. In addition, H3K4me3 was increased at the VEGFa gene and protein expression of VEGF was increased in astrocytes from adult females relative to aging females following ischemia (Chisholm et al., 2015) (Fig. 1). This suggests that increased astrogliosis in aged animals discussed previously is not due to overproduction of VEGF. However, the reduced VEGF levels in aging astrocytes may mediate a loss of neuroprotection and consequently, increase brain vulnerability to ischemia. Some of the age-related changes mentioned earlier appear to be more plastic and independent of H3K4me3. For example, the age-related changes that were previously observed in astrocytic IGF-1 secretion and glutamate clearance (Lewis et al., 2012) occur without changes in H3K4me3 at the IGF-1, GLT1 and GLAST genes (Chisholm et al., 2015). Functioning of the glutamate transporter is known to be improved by IGF-1 (Suzuki et al., 2001) and thus dysfunction in these systems may be attenuated with IGF-1 treatment rather than altering gene expression.
The finding that astrocytes from adult females have more genes that are differentially enriched for H3K4me3 following ischemia (Chisholm et al., 2015), suggests that future therapies that target epigenetic modifications may confer neuroprotection during aging. Indeed, recent research has shown beneficial effects of the pan HDAC inhibitor, resveratrol, in astrocyte cultures exposed to stressors (Venturelli et al., 2013). Resveratrol was able to attenuate the increase in reactive astrocytes as well as decrease the production of pro-inflammatory mediators following astrocyte activation with beta amyloid 1–42 (Scuderi et al., 2014). Importantly, a separate study found that resveratrol was able to decrease the levels of TNF-α and IL-1β and increase the glutamine synthetase activity and glutathione levels in astrocytes from adult and aged male rats (Bellaver et al., 2014) suggesting that the neuroprotective properties of resveratrol are mediated through astrocytes and are maintained during aging. In order to develop new effective therapies for stroke, a better understanding of age-related epigenetic changes, and how these are influenced by sex and ischemia, is necessary.
Acknowledgments
The authors would like to thank Annette Fincher for her generous help designing and creating the artwork. This study was supported by NIH/AG042189 and NIH/NS074895 to FS.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. (discussion 166) [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Acaz-Fonseca E, Azcoitia I, Garcia-Segura LM. Gonadal hormones and the control of reactive gliosis. Horm Behav. 2013;63:216–221. doi: 10.1016/j.yhbeh.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Arias C, Zepeda A, Hernandez-Ortega K, Leal-Galicia P, Lojero C, Camacho-Arroyo I. Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus. Horm Behav. 2009;55:257–263. doi: 10.1016/j.yhbeh.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Arimoto JM, Wong A, Rozovsky I, Lin SW, Morgan TE, Finch CE. Age increase of estrogen receptor-alpha (ERalpha) in cortical astrocytes impairs neurotrophic support in male and female rats. Endocrinology. 2013;154:2101–2113. doi: 10.1210/en.2012-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S, de Araujo GW, Beyer C. Gender-specific regulation of mitochondrial fusion and fission gene transcription and viability of cortical astrocytes by steroid hormones. J Mol Endocrinol. 2008;41:289–300. doi: 10.1677/JME-08-0085. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci. 2010;32:1995–2002. doi: 10.1111/j.1460-9568.2010.07516.x. [DOI] [PubMed] [Google Scholar]
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- Baltan S. Histone deacetylase inhibitors preserve function in aging axons. J Neurochem. 2012;123(Suppl 2):108–115. doi: 10.1111/j.1471-4159.2012.07949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Moinuddin A, Morley JE. Regional transport of TNF-alpha across the blood-brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol Aging. 2001;22:671–676. doi: 10.1016/s0197-4580(01)00220-2. [DOI] [PubMed] [Google Scholar]
- Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol In Vitro. 2014;28:479–484. doi: 10.1016/j.tiv.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105:15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell. 2011;10:466–482. doi: 10.1111/j.1474-9726.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga AM, Sascau M, Pisoschi C, Herndon JG, Kessler C, Popa-Wagner A. The genomic response of the ipsilateral and contralateral cortex to stroke in aged rats. J Cell Mol Med. 2008;12:2731–2753. doi: 10.1111/j.1582-4934.2008.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga AM, Scholz CJ, Kumar S, Herndon JG, Alexandru D, Cojocaru GR, Dandekar T, Popa-Wagner A. Identification of new therapeutic targets by genome-wide analysis of gene expression in the ipsilateral cortex of aged rats after stroke. PLoS One. 2012;7:e50985. doi: 10.1371/journal.pone.0050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res. 2009;87:2484–2497. doi: 10.1002/jnr.22074. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Castellano JF, Fletcher BR, Kelley-Bell B, Kim DH, Gallagher M, Rapp PR. Age-related memory impairment is associated with disrupted multivariate epigenetic coordination in the hippocampus. PLoS One. 2012;7:e33249. doi: 10.1371/journal.pone.0033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2010;58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hu W, Toung TJ, Zhang Z, Parker SM, Roselli CE, Hurn PD. Age-dependent effects of testosterone in experimental stroke. J Cereb Blood Flow Metab. 2009;29:486–494. doi: 10.1038/jcbfm.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm N, Henderson M, Selvamani A, Park MJ, Dindot S, Miranda R, Sohrabji F. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 0. 2015 doi: 10.1080/15592294.2014.1001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copen WA, Schwamm LH, Gonzalez RG, Wu O, Harmath CB, Schaefer PW, Koroshetz WJ, Sorensen AG. Ischemic stroke: effects of etiology and patient age on the time course of the core apparent diffusion coefficient. Radiology. 2001;221:27–34. doi: 10.1148/radiol.2211001397. [DOI] [PubMed] [Google Scholar]
- Cordeau P, Jr, Lalancette-Hebert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke. 2008;39:935–942. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- De Butte-Smith M, Nguyen AP, Zukin RS, Etgen AM, Colbourne F. Failure of estradiol to ameliorate global ischemia-induced CA1 sector injury in middle-aged female gerbils. Brain Res. 2007;1153:214–220. doi: 10.1016/j.brainres.2007.03.082. [DOI] [PubMed] [Google Scholar]
- Dhungana H, Malm T, Denes A, Valonen P, Wojciechowski S, Magga J, Savchenko E, Humphreys N, Grencis R, Rothwell N, Koistinaho J. Aging aggravates ischemic stroke-induced brain damage in mice with chronic peripheral infection. Aging Cell. 2013;12:842–850. doi: 10.1111/acel.12106. [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood–brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Dong P, Zhao J, Zhang Y, Dong J, Zhang L, Li D, Li L, Zhang X, Yang B, Lei W. Aging causes exacerbated ischemic brain injury and failure of sevoflurane post-conditioning: role of B-cell lymphoma-2. Neuroscience. 2014;275:2–11. doi: 10.1016/j.neuroscience.2014.05.064. [DOI] [PubMed] [Google Scholar]
- Dos Santos Sant’Anna G, Rostirola Elsner V, Moyses F, Reck Cechinel L, Agustini Lovatel G, Rodrigues Siqueira I. Histone deacetylase activity is altered in brain areas from aged rats. Neurosci Lett. 2013;556:152–154. doi: 10.1016/j.neulet.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Dotson AL, Zhu W, Libal N, Alkayed NJ, Offner H. Different immunological mechanisms govern protection from experimental stroke in young and older mice with recombinant TCR ligand therapy. Front Cell Neurosci. 2014;8:284. doi: 10.3389/fncel.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Duncan KA, Moon J, Vartosis D, Zee I. Injury-induced expression of glial androgen receptor in the zebra finch brain. J Neurotrauma. 2013;30:1919–1924. doi: 10.1089/neu.2013.2951. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood–brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Figley CR, Stroman PW. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci. 2011;33:577–588. doi: 10.1111/j.1460-9568.2010.07584.x. [DOI] [PubMed] [Google Scholar]
- Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, Schwamm LH, Get With the Guidelines-Stroke Steering Committee Investigators Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern Med. 2009;48:967–973. doi: 10.2169/internalmedicine.48.1757. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Luquin S, Fernandez AM, Garcia-Segura LM. Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. Int J Dev Neurosci. 1999;17:145–151. doi: 10.1016/s0736-5748(98)00065-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Wehner S, Reeves MJ. Presenting symptoms and onset-to-arrival time in patients with acute stroke and transient ischemic attack. J Stroke Cerebrovasc Dis. 2011;20:494–502. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Gattringer T, Ferrari J, Knoflach M, Seyfang L, Horner S, Niederkorn K, Culea V, Beitzke M, Lang W, Enzinger C, Fazekas F. Sex-related differences of acute stroke unit care: results from the Austrian stroke unit registry. Stroke. 2014;45:1632–1638. doi: 10.1161/STROKEAHA.114.004897. [DOI] [PubMed] [Google Scholar]
- Genis L, Davila D, Fernandez S, Pozo-Rodrigalvarez A, Martinez-Murillo R, Torres-Aleman I. Astrocytes Require Insulin-like Growth Factor I to Protect Neurons Against Oxidative Injury. eCollection. 2014;2014 doi: 10.12688/f1000research.3-28.v1. F1000Res 3:28-28.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134:2125–2133. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits on-going autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc Natl Acad Sci U S A. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Duckles SP, Weiss JH, Li X, Krause DN. 17Beta-estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen–glucose deprivation/reperfusion. Free Radic Biol Med. 2012;52:2151–2160. doi: 10.1016/j.freeradbiomed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Etgen AM. Neuroprotective action of acute estrogens: animal models of brain ischemia and clinical implications. Steroids. 2013;78:597–606. doi: 10.1016/j.steroids.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell. 2014;13:1059–1067. doi: 10.1111/acel.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Lowry NC. Neuroanatomical changes associated with cognitive aging. Curr Top Behav Neurosci. 2012;10:137–162. doi: 10.1007/7854_2011_137. [DOI] [PubMed] [Google Scholar]
- Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM, Investigators of the Registry of the Canadian Stroke Network Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Li X, Tan Z, VanGilder RL, Rosen CL, Huber JD. NOX2 inhibition with apocynin worsens stroke outcome in aged rats. Brain Res. 2009;1292:165–172. doi: 10.1016/j.brainres.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YD, Sohn NW, Kang C, Soh Y. DNA array reveals altered gene expression in response to focal cerebral ischemia. Brain Res Bull. 2002;58:491–498. doi: 10.1016/s0361-9230(02)00823-7. [DOI] [PubMed] [Google Scholar]
- Kim JB, Piao CS, Lee KW, Han PL, Ahn JI, Lee YS, Lee JK. Delayed genomic responses to transient middle cerebral artery occlusion in the rat. J Neurochem. 2004;89:1271–1282. doi: 10.1111/j.1471-4159.2004.02429.x. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010a;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol Sex Differ. 2010b;1 doi: 10.1186/2042-6410-1-7. (7-6410-1-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kury P, Schroeter M, Jander S. Transcriptional response to circumscribed cortical brain ischemia: spatiotemporal patterns in ischemic vs. remote non-ischemic cortex. Eur J Neurosci. 2004;19:1708–1720. doi: 10.1111/j.1460-9568.2004.03226.x. [DOI] [PubMed] [Google Scholar]
- Latour A, Grintal B, Champeil-Potokar G, Hennebelle M, Lavialle M, Dutar P, Potier B, Billard JM, Vancassel S, Denis I. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell. 2013;12:76–84. doi: 10.1111/acel.12026. [DOI] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon RL, Li X, Huber JD, Rosen CL. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology. 2012;153:3386–3393. doi: 10.1210/en.2011-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DK, Thomas KT, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol Aging. 2012;33:1123.e1–1123.16. doi: 10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27:135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J Neurosci Methods. 2008;171:214–217. doi: 10.1016/j.jneumeth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014a;62:2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ni JJ, Huang JJ, Kou ZW, Sun FY. VEGF overexpression enhances the accumulation of phospho-S292 MeCP2 in reactive astrocytes in the adult rat striatum following cerebral ischemia. Brain Res. 2015;1599:32–43. doi: 10.1016/j.brainres.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Ma YL, Qin P, Feng DY, Li Y, Zhang LX, Liu ZY, Yin AQ, Tang WH, Dong HL, Meng LZ, Hou WG, Xiong LZ. Estrogen regulates the expression of Ndrg2 in astrocytes. Brain Res. 2014;1569:1–8. doi: 10.1016/j.brainres.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Kastin AJ, Zadina JE. Passage of interleukin-1-beta across the blood–brain barrier is reduced in aged mice: a possible mechanism for diminished fever in aging. Neuroimmunomodulation. 2000;8:148–153. doi: 10.1159/000054275. [DOI] [PubMed] [Google Scholar]
