Abstract
Introduction
Comparing patient-reported outcomes such as urinary and erectile function across institutions is critical for prostate cancer research and quality assurance. Such comparisons are complicated by the use of different questionnaires. We aimed to develop a method to convert scores between four commonly used instruments.
Materials and Methods
Patient-reported data on urinary and sexual function were collected from 1,284 men with localized prostate cancer using the Expanded Prostate Index Composite (EPIC-26), UCLA Prostate Cancer Index (PCI), Sexual Health Inventory for Men (SHIM) and International Prostate Symptom Scale (IPSS) questionnaires. We investigated several methods to convert scores between questionnaires.
Results
Conversion between EPIC and PCI urinary and sexual function subscales is best achieved using only the subset of questions that were asked on both questionnaires. For the conversion between EPIC or PCI erectile function scores and the SHIM scores, we defined thresholds of poor, intermediate or good function respectively: EPIC/PCI 0 – 40 and SHIM 1–7; EPIC/PCI 41 – 59 and SHIM 8–16; EPIC/PCI 60 – 100 and SHIM 17–25. Urinary continence scores are highly correlated between PCI and EPIC (r=0.94). No comparison was possible between IPSS with EPIC and PCI due to differences in domains addressed by these questionnaires.
Conclusions
We have introduced methods for converting scores between the EPIC, PCI and SHIM questionnaires. While these conversion methods may introduce a minor amount of imprecision, they represent the best available tools for combining and comparing patient-reported outcomes assessed using different instruments among men undergoing radical prostatectomy or on active surveillance.
Keywords: patient outcome assessment, prostatic neoplasms, urinary incontinence, sexual dysfunction
Introduction
Adequately determining quality of medical care involves assessing the effectiveness of treatment, adverse effects related to treatment, and patient quality of life (QOL). In the case of prostate cancer, both the tumor and its treatment can affect urinary and erectile dysfunction, and consistently collecting outcomes data for these domains is essential both for comparisons between treatments and for provider-level quality assessments. For a variety of reasons, physician determination of patient urinary and sexual function is consistently inadequate, and these outcomes must be evaluated by direct patient report, using validated QOL questionnaires.1
A variety of instruments exist to measure patient-reported outcomes (PROs) in prostate cancer patients. This can make comparisons for both research and quality assurance difficult. Imagine, for example, a scenario in which two hospitals would like to implement a program comparing outcomes after radical prostatectomy so that surgeons can share knowledge and improve surgical technique and outcomes. One hospital measures post-operative sexual function using the EPIC2, which is scored from 0 to 100 and includes questions on sexual desire and erectile function, while the other hospital uses the Sexual Health Inventory for Men SHIM, which is scored from 1 to 25 and includes questions about erectile function only. If the average baseline-adjusted score one year post-surgery is a SHIM of 16 at one institution and EPIC of 52 at the other, there is no obvious way to know which is obtaining better results.
Several previous publications have compared different prostate cancer outcome instruments attempting to derive a numerical conversion between raw scores (e.g. SHIM = EPIC ÷ 4 + 1).3–6 A typical finding has been that because of variation in the domains included - for instance, that EPIC measures both erectile function and sexual desire while SHIM measures only function - conversion between these two scales cannot be done. However, an alternative would be to use a subset of the EPIC questions, compare those to SHIM, and create a conversion factor that allows comparison for the specific endpoint of erectile function. We aimed to develop an appropriate and easily interpretable conversion method to facilitate comparisons between different prostate cancer PRO questionnaires, without the constraint that all questions in an instrument need be included.
Materials and Methods
The Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry is a prospective disease registry collecting data on men treated for prostate cancer at primarily community-based clinical sites across the United States. Men are treated according to local urologists’ practices, and are followed, under central and/or local institutional review board supervision, until death or study withdrawal. Participating clinicians report diagnostic, risk stratification, and clinical outcomes data, and patients directly report their quality of life before first treatment and at least annually in followup using validated PRO questionnaires. Additional details have been published previously.7 Until 2013, prostate cancer specific QOL was measured using the PCI8, which includes domains for urinary continence, sexual function, and bowel function as well as corresponding bother domains. In 2013, to better reflect the differential impact of non-surgical treatments on QOL, the PCI was replaced with the EPIC-26, which specifically includes urinary irritation and hormonal impact questions.
In anticipation of this switch, we conducted a one-time cross-sectional sub-study. Men were invited irrespective of primary treatment or duration of followup since treatment and answered an extended QOL survey which included the full length PCI, the EPIC-26, the SHIM, and the International Prostate Symptom Scale (IPSS). There is considerable but incomplete overlap between the urinary questions in the PCI and the urinary continence questions in the EPIC-26, and likewise between the sexual function questions included in both instruments, and both are scaled 0–100 in each domain. The SHIM, as noted above, includes only questions on erectile function and is scaled 1–25. Finally, the IPSS focuses on urinary obstructive and irritative symptoms, and is scored 1–35, with a single additional question about overall urinary QOL. A principal goal of this sub-study was therefore to determine the extent to which scores from each of these instruments are interchangeable, and to find ways to convert urinary function scores among EPIC-26, PCI and IPSS, and sexual function scores between EPIC-26, PCI and SHIM.
