Abstract
Hypertension is a major risk factor for the development and progression of chronic kidney disease (CKD). Mineralocorticoid receptor antagonists (MRAs) are effective in the management of resistant hypertension but are not widely used in CKD because of the risk of hyperkalemia. We retrospectively evaluated the long-term effects and safety of MRAs added to a pre-existing antihypertensive regimen in subjects with resistant hypertension associated with stage 3 CKD. In all, 32 patients were treated with spironolactone and 4 with eplerenone for a median follow-up of 312 days. MRAs induced a significant decrease in systolic blood pressure from 162±22 to 138±14 mm Hg (P<0.0001) and in diastolic blood pressure from 87±17 to 74±12 mm Hg (P<0.0001). Serum potassium increased from 4.0±0.5 to 4.4±0.5 mEq l−1 (P = 0.0001), with the highest value being 5.8 mEq l−1. The serum creatinine increased from 1.5±0.3 to 1.8±0.5 mg dl−1 (P = 0.0004) and the estimated glomerular filtration rate decreased from 48.6±8.7 to 41.2±11.5 ml min−1 per 1.73 m2 (P = 0.0002). One case of acute renal failure and three cases of significant hyperkalemia occurred. MRAs significantly reduced blood pressure in subjects with resistant hypertension associated with stage 3 CKD, although close biochemical monitoring is recommended because of an increased risk of hyperkalemia and worsening of renal function.
Keywords: aldosterone, chronic kidney disease, resistant hypertension, hyperkalemia, mineralocorticoid receptor antagonists
Introduction
Hypertension is a strong and independent risk factor for the development and progression of both cardiovascular disease and chronic kidney disease (CKD).1–4 Controlling hypertension is critical to avoid progression to end-stage renal disease and to minimize the risk of cardiovascular events in CKD patients.3,5,6 The target blood pressure is generally considered <130/80 mm Hg in CKD patients; however, this is achieved in <30% of the patients.7 Mineralocorticoid receptor antagonists (MRAs), such as spironolactone and eplerenone, have been effective as add-on therapy for improving blood pressure control in subjects with resistant hypertension without CKD.8,9 However, their use has been limited in patients with moderate-to-advanced CKD mostly because of the risk of hyperkalemia.10 This study was undertaken to examine the long-term effects and tolerability of MRAs added to preexisting antihypertensive regimens that included a diuretic and a renin–angiotensin–aldosterone system (RAAS) blocker in subjects with resistant hypertension associated with CKD stage 3 (estimated glomerular filtration rate (eGFR) 30–59 ml min−1 per 1.73 m2).
Materials and methods
The patients included in this retrospective analysis were seen between February 2001 and June 2009 at a university-based clinic staffed by hypertension specialists (University of Alabama at Birmingham, Birmingham, AL, USA). All patients with resistant hypertension and CKD stage 3 who received either spironolactone or eplerenone during the study period were included in this analysis. Resistant hypertension was defined by failure to achieve the goal blood pressure of <130/80 mm Hg despite adherence to a stable antihypertensive drug regimen of three or more medications, including a RAAS blocker (angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB)) and a diuretic appropriate for the level of kidney function. For each patient, the RAAS blocker had been titrated to the maximum recommended/tolerated dose.
Demographics, laboratory and medication data were extracted from the electronic medical records for baseline (before MRA treatment) and the most recent follow-up visit. The end points were change in systolic blood pressure (SBP), serum potassium and creatinine, eGFR by MDRD four-variable formula, diastolic blood pressure (DBP) and tolerability.11 Office blood pressure readings were obtained following the American Heart Association guidelines.2 The University of Alabama Institutional Review Board approved this analysis.
Values between groups were compared by a two-tailed t-test or Wilcoxon’s test, where applicable, and categorical values were compared using a χ2-test or Fisher’s exact test. Values are expressed as mean±s.d., unless otherwise noted. Baseline SBP, body mass index, medication type, gender, race and age were evaluated as independent predictors of follow-up SBP values by multivariate analysis. P-values were reported for descriptive purposes and P<0.05 was considered statistically significant. A logistic regression analysis was performed to determine factors that may lead to hyperkalemia and an increase in serum creatinine >30% from baseline, with factors such as age, body mass index, baseline eGFR, baseline serum potassium and creatinine, baseline SBP and DBP, medication type, gender, race, weight, diabetes and smoking status being considered. Because of the observational hypothesis-generating nature of this study, no adjustments were made for multiple assessments.
