Abstract
Introduction
The virulence factors of Pseudomonas aeruginosa are under the control of quorum sensing (QS) signals. Hence, interference with QS prevents its pathogenesis.
Objective
The aim of the present research is to assess the influence of some β-lactam antibiotics on cell communication and the release of different virulence factors.
Methods
The minimal inhibitory concentrations of ceftazidime, cefepime and imipenem were evaluated by microbroth dilution method. The effect of sub-inhibitory concentration of the tested antibiotics on QS signals was investigated using reporter strain assay. In addition, different virulence factors (elastase, protease, pyocyanin and hemolysin) were estimated in the presence of their sub-inhibitory concentrations.
Results
Low concentrations of ceftazidime, cefepime and imipenem caused significant elimination of the QS signals 3OH-C12-HSL and C4-HSL up to 1/20 MIC. Furthermore, low concentrations of the tested antimicrobials suppressed virulence factors elastase and hemolysin. Moreover, 1/20 of their MICs reduced elastase, protease, pyocyanin and hemolysin.
Conclusion
Utilization of β-lactam antibiotics at low concentrations could be an effective approach for prevention and treatment of P. aeruginosa infection.
Keywords: Quorum sensing inhibition, β-lactams, Pseudomonas aeruginosa
Introduction
Pseudomonas aeruginosa is an opportunistic human pathogen with remarkable metabolic versatility. Infections with P. aeruginosa are common in compromised patients suffering from cystic fibrosis, severe burns, deep wounds, in addition to patients having urinary tract infections. P. aeruginosa produces various virulence attributes, including biofilm, toxins and enzymes such as pyocyanin, protease, elastase, and rhamnolipids1.
P. aeruginosa exhibits its virulence behavior via quorum sensing (QS)2. The common QS systems in P. aeruginosahave been assigned las and rhl. QS circuits in P. aeruginosa are connected by signaling molecules called autoinducers. The las system is composed of the synthase gene lasI and its transcriptional regulatory protein LasR. Its auto-inducer is called N-(3-oxododecanoyl) homoserine lactone (3OH-C12-HSL). Similarly, the rhl system consists of rhlI synthase, and its transcriptional regulatory protein LasR. Also, it possesses autoinducer molecule N-butyryl homoserine lactone (C4-HSL)2,3. When bacterial growth reaches a specific threshold, the signals acyl homoserine lactones (AHL) are released and stimulates the expression of virulence genes4. Both the las and rhl systems are coregulated, and las system is dominant over the rhl pathway. Hence, inhibition of these signaling molecules hinders the pathogenicity of P. aeruginosa, and could be used for prevention or treatment of its infections5.
Several plants have QS inhibiting activities. Medicinal plants6 and edible plants7 exhibited QSI effects. Also, Streptomyces coelicoflavus isolated from soil microbiota produced 1 H-pyrrole-2-carboxylic acid with QSI effect8. Synthetic molecules and peptides exhibited QSI activity9. Previous studies focused on the effect of some antimicrobials such as aminoglycosides and quinolones on QS of P. aeruginosa. Azithromycin showed significant elimination of QS and virulence factors of P. aeruginosa10. The present study focused on the effect of sub-inhibitory concentrations of some β-lactam antibiotics on QS of P. aeruginosa. Furthermore, virulence factors of P. aeruginosa were assessed in the presence of sub-inhibitory concentrations of the tested antibiotics.
Materials and methods
Bacterial strains, growth media and conditions
The wild strain P. aeruginosa PAO1 was used for the assay of QSI effects of the tested antibiotics. Two reporter strains; P. aeruginosa pME3846 (rhlI-lacZ; Tcr) and E. coli MG4/pKDT17 (lasB::lacZplac-lasR Apr)2,3 were used for the assessment of rhlI and lasI/R, respectively in the presence and absence of the tested antibacterials. The QS deficient P. aeruginosa isolate PAO-JP2 double mutant (Δ lasI::Tn10,Tcr; Δ rhlI::Tn5012, Hgr) was included as a negative control11. All bacterial cultures were grown in Luria Bertani medium (LB broth; tryptone 1%, yeast extract 0.5%, and NaCI 1%) at 37° C.