After investigating several potential methods for converting questionnaire scores (see supplementary Methods), we decided to convert between the EPIC and PCI scales by using questions that were asked in both questionnaires and then finding the appropriate conversion factor between the scores. Modified urinary function scores on a scale of 0–100 were calculated using only questions common to both the EPIC-26 and PCI questionnaires. Since there was no overlap in questions between the EPIC-26 or PCI and the IPSS, we investigated whether questions on overall urinary bother from the EPIC-26 and PCI questionnaires could be compared to the IPSS question regarding quality of life with urinary symptoms.
We converted sexual function scores in the EPIC-26 and PCI questionnaires in the same way we had converted urinary function scores, by using questions that were common to both questionnaires. Since the PCI but not the EPIC-26 asks about libido, only questions regarding erectile function were included in the modified sexual function score.
Scores from the SHIM questionnaire are often categorized,9 and we predicted that, given the different range of SHIM and PCI/EPIC scores, it would be preferable to ensure consistent categorization between scales than attempt a direct numeric conversion. Hence we opted to determine cut points for EPIC-26 and PCI scores that would allow for consistent categorization of sexual function.
Additional details regarding the methodology are included in the supplemental material. All analyses were performed using Stata 13 (Stata Corporation, College Station, TX).
Results
The cohort consisted of 1,284 men who completed all four questionnaires after prostate cancer treatment or while on active surveillance. Patient characteristics are summarized in Table 1. Patients who did not complete all surveys were older and a greater number underwent radical prostatectomy as compared to those who did not complete the surveys, or did not respond (p<0.01).
Table 1.
Patient characteristics for those with complete response to survey, those with incomplete response to survey, and those who declined or did not respond. Data are presented as median (IQR) or frequency (percent).
| Complete response to survey (N=1284) | Incomplete response to survey (N=598) | Declined or no response (N=1011) | p value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 63 (58, 68) | 67 (61, 72) | 63 (58, 69) | <0.01 |
| Age at survey (years) | 73 (67, 79) | 78 (72, 83) | - | <0.01 |
| Treatment to survey (years) | 9 (7, 11) | 9 (7, 12) | - | <0.01 |
| Treatment | <0.01 | |||
| Radical Prostatectomy | 908 (71) | 267 (45) | 598 (59) | |
| Brachytherapy | 155 (12) | 80 (13) | 97 (9.6) | |
| External beam radiation | 70 (5.5) | 53 (8.9) | 81 (8.0) | |
| Primary androgen deprivation therapy | 56 (4.4) | 51 (8.5) | 104 (10) | |
| Active surveillance/watchful waiting | 52 (4.1) | 31 (5.2) | 91 (9.0) | |
| Cryotherapy | 41 (3.2) | 34 (5.7) | 40 (4.0) | |
| Focal therapy | 2 (0.2) | 0 (0) | 0 (0) | |
| Other | 0 (0) | 82 (14) | 0 (0) |
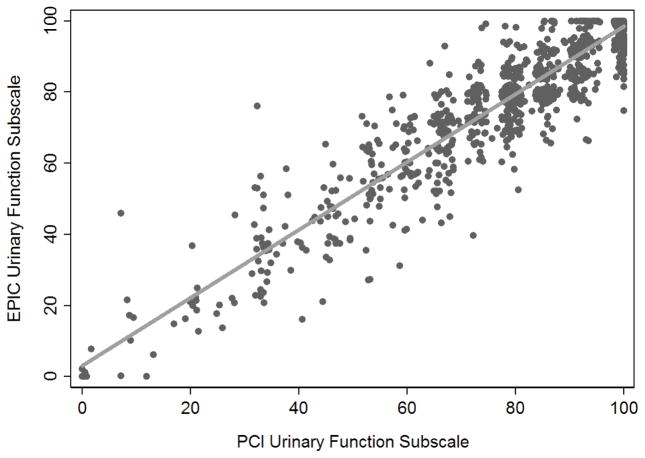
A modified urinary score was calculated using the four questions asked on both the EPIC-26 and PCI questionnaires. The modified urinary function score on a scale of 0–100 was calculated as the mean score of all questions answered for those who answered at least three out of the four included questions (Table 2). Median and quartiles of the modified EPIC-26 urinary score (n= 1,284) and PCI score (n=1,249) were identical (85.5; 73, 100), with high correlation between the two scores (concordance correlation coefficient r=0.94; Figure 1). Due to the overlapping distribution of the urinary continence scores and the similarity in questions between EPIC-26 and PCI, we believe the best way to convert between EPIC-26 and PCI scores is as a 1:1 conversion of the urinary continence score calculated using only the questions common to both questionnaires. We stress this conversion should be restricted to use for radical prostatectomy and active surveillance patients, since questions about irritative urinary symptoms commonly caused by radiation are not included in the PCI questionnaire and therefore were not included in the modified urinary continence scores.
