Abstract
Background
Tetramethyl-piperidine-substituted, B4119 and B4158 have been shown to exhibit antiplasmodial activity.
Objectives
The in vitro antiplasmodial, cytotoxic and oxidative activities of clofazimine and its analogues, all TMP (tetramethylpiperidyl)-substituted phenazines except B669, were evaluated in this study.
Methods
The antiplasmodial activity of the compounds against RB-1 and pfUP10 laboratory strains of Plasmodium falciparum was investigated by flow cytometry. The cytotoxic activity against HeLa cells and oxidative activity were studied employing colorimetric and cytochrome C reduction assays respectively.
Results
The riminophenazine agents exhibited antiplasmodial action of varying degrees: B669, B4100 and B4103 showed the best activity while B4121 and B4169 exhibited significant activity at 2µg/ml. Clofazimine had no antiplasmodial activity. The compounds B4100, B4103, B4121 and B4169 exhibited significant cytotoxic activity against HeLa cells at concentrations of 0.5µg/ml and above while B669 was active at 2µg/ml. Clofazimine and B669 tested at a concentration of 0.5µg/ml caused enhancement (p ≤ 0.05) of neutrophil superoxide production when compared to the FMLP control while all the other TMP-derivatives had no effect (p ≥ 0.05).
Conclusion
Tetramethylpiperidyl-subsituted phenazines may potentially be useful antimalarial/antitumor agents with no pro-oxidative properties. In vivo studies on the agents relative to these properties are recommended.
Keywords: Riminophenazines, malaria, cytotoxicity and superoxide anions
Introduction
The World Health Organization reported around 207 million cases of malaria in 20121. More than 80% of the world's malaria casualties occur in Africa, involving mostly children under the age of five and pregnant women2,3. Interestingly, recent reports indicate a decline in malaria cases mitigated by use of artemisinin-based combination therapy (ACT), notably administration of Coartem™ in malaria hit regions, and introduction of long-lasting insecticidal nets (LLIN)4,5,6,7. However, there are reports of emergence of ACT resistance in South-East Asia8,9. The situation thus calls for augmented efforts directed at developing new and novel antimalarial agents and other drugs that can potentiate the activity of existing antimalarials10.
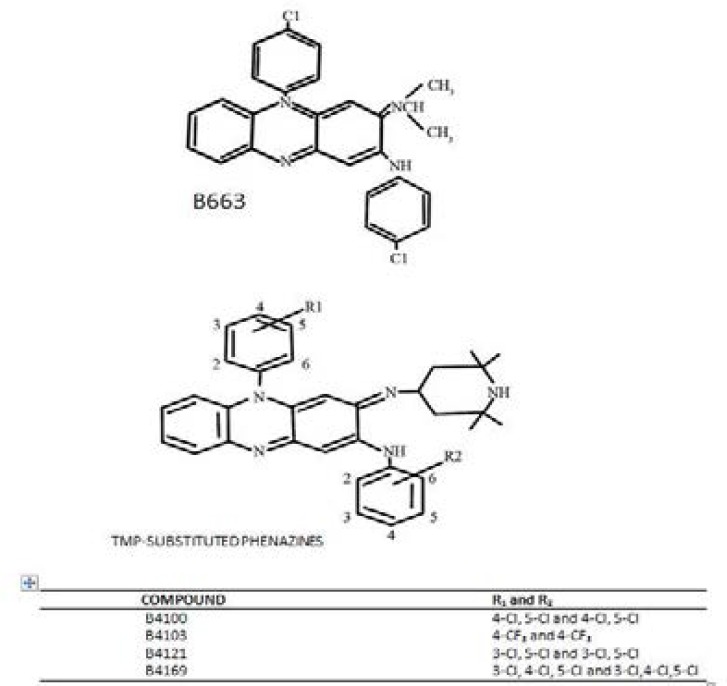
Clofazimine or Lamprene™ is a riminophenazine compound used with dapsone and rifampicin for the treatment of leprosy11. Clofazimine is highly lipophilic and exhibits dose-limiting gastrointestinal toxicity as well as dose-related skin pigmentation12. In an effort to minimize side-effects and to improve activity, derivatives of clofazimine, called TMP (tertramethyl-piperidyl) substituted phenazines were designed. They differ from clofazimine structurally as they contain the TMP group which have been extensively studied for a variety of biological activity among which are anti-tumor, antimicrobial, antimycobacterial and antimalarial activities. The antimycobacterial activity (not isopropyl group in case of clofazimine) is consequent upon substituting on the imino nitrogen at position 2 of the phenazine nucleus13,14 (Fig. 1).