Determination of minimal inhibitory concentration
Minimal inhibitory concentrations (MICs) of the studied β-lactams: cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp), were estimated by broth microdilution method (CLSI, 2014). Two-fold serial dilutions of each antibiotic were prepared and inoculated with 0.1 ml of PAO1 inoculum contained 5×106 CFU/ ml and incubated at 37 °C for 24 h. Values of MIC were recorded as the lowest concentration of the antibiotic at which there was no visible growth of the organism12.
Determination of the viable count of P. aeruginosa PAO1
The viability of P. aeruginosa PAO1 wild type was examined in the presence of sub-inhibitory concentrations (1/4 MIC) of the tested β-lactams using pour plate counting method and cell proliferation was checked before supernatant collection13. Similarly, the viable count of the untreated cells was performed and compared to the treated cultures.
Preparation of the supernatant
P. aeruginosa PAO1 was propagated in LB broth containing 1/4, 1/8 and 1/20 MIC of each antibiotic. P. aeruginosa PAO1 was also grown without antimicrobial agents as the positive control and PAO-JP2 was propagated under the same conditions as the negative control14. The supernatants of the treated and untreated Pseudomonas cultures were separated by centrifugation at 8.000 rpm for 10 min at 4°C. Cell-free supernatants were then stored at −20°C to be used for estimation of AHLs and assay of various virulence factors15.
Effect of β-lactams on QS signal molecules
The QS signals 3OH-C12-HSL and C4-HSL were detected in treated and untreated cultures of PAO1, respectively. The overnight growth of the reporter strains E. coli MG4 (pKDT17) and P. aeruginosa (pME3846) were diluted up to OD600 of 0.1. The previously prepared cell-free supernatant (1 ml) was mixed with 0.5 ml of E. coli MG4 and 1 ml P. aeruginosa pME3846. Cells were propagated till they reached 0.3–0.4 at OD600 then pelleted. β-galactosidase was estimated according to Miller assay method16.
Effect on virulence factors
The effect of the tested β-lactams on the virulence determinants of P. aeruginosa PAO1 was estimated using the prepared supernatants both in the presence and absence of tested β-lactams.
Evaluation of LasB elastase
The supernatant of P. aeruginosa was mixed with 10 mg of elastin Congo red (ECR, Sigma Chemicals, St. Louis, USA) in ECR buffer (100 mM Tris buffer, pH 7.2). The insoluble substrate was centrifuged and the absorbance of the supernatant was estimated at 495 nm17.
Total protease production
Total proteolytic effects of the treated and untreated cells were detected using modified skim milk method18. Cultures supernatants of PAO1 were mixed with skim milk (1.25%) at 1:2 ratio and reaction was incubated at 37°C for 30 min. The turbidity of the solution was determined at OD 600nm. The results were calculated as relative protease production to the untreated PAO1 strain.
Determination of hemolysin production
The hemolysin test was performed by incubating 700 µl of 2% erythrocytes suspension with 0.5 ml of PAO1 supernatant for 2 h at 37°C. The suspension was centrifuged and the produced hemoglobin was estimated by measuring the OD at 540nm. The negative control; RBCs in LB broth was evaluated under the same conditions. The total RBCs lysed were detected using 0.1% SDS19.
Pyocyanin assay
King A media (peptone 2%, K2SO4 1%, and MgCl2 0.14%) supplied with sub-MICs of β-lactams was inoculated and propagated at 37 °C for 48 h in triplicates. Media without antibiotics was also inoculated with PAO1 and propagated as a positive control. The culture was centrifuged and pyocyanin was extracted using chloroform in acidic pH and the red layer was separated. Its absorbance was measured at 520 nm and pyocyanin concentration was expressed as µg/ml by multiplying the OD520 by 17.07220.
Statistical analysis
The mean of three independent experiments and the standard deviation of the mean were calculated by Excel data package. P. aeruginosa treated with β-lactams were compared to the untreated control using GraphPad Instate software (version 3.05) and Tukey-Kramer multiple-comparison test. Significant difference between treated and untreated culture was assigned when the probability value was P< 0.05 or P< 0.01.
Results
Growth inhibitory concentrations for P. aeruginosa PAO1 Table (1) showed the MIC values of tested β-lactams and the mean value for their sub-inhibitory concentrations used for testing QSI effect and virulence factors.
Table (1).