- Miao J, Shen LH, Tang YH, Wang YT, Tao MX, Jin KL, Zhao YJ, Yang GY. Overexpression of adiponectin improves neurobehavioral outcomes after focal cerebral ischemia in aged mice. CNS Neurosci Ther. 2013;19:969–977. doi: 10.1111/cns.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizawa Y, Sato K, Takaki J, Kawasaki A, Shibata K, Suzuki T, Ohta S, Koizumi S. Cell-autonomous enhancement of glutamate-uptake by female astrocytes. Cell Mol Neurobiol. 2012;32:953–956. doi: 10.1007/s10571-012-9829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Tessler S, Gitay-Goren H, Cohen T, Levi BZ. Vascular endothelial growth factor and its receptors. Prog Growth Factor Res. 1994;5:89–97. doi: 10.1016/0955-2235(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2014;35:1–14. doi: 10.1016/j.neurobiolaging.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG, American Heart Association Advocacy Coordinating Committee and Stroke Council Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005a;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Karolczak M, Krust A, Chambon P, Beyer C. Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia. 2005b;50:270–275. doi: 10.1002/glia.20162. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Pelegri C, Canudas AM, del Valle J, Casadesus G, Smith MA, Camins A, Pallas M, Vilaplana J. Increased permeability of blood–brain barrier on the hippocampus of a murine model of senescence. Mech Ageing Dev. 2007;128:522–528. doi: 10.1016/j.mad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Perez-Alvarez MJ, Maza Mdel C, Anton M, Ordonez L, Wandosell F. Post-ischemic estradiol treatment reduced glial response and triggers distinct cortical and hippocampal signaling in a rat model of cerebral ischemia. J Neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-157. (157-2094-9-157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–293. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A. Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging. 2014;35:15–23. doi: 10.1016/j.neurobiolaging.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Rosen CL, Dinapoli VA, Nagamine T, Crocco T. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg. 2005;103:687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17Beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ. 2011;2 doi: 10.1186/2042-6410-2-7. (7-6410-2-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C, Stecca C, Bronzuoli MR, Rotili D, Valente S, Mai A, Steardo L. Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer’s disease. Front Pharmacol. 2014;5:89. doi: 10.3389/fphar.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010a;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010b;31:1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Williams MH, Miranda RC, Sohrabji F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin Sci (Lond) 2014;127:77–89. doi: 10.1042/CS20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Gao H, Shi X, Wang N, Ai D, Li J, Ouyang L, Yang J, Tian Y, Lu J. Glutamine synthetase plays a role in D-galactose-induced astrocyte aging in vitro and in vivo. Exp Gerontol. 2014;58:166–173. doi: 10.1016/j.exger.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Sieber MW, Guenther M, Jaenisch N, Albrecht-Eckardt D, Kohl M, Witte OW, Frahm C. Age-specific transcriptional response to stroke. Neurobiol Aging. 2014;35:1744–1754. doi: 10.1016/j.neurobiolaging.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Estrogen–IGF-1 interactions in neuroprotection: ischemic stroke as a case study. Front Neuroendocrinol. 2015;36C:1–14. doi: 10.1016/j.yfrne.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Stipursky J, Romao L, Tortelli V, Neto VM, Gomes FC. Neuron-glia signaling: implications for astrocyte differentiation and synapse formation. Life Sci. 2011;89:524–531. doi: 10.1016/j.lfs.