Table 2.
Questions common to both EPIC-26 and PCI questionnaires.
| Question Text | EPIC-26 | PCI v1 |
|---|---|---|
| Urinary domain | ||
| Which of the following best describes your urinary control during the last 4 weeks? | Question 2 | Question 13 |
| How many pads or adult diapers per day did you usually use to control leakage during the last 4 weeks? | Question 3 | Question 14 |
| How big a problem has dripping urine or wetting your pants been for you? | Question 4a | Question 15b |
| Overall, how big a problem has your urinary function been for you during the last 4 weeks? | Question 5 | Question 16 |
| Erectile function domain | ||
| How would you rate your ability to have an erection during the last 4 weeks? | Question 8a | Question 22b |
| How would you describe the usual quality of your erections? | Question 9 | Question 23 |
| How would you describe the usual frequency of your erections? | Question 10 | Question 24 |
| Overall, how would you rate your ability to function sexually during the last 4 weeks? | Question 11 | Question 27 |
| Overall, how big a problem has your sexual function been for you during the last 4 weeks? | Question 12 | Question 28 |
Figure 1.
Correlation between EPIC-26 and PCI urinary function subscale scores (r=0.94).
Conversely, questions about urinary function or symptoms were not comparable between IPSS and EPIC-26 or PCI. The PCI questionnaire focuses exclusively on urinary continence and bother, and the EPIC on urinary continence and irritation, while the IPSS asks about a variety of general urinary symptoms, focusing primary on obstruction. We considered including obstructive or irritative symptoms only, since these domains are addressed on both the EPIC-26 and IPSS. However, correlations between these questions were weak (0.61 for incomplete emptying, 0.70 for weak stream, 0.60 for frequency). We then explored the one question about general urinary bother from each questionnaire, but found no cut points that identified bothersome urinary symptoms with good concordance between questionnaires (further details in the supplement). Based on these analyses, we concluded that it is inappropriate to convert scores between the IPSS and either EPIC-26 or PCI.
We next explored ways to convert sexual function scores between the EPIC-26, PCI and SHIM questionnaires. As with urinary function, several sexual function questions were identical on both the EPIC-26 and PCI questionnaires. Five questions that occurred on both questionnaires and addressed erectile function were included in the modified sexual function subscale (Table 2). Questions on libido or sexual desire were excluded from this score. The modified sexual function score was calculated on a scale of 0–100 as the mean score of all non-missing answers for patients who answered at least 3 out of the 5 included questions.
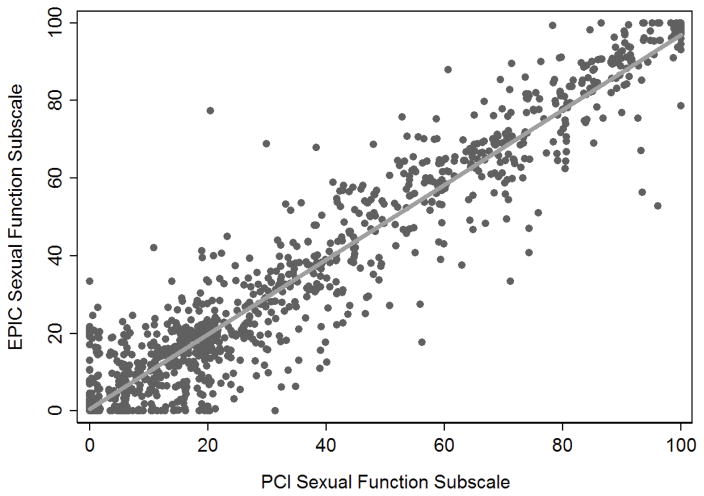
The median modified sexual function score for the EPIC-26 was 20 (IQR 5, 55). For the PCI questionnaire, the median score was 20 (IQR 6.6, 55). As seen with urinary function scores, the distribution of the sexual function scores in EPIC-26 and PCI questionnaires overlap, and all questions included in this score were asked on both questionnaires. Again, correlation was excellent (concordance correlation coefficient r=0.96), as illustrated in figure 2. As such, we believe the best way to convert between EPIC-26 and PCI scores for sexual function is to do a 1:1 conversion of the scores, including only the five questions common to both questionnaires.
Figure 2.