Results
A total of 36 patients who received a MRA as add-on therapy were included in this analysis. In all, 32 patients received spironolactone and 4 were prescribed with eplerenone (mean dose 23.6±10.5 and 60.4±33.9 mg per day, respectively). Two patients were switched from spironolactone to eplerenone because of adverse effects (breast tenderness). The median time between baseline (before MRA treatment) and the most recent follow-up visit was 312 days. The baseline demographics and laboratory data for all evaluated subjects are shown in Table 1. Of note, 64% were male, 53% African-American, 56% obese, 31% diabetic and 25% were diagnosed previously with coronary artery disease. Blood pressure was uncontrolled despite being on an average of 4.9 different antihypertensive agents.
Table 1.
Baseline characteristics of the study population
| Variables | Mean±s.d. |
|---|---|
| Age (years) | 63±11 |
| Males (%) | 64 |
| African-Americans (%) | 53 |
| BMI, kg m−2 | 31.5±6.4 |
| Obesity (%) | 56 |
| Diabetes (%) | 31 |
| Coronary artery disease (%) | 25 |
| Hyperlipidemia (%) | 81 |
| Antihypertensive agents (n) | 4.9±1.6 |
| Systolic blood pressure (mm Hg) | 162±22 |
| Diastolic blood pressure (mm Hg) | 87±17 |
| Serum creatinine (mg dl−1) | 1.5±0.3 |
| eGFR (ml min−1 per 1.73 m2) | 48.6±8.7 |
| Serum potassium (mEq l−1) | 4.0±0.5 |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate.
Effects of MRA add-on therapy on blood pressure
MRAs induced a significant decrease in SBP from 162±22 to 138±14 mm Hg (P<0.0001) and in DBP from 87±17 to 74±12 mm Hg (P<0.0001; Figure 1). The mean number of antihypertensive agents increased from 4.9±1.6 at baseline to 5.4±1.5 (MRA included) at the last follow-up visit (P<0.01). Forty-four percent of the patients discontinued at least one antihypertensive medication during the follow-up, while 19% of the patients had at least one antihypertensive medication added besides the MRA during the follow-up. A multiple regression analysis was performed to determine the predictors of SBP response to MRAs. Of the biological risk factors, higher baseline SBP measure was the only statistically significant predictor (P<0.0001). Older subjects were less likely to have a change in SBP than younger subjects, with a trend toward an association between the patient’s age and follow-up SBP (P = 0.1095).
Figure 1.
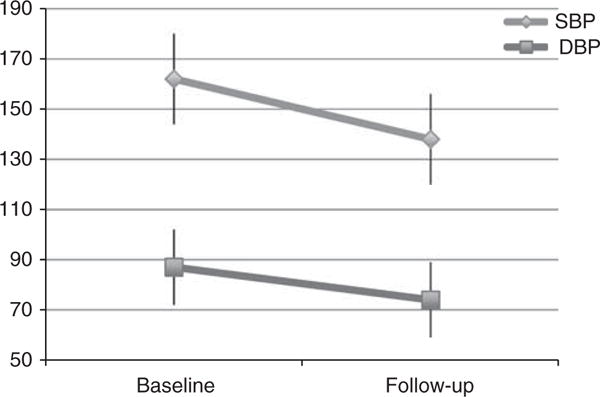
Changes in SBP and DBP (mm Hg) after a median of 312 days of follow-up.