Figure 1.
*Note that agent B669 is not a TMP-riminophenazine.
Their mechanisms of action in the various experimental systems have also been shown15,16,17,18. However, antiplasmodial and/or antitumor activity of other TMP phenazines such as B4100, B4103, 4121 and B4169 are yet to be fully explored and herein is the backdrop of the present study. Thus, the current study expands on the existing knowledge on determining the antimalarial, antitumor and oxidative activities of the substituted TMP-phenazines to further investigate their antimalarial efficacy and antitumor drug property.
Materials and methods
Test agents. The molecular structures of clofazimine and TMP-phenazines are shown in Figure 1. The riminophenazine agents were synthesised by Dr JF O'Sullivan (Department of Chemistry, University College Belfield, Dublin, Republic of Ireland). All the agents were dissolved in DMSO to a concentration of 2mg/ml and diluted further in culture medium supplemented with 10% heat-activated FCS (complete culture medium) directly before use. Control systems contained the corresponding DMSO concentrations only. Chloroquine disulphate was obtained from Sigma Chemicals Co (St. Louis, MO), diluted and filter-sterilised in distilled water to a final concentration of 10mM.
Chemicals and reagents.
Unless indicated, these were obtained from Sigma Chemical Co. (St. Louis, MO).
Parasite strains and culturing conditions. The chloroquine-sensitive (pfUP10) and low grade-resistant (RB-1) laboratory strains of Plasmodium falciparum were maintained in a continuous culture system of human blood group O+ erythrocytes and RPMI-140 medium containing human AB+ serum as described elsewhere18. Parasite cultures were synchronised to the ring stage by treatment with 5% sorbitol18.
In vitro antiplasmodial assay.
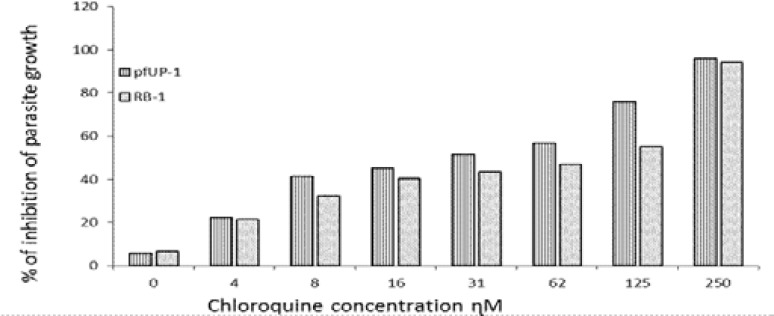
Ring-stage parasite culture suspension (2% parasitemia and 0.5% final haematocrit) were exposed to chloroquine disulphate (0–250 Ƞ M final concentration). Parasites in culture medium and complete culture medium alone respectively served as positive and negative controls. After 48h incubation at 37°C in an atmosphere of 5% O2/5% CO2/ 90% N2, parasite growth was evaluated by a modified flow cytometric method described elsewhere19. Levels of parasite growth were expressed as percentage inhibition of parasite growth ± SEM.
In vitro chloroquine-sensitizing activities of riminophenazine agents. The ring-stage parasites cultures of RB-1 strain was used in this assay. A combination of suboptimal concentrations of chloroquine (31 and 61 Ƞ M) with the experimental agents (0.125–2 µg/ml) were plated and evaluated as indicated above19. The levels of parasitemia were expressed as percentage inhibition of parasite growth.
Cytotoxicity assay.
