Abstract
Background
Obesity is a modifiable risk factor for hypertension and T2D. Objective(s): We examined relations between fasting plasma adiponectin (ADIP), C-reactive protein (CRP) concentrations and markers of T2D in African Americans (AA).
Methods
Fasting plasma ADIP, CRP, Insulin (IN), HOMA-IR, lipid profiles, body fat percent (%BF), waist circumference (WC), body mass index (BMI) and blood pressure measures were determined in AA women (W: n=77) and men (M: n=34). Participants were classified into: 1) Normal fasting glucose (FG) and Normal %BF; 2) Normal FG and High %BF; and 3) High FG.
Results
Compared to men, women had significantly higher mean ADIP (W: 31.4±2.9 vs. M: 18.0±4.4 ng/L), CRP (W: 3.2±0.3 vs. M: 2.0±0.5 mg/L), %BF (W: 41.2±0.9 vs. M: 27.2±1.3), and BMI (W: 32.3±0.7 vs. M: 29.2±1.1 kg/m2). Women with normal FG and %BF had significantly higher ADIP (64.0±6.0) and lower CRP (1.3±0.6) concentrations than normal FG/ high %BF (ADIP: 37.0±5.0 and CRP: 3.1 ±0.5) and high FG (ADIP: 15.1±4.1 and CRP: 4.0 ± 0.5) groups. Women with high ADIP to CRP ratio had favorable metabolic and anthropometric profiles.
Conclusion
Low ADIP and high CRP are associated with excessive %BF and FG in AA women. ADIP/CRP, may be useful for detecting metabolic dysregulation.
Keywords: Obesity, type 2 diabetes, inflammation
Introduction
Obesity, defined as an accumulation of excess body fat1,2, increases the risk of developing insulin resistance (IR) and type 2 diabetes (T2D)3. Since these conditions reflect metabolic dysregulation and likely systemic inflammation, interest in various bio-active proteins has emerged. Two proteins of interest are adiponectin and C reactive protein (CRP), produced by adipose tissue and the liver, respectively4. The significant inverse correlation between anti-inflammatory adiponectin and pro-inflammatory CRP reported in certain obese, diabetic and coronary artery disease (CAD) populations5, underscores the importance of investigating adiponectin and CRP together to elucidate their opposing relationships in obesity and T2D.
Adipose tissue has traditionally been seen as a storage place for fatty acids; however this notion has been replaced over the last years and adipose tissue has been recognized to play a central role in lipid and glucose metabolism and production of various hormones and cytokines6. Adipose tissue is an active endocrine organ that secretes adipocytokines, such as adiponectin. Adiponectin has anti-diabetic and anti-inflammatory properties7 and appears to be higher in women than men8–10. Discovered in the mid-1990s by four different research groups, it is also referred to as Acrp30, AdipoQ, ApM1, and GBP2811. Within the general population, high adiponectin levels have been associated with positive cardiovascular benefits; whereas low values of adiponectin have been linked to a variety of health risks - obesity, metabolic syndrome (MS), IR, T2D and unfavorable lipid profiles12–15. Other studies have reported that individuals with CAD and/or T2D have lower plasma adiponectin levels than age- and body mass index- (BMI) matched, non-diabetic individuals without CAD16. Little is known about the regulation of adiponectin, especially in African Americans (AA) and most of the factors and mechanisms affecting adiponectin levels are poorly described17.
CRP is another important systemic marker for inflammation and high CRP levels have also been linked to multiple health risks - obesity, T2D, cardiovascular disease (CVD), and hyperlipidemia18–20. Due to the apparent strong associations between CRP and health risks, the American Heart Association and Centers for Disease Control and Prevention evaluated CRP as a risk assessment tool and proposed cut points of <1 mg/L, 1–3 mg/L and >3 mg/L be used to identify those at low, average, and high relative risk, respectively for CVD21. Plasma CRP correlates positively with body fat mass, visceral adipose tissue accumulation and plasma insulin22, and is associated with T2D23. Conflicting results have been found with regard to gender differences in CRP. Whereas some studies have reported higher CRP levels in women24,25, others have found no gender differences26. Ethnic differences in CRP concentrations have also been noted: two large cohort studies found that CRP tends to be higher in AA and Hispanic women than Caucasians and Asian women27,28.