Correlation between EPIC-26 and PCI sexual function subscale scores (r=0.96).
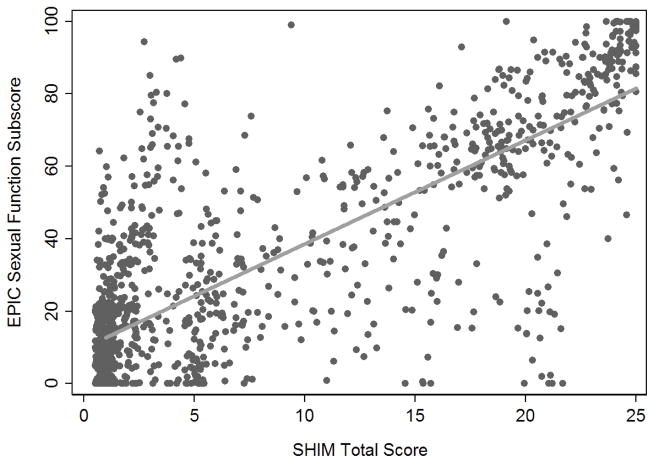
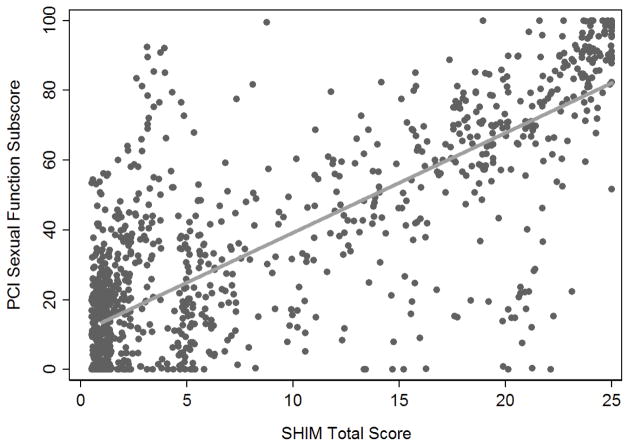
To convert between SHIM and EPIC-26 or PCI scores (Figures 3 and 4), we searched for score cut points that would allow us to classify patients as having good, intermediate or poor sexual function, corresponding to the categorization of SHIM scores. A threshold of 40 for poor function for both EPIC-26 and PCI questionnaires (see supplemental materials for further details) concordantly classified 86% of all patients based on the modified EPIC-26 score (Table 3). A cut point of 59 was chosen for good function in both questionnaires, which led to concordant classifications from the EPIC-26 for 92% of all patients. Concordance rates for the PCI scores were similar to EPIC concordance rates (Table 3). While these cut points accurately classify patients who report good or poor function on the SHIM questionnaire, they are less accurate for classifying patients who reported SHIM scores in the intermediate function range. However, these patients represented only 10% of the cohort.
Figure 3.
Correlation between EPIC-26 sexual function scale scores and SHIM scores (r=0.80).
Figure 4.
Correlation between PCI sexual function scale scores and SHIM scores (r=0.80).
Table 3.
Concordance between SHIM categories and categorized EPIC-26 or PCI sexual function scores.
| SHIM Poor Function (1–7) | SHIM Intermediate Function (8–16) | SHIM Good Function (17–25) | Total | |
|---|---|---|---|---|
| EPIC-26 Poor Function (0–40) | 782 (91%) | 68 (52%) | 30 (10%) | 880 |
| EPIC-26 Intermediate Function (41–59) | 53 (6.1%) | 40 (31%) | 21 (7.2%) | 114 |
| EPIC-26 Good Function (60–100) | 29 (3.4%) | 22 (17%) | 239 (82%) | 290 |
| Total | 864 | 130 | 290 | 1,284 |
| PCI Poor Function (0–40) | 751 (91%) | 54 (42%) | 28 (10%) | 833 |
| PCI Intermediate Function (41–59) | 54 (6.5%) | 49 (38%) | 22 (8.0%) | 125 |
| PCI Good Function (60–100) | 25 (3.0%) | 26 (20%) | 226 (82%) | 277 |
| Total | 830 | 129 | 276 | 1,235 |
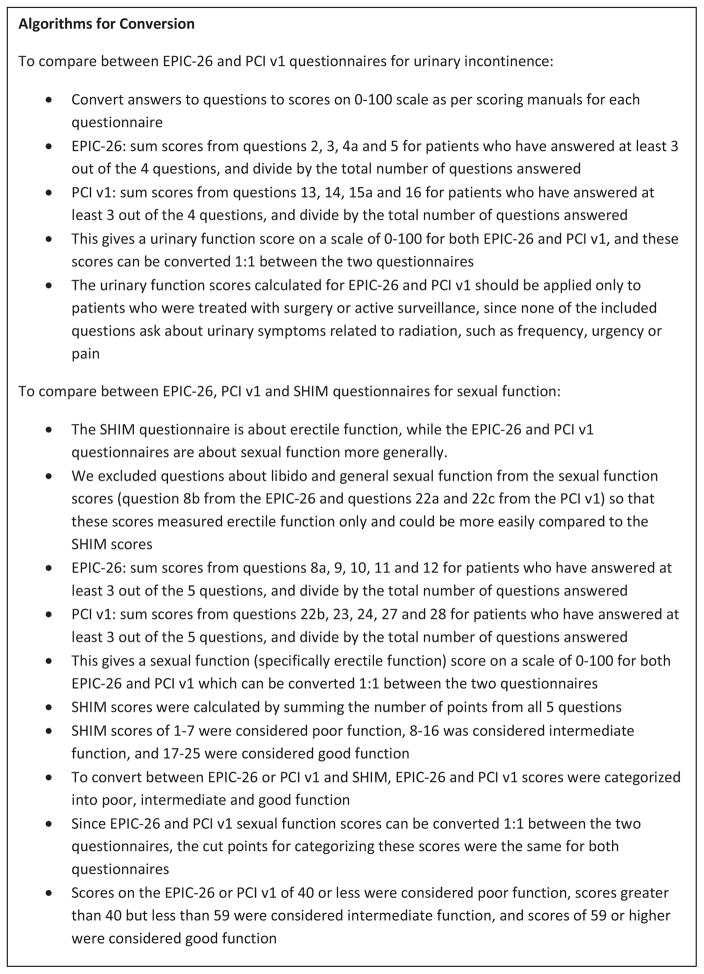
Psychometric properties of the modified EPIC and PCI urinary continence and sexual scores were good (details are available in the appendix). Details on how to calculate the scores are presented in figure 5.
Figure 5.
Algorithm for interconversion between questionnaires
Discussion
The ability to document and compare outcomes, including PROs, is vital to ongoing research and to a wide range of quality improvement efforts in medicine. Multiple questionnaires are used to measure PROs after prostate cancer treatment. These questionnaires were developed with different goals and methodologies, ask different questions, and are scored in different ways. Such differences complicate comparisons of prostate cancer PROs between or within institutions using different questionnaires.