Minimal inhibitory concentration (MIC) values of the tested β-lactam and their sub-inhibitory concentrations
| Compounds | MIC value | 1/4 MIC | 1/8 MIC | 1/20 MIC |
| Cefepime (Cfp) | 200 (µg/ml) | 50 | 25 | 10 |
| Ceftazidime (Cft) | 1.0 (µg/ml) | 0.2 | 0.1 | 0.05 |
| Imipenem(Imp) | 1.0 (µg/ml) | 0.2 | 0.1 | 0.04 |
Effect of sub-inhibitory concentrations of the tested β-lactams on the growth of P. aeruginosa PAO1
Viable counts of P. aeruginosa PAO1 treated with sub-inhibitory concentrations of cefepime, ceftazidime, and imipenem was performed using pour plate method. The tested concentrations (1/4 MIC) did not alter bacterial growth compared to the control untreated cultures and the same bacterial count of untreated PAO1 (161±21×107 CFU/ml).
Inhibition of quorum sensing signal molecules Effect on 3OH-C12-HSL
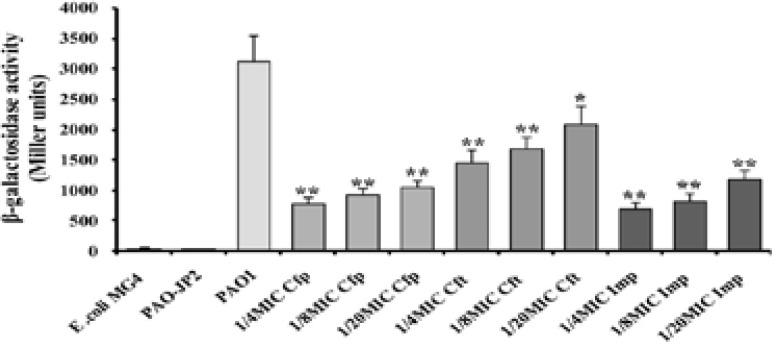
Untreated P. aeruginosa PAO1 produced the highest β-galactosidase activity (3125 Miller units). Double mutant strain devoid of QS genes (PAO-JP2) showed the lowest level of β-galactosidase (39 Miller units). The level of 3OH-C12-HSL was significantly reduced by all sub-MICs of cefepime (66%–76% inhibition) and imipenem (63%–78%). However, ceftazidime exhibited less significant effect ranged from 54% at 1/4 MIC to 47% at 1/8 MIC, while ceftazidime (1/20 MIC) did not affect 3OH-C12HSL yield (Fig. 1) as compared to the untreated PAO1 strain.
Figure (1).
β-galactosidase assay of 3OH-C12-HSL. Effect of sub-MICs of cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp) on 3OH-C12-HSL production (**, highly significant P<0.01 and *, significant P<0.05).
Effect on C4-HSL
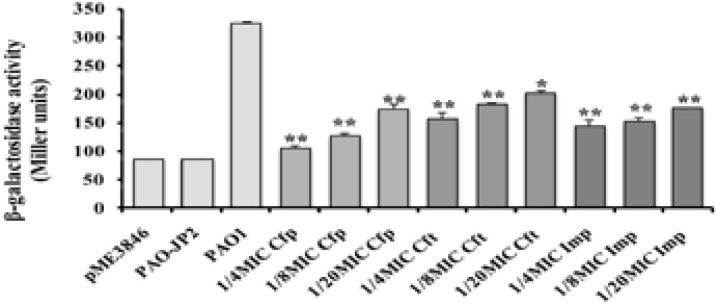
C4-HSL level was significantly reduced (P<0.01) by the assayed antibiotics at their sub-inhibitory concentrations with different degrees of signals inhibition (Fig. 2) as compared to the untreated P. aeruginosa PAO1. C4-HSL levels were significantly lowered by sub-MICs of cefepime by 47–68% inhibition, ceftazidime by (40%–52%), and imipenem by (47–56%). Double mutant strain showed the lowest level of β-galactosidase activity (85 Miller units). All tested antibiotics produced no effect on both reporter strains.
Figure (2).
β-galactosidase assay of C4-HSL. Effect of sub-MICs of cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp) on the release of C4-HSL (**, highly significant P<0.01 and *, significant P<0.05).