However, some obese individuals are free from obesity-related metabolic complications29. The aim of this study was to evaluate associated patterns of plasma adiponectin and high sensitivity CRP (hs-CRP) concentrations as a function of metabolic risk factors - fasting blood glucose (FG), insulin (IN), waist circumference (WC), percent body fat (% BF), mean arterial blood pressure (MAP) and plasma lipids (high density lipoprotein cholesterol/HDL-C and triglycerides/TG). We hypothesized that, among individuals with increased FG and %BF, combining adiponectin and CRP would provide a valuable index of obesity related inflammatory and metabolic markers. Therefore, we examined mean differences in metabolic characteristics based on the combined measurements of adiponectin and CRP in AA men and women after classifying participants into three metabolic risk groups: (a) normal FG and %BF; (b) normal FG and high %BF; and (c) high FG. In addition we determined whether the plasma adiponectin to CRP ratio level would be a predictive measure of metabolic risk.
Methods
Study population
Participants with complete dataset included 111 AA men (n= 34) and women (n = 77) between 18 to 60 years of age who were recruited between January 2008 and September 2009 through public advertisements and announcements at local churches in the Greater Washington DC area. Participants who were pregnant or taking steroid medications were excluded for distinct hormonal profiles associated with circulating levels of adiponectin. The study was approved by the Institutional Review Board of the Uniformed Services University of the Health Sciences (USUHS), and written informed consent was obtained from all participants.
Procedures
Participants visited the laboratory between 0700 and 0900 AM, wherein after obtaining informed consent, blood pressure (BP) and anthropometric evaluations were obtained. Baseline blood collections were obtained after an overnight fast for determination of glucose, insulin, HDL-C, TG, hs-CRP and adiponectin.
Anthropometric measurements
Body weight was measured with a calibrated balance beam metric scale to the nearest 0.1kg and height was measured with a stadiometer to the nearest 0.1cm. BMI was calculated from height and weight (kg/m2). %BF was estimated by using bioelectric impedance and calculated with the NHANES III prediction formula. WC was measured at the midpoint between the lower rib margin and the iliac crest by using a non-elastic measuring tape. Baseline BP was recorded with a standard BP monitor: measurements were obtained on at least two occasions with the participant in a seated position to ensure accurate assessment. Mean arterial pressure (MAP) was calculated using the formula ((2*Diastolic BP) + (Systolic BP))/330.
Physiological and biochemical measurements
Fasting blood glucose concentration was measured with One Touch Ultra monitoring system, hs-CRP analyses were carried out with an Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Erlanger, Germany), Insulin was detected using the Human Insulin ELISA kit from Millipore (St.Charles, Missouri, USA), Adiponectin was detected using the Human Adiponectin sandwich immunoassay kit from Meso Scale Discovery (Gaithersburg, MD, USA) and lipid profiles (Total Cholesterol, HDL-C, LDL-C and TG) were determined in the Clinical Laboratory at the National Institutes of Health Department of Laboratory Medicine using an LX-20 analyzer (Beckman, San Diego, CA). Mean values are expressed in mm/L, µU/mL, mg/L, ng/mL and mmol/L respectively. Insulin Resistance was defined by using homeostasis model asessment method of insulin resistance (HOMA-IR) (glucose [in millimoles per liter] × insulin [in microunits per milliliter]/22.5).
Statistical analysis
The frequency and distributions of variables were examined to determine normality of distribution and identify outliers. Distributions of plasma adiponectin and CRP levels were skewed, so natural log transformed values were used to normalize the variables for analyses. Men and women with a %BF ≥ 25.1 and 35.1 respectively, were categorized as high %BF and participants with FG ≥ 6.1 mmL were considered as diabetic. Three groups were then formed based on FG and %BF: 1) low risk - normal FG and %BF; 2) moderate risk - normal FG and high %BF; and 3) high risk - high FG. Covariates, such as age and income, were controlled for in all analyses.
In addition, the ratio of adiponectin (values were multiplied by 1,000 to generate whole numbers greater than 0.1) to CRP was calculated (mg/L). Cut points were made for 2 groups and participants in the lower point of adiponectin to CRP ratio were classified as “high risk”, participants in the upper point were classified as “low risk”. Multivariate analyses of variance (MANOVA) were used to determine differences in (1) metabolic (FG, IN, HOMA-IR), lipid profiles (TC, TG, LDL-C and HDL-C) and physical variables (BMI, %BF, WC, MAP) as a function of metabolic grouping low and high adiponectin to CRP ratio and (2) variations in plasma adiponectin, CRP and adiponectin to CRP ratio in the three %BF and diabetes status groups. Partial correlations were calculated to find associations between adiponectin and CRP with physical, lipid and metabolic measurements.