Effects of MRA add-on therapy on serum potassium
Serum potassium increased significantly from 4.0±0.5 at baseline to 4.4±0.5 mEq l−1 (P =.0001) during follow-up (Figure 2). Eight patients (22%) developed hyperkalemia (serum potassium >5.0 mEq l−1); of these 3 (8%) had a serum potassium >5.5 mEq l−1, with the highest value observed being 5.8 mEq l−1. Hyperkalemia was detected in 7% of all blood draws (19/270) during the observation period, and serum potassium >5.5 mEq l−1 in 2.6% (7/270). A logistic regression analysis was performed to determine which factors might lead to hyperkalemia. Higher baseline DBP was the only significant predictor of hyperkalemia (P<0.0001).
Figure 2.
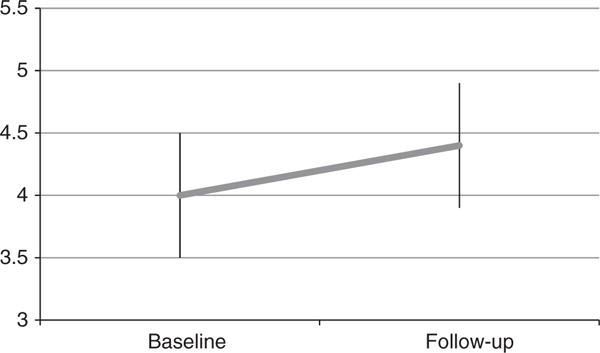
Changes in serum potassium (mEq l−1) after a median of 312 days of follow-up.
Effects of MRA add-on therapy on serum creatinine and Egfr
Serum creatinine increased from 1.5±0.3 to 1.8±0.5 mg dl−1 (P = 0.0004) and eGFR decreased from 48.6±8.7 to 41.2 ±11.5 ml min−1 per 1.73 m2 (P = 0.0002). Eleven patients (31%) had a serum creatinine increase of >30%. A logistic regression analysis was performed and no factors were found to be associated with a serum creatinine increase of >30% from baseline or with a decrease in eGFR.
Tolerability of MRAs
One patient developed acute renal failure that completely resolved with discontinuation of spironolactone. His creatinine increased from 1.1 to 2.1 mg dl−1 on spironolactone 25 mg daily, with an increase in serum potassium from 3.0 to 5.3 mEq l−1. Of note, this was associated with a decrease in SBP and DBP by 48 and 46 mm Hg, respectively. One patient developed symptomatic hypotension that resolved with discontinuation of spironolactone 12.5 mg daily. Three patients developed significant hyperkalemia. One of them had spironolactone 12.5 mg daily discontinued because of a serum potassium level of 5.7 mEq l−1, and another had spironolactone reduced from 25 to 12.5 mg daily because of a serum potassium level of 5.8 mEq l−1. Two patients developed breast tenderness that resolved when switched to eplerenone. No cases of gynecomastia or impotence were observed.
Discussion
This study shows that the long-term addition of a low-dose of MRAs to pre-existing antihypertensive regimens that include a RAAS blocker and a diuretic significantly decreased SBP and DBP and had few adverse effects in patients with resistant hypertension associated with stage 3 CKD. These results are novel in that prior evaluations of MRA use in CKD have mainly focused on proteinuria reduction as a way to preserve kidney function and have generally included patients with early stages of CKD and adequately controlled hypertension.12–15
The decrease in blood pressure achieved by MRAs in our study population was significant, and 44% of the patients discontinued at least one antihypertensive medication during the follow-up once a MRA was added. This might be explained by inadequate suppression of aldosterone secretion despite patients already being on a RAAS inhibitor.16 Of note, the patients with higher blood pressure at baseline were the ones who benefited the most from the MRA addition, as is typical of other antihypertensives.17 We also found that the reduction in SBP induced by MRAs was greater in younger subjects than in elders. This finding might be secondary to more diffuse vascular disease in the elderly, making them less responsive to the effects of MRAs.
The increase in serum potassium associated with addition of MRAs was statistically significant but clinically modest. The incidence of hyperkalemia was low in our study (7%) despite the concurrent use of an ACE-I or ARB in the setting of moderately advanced CKD, and for some patients, diabetes mellitus. This might be related to the concomitant use of an appropriately dosed diuretic, and to the fact that none of the patients had baseline serum potassium >4.7 mEq l−1. The baseline DBP was the only significant predictor of hyperkalemia. This finding has not been described previously.18 The physiological explanation for such relation is not obvious and will need to be confirmed in additional studies involving larger populations.