Given their brevity and ease of calculation, the IPSS and SHIM are very widely used in clinical practice. The PCI was the first comprehensive QOL instrument specifically validated for prostate cancer patients, though its questions tend to focus on adverse effects of prostatectomy rather than radiation or other treatments. The EPIC was developed from the PCI to capture these differential effects more completely, and the shortened EPIC-26 is perhaps most frequently used in contemporary research, and has been endorsed for outcomes assessment by the International Consortium for Health Outcomes Measurement (ICHOM) group1—with two important modifications: the addition of a question specifically assessing libido, and a question on the use of phosphodiesterase inhibitors or other erectile aids.1 The latter is a particularly important addition since the question is not asked on many standard PRO questionnaires, with the exception of the Memorial Sloan Kettering Cancer Center questionnaire10, leaving patients using such aids confused as whether to answer assuming use or non-use.
The lack of standardized methods for collecting and comparing prostate cancer PROs has been known for some time. An attempt by the American Urological Association to synthesize multiple QOL instruments in 2007 was unsuccessful due to differences between instruments.5 Several studies have been published which investigated comparing or converting between QOL questionnaires. Of two studies that attempted to compare sexual function scores between instruments, one was unable to find an appropriate conversion11 while the other was only able to identify a threshold value for defining men as either potent or impotent.12 While Namiki et al. published conversions between total EPIC and PCI urinary and sexual function scores, we believe that these are inappropriate given that the EPIC and PCI questionnaires address different domains of sexual and urinary function.4 Hedgepeth et al. aimed to compare scores from multiple instruments by breaking down some instruments into subscales and including individual questions from other instruments in their analysis, although they did not attempt to convert scores between instruments or provide a method for standardizing score comparisons.6
Our approach differs from these generally unsuccessful attempts to convert between different prostate cancer PROs using total questionnaire scores, including those of the AUA effort5. Instead of treating existing PRO questionnaires as inviolable, with the only option being the comparison of total domain scores, we investigated the use of subsets of questions addressing comparable domains. We have developed a methodology to convert between urinary and sexual function scores on the EPIC-26, PCI and SHIM questionnaires. We believe the key to an appropriate conversion between quality of life instruments is converting only between symptom domains that are addressed in both instruments.
While these conversion methods provide an appropriate comparison between instruments, we acknowledge certain limitations. The majority of patients in our sample have undergone radical prostatectomy. While we did assess the conversions for radical prostatectomy and radiation patients separately (see supplementary material), the use of this conversion may be less accurate in patients who were treated with methods other than surgery given the explicit focus of the PCI questions on continence rather on other urinary symptoms. We plan to conduct further research examining our approach for patients treated non-surgically. An additional limitation is that the combined survey was administered as a one-time cross-sectional sub-study, so we cannot specifically analyze relative stability of any of the instruments over time.