The sample size of 111 (69% female) was adequate to detect moderate group differences across two independent groups of different sizes (d=0.6), and across three sized groups (f=0.30). Participants with complete data were in cluded in the present analysis. Results are expressed as mean ± SEM and the statistical significance was set at p ≤ 0.05. Statistical analyses were performed with IBM SPSS 20.0 for Windows (SPSS Inc., Chicago, Ill., USA).
Results
Compared to men, women had higher plasma hs-CRP (Women: 3.2 ± 0.3 mg/L, Men: 2.0 ± 0.5 mg/L; p ≤ 0.05), adiponectin (Women: 31.4 ± 2.9 ng/mL, Men: 18.0 ± 4.4 ng/mL; p ≤ 0.05), %BF (Women: 41.2 ± 0.9, Men: 27.2 ± 1.3; p ≤ 0.001) and BMI (Women: 32.3 ± 0.7, Men: 29.2 ± 1.1; p = 0.05), as shown in Table 1.
Table 1.
Metabolic and physical characteristics by gender (Mean ± S.E.M)
| Variables | Women (n=77) | Men (n=34) |
| CRP (mg/L)* | 3.2 ± 0.3 | 2.0 ± 0.5 |
| Adiponectin (ng/mL)** | 31.4 ± 2.9 | 18.0 ± 4.4 |
| 1Adiponectin/CRP ratio | 43.2 ± 7.5 | 28.0 ± 11.3 |
| Total Cholesterol (mmol/L) | 4.1 ± 0.1 | 4.0 ± 0.1 |
| Triglycerides (mmol/L) | 1.0 ± 0.1 | 1.2 ± 0.1 |
| LDL-C (mmol/L) | 2.4 ± 0.1 | 2.3 ± 0.1 |
| HDL-C (mmol/L) | 1.3 ± 0.04 | 1.2 ± 0.06 |
| Fasting Glucose (mmol/L) | 6.2 ± 0.2 | 6.3 ± 0.2 |
| Insulin (µU/mL) | 7.5 ± 0.9 | 7.4 ± 1.3 |
| HOMA-IR | 2.2 ± 0.3 | 2.2 ± 0.4 |
| Waist Circumference (cm) | 99.0 ± 2.0 | 98.5 ± 3.0 |
| Percent Body Fat*** | 41.2 ± 0.9 | 27.2 ± 1.3 |
| Body Mass Index (kg/m2)* | 32.3 ± 0.7 | 29.2 ± 1.1 |
| MAP (mm Hg) | 99.0 ± 2.0 | 101.0 ± 2.3 |
Note: Mean Differences significant at
p≤ 0.05,
p ≤0.01,
p≤0.001 (controlled for age and income;
ratio of adiponectin (values multiplied by 1000 to generate whole numbers) to CRP.
Abbreviations: C reactive protein (CRP); Low-Density Lipoprotein Cholesterol (LDL-C); High-Density Lipoprotein Cholesterol (HDL-C); Homeostasis Model Assessment Method of Insulin Resistance (HOMA-IR); Mean Arterial Pressure (MAP).
Table 2 presents correlation coefficients for plasma adiponectin and CRP with physical (BMI, WC, MAP and %BF), metabolic and lipid (FG, IN, HOMA-IR, TC, TG, LDL-C and HDL-C) variables. A significant negative correlation was observed between plasma adiponectin and CRP in AA men (r = −0.39, p ≤ 0.05) and women (r = −0.32, p ≤ 0.01). Adiponectin was significantly and negatively associated with BMI and WC in both men and women, whereas in women only, adiponectin was also associated with %BF, IN, FG and HOMA-IR. In men, there was a significant negative association between CRP and HDL-C; a positive association between CRP and BMI in both men and women and with WC, %BF, FG, IN and HOMA-IR, in women only.
Table 2.