Cytotoxic assays were performed using round-bottom 96-well tissue culture plates using a Hela (human cervix epithelioid carcinoma) cell line. Cultured cells were harvested from 75% confluent tissue culture flasks with 0.2% trypsin, washed, and resuspended in MEM supplemented with 10% heat-inactivated FCS. To each well were added 20 µl of tissue culture cells (2×106 cells/ml) and the volumes were brought to 200 µl with complete culture medium, containing the various solvent controls or concentrations (0.0625–2 µg/ml) of the various test agents. The plates were incubated for 72 h at 37°C in an atmosphere of 5% CO2. At the end of the incubation period, culture supernatants were discarded and adherent cells were fixed with 10% phosphate-buffered formalin, washed with PBS, and stained with 0.02% crystal violet dye. Plates were washed in water and left to dry on paper towels. The stain was extracted with 10% SDS at 37°C for 18 h. The absorbance was measured at 620 nm on a Titertrek Multiskan plate reader. Background values (medium only) were subtracted from each reading. Results are expressed as percentage inhibition of cell growth.
Separation of neutrophils from human peripheral blood (Ficoll method).
Purified neutrophils were prepared from heparinized venous blood of healthy adult human volunteers and were separated from mononuclear leukocytes by centrifugation on Histopague-1077 at 300 g for 25 min at 4°C. After removal of the plasma and mononuclear cell layers, the neutrophil and erythrocyte fractions were sedimented in an abundance of 3% gelatin for 15 minutes at 37°C to remove the red blood cells. The neutrophil-rich supernatant was centrifuged at 160 g for 10 min. The cells were washed with PBS at 110 g for 10 min. at room temperature and the residual red cells were lysed with 0.83% NH4Cl solution on ice for 10 min. The cells were washed with PBS at 110 g and the resultant pellet resuspended in indicator-free HBSS before use. Neutrophils of high purity (>90%) and viability (>95%) as determined by the Trypan blue dye-exclusion test were diluted to 1–2 ×107cells/ml and held on ice before assaying for superoxide anion production.
Super oxide anion production.
The generation of superoxide anion by neutrophils was assessed by the superoxide dismutase inhibitable reduction of cytochrome c as described by Babior and colleagues19. Each assay was performed in triplicates. The riminophenazine agents (0.5 µg/ml, final concentration) were added to pre-warmed neutrophils (1×106cells/ml) in indicator-free HBSS. After a 5 min. incubation, 100 µl of SOD (100 units/ml final concentration) were added into appropriate tubes followed by 100 µl cytochrome c (0.1 mM final concentration). After a further 5 min. incubation, FMLP (0.1 µM) was added as stimulant to experimental systems. The reactions were terminated after 5 min. by addition of ice-cold PBS. A set of control tubes for FMLP alone, solvent systems and baseline parameters were included. The tubes were centrifuged for 10 min. at 4°C at 100 g. The optical density of the supernatants was measured at 550nm using a Pye Unicam SP1700 ultraviolet spectrophotometer. Results were expressed as nanomoles reduced cytochrome c/ 106 neutrophils / 5 min ± SEM.
Statistical analysis
The data obtained were analysed using Excel and Graph Pad Prism 5.00. The results are represented as mean ± SD for four experiments conducted in triplicate. Levels of statistical significance were calculated using Student's t test, with p ≤ 0.05 considered significant.
Results
Antimalarial activity by chloroquine and TMP-phenazines.