Effect of sub-inhibitory concentrations on virulence factors of P. aeruginosa PAO1
Effect on elastase enzyme
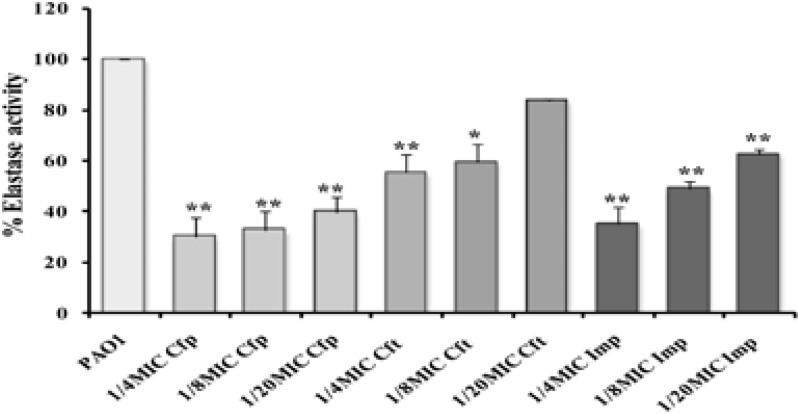
Significant reduction in elastase production was detected when P. aeruginosa PAO1 was grown in presence of sub-inhibitory concentrations of cefepime (61%–70% inhibition) and imipenem (52%–66%) with P<0.01. However, ceftazidime exhibited significant effect ranging from 54% at 1/4 MIC with P<0.01 to 42% at 1/8 MIC (P<0.05), while 1/20 MIC ceftazidime did not affect the elastase level (Fig. 3).
Figure (3).
Relative production of elastase from P. aeruginosa PAO1 grown with sub-MICs of cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp) (**, highly significant P<0.01 and *, significant P<0.05).
Effect on total protease
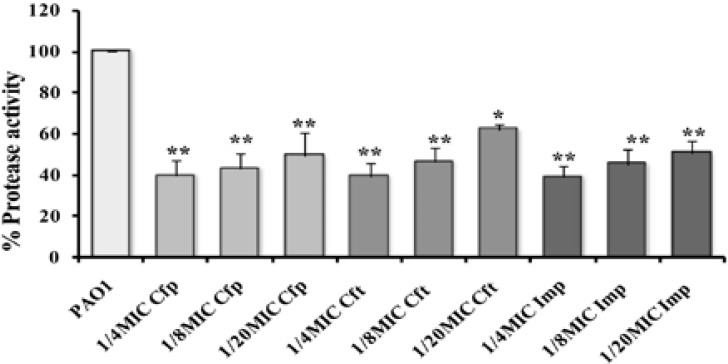
There was significant lowering in total protease production in cultures supplied with sub-MICs of cefepime (51–61% decrease) and imipenem (50–62%) with P<0.01. However, ceftazidime exhibited variable reduction levels ranged from 62% to 54% at 1/4 and 1/8 MIC, respectively (P<0.01). However, it showed less effect at 1/20 MIC (39%, P<0.05) (Fig. 4).
Figure (4).
Effect on the production of total protease in PAO1 by sub-MICs of cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp) (**, highly significant P<0.01 and *, significant P<0.05).
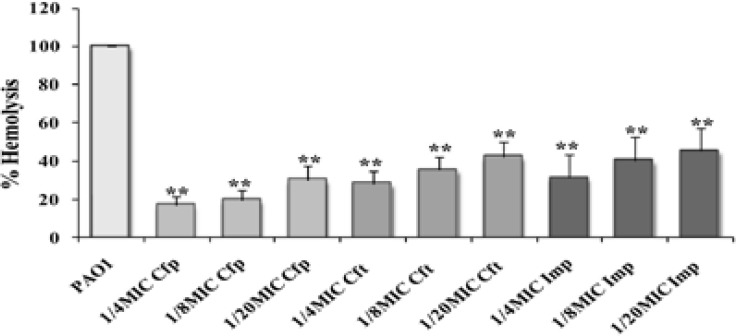
Effect on hemolysin production
The effect on hemolytic activity was expressed as percentage lysis of RBCs by PAO1culture supernatants treated with sub-inhibitory concentrations, as compared to lysis obtained by the untreated PAO1 supernatant19. Significant reduction in hemolysin production (P<0.01) was recorded with sub-MICs of cefepime (69–83%), ceftazidime (58–72%), and imipenem (55–69%) (Fig. 5).
Figure (5).