Partial correlations between adiponectin, CRP, physical and metabolic variables by gender (Women: n = 77; Men: n = 34)
| Variables | Adiponectin | CRP | ||
| Women | Men | Women | Men | |
| Adiponectin (mg/L) | − | − | −0.32** | −0.34* |
| CRP (mg/L) | −0.32** | −0.39* | − | − |
| BMI (kg/m2) | −0.23* | −0.40* | 0.51*** | 0.45** |
| WC (cm) | −0.30** | −0.34* | 0.57*** | 0.32 |
| MAP (mm Hg) | 0.13 | −0.13 | 0.16 | 0.002 |
| Body Fat (%) | −0.24* | −0.26 | 0.50*** | 0.33 |
| FG (mmol/L) | −0.34** | −0.05 | 0.30** | −0.10 |
| IN (µU/mL) | −0.33** | −0.11 | 0.30* | 0.17 |
| HOMA-IR | −0.33** | −0.12 | 0.30** | 0.15 |
| TC (mmol/L) | −0.10 | −0.15 | 0.12 | 0.04 |
| TG (mmol/L) | −0.22 | −0.23 | 0.16 | 0.13 |
| LDL-C (mmol/L) | −0.13 | −0.15 | 0.16 | 0.17 |
| HDL-C (mmol/L) | 0.16 | 0.17 | −0.11 | −0.36* |
Note: Significance of correlation coefficients shown at
p≤ 0.05,
p ≤0.01,
p≤0.001 (controlled for age and income).
Abbreviations: C reactive protein (CRP); Body Mass Index (BMI); Waist Circumference (WC); Mean Arterial Pressure (MAP); Fasting Glucose (FG); Insulin (IN); Homeostasis Model Assessment of Insulin Resistance (HOMA-IR); Total Cholesterol (TC); Triglycerides (TG); Low-Density Lipoprotein Cholesterol (LDL-C); High-Density Lipoprotein Cholesterol (HDL-C).
Table 3 shows metabolic and physical baseline differences based on low and high adiponectin to CRP ratio. AA women with a high adiponectin to CRP ratio had significantly lower FG, IN, HOMA-IR, WC, %BF and BMI while for men, only HDL-C, BMI and %BF were significantly different between the two ratio groups.
Table 3.
Metabolic and physical characteristic based on low and high a diponectin to CRP ratio (Mean ± S.E.M)
| Variables | Adiponectin/CRP ratio WOMEN |
Adiponectin/CRP ratio MEN |
||
| Low (n= 39) | High (n=38) | Low (n=17) | High (n=17) | |
| TC (mmol/L) | 4.2 ± 0.1 | 4.0 ± 0.1 | 4.1 ± 0.2 | 4.1 ± 0.2 |
| LDL-C (mmol/L) | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.2 |
| TG (mmol/L) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.0 ± 0.1 |
| HDL-C (mmol/L) | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1* | 1.3 ± 0.1 |
| FG (mmol/L) | 6.4 ± 0.1** | 6.0 ± 0.2 | 7.0 ± 0.5 | 6.2 ± 0.5 |
| IN (µU/mL) | 10.0 ± 1.0** | 5.0 ± 1.0 | ||
| HOMA-IR | 3.0 ± 0.3** | 1.4 ± 0.3 | 3.0 ± 0.7 | 1.4 ± 0.7 |
| WC (cm) | 104.1 ± 2.6** | 92.1 ± 3.0 | 104.1 ± 3.7 | 94.1 ± 4.0 |
| BF (%) | 44.0 ± 1.2** | 39.0 ± 1.3 | 30.0 ± 1.3* | 25.2 ± 1.3 |
| BMI (kg/m2) | 34.4 ± 1.1*** | 30.1 ± 1.1 | 31.2 ± 1.2* | 27.3 ± 1.1 |
| MAP (mm Hg) | 99 ± 2.0 | 97 ± 2.3 | 101 ± 3.3 | 102 ± 3.3 |
Note: Mean Differences significant at
p≤ 0.05,
p ≤0.01,
p≤0.001; (controlled for age and income; participants who were in the lower 50 percentile of adiponectin to CRP ratio were classified as “low”, participants in the upper 50 percentile were classified as “high”).
Abbreviations: Total Cholesterol (TC); Low-Density Lipoprotein Cholesterol (LDL-C); Triglycerides (TG); High-Density Lipoprotein Cholesterol (HDL-C); Fasting Glucose (FG); Insulin (IN); Homeostasis Model Assessment of Insulin Resistance (HOMA-IR); Waist Circumference (WC); Body Mass Index (BMI); Mean Arterial Pressure (MAP).