Our proposed conversion does introduce a degree of imprecision, as the conversion algorithms we propose do not led to 100% concordance between instruments. We believe, however, that this is minor compared to the other sources of imprecision associated with quality assurance efforts, such as incomplete adjustment for case mix or post-surgical care, or statistical variation: for instance, the 95% C.I. around a functional recovery rate of 50% for a surgeon with 100 prostatectomy cases is ± 10%.
Our proposed conversions provide appropriate and straightforward ways to compare surgeons and institutions that measure outcome using different PRO instruments. This method would allow historical data collected on the PCI to be combined with more contemporary data using EPIC-26 and allow sexual function scores collected on the SHIM to be compared to scores from the EPIC-26 and PCI. Although we intend to conduct further research in this area, we believe that our approach can be used for other research projects, and can be implemented into current and emerging quality assurance programs.
Supplementary Material
Acknowledgments
Funding
This work was supported by David H. Koch provided through the Prostate Cancer Foundation; the Sidney Kimmel Center for Prostate and Urologic Cancers; SPORE grant from the National Cancer Institute to Dr. H. Scher (grant number P50-CA92629); a National Institutes of Health/National Cancer Institute Cancer Center Support Grant to MSKCC (grant number P30-CA008748); a Movember grant to Dr. Vickers; and the Department of Defense Prostate Cancer Research Program (grant number W81XWH-13-2-0074).
Key of Abbreviations
- EPIC-26
Expanded Prostate Index Composite
- PCI
Prostate Cancer Index
- IPSS
International Prostate Symptom Scale
- SHIM
Sexual Health Inventory for Men
- QOL
Quality of life
- PROs
patient-reported outcomes
- ED
erectile dysfunction
Appendix
Further details of methods
The responses to questions on the EPIC-26 and PCI questionnaires were converted to scores on a scale of 0 to 100 based on the respective scoring manuals. SHIM scores were summed and categorized: the SHIM questionnaire classifies scores of 1 to 7 as “severe erectile dysfunction (ED)” which we considered “poor sexual function”. We combined the categories of “moderate ED” (8 to 11) and “mild to moderate ED” (12 to 16) into one category, which we called “intermediate sexual function”. “Good sexual function” corresponded to scores between 17 and 25 (“mild ED”).
IPSS scores were summed per the IPSS scoring instructions. IPSS scores 0–7 correspond to mild irritative/obstructive voiding symptoms; 8–20 indicates moderate symptoms; and 21–35 indicates severe symptoms.
One way to convert questionnaire scores would be to find one or more questions on each questionnaire that address the same concepts or symptoms and include only these questions in the conversion. Another way would be to determine a constant that would allow for the total score of one questionnaire to be recalibrated to match the scale of another questionnaire. A third way would be to determine appropriate cut points to categorize total scores into two or more groups and compare these groups between questionnaires. This approach is useful when the distribution of the data for one or more score is skewed or asymmetric.
Urinary function
Due to the large overlap in urinary incontinence questions between the EPIC-26 and PCI questionnaires, we converted between scores using a subset of questions that were asked on both questionnaires. Question 1 from the EPIC-26 and question 12 from the PCI asked the same question about urine leakage, although they provided different answer choices. The EPIC-26 question had a median score of 75 (IQR 25, 100) while the PCI question had a median score of 67 (IQR 0, 100). Since there was no meaningful way to convert between the two sets of answer choices and there was a difference in the distribution of scores between questionnaires, we excluded this question from our modified urinary incontinence score. Question 15b from the PCI did not have a corresponding question asked in the EPIC-26 and so was excluded. The EPIC-26 asked about irritative urinary symptoms, while the PCI did not, so these questions were also excluded (questions 4b, 4c, 4d and 4e). Incontinence questions 2, 3, 4a and 5 from the EPIC-26 correspond to questions 13, 14, 15a and 16 from the PCI, respectively. These four questions had the same question text, and the answer choices were largely consistent between questionnaires. The only exception, regarding pad use, was question 3 (EPIC-26) which had four answer choices where PCI question 14 had three answer choices. Despite minor differences in answer choices between the two questionnaires, modified urinary incontinence scores were highly comparable between the questionnaires, with the EPIC-26 questions having a mean of 90.4 (SD 22.7) and the PCI questions having a mean of 89.8 (SD 23.4). For both questions, the median was 100 (IQR 100, 100). We also assessed whether a conversion factor would be needed between the two scores. However, these modified urinary scores can be compared 1:1 between EPIC-26 and PCI. Cronbach’s alpha was 0.89 for the four selected EPIC questions and 0.88 for the four selected PCI questions on urinary incontinence. As age increased, scores for all EPIC urinary questions and scores for the four