Inhibition of hemolytic activity in P. aeruginosa PAO1 using sub-inhibitory concentrations of cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp) (**, highly significant P<0.01 and *, significant P<0.05).
Elimination of pyocyanin production
The effect of the tested antibiotics on the production of pyocyanin pigment from PAO1 cells was assessed. Pyocyanin production was significantly reduced by sub-MICs of cefepime (63–73%), 1/4 and 1/8 MIC of imipenem (51–57%) with P<0.01, and 1/20 MIC of imipenem by 40% (P<0.05). However, ceftazidime exhibited significant reduction ranged from 57% at 1/4 MIC to 37% at 1/8 MIC, while 1/20 MIC did not affect pyocyanin yield (Fig. 6). On the other hand, PAO-JP2 showed the lowest pyocyanin level (0.6 µg/ml)
Figure (6).
Pyocyanin production; cefepime (Cfp), ceftazidime (Cft), and imipenem (Imp) compared to untreated standard strain PAO1. (**, highly significant P<0.01 and *, significant P<0.05).
Discussion
P. aeruginosa is one of the main causes of the persistent urinary tract, respiratory tract, burn and wound infections21. Pathogenesis of P. aeruginosa is attributed to secreted virulence factors such as elastase, proteases, and pyocyanin. Its pathogenesis is also disseminated through bacterial adhesion and biofilm formation5. QS is the key regulator for Pseudomonas virulence and biofilm formation. Hence, reduction in bacterial communication by QS inhibitors results in reduced levels of virulence factors22. Therefore, targeting QS cascade developed a new therapeutic approach for control of Pseudomonas infections.
This study showed that the sub-inhibitory concentrations of the three tested β-lactams exhibited significant elimination of QS signals. Quantitative assessment of both QS signals has clearly indicated that anti-QS activity of the tested drugs is concentration dependent. Regarding the autoinducers 3OH-C12-HSL and C4-HSL, they significantly decreased (P<0.01) by sub-inhibitory concentrations of cefepime, ceftazidime and imipenem compared to the untreated PAO1 strain (Figs. 1 and 2). In line with these findings, certain macrolide antibiotics (azithromycin) are capable of repressing the synthesis of P. aeruginosa AHLs signal molecules when applied at sub-MICs23. Furthermore, sub-inhibitory concentrations of tobramycin, ciprofloxacin, and ceftazidime were effective in lowering the AHL levels in P. aeruginosa24. The QSI of some antimicrobials is attributed to the alteration in membrane permeability, and subsequent elimination of the flux of N-3-oxo-dodecanoyl-l-homoserine lactone25. Also, efflux inhibitor phenylalanine arginyl β-naphthylamide caused significant elimination of QS signals and related virulence factors among multidrug resistant P. aeruginosa clinical isolates26.
To confirm that the elimination of QS signals and virulence was not related to the antimicrobial activities of the tested β-lactams, viable counts of the treated cells were performed and our data have shown no change in the cell growth. Bacterial growth in the presence of Sub-MICs of tested β-lactams produced viable bacterial count similar to the positive control (untreated P. aeruginosa PAO1).
The effect of sub-MIC concentrations on P. aeruginosa-virulence has been verified. One of the main pathogenic factors in P. aeruginosa is LasB elastase. LasB elastase and proteases interfere with host defense mechanisms and destroy tissue components27. Most of sub-MICs of the tested compounds resulted in a significant decrease in LasB elastase and proteases enzymes (P<0.01 and P<0.05) (Figs. 3–4). The inability of PAO1 strain to produce elastase and protease has been linked with their reduced level of associated QS molecules released under the influence of the assayed antibiotics. Much lower concentration (1/20 MIC) of some of these antibiotics did not affect bacterial QS signaling network and hence, showed no remarkable effect on elastase and protease activities. QS-deficient strains were non-virulent and lose the ability to produce elastase and protease28.
Hemolysin is another aspect of P. aeruginosa virulence which enhances its pathogenicity and helps in the survival of pathogen by inhibiting the host defense factors. Significantly reduced levels (P<0.01) of hemolysin were observed after treating P. aeruginosa PAO1 with sub-inhibitory concentrations of cefepime, ceftazidime, and imipenem (Fig. 5). Similarly, a low hemolytic activity of P. aeruginosa was reported with sub-inhibitory concentrations of erythromycin29, eugenol30 and the 7-fluoroindole17.