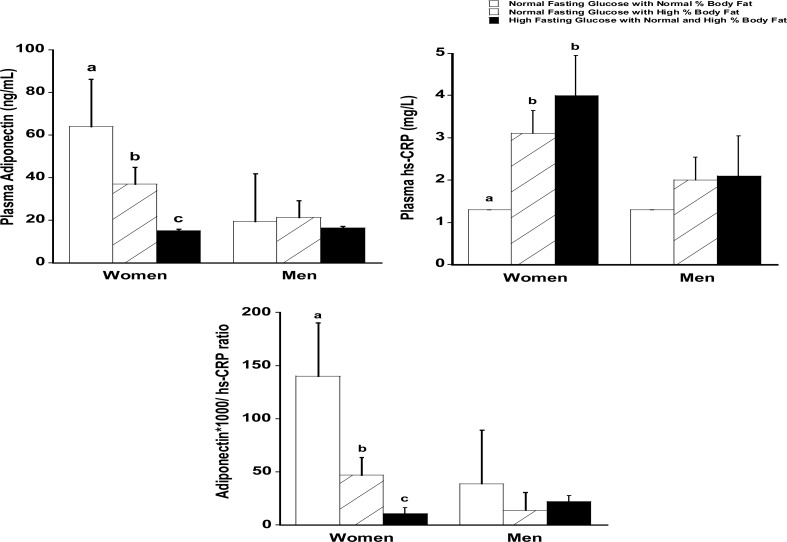
Figure 1 presents mean plasma adiponectin, CRP and adiponectin to CRP ratio by the three metabolic groupings and gender. Women with normal FG/%BF had significantly higher adiponectin (64±6.0 ng/mL) and lower CRP (1.3±0.6 mg/L) concentrations than women with normal FG and high %BF (adiponectin: 37.0 ±5.0 ng/ mL and CRP: 3.1±0.5 mg/L) and high FG (adiponectin: 15.1 ± 4.1 ng/mL and CRP: 4.0 ± 0.5mg/mL). Additionally, adiponectin to CRP ratio differed significantly in all three groups among women. This pattern did not hold in AA men.
Figure 1.
Plasma adiponectin, CRP, and the adiponectin to CRP ratio based on normal %body fat and fasting glucose (Women = 23; Men = 16), high %body fat and normal fasting glucose (Women = 30; Men = 12) and normal/high % body fat/high fasting glucose (Women = 43; Men = 21) (Mean ± S.E.M)
Note: Means with different superscript letters differ significantly; p≤ 0.05 (controlled for age and income; ratio of adiponectin to CRP values were multiplied by 1000 to generate whole numbers). Abbreviations: CRP: C-Reactive Protein).
Discussion
Excessive body fat is a major risk for cardiovascular disease and increases the risk of hypertension, dyslipidemia, hyperglycemia and low-grade inflammation process. Two proteins that are associated with T2D and obesity are adiponectin and CRP. Adiponectin is exclusively produced by adipocytes, circulates in plasma, and is reduced in obesity, IR, MS and T2D. Adiponectin helps to regulate the release of various inflammatory and anti-inflammatory cytokines13,31. Whereas CRP released from the liver in response to the inflammatory cytokines - IL-1 beta, IL-6 and TNF-alpha is a risk factor for cardiovascular events and is related to an increased risk of incident T2D. Although CRP and adiponectin were negatively related, results from the present study strongly suggests that AA women with low adiponectin and high CRP are likely to have excessive body fat and glucose dysregulation as compared to AA women with high adiponectin and low CRP values. In contrast, women who are obese, but not pre-diabetic, are likely to have moderately high adiponectin and CRP values. A predictive cut-off for adiponectin could be developed, but an adiponectin/CRP ratio may preclude the need for such a cut-off.
Previous studies have shown that circulating CRP is a predictor of CVD32 whereas adiponectin levels are reflective of IR, central obesity and intra-abdominal fat accumulation16,33,34. In the present study, adiponectin was negatively correlated with many markers (BMI, WC, %BF, and FG), whereas CRP was positively correlated with various metabolic markers (BMI, WC, %BF, IN, FG and HOMA-IR) in AA women. Although CRP and adiponectin were negatively related, the strength of the relationship was low; combining the two variables may provide more valuable information regarding obesity and diabetes than any of the individual variables. Interestingly, the observed trends were not seen in AA men.
Adiponectin and CRP are independent risk factors of CVD, together serve a synergistic role in metabolic regulation in the general population and T2D subjects35–37. We found that AA women with high adiponectin and low CRP levels, which translated into a high ratio, had significantly lower FG, IN, HOMA-IR, WC, %BF and BMI relative to those with a low ratio. A combined measurement of these markers may be more useful for detecting persons at high risk for cardiovascular disease and diabetes, than one of the measure alone.