The observed increases in serum creatinine and reductions in eGFR were significant, with 31% of the patients experiencing >30% increase in serum creatinine after being started on MRAs. Whether this might be beneficial or detrimental over the long term cannot be established because of the lack of a control group and the retrospective nature of this analysis. A fall in eGFR 4 weeks after addition of a MRA to ACE-I and/or ARB-treated patients with CKD was described by Bianchi et al.13 The eGFR in these patients remained then stable whereas the eGFR of the control patients continued to deteriorate slowly over time. At the end of the 1-year treatment period, the percent decline in eGFR was lower in the MRA-treated group than in the controls, suggesting a possible reno-protective effect of MRAs despite the initial drop in eGFR.13
Increases in serum creatinine have been commonly described in subjects with CKD after being started on a RAAS inhibitor, particularly when their blood pressures were reduced.19 However, the percentage of patients experiencing an increased serum creatinine following MRA administration in our study was higher than that observed in other studies utilizing MRAs. For example, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm study, 7% of the patients receiving spironolactone had an increase in serum creatinine >1.7 mg dl−1.8 This might be related to MRA-induced reductions in blood pressure and associated decreases in intraglomerular pressure in patients with an impairment of renal autoregulation because of CKD. Alternatively, the reduction in eGFR may have occurred secondary to a direct renal hemodynamic effect of aldosterone blockade and/or slight volume depletion because of the combined use of a thiazide or loop diuretic and a MRA.20,21 We cannot rule out that the cause of the observed eGFR decline might be independent of the use of MRA and instead be related to the progression of CKD.
We presume that most of our patients have hypertensive renal disease based on the long-term history of resistant hypertension and the low level of urinary protein excretion in the 10 patients for whom 24-h urine collection was available (average proteinuria 433 mg every 24 h; median 566 mg every 24 h; range 0–2144 mg every 24 h). Biopsy data are not available.
In the African American Study of Kidney Disease and Hypertension, which involved patients with characteristics similar to the current study cohort (patients with hypertensive renal disease), the mean decline in GFR was faster in the first 3 months of intensive blood pressure control treatment but overall was less than that observed in our study (~ 2 ml min−1 per 1.73 m2 per year).22 The absence of a control group and of precise quantification of proteinuria in the entire patient cohort precludes the current analysis for addressing this question.
To limit the risk of decreases in GFR, we suggest patients to be started on a low dose of MRAs, for example, spironolactone 12.5 daily or eplerenone 25 mg daily, advised to avoid dehydration, warned against the use of non-steroidal anti-inflammatory medications, and have their blood pressure, serum creatinine and potassium closely monitored. The dose of the MRAs can then be titrated according to the achieved blood pressure response and their effect on serum creatinine and potassium. We do not recommend exceeding doses of 50 mg day−1 for spironolactone or 100 mg day−1 for eplerenone based on our experience of minimal additive antihypertensive efficacy beyond these dosages in patients with resistant hypertension.
Our results confirm the short-term antihypertensive effects of spironolactone as add-on therapy in patients with resistant hypertension associated with stage 2 and 3 CKD as recently reported by Khosla et al.23 Our results extend those of Khosla et al. in that we report sustained antihypertensive benefit and tolerability during long-term follow-up (median 312 vs 45 days), in patients with more advanced CKD (mean eGFR 48.6±8.7 vs 56.5±16.2 ml min−1 per 1.73 m2), in a more racially balanced cohort (Caucasians 47% vs 18%) and in both diabetics and non-diabetics (non-diabetics 69% vs 13%).23
Our study is limited by its retrospective design and inclusion of a relatively small number of subjects. Of interest, but lacking with the current study design, would have been the effect of MRA use on albuminuria/proteinuria.