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ikegaya Y, Matsuura S, Kanai Y, Endou H, Matsuki N. Transient up-regulation of the glial glutamate transporter GLAST in response to fibroblast growth factor, insulin-like growth factor and epidermal growth factor in cultured astrocytes. J Cell Sci. 2001;114:3717–3725. doi: 10.1242/jcs.114.20.3717. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Tan Z, Li X, Kelly KA, Rosen CL, Huber JD. Plasminogen activator inhibitor type 1 derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res. 2009;1281:84–90. doi: 10.1016/j.brainres.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant KA, Kerr AL, Adkins DL, Donlan N, Thomas N, Kleim JA, Jones TA. Age-dependent reorganization of peri-infarct “premotor” cortex with task-specific rehabilitative training in mice. Neurorehabil Neural Repair. 2014;29(2):193–202. doi: 10.1177/1545968314541329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Akiguchi I, Yagi H, Naiki H, Fujibayashi Y, Kimura J, Takeda T. Age-related changes in barrier function in mouse brain I. Accelerated age-related increase of brain transfer of serum albumin in accelerated senescence prone SAM-P/8 mice with deficits in learning and memory. Arch Gerontol Geriatr. 1993;16:233–248. doi: 10.1016/0167-4943(93)90035-g. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Liu K, Ardeshiri A, Cheng J, Murphy SJ, Hurn PD, Herson PS. Poly (ADP-ribose) polymerase-1 initiated neuronal cell death pathway—do androgens matter? Neuroscience. 2010;166:476–481. doi: 10.1016/j.neuroscience.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli S, Berger A, Bocker A, Busch C, Weiland T, Noor S, Leischner C, Schleicher S, Mayer M, Weiss TS, Bischoff SC, Lauer UM, Bitzer M. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS One. 2013;8:e73097. doi: 10.1371/journal.pone.0073097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal–glial transmission. J Anat. 2007;210:651–660. doi: 10.1111/j.1469-7580.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorbrodt AW, Dobrogowska DH, Meeker HC, Carp RI. Immunogold study of regional differences in the distribution of glucose transporter (GLUT-1) in mouse brain associated with physiological and accelerated aging and scrapie infection. J Neurocytol. 1999;28:711–719. doi: 10.1023/a:1007034003114. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, Leeds P, Chuang DM. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke. 2012;43:2430–2436. doi: 10.1161/STROKEAHA.112.652545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jie C, Dai X. Possible roles of astrocytes in estrogen neuroprotection during cerebral ischemia. Rev Neurosci. 2014;25:255–268. doi: 10.1515/revneuro-2013-0055. [DOI] [PubMed] [Google Scholar]
- Wong R, Renton C, Gibson CL, Murphy SJ, Kendall DA, Bath PM, Progesterone Pre-Clinical Stroke Pooling Project Collaboration Progesterone treatment for experimental stroke: an individual animal meta-analysis. J Cereb Blood Flow Metab. 2013;33:1362–1372. doi: 10.1038/jcbfm.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Han J, Huang G, Ying W. Inflammation in ischaemic brain injury: current advances and future perspectives. Clin Exp Pharmacol Physiol. 2010;37:253–258. doi: 10.1111/j.1440-1681.2009.05279.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H, Zhang P, Ibe C, Zonderman AB, Longo DL, Poosala S, Becker KG, Mattson MP. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007;8:R234. doi: 10.1186/gb-2007-8-11-r234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Zhu SW, Duan RS, Mohammed AH, Winblad B, Zhu J. Gender differences in susceptibility to kainic acid-induced neurodegeneration in aged C57BL/6 mice. Neurotoxicology. 2008;29:406–412. doi: 10.1016/j.neuro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Tang H, Dong Y, Chan A, Sareddy GR, Vadlamudi RK, Brann DW. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol Cell Endocrinol. 2014;389:84–91. doi: 10.1016/j.mce.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Watts LT, Holstein DM, Prajapati SI, Keller C, Grass EH, Walter CA, Lechleiter JD. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5:e14401. doi: 10.1371/journal.pone.0014401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Talley Watts L, Holstein DM, Wewer J, Lechleiter JD. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab. 2013;33:600–611. doi: 10.1038/jcbfm.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]