Although obesity is a risk factor for insulin resistance, CVD and T2DM, not every obese individual suffers the same metabolic dysregulation. In the 1980s, a subgroup of metabolically normal, but obese, individuals with relatively high insulin sensitivity and a favorable metabolic profile was reported by several investigators38. A unique subset of obese individuals appears to be protected from developing the metabolic disturbances typically associated with obesity. These individuals, despite being obese display normal to high insulin sensitivity and favorable cardiovascular risk profiles39–43. Thus, it is important to characterize differences between these groups to uncover additional mechanisms connecting obesity and health risk. In our study, we examined differences in adiponectin and CRP and the adiponectin to CRP ratio in AA men and women with normal %BF/FG, high %BF/normal FG and normal and high %BF with high FG. Whereas no differences were noted between the men by metabolic group assignment, plasma adiponectin was consistently lower and CRP higher in women in the high FG group compared to women in the normal %BF/FG and in the high %BF/normal FG group. The high FG also had a significantly lower adiponectin to CRP ratio relative to the other metabolic groups of women. Our findings are also consistent with epidemiological studies showing that circulating levels of adiponectin are lower in diabetic than in healthy individuals44,45. Additionally, studies have reported that heavier individuals had greater health benefit and lower health risks from high levels of adiponectin than their lean counterparts46,47. Whether a combined measure of these two markers, such as adiponectin/CRP ratio, would be useful for detecting and monitoring metabolic and low-grade inflammation health risk in AA women remains to be fully understood.
Gender differences in adiponectin and CRP levels have previously been reported23,48,49, and our study provides further evidence of these gender differences because AA women had significantly higher adiponectin and CRP levels than AA men. Additionally, we found a significant negative association between plasma adiponectin and CRP in both genders.
Limitations
We had data on fewer men than women, and this may have limited our findings among men. The present study examined only AA and it would be interesting to examine differences across other ethnic overweight/obese and diabetic/non diabetic groups by gender as well. Longitudinal research with more participants will be necessary to determine the independent and interactive relationships between adiponectin and hs-CRP, and the health outcomes they reflect.
Conclusion
A statistically significant negative association was observed between plasma adiponectin and CRP in both AA men and women; AA women had higher adiponectin and CRP levels compared to AA men. Overall, AA women with high fasting glucose and HOMA-IR had lower concentrations of adiponectin and higher CRP levels than AA women with normal glucose. Measures of obesity and metabolic dysregulation are more strongly related to CRP and adiponectin in AA women than in AA men. Finally, the ratio of circulating levels of adiponectin to CRP was examined and found to discriminate between AA women who were diabetic versus those who were not, regardless of obesity status, which suggests that adiponectin may have a protective effect against obesity related metabolic derangements in AA women. Prospective studies will be needed to confirm this finding.
Acknowledgements
The project was funded by NCMHD, National Institutes of Health, Bethesda, MD, Establishing Exploratory NCMHD Research Centers of Excellence (P20), RFAMD-07-001. We thank Dr. Alan Remaley's team at NIH for their contributions.
Conflict of interest and disclaimer
The authors have no conflict of interests in the present study. The views expressed are those of the authors and do not reflect the official policy position of the USUHS, Department of the Army, Department of the Navy, Department of Air Force, the United States Department of Defense or the United States Government.
Author contributions
Preetha Anna Abraham: drafted and wrote the present manuscript, contributed to the conception and design of research, acquisition of data, prepared figures, performed experiments, and conducted statistical analysis; Selasi Attipoe: assisted with performing experiments, acquisition of data, and manuscript preparation; Josh Kazman: assisted with statistical analyses and interpretation of results; Stacey Zeno: supervision of study execution, acquisition of data, and assisted with manuscript preparation; Merrily Poth and Patricia Deuster: acquired funding for the research, supervision of study execution, contributed to the study design, edited and approved the final version of the manuscript.