selected PCI urinary questions decreased, although there was no difference in the effect of age on the two different scores (p=0.6). We also planned to look at the association between scores and time from treatment, but the median time to survey was 9 years and few patients were in the first year after treatment, when scores change rapidly. Urinary scores were higher for patients undergoing radiation compared to those undergoing radical prostatectomy. While there was some evidence that the difference in scores between radiation and surgery patients was smaller on the subset of four EPIC questions and larger when calculating the total EPIC urinary score (p=0.059), this was likely due to the fact that the total EPIC urinary score includes questions on irritative symptoms commonly caused by radiation. For the PCI question, results for age (p=0.6) were similar to those seen for EPIC. There was no evidence of a difference in total PCI score and the subset of PCI questions among radiation and surgery patients, likely because the PCI questionnaire did not ask questions on irritative urinary symptoms (p=0.16)
For the conversion in urinary scores using the IPSS, we investigated whether any questions on the IPSS were similar to questions on the EPIC-26 or PCI. We originally considered converting between IPSS, EPIC-26 and PCI questionnaires by matching questions which dealt with the same symptoms. However, similar questions were not asked across all questionnaires. For example, IPSS asks about weak urinary stream and incomplete emptying as two questions, while the EPIC-26 combines these two symptoms into one question and the PCI questionnaire does not address these symptoms at all.
Urinary bother
Since we found that no questions were highly similar between all three questionnaires, we examined whether questions on overall urinary bother from the EPIC-26 or PCI questionnaires could be converted to correspond with the IPSS question regarding quality of life with urinary symptoms (question 8). EPIC-26 question 5 and PCI question 16 are identical, addressing overall urinary bother (“Overall, how big a problem has your urinary function been for you during the last 4 weeks?”) with answers on a 1 to 5 scale (“no problem” to “big problem”). IPSS question 8 addresses overall quality of life related to urinary symptoms (“If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?”) with answers on a 0 to 6 scale (“delighted” to “terrible”).
Since there was no straightforward way to convert between the answer choices for these questions, we investigated several cut points to classify patients into two groups, based on whether they reported significant urinary problems. We first categorized patients as having bothersome urinary symptoms as those who scored 2 or higher on IPSS question 8 and those who scored 3 or higher on EPIC-26 question 5 or PCI question 16. Only 5% of these patients with bothersome symptoms on the EPIC-26 were misclassified as having no bothersome symptoms on the IPSS. However, 69% of patients reporting bothersome symptoms on the IPSS were misclassified as having no symptoms on the EPIC-26. Changing the IPSS cut point from 2 to 3 reduced the number of patients who were misclassified as having no symptoms on the EPIC-26 to 45%, but increased the number of patients with bothersome symptoms on the EPIC-26 who were misclassified as no bothersome symptoms on the IPSS to 27%. Results were similar when testing various other cut points and when comparing PCI and IPSS scores.
Sexual function and bother
The same method that was used to convert urinary function scores between EPIC-26 and PCI questionnaires was also used to convert sexual function scores. Questions that occurred on both questionnaires were summed into a modified sexual function score. Questions 22a, 25 and 26 from the PCI about desire, waking with erection, and intercourse were excluded since there were no corresponding EPIC-26 questions. Question 8b from EPIC-26 and question 22c from PCI were the same, although we excluded these questions since we were interested specifically in erectile function. Questions 8a, 9, 10, 11 and 12 on the EPIC-26 corresponded to questions 22b, 23, 24, 27 and 28 on the PCI, respectively. All question text and answer choices were the same for these questions on both questionnaires. Cronbach’s alpha was 0.90 for the selected EPIC questions and 0.89 for the selected PCI questions on sexual function. EPIC total sexual function score and our modified EPIC sexual function score decreased with age with no difference in the effect on the two scores (p=0.7). Both EPIC scores were higher for radiation patients as compared to surgery patients, but again there was no difference in the effect between total and modified sexual function score (p=0.8). Results were consistent for the total PCI and modified PCI sexual function scores, with no evidence of a difference in how the scores changed based on the effect of age (p=0.6) or when comparing radiation to surgery (p=0.6).
EPIC-26 question 12 (“Overall, how big a problem has your sexual function or lack of sexual function been for you during the last 4 weeks?”) and PCI question 16 (“Overall, how big a problem has your sexual function been for you during the last 4 weeks?”) are nearly identical, addressing overall sexual bother with answers on a 1 to 5 scale (“no problem” to “big problem”).