On the same instance, QS system coordinates the release of secondary metabolites such as pyocyanin15. Direct antagonists of QS in P. aeruginosa have a great impact on the production of pyocyanin31. In this study, the level of pyocyanin was significantly reduced (P<0.01) with sub-MICs of cefepime, ceftazidime and imipenem (Fig. 6).
These results indicated that controlling the production of QS molecules hinders most of the associated virulence agents. Also, inhibition of AHL signaling in Pseudomonas caused a reduction in the related virulence factors and attenuation of this pathogen when tested on animal models of pneumonia. On the same instance, synergistic effect has been achieved upon treating mice with both the QSI furanone C-30 and tobramycin, with increase in the clearance of P. aeruginosa in a foreign-body infection model32.
Conclusion
The fact that these antibiotics are already approved drugs for human use is a significant benefit in the future application and development of anti-pathogenic drugs. Hence, further investigations are necessary to detect anti-quorum sensing potential of antimicrobial combinations. Also, further in-vivo studies are required to confirm their possible utilization as anti-pathogenic agents for competing with P. aeruginosa infections.
Acknowledgements
All appreciation to Prof. Martin Schuster, Department of Microbiology, Oregon State University, Nash Hall, Corvallis, OR 97331 for the E. coli reporter strain MG4/pKDT17 and P. aeruginosa PAO-JP2. All thanks to Prof. Paul Williams, Department of Molecular Medical Science, Centre for Bimolecular Science, University of Nottingham, UK, for P. aeruginosa/pME3846.
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Wagner VE, Filiatrault MJ, Picardo KF, Iglewski BH. Pseudomonas aeruginosa virulence and pathogenesis issues. In: Cornelis P, editor. Pseudomonas genomics and molecular biology. 1st edn. Norfolk: Caister Academic Press; 2008. pp. 129–158. [Google Scholar]
- 2.Pessi G, Williams F, Hindle Z, Heurlier K, Holden MT, Camara M, et al. The global post transcriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in P. aeruginosa. J Bacteriol. 2001;183:6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH, et al. Structure of the autoinducer required for expression of P. aeruginosa virulence genes. Proc Natl AcadSci USA. 1994;91(1):197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in P. aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaki AA, Shaaban MI, Hashish NE, Amer MA, Lahloub MF. Assessment of Anti-Quorum Sensing Activity for Some Ornamental and Medicinal Plants Native to Egypt. Sci Pharm. 2013;81:251–258. doi: 10.3797/scipharm.1204-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Haidari RA, Shaaban MI, Ibrahim S, Mohamed G. Anti-quorum sensing activity of some medicinal plants. Afr J Tradit Complement Altern Med. 2016;13(5):67–71. doi: 10.21010/ajtcam.v13i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Shaaban MI, Abdel Bar FM, El-Mahdy AM, Shokralla S. Quorum Sensing Inhibiting Activity of Streptomyces coelicoflavus Isolated from Soil. Front Microbiol. 2016;7:659. doi: 10.3389/fmicb.2016.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabr MT, El-Gohary NS, El-Bendary ER, El-Kerdawy MM, Ni N, Shaaban MI. Synthesis, antimicrobial, antiquorum-sensing and cytotoxic activities of new series of benzothiazole derivatives. CCL. 2015;26:1522–1528. [Google Scholar]
- 10.Nalca Y, Jänsch L, Bredenbruch F, Geffers R, Buer J, Häussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother. 2006;50:1680–1688. doi: 10.1128/AAC.50.5.1680-1688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in the control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI Clinical and Laboratory Standards Institute, author. Performance standards for antimicrobial susceptibility testing: 24 informational supplement M100-S24. Wayne: PA: CLSI; 2014. [Google Scholar]
- 13.Standards Australia, author. Water Microbiology-Heterotrophic Colony Count Methods - Pour Plate Method using Plate Count Agar AS 427631 Committee FT/20, Water Microbiology. Homebush, Australia: Council of Standards Australia; 1995. PubMed. [Google Scholar]