References
- 1.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2002;3(3):141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 2.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(1):85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 3.Maggio CA, Pi-Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes care. 1997;20(11):1744–1766. doi: 10.2337/diacare.20.11.1744. [DOI] [PubMed] [Google Scholar]
- 4.Al-Daghri NM, Al-Attas OS, Al-Rubeaan K, Sallam R. Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria. Diabetes/metabolism research and reviews. 2008;24(1):52–58. doi: 10.1002/dmrr.763. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Zhang H, et al. Inverse association between adiponectin and C-reactive protein in substantially healthy Japanese men. Atherosclerosis. 2006;188(1):184–189. doi: 10.1016/j.atherosclerosis.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European Heart Journal. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107(5):671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin GA, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes (Lond) 2007;31(3):457–465. doi: 10.1038/sj.ijo.0803427. [DOI] [PubMed] [Google Scholar]
- 9.Tabatabaei-Malazy O, Hasani-Ranjbar S, Amoli MM, Heshmat R, Sajadi M, Derakhshan R, et al. Gender-specific differences in the association of adiponectin gene polymorphisms with body mass index. The review of diabetic studies: RDS. 2010;7(3):241–246. doi: 10.1900/RDS.2010.7.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarrow JF, Beggs LA, Conover CF, McCoy SC, Beck DT, Borst SE. Influence of androgens on circulating adiponectin in male and female rodents. PloS one. 2012;7(10):e47315. doi: 10.1371/journal.pone.0047315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54(9):2772–2778. doi: 10.2337/diabetes.54.9.2772. [DOI] [PubMed] [Google Scholar]
- 12.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochemical and biophysical research communications. 2012;425(3):560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes care. 2003;26(8):2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 14.Davis SK, Gebreab SY, Xu R, Riestra P, Khan RJ, Sumner AE, et al. Association of adiponectin with type 2 diabetes and hypertension in African American men and women: the Jackson Heart Study. BMC cardiovascular disorders. 2015;15:13. doi: 10.1186/s12872-015-0005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA: The Journal of the American Medical Association. 2009;302(2):179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 16.Goropashnaya AV, Herron J, Sexton M, Havel PJ, Stanhope KL, Plaetke R, et al. Relationships between plasma adiponectin and body fat distribution, insulin sensitivity, and plasma lipoproteins in Alaskan Yup'ik Eskimos: the Center for Alaska Native Health Research study. Metabolism: clinical and experimental. 2009;58(1):22–29. doi: 10.1016/j.metabol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolland YM, Haren MT, Patrick P, Banks WA, Malmstrom TK, Miller DK, et al. Adiponectin levels in obese and non-obese middle-aged African-American women. Obes Res Clin Pract. 2007;1(1):1–78. doi: 10.1016/j.orcp.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108(24):2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 19.Lear SA, Chen MM, Birmingham CL, Frohlich JJ. The relationship between simple anthropometric indices and C-reactive protein: ethnic and gender differences. Metabolism: clinical and experimental. 2003;52(12):1542–1546. doi: 10.1016/j.metabol.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA: The Journal of the American Medical Association. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 21.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008;54(6):1027–1037. doi: 10.1373/clinchem.2007.098996. [DOI] [PubMed] [Google Scholar]
- 22.Ong KL, Tso AW, Xu A, Law LS, Li M, Wat NM, et al. Evaluation of the combined use of adiponectin and C-reactive protein levels as biomarkers for predicting the deterioration in glycaemia after a median of 5.4 years. Diabetologia. 2011;54(10):2552–2560. doi: 10.1007/s00125-011-2227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahonen T, Vanhala M, Kautiainen H, Kumpusalo E, Saltevo J. Sex differences in the association of adiponectin and low-grade inflammation with changes in the body mass index from youth to middle age. Gender medicine. 2012;9(1):1–8. doi: 10.1016/j.genm.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Arena R, Arrowood JA, Fei DY, Helm S, Kraft KA. The relationship between C-reactive protein and other cardiovascular risk factors in men and women. J Cardiopulm Rehabil. 2006;26(5):323–327. doi: 10.1097/00008483-200609000-00009. quiz 8–9. [DOI] [PubMed] [Google Scholar]
- 25.Williams MJ, Milne BJ, Hancox RJ, Poulton R. C-reactive protein and cardiorespiratory fitness in young adults. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2005;12(3):216–220. doi: 10.1097/01.hjr.0000166453.15722.7b. [DOI] [PubMed] [Google Scholar]