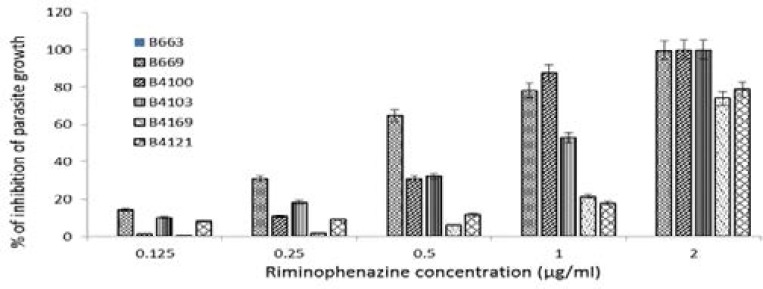
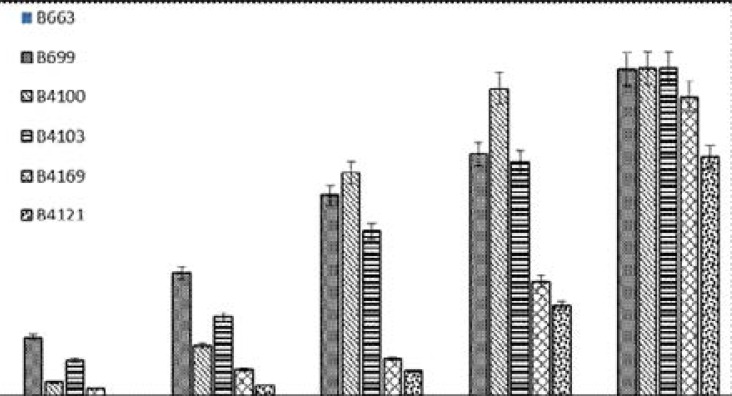
The anti-growth effect of chloroquine disulphate (0–250 Ƞ M, final concentration) and TMP-phenazines (0.125–2 µg/ml) of the pfUP10 and RB-1 laboratory strains of Plasmodium falciparum was measured by a flow cytometric method. The results are shown in Figure 2, 3, and 4 respectively.
FIGURE 2.
FIGURE 3.
FIGURE 4.
As shown, chloroquine exhibited significant activity (p ≤ 0.05) from 31 ȠM and higher with IC50 concentrations of 24 Ƞ M and 94 Ƞ M for the pfUP10 and RB-1 strains respectively. The antimalarial effects of a combination of chloroquine (31 and 61 Ƞ M) with the experimental agents (0.125–2µg/ml) did not increase sensitivity of the malaria parasite to chloroquine, but an additive effect was observed in chloroquine (62 Ƞ M) combinationswith 0.25 µg/ml of B4103 (Results not shown). Riminophenazine compounds (B669, B4100 and B4103; at concentrations of 0.5 µg/ml and higher) showed significant (p<0.05) antimalarial activity against the RB-1 strain (Fig. 3), whereas B4121 and B4169 were only active at the highest concentration of 2 µg/ml. Clofazimine did not exhibit any antiplasmodial activity at any of the concentrations tested. Similar results were obtained with the pfUP10 strain of Plasmodium falciparum Figure 4). The 50% inhibitory concentration (IC50) for the agents used in this study are shown in Table 1.
Table 1.
| Riminophenazine molecule |
RB-1 | PfUP10 |
| B663 (µg/ml) | >2 | >2 |
| B669 (µg/ml) | 0.4± 0.9 | 0.2± 1 |
| B4100 (µg/ml) | 0.75± 1 | 0.37± 3 |
| B4103 (µg/ml) | 0.9± 2.1 | 0.3± 2.2 |
| B4169 (µg/ml) | 1.4± 0.8 | 1.3± 07 |
| B4121 (µg/ml) | 1.5± 2 | 1.7± 0.5 |
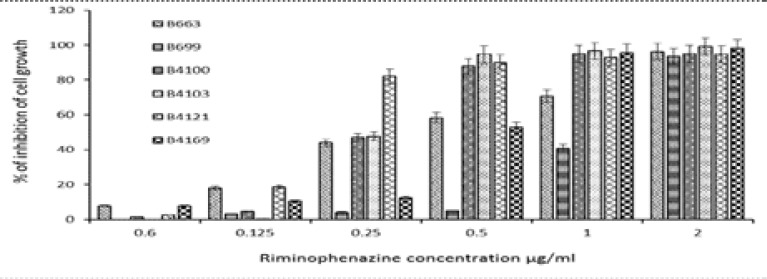
Inhibition of tumor cell proliferation by TMP-phenazines. The cytotoxic effect of the TMP-phenazines (0.0625–2 µg/ml) against a Hela cell line were evaluated using a colorimetric procedure. The results are shown in Figure 5. Experimental agents B4100, B4103 and B4121 exhibited antitumor activity at concentrations of 0.25 µg/ml and higher while B663, B669 and B4169 showed the similar activity at concentrations of 0.5 µg/ml and higher. The 50% inhibitory concentrations (IC50) are indicated in Table 2.
FIGURE 5.