- 14.El-Mowafy SA, Abd El-Galil KH, El-Messery SM, Shaaban MI. Aspirin is an efficient inhibitor of quorum sensing, virulence and toxins in Pseudomonas aeruginosa. Microb Pathog. 2014;74:25–32. doi: 10.1016/j.micpath.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RK, Setia S, Harjai K. Expression of Quorum Sensing and Virulence Factors Are Interlinked in P. aeruginosa: an in vitro Approach. Am J Biomed Sci. 2011;3(2):116–125. PubMed. [Google Scholar]
- 16.Miller JA. Experiments in molecular genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 17.Ohman DE, Cryz SJ, Iglewski Isolation and characterization of a P. aeruginosa PAO1 mutant that produces altered elastase. J Bacteriol. 1980;142:836–884. doi: 10.1128/jb.142.3.836-842.1980. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Mowafy SA, Shaaban MI, Abd El-Galil KH. Sodium Ascorbate as a Quorum Sensing Inhibitor of Pseudomonas aeruginosa. J Appl Microbiol. 2014;117(5):1388–1399. doi: 10.1111/jam.12631. [DOI] [PubMed] [Google Scholar]
- 19.Rossignol G, Merieau A, Guerillon J, Veron W, Lesouhaitier O, Feuilloley MG, et al. Involvement of a phospholipase C in the hemolytic activity of a clinical strain of P. fluorescens. BMC Microbiology. 2008;8:189. doi: 10.1186/1471-2180-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ra'oof WM, Latif IA. In vitro study of the swarming phenomena and antimicrobial activity of pyocyanin produced by P. aeruginosa isolated from different human infections. Europ J of scientific research. 2010;47(3):405. [Google Scholar]
- 21.Oberhardt MA, Puchalka J, Fryer KE, Martins dos Santos VA, Papin JA. Genome-Scale Metabolic Network Analysis of the Opportunistic Pathogen Pseudomonas aeruginosa PAO1. J Bacteriol. 2008;190:2790–2803. doi: 10.1128/JB.01583-07. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The Multiple Signaling Systems Regulating Virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76(1):46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pechere JC. Azithromycin reduces the production of virulence factors in Pseudomonas aeruginosa by inhibiting quorum sensing. Jpn J Antibiot. 2001;54:87–89. [PubMed] [Google Scholar]
- 24.Garske LA, Beatson SA, Leech AJ, Walsh SL, Bell SC. Sub-inhibitory concentrations of ceftazidime and tobramycin reduce the quorum sensing signals of P. aeruginosa. Pathology. 2004;36(6):571–575. doi: 10.1080/00313020400011300. [DOI] [PubMed] [Google Scholar]
- 25.Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, et al. Effects of Antibiotics on Quorum Sensing in P. aeruginosa. Antimicrob Agents Chemother. 2008;52:3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Shaer S, Shaaban M, Barwa R, Hassan R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl-naphthylamide. J Med Microbiol. 2016 doi: 10.1099/jmm.0.000327. in press. [DOI] [PubMed] [Google Scholar]
- 27.Khajanchi BK, Sha J, Kozlova EV, Erova TE, Suarez G, Sierra JC, et al. N-acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology. 2009;155:3518–3531. doi: 10.1099/mic.0.031575-0. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilder CN, Allada G, Schuster M. Instantaneous within-patient diversity of P. aeruginosa quorum-sensing populations from cystic fibrosis lung infections. Infect Immun. 2009;77(12):5631–5639. doi: 10.1128/IAI.00755-09. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sofer D, Gilboa-Garber N, Belz A, Garber NC. Sub-inhibitory' erythromycin represses production of Pseudomonas aeruginosa lectins, autoinducer and virulence factors. Chemotherapy. 1999;45:335–341. doi: 10.1159/000007224. [DOI] [PubMed] [Google Scholar]
- 30.Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunology Medical Microbiology. 2010;58:161–168. doi: 10.1111/j.1574-695X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 31.Morkunas B, Galloway WR, Wright M, Ibbeson BM, Hodgkinson JT, O'Connell KM, et al. Inhibition of the production of the P. aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics. Organic Biomol Chem. 2012;42:8452–8464. doi: 10.1039/c2ob26501j. [DOI] [PubMed] [Google Scholar]
- 32.Christensen LD, van Gennip M, Jakobsen TH, Alhede M, Hougen HP, Høiby N, et al. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J Antimicrob Chemother. 2012;67:1198–1206. doi: 10.1093/jac/dks002. [DOI] [PubMed] [Google Scholar]