- 26.Thorand B, Baumert J, Doring A, Herder C, Kolb H, Rathmann W, et al. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184(1):216–224. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr, et al. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. American Heart Journal. 2006;152(3):593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN) American Heart Journal. 2005;149(6):1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35(7):971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 30.Ghazzi MN, Perez JE, Antonucci TK, Driscoll JH, Huang SM, Faja BW, et al. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. The Troglitazone Study Group. Diabetes. 1997;46(3):433–439. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- 31.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature reviews Immunology. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 32.Liao CH, Li HY, Yu HJ, Chiang HS, Lin MS, Hua CH, et al. Low serum sex hormone-binding globulin: marker of inflammation? Clinica chimica acta. International Journal of clinical chemistry. 2012;413(7–8):803–807. doi: 10.1016/j.cca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Mohammadzadeh G, Zarghami N. Hypoadiponectinemia in obese subjects with type II diabetes: A close association with central obesity indices. Journal of research in medical sciences: The Official Journal of Isfahan University of Medical Sciences. 2011;16(6):713–723. [PMC free article] [PubMed] [Google Scholar]
- 34.Salmenniemi U, Ruotsalainen E, Vanttinen M, Vauhkonen I, Pihlajamaki J, Kainulainen S, et al. High amount of visceral fat mass is assoc iated with multiple metabolic changes in offspring of type 2 diabetic patients. Int J Obes (Lond) 2005;29(12):1464–1470. doi: 10.1038/sj.ijo.0803041. [DOI] [PubMed] [Google Scholar]
- 35.Saisho Y, Hirose H, Seino Y, Saito I, Itoh H. Usefulness of C-reactive protein to high-molecular-weight adiponectin ratio to predict insulin resistance and metabolic syndrome in Japanese men. Journal of atherosclerosis and thrombosis. 2010;17(9):944–952. doi: 10.5551/jat.4234. [DOI] [PubMed] [Google Scholar]
- 36.Saisho Y, Hirose H, Yamamoto Y, Nakatani H, Itoh H. Combination of C-reactive protein and high molecular weight (HMW)-adiponectin reflects further metabolic abnormalities compared with each of them alone in Japanese type 2 diabetic subjects. Endocrine Journal. 2008;55(2):331–338. doi: 10.1507/endocrj.k07e-032. [DOI] [PubMed] [Google Scholar]
- 37.Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetology & metabolic syndrome. 2014;6(1):99. doi: 10.1186/1758-5996-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? The Journal of clinical endocrinology and metabolism. 2001;86(3):1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 39.Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A, et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. The Journal of clinical endocrinology and metabolism. 2004;89(10):5013–5020. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 40.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 41.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. The Journal of clinical endocrinology and metabolism. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 42.Pataky Z, Bobbioni-Harsch E, Golay A. Open questions about metabolically normal obesity. Int J Obes (Lond) 2010;(34 Suppl 2):S18–S23. doi: 10.1038/ijo.2010.235. [DOI] [PubMed] [Google Scholar]
- 43.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. The American Journal of clinical nutrition. 1981;34(8):1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 44.Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes care. 2003;26(7):2015–2020. doi: 10.2337/diacare.26.7.2015. [DOI] [PubMed] [Google Scholar]
- 45.Nayak S, Soon SQ, Kunjal R, Ramadoo R, Baptiste O, Persad J, et al. Relationship between adiponectin, inflammatory markers and obesity in type 2 diabetic and non-diabetic Trinidadians. Archives of physiology and biochemistry. 2009;115(1):28–33. doi: 10.1080/13813450902758785. [DOI] [PubMed] [Google Scholar]
- 46.Aguilar-Salinas CA, Garcia EG, Robles L, Riano D, Ruiz-Gomez DG, Garcia-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. The Journal of clinical endocrinology and metabolism. 2008;93(10):4075–4079. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 47.Morrison JA, Glueck CJ, Daniels S, Wang P, Horn P, Stroop D. Paradoxically high adiponectin and the healthy obese phenotype in obese black and white 16-year-old girls. Translational research: The Journal of laboratory and clinical medicine. 2010;156(5):302–308. doi: 10.1016/j.trsl.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bo S, Gentile L, Ciccone G, Baldi C, Benini L, Dusio F, et al. The metabolic syndrome and high C-reactive protein: prevalence and differences by sex in a southern-European population-based cohort. Diabetes/metabolism research and reviews. 2005;21(6):515–524. doi: 10.1002/dmrr.561. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda Y, Tanioka T, Yoshioka T, Nagano T, Hiroi T, Yoshikawa K, et al. Gender differences in association of plasma adiponectin with obesity reflect resultant insulin resistance in non-diabetic Japanese patients with schizophrenia. Psychiatry and clinical neurosciences. 2005;59(3):266. doi: 10.1111/j.1440-1819.2005.01370.x. [DOI] [PubMed] [Google Scholar]