Table 2.
| Riminophenazine agent | IC50 value (µg/ml) |
| B663 | 0.38 ± 0.8 |
| B669 | 0.75 ± 0.9 |
| B4100 | 0.32 ± 2.1 |
| B4103 | 0.3 ± 3 |
| B4121 | 0.2 ± 0.7 |
| B4169 | 0.4 ± 0.3 |
Superoxide anion production by neutrophils.
Results in Figure 6 show that only B669 and clofazimine tested at a concentration of 0.5 µg/ml caused a significant (p < 0.05) enhancement of neutrophil superoxide anion production when compared to the FMLP control. The other riminophenazine agents, all TMP-derivatives (B4100, B4103, B4121 and B4169), had no significant effect on cellular superoxide anion release (p ≥ 0.05).
Discussion
The results of the present study show that the TMP-phenazines, B4100, B4103, 4121 and B4169 studied also exhibit in vitro antiplasmodial, antitumor with no pro-oxidative activities. The classical riminophenazine agent (B663) does not show activity against malaria parasite and the inclusion of the TMP group in the phenazine nucleus seems to have modified the chemical nature of the classical riminophenazines, hence the antiplasmodial, antitumor activity recorded. The present result is consistent with another study where the TMP-phenazine agents B4119 and B4158 possessed activity against multidrug resistant strains of Plasmodium falciparum by interfering with the parasite haemoglobin degradation (to acquire iron for nutritional purposes) in erythrocytes17.
Another study has recently documented that a series of riminophenazine analogues, (not TMP-phenazines in this case), also exhibited in vitro activity against sensitive and resistant strains of Plasmodium falciparum as well as against different species of Leishmania promastigotes. Their antiprotozoal activity in vitro is observable with IC50 in submicromolar concentrations20. Their result which corroborates the need for further explorative studies on riminophenazines analogues anti-plasmodium, antitumor activities among others augments the basis for the present study
Van Niekerk and colleagues have also documented the potential of tetramethylpiperidine-substituted phenazines to inhibit the proliferation of intrinsically multidrug resistant carcinoma cell lines by a dual mechanism of action13. The methodology of the present study on anti-tumor activity of these experimental agents differs from the methods employed by others on various aspects of the experimental setups (cells, agents concentrations, incubation period and cell proliferation assay method), and is suggestive of better antitumor potential of these analogues studied.
The lack of pro-oxidative activity may be attributable to the following: (i) agents were screened at fixed concentrations and might be active at higher or lower concentrations, (ii) agents may require a longer pre-incubation period, (iii) use of other stimuli of membrane-associated oxidative metabolism such as PMA, LPS, calcium ionophore and opsonized zymosan may be necessary and (v) it might be essential to evaluate the superoxide scavenging property of the TMP-derivatives.
Conclusion
The antiplasmodial and anti-tumor efficacy of these experimental agents at pharmaceutically relevant concentrations, suggest that they are promising candidates for intensive evaluation in experimental animal models for malaria and cancer treatments which study is currently in progress.
Acknowledgements
The research was funded by the South African Medical Research Council (SAMRC) and Adcock Ingram Phar- maceuticals (Johannesburg, SA). The pfUP10 and RB-1 laboratory strains of Plasmodium falciparum were kindly provided by Profs, Abram Louw (Department of Bio- chemistry, University of Pretoria) and Peter Smith (De- partment of Clinical Pharmacology, University of Cape Town, South Africa) respectively.
Conflict of interest
None to declare.