This analysis indicates that the addition of a low-dose MRA to ongoing treatment with an ACE-I or ARB and a thiazide or loop diuretic is an effective antihypertensive treatment strategy in patients with resistant hypertension associated with stage 3 CKD. However, a significant number of patients experienced increases in serum creatinine concentration (31%) or severe hyperkalemia (8%), indicating a need for close biochemical monitoring. As this study only included patients with stage 3 CKD, similar levels of benefit and tolerability should not be presumed in more advanced (stage 4 and 5) CKD without separate testing. Prospective, randomized studies are needed to determine whether the observed beneficial antihypertensive effects of addition of MRAs to ACE-Is or ARBs are associated with a slower decline of GFR and/or fewer cardiovascular events.
What is known about this topic
Controlling HTN is critical to avoid/slow progression to ESRD and minimize the risk of cardiovascular events in CKD patients.
Target blood pressure control is rarely achieved in CKD patients.
MRAs are effective in the management of resistant hypertension as add-on therapy, but are not widely used in CKD patients mainly because of the risk of hyperkalemia.
What this study adds
MRAs are extremely effective in controlling HTN when used as add-on therapy to a regimen including an ACE-I or ARB and a thiazide/loop diuretic in subjects with resistant hypertension and associated with stage 3 CKD.
Close biochemical monitoring is recommended when MRAs are used as add-on therapy in subjects with resistant hypertension associated with stage 3 CKD because of the risk of hyperkalemia and worsening of renal function.
Acknowledgments
This study was supported by NHLBI RO1-HL79040 (David A Calhoun) and T32 HL007457 (Roberto Pisoni)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G. Urinary protein excretion rate is the best independent predictor of esrf in non-diabetic proteinuric chronic nephropathies. ‘Gruppo italiano di studi epidemiologici in nefrologia’ (gisen) Kidney Int. 1998;53:1209–1216. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of high blood pressure. The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 6.Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005;16:2170–2179. doi: 10.1681/ASN.2004090763. [DOI] [PubMed] [Google Scholar]
- 7.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, et al. Independent and additive impact of blood pressure control and angiotensin ii receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 8.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 9.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 10.Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med. 2001;110:438–441. doi: 10.1016/s0002-9343(01)00642-8. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Tarnow L, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68:2829–2836. doi: 10.1111/j.1523-1755.2005.00756.x. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–2123. doi: 10.1038/sj.ki.5001854. [DOI] [PubMed] [Google Scholar]
- 14.Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ. Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin ii receptor blocker. Clin J Am Soc Nephrol. 2006;1:256–262. doi: 10.2215/CJN.01040905. [DOI] [PubMed] [Google Scholar]
- 15.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ace inhibitors or angiotensin receptor blockers in ckd: a systematic review. Am J Kidney Dis. 2008;51:199–211. doi: 10.1053/j.ajkd.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor GA, Markandu ND, Rotellar C, Smith SJ, Sagnella GA. Different effect of nifedipine in normotensive and hypertensive individuals: a functional anomaly of vascular smooth muscle in essential hypertension? Rev Esp Cardiol. 1983;36:473–477. [PubMed] [Google Scholar]
- 18.Ahuja TS, Freeman D, Jr, Mahnken JD, Agraharkar M, Siddiqui M, Memon A. Predictors of the development of hyperkalemia in patients using angiotensin-converting enzyme inhibitors. Am J Nephrol. 2000;20:268–272. doi: 10.1159/000013599. [DOI] [PubMed] [Google Scholar]
- 19.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 20.Arima S. Aldosterone and the kidney: rapid regulation of renal microcirculation. Steroids. 2006;71:281–285. doi: 10.1016/j.steroids.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Loutzenhiser R, Griffin KA, Bidani AK. Systolic blood pressure as the trigger for the renal myogenic response: protective or autoregulatory? Curr Opin Nephrol Hypertens. 2006;15:41–49. doi: 10.1097/01.mnh.0000199011.41552.de. [DOI] [PubMed] [Google Scholar]
- 22.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. African American Study of Kidney Disease and Hypertension Study Group: effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 23.Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am J Nephrol. 2009;30:418–424. doi: 10.1159/000237742. [DOI] [PubMed] [Google Scholar]