Treatment-specific sensitivity analyses
We noted that the distribution of EPIC-26 and PCI sexual function scores was highly skewed. In this cohort, 67% of men had poor sexual function, 10% had intermediate sexual function, and 23% had good sexual function as defined by SHIM scores. We took the 67th centile of EPIC-26 and PCI scores as a starting point for identifying an appropriate threshold for poor function. The 67th centile for EPIC-26 was 38.4 and 40 for PCI, so we tested cut points from 34 to 44. The 77th centile was 58.4 for both the EPIC-26 and the PCI, so cut points between 54 and 62 were tested.
As a sensitivity analysis, we assessed urinary incontinence and sexual function scores and conversions among only men who underwent radical prostatectomy (RP) (71% of patients). The distribution of EPIC-26 and PCI urinary function scores was similar to scores in the entire cohort. The median EPIC-26 score in RP patients was 85.5 (IQR 71, 100), with a mean of 80.4 (SD 21.0). The median PCI score in these patients was also 85.5 (IQR 73, 100), with a mean of 81.1 (SD 20.5). Similar, sexual function scores among RP patients were similar to those in the full cohort, with a median of 20 (IQR 5, 59.2) and a mean of 32.9 (SD 31.1) on the EPIC-26. Median PCI scores for sexual function in RP patients were 20 (IQR 6.6, 58.4), with a mean of 33.9 (SD 31.1). Using the same cut points for good function (59) and poor function (40), concordance was 92% and 86% for EPIC-26 scores and 91% and 88% for PCI scores, respectively. Overall concordance for all three categories was 82% for EPIC-26 and 83% for PCI, compared to 83% for both in the entire cohort.
We also assessed the urinary incontinence and sexual function scores and conversions among patients who underwent external beam radiation or brachytherapy (17.5% of patients). Median EPIC-26 and PCI subscale urinary incontinence scores were higher among men undergoing radiation than in the overall cohort (93.75, IQR 79.25, 100 for both EPIC-26 and PCI). Patients receiving radiation had a mean of 85.9 (SD 17.7) for EPIC-26 and 86.7 (SD 18.8) on PCI. Although scores were higher among these patients since questions about irritative symptoms were excluded, the conversion between EPIC-26 and PCI urinary incontinence subscales remained valid for patients undergoing radiation. The conversion between the EPIC-26 and PCI sexual function subscales was also valid for these patients, with similar means and distributions of scores. The median sexual function score was 20 (IQR 6.6, 53.4) for EPIC-26 and 20 (IQR 10.0, 46.2) for PCI. Mean scores were 31.6 (SD 28.7) for EPIC-26 and 31.2 (SD 28.4) for PCI.
Footnotes
Conflict of Interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin NE, Massey L, Stowell C, et al. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. European urology. 2015 Mar;67(3):460–467. doi: 10.1016/j.eururo.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 2.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000 Dec 20;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan R, Mulhall J, Rao S, et al. Predictive correlation between the International Index of Erectile Function (IIEF) and Sexual Health Inventory for Men (SHIM): implications for calculating a derived SHIM for clinical use. The journal of sexual medicine. 2007 Sep;4(5):1336–1344. doi: 10.1111/j.1743-6109.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 4.Namiki S, Takegami M, Kakehi Y, Suzukamo Y, Fukuhara S, Arai Y. Analysis linking UCLA PCI with Expanded Prostate Cancer Index Composite: an evaluation of health related quality of life in Japanese men with localized prostate cancer. The Journal of urology. 2007 Aug;178(2):473–477. doi: 10.1016/j.juro.2007.03.113. discussion 477. [DOI] [PubMed] [Google Scholar]
- 5.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. The Journal of urology. 2007 Jun;177(6):2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Hedgepeth RC, Labo J, Zhang L, Wood DP., Jr Expanded Prostate Cancer Index Composite versus Incontinence Symptom Index and Sexual Health Inventory for Men to measure functional outcomes after prostatectomy. The Journal of urology. 2009 Jul;182(1):221–227. doi: 10.1016/j.juro.2009.02.155. discussion 227–228. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. The Journal of urology. 2004 Apr;171(4):1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 8.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Medical care. 1998 Jul;36(7):1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. International journal of impotence research. 1999 Dec;11(6):319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 10.Vickers AJ, Savage CJ, Shouery M, Eastham JA, Scardino PT, Basch EM. Validation study of a web-based assessment of functional recovery after radical prostatectomy. Health and quality of life outcomes. 2010;8:82. doi: 10.1186/1477-7525-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levinson AW, Ward NT, Sanda MG, et al. Comparison of validated instruments measuring sexual function in men. Urology. 2010 Aug;76(2):380–386. doi: 10.1016/j.urology.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Schroeck FR, Donatucci CF, Smathers EC, et al. Defining potency: a comparison of the International Index of Erectile Function short version and the Expanded Prostate Cancer Index Composite. Cancer. 2008 Nov 15;113(10):2687–2694. doi: 10.1002/cncr.23887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.