References
- 1.WHO, author. World Malaria Report 2013. Geneva. Switzerland: [Google Scholar]
- 2.WHO, author. World Malaria Report. Geneva Switzerland: 2014. [Google Scholar]
- 3.Ceesay SJ, Koivogu L, Nahum A, et al. Malaria prevalence among young infants in different transmission settings, Africa. Emerging Infectious Diseases. 2015;21:1114–1120. doi: 10.3201/eid2107.142036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wongsrichanalai C, Sibley CH. Fighting drug-resistant Plasmodium falciparum: the challenge of artemisinin-resistance. Clinical Microbiology and Infection. 2013;14:908–14916. doi: 10.1111/1469-0691.12316. [DOI] [PubMed] [Google Scholar]
- 5.Zarocostas J. Malaria death falls by 47% globally between 2000 and 2013, WHO says. The Pharmaceutical Journal. 2014:293. doi: 10.1211/PJ.2014.20067360. [DOI] [Google Scholar]
- 6.C Njunju EM, Virtanen M, Hamed K, Gomes M, Van Geertuyden JP. Exposure to arte-mether-lumefantrine (Coartem®) in first trimester pregnancy in an observational study in Zambia. Malaria Journal. 2015;14:77–86. doi: 10.1186/s12936-015-0578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dondorp AM, Nosten F, Yi P, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ. Artermisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, Von Seidlen L. Artermisinin resistance: current status and scenarios for containment. Nature Reviews Microbiology. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 9.Dahl EL, Rosenthal PJ. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrobial Agents and Chemotherapy. 2007;51:3485–3490. doi: 10.1128/AAC.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.18th “WHO Model List of Essential Medicines”. World Health Organization; 2013. http:/www.who.int./medicine/publication/essentialmedicines/en/ (03/May/2016, day last assessed) [Google Scholar]
- 11.Franzblau SG, O'Sullivan JF. Structure-activity relationship of selected phenazine agents against Mycobacterium leprae in vitro. Antimicrobial Agents and Chemotherapy. 1988;32:1583–1585. doi: 10.1128/aac.32.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matlola NM, Steel H, Anderson R. Antimycobacterial action of B4128, a novel thetramethylpiperidyl-substituted phenazine. Journal of Antimicrobial Chemotherapy. 2001;47:199–202. doi: 10.1093/jac/47.2.199. [DOI] [PubMed] [Google Scholar]
- 13.Van Niekerk E, O'Sullivan JF, Joone GK, Van Rensburg CEJ. Tetramethylpiperidine-substituted phenazines inhibit the proliferation of intrinsically multidrug resistant carcinoma cell lines. Investigational New Drugs. 2001;19:211–217. doi: 10.1023/a:1010691714635. [DOI] [PubMed] [Google Scholar]
- 14.Van Rensburg CEJ, Anderson R, O'Sullivan JF. Riminophenazines compounds: pharmacology and anti-neoplastic potential. Critical Reviews in Oncology/Hematology. 1997;25:55–67. doi: 10.1016/s1040-8428(96)00229-6. [DOI] [PubMed] [Google Scholar]
- 15.Reddy VM, O'Sullivan JF, Gangadharam RJ. Antimycobacterial activities of riminophenazines. Journal of Antimycobacterial Agents and Chemotherapy. 1999;43:615–623. doi: 10.1093/jac/43.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Moloko CC, Helen CS, Fourie FB, Germishuizen WA, Anderson R. Clofazimine: current status and future prospects. Downloaded from http://jac.oxfordjournals.org/ [DOI] [PubMed]
- 17.Makgatho ME, Anderson R, O'Sullivan JF, Egan TJ, Freeze JA, Cornelius N, Van Rensburg CEJ. Tetramethylpiperidine-substituted phenazines as novel anti-plasmodial agents. Drug Development Research. 2000;50:195–202. [Google Scholar]
- 18.Schulze DLC, Makgatho EM, Coetzer TL, Louw AI, Van Rensburg CEJ, Visser L. Development and application of a modified flow cytometric procedure for rapid in vitro quantification of malaria parasitemia. South African Journal of Sciences. 1991;93:156. [Google Scholar]
- 19.Cockeran R, Theron AJ, Steel HC, Feldman C, Mitchell TJ, Anderson R. Proinflammatory interactions of pneumolysin with human neutrophils. Journal of Infectious Diseases. 2001;183:604–611. doi: 10.1086/318536. [DOI] [PubMed] [Google Scholar]
- 20.Barteselli A, Casagrande M, Basilico N, Parapini S, Rusconi CM, Tonelli M, Boido V, Taramelli D, Sparatore F, Sparatore A. Clofazimine analogs with anti-leish-manial and antiplasmodial activity. Bioorganic and Medicinal Chemistry. 2015;23:55–65. doi: 10.1016/j.bmc.2014.11.028. [DOI] [PubMed] [Google Scholar]