Abstract
Objective
Resistant hypertension (RHTN), blood pressure (BP) at least 140/90 mmHg despite using at least three different medications, including a diuretic, is associated with high dietary sodium and hyperaldosteronism. Mineralocorticoid receptor antagonists are recommended for treatment of RHTN, however, BP response to these agents varies widely. In the current analysis, we assessed predictors of BP response to spironolactone in patients with RHTN.
Methods
We retrospectively evaluated the BP response to adding spironolactone 12.5–25 mg to existing medications. A favorable BP response was defined as a reduction in SBP of at least 10 mmHg. Tested variables included baseline characteristics and biochemical parameters.
Results
A total of 79 patients with RHTN were included in the analysis. Evaluated patients were more likely women (53.2%) and African-American (55.8%); were generally obese (76%) and were prescribed an average of four antihypertensive medications. Baseline SBP was 153.6 ± 22.3 mmHg; addition of spironolactone resulted in a mean reduction of 15.5 ± 20.7 mmHg. Patients with high urinary sodium excretion (≥ 200mEq/24h) had a significantly greater BP reduction compared with patients with normal excretion (< 200mEq/24h) (P = 0.008). Multivariable analysis identified 24 h urinary sodium excretion as a significant predictor of BP response (P = 0.021) after controlling for potential confounders, including primary aldosteronism.
Conclusion
The antihypertensive effect of spironolactone is positively related to urinary sodium excretion regardless of aldosterone status. These findings suggest that mineralocorticoid receptor antagonists may be of preferential benefit in counteracting the BP effects of high dietary sodium.
Keywords: primary aldosteronism, resistant hypertension, spironolactone, urinary sodium
INTRODUCTION
Resistant hypertension (RHTN), defined as blood pressure (BP) at least 140/90mmHg despite using at least three medications, including a diuretic, is a common clinical problem [1–2]. Crosssectional studies have shown that 10–15% of the hypertensive population have RHTN [3]. Despite lifestyle modifications and use of combination therapies, 30–50% of patients in hypertension specialty clinics do not achieve BP goal [4].
High dietary sodium intake and hyperaldosteronism are commonly associated with RHTN [5]. In addition, miner-alocorticoid receptor antagonists (MRAs) are widely recognized as an effective add-on therapy for patients with poorly controlled RHTN, including those with normal aldosterone levels [1–6].
As with other classes of antihypertensive agents, the BP response to MRAs varies widely, in patients with RHTN [6]. Identification of predictors of BP response with MRA use would serve to maximize and potentially broaden their antihypertensive benefit. The purpose of this study was to identify patient characteristics, hemodynamic parameters, and biochemical variables that predict a favorable BP response to adding spironolactone to existing multidrug regimens in patients with uncontrolled RHTN.
METHODS
The study is a retrospective analysis of consecutive patients referred to the University of Alabama at Birmingham (UAB) Hypertension Clinic for evaluation and treatment of apparent RHTN between 2012 and 2014.
Study population
The analysis included 79 patients with apparent RHTN defined as having clinic BP at least 140/90 mmHg despite use of at least three drug regimen, including a diuretic. The majority of patients were on a four drug treatment regimen comprised of an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker, a long-acting dihydropyridine-type calcium channel blocker (amlodipine), a diuretic (chlorthalidone 25 mg), and a β blocker (carvedilol). A total of 33 patients were receiving clonidine or hydralazine as a fourth drug. As part of our routine care, all patients were advised to ingest a low-sodium/high-fiber diet [1].
Baseline parameters
All baseline assessments were done prior to administration of spironolactone. Assessed variables included demographics (age, sex, height, weight, BMI, obesity defined as BMI ≥ 30kg/m2 [7]), medical history (duration of hypertension, comorbidities, number and class of medications), and biochemical analysis [serum potassium, creatinine, estimated glomerular filtration rate (GFR, ml/min per 1.73 m2) [8], serum aldosterone concentration (SAC, ng/dl), plasma renin activity (PRA, ng/ml per h), aldosterone-to-renin ratio (ARR)], and 24 h urinary excretion of aldosterone (U-Aldo, ug/24h), sodium (U-Na+, mEq/24h), potassium (U-K+, mEq/24h), cortisol (U-C, ug/24h), and creatinine (U-Cr, mg/24h).
Blood pressure management algorithm
The BP response was determined after adding spironolactone 12.5 or 25 mg once daily as a fourth or fifth drug to the above three to four-drug regimen in all patients. The follow-up assessment was 4–6 weeks after adding spironolactone in all patients. Follow-up assessment, as per standard of care at the UAB hypertension clinic, includes BP measurement, serum electrolytes, and assessing for breast tenderness and/or gynecomastia.
Exclusion criteria
Exclusion criteria for this analysis included: receiving an MRA upon referral, suspicion of being nonadherent with prescribed medications, failure to report for follow-up within the 4–6 week time period after starting spironolactone, reporting adverse effects with spironolactone necessitating withdrawal of the medication, addition of eplerenone or other new medications along with spironolactone or dosage adjustments of existing were excluded from analysis, and inadequate urine collection (based on urine creatinine [9]).
Blood pressure assessment
BP in the clinic was measured using the auscultatory method with Welch Allyn Tycos 509 Wall and Mobile Aneroid Sphygmomanometers by a trained physician according to published guidelines [10]. At the first clinic visit, BP was taken in both arms with the higher BP reading was used in future visits. Patients were seated for 5 min prior to measurement, with feet on the floor, arm supported at heart level and with use of a correctly sized cuff (encircling at least 80% of arm circumference). Three BP readings were taken at 2 min intervals and with the second and third readings being averaged to obtain the clinic BP.
Secondary causes of RHTN, such as renovascular disease, obstructive sleep apnea, pheochromocytoma, or Cushing’s syndrome [6] were assessed by biochemical evaluation, imaging, or a sleep study as clinically indicated.
Outcome
Patients were considered to have a favorable BP response to spironolactone if they had at least 10 mmHg reduction in SBP on their follow-up clinic visit. Primary aldosteronism was defined as PRA less than 1 ng/ml per h and U-Aldo more than 12ug/24h. For purpose of analysis, U-Na+ was dichotomized at 200 mEq/24 h, the recommended upper limit of normal for our hospital laboratory.
The study was approved by the UAB Institutional Review Board and conducted according to institutional guidelines.
Statistical analysis
Demographics and clinical characteristics were summarized using descriptive statistics (mean ± SD or percentage). Student’s t-test and χ2 test (or Fisher’s exact test) were used to test for associations between demographics/clinical characteristics and favorable BP response to spironolactone. Specifically the SBP reduction was compared between patients with U-Na+ at least 200 mEq and U-Na+ less than 200 mEq/24 h. Variables that did not meet the normality assumption were tested using a nonparametric method. To identify variables that were predictive of favorable BP response to spironolactone, while adjusting for potential confounders, a multivariable logistic regression was performed. To avoid overfitting the model, only variables with P less than 0.20 [11] in the bivariate analysis were selected for inclusion into the final model. Multicollinearity was assessed using Spearman correlation matrix and variance inflation factor [12]. The inclusion criteria for the multivariable model were based on clinical importance, correlation ρ less than 0.40 and variance inflation factor less than 5 [13–15]. The goodness-of-fit of this model was verified by c statistic. A P value of less than 0.05 was considered statistically significant in two-tailed statistical tests. All analyses were conducted using SAS 9.4 software (SAS Institute, Cary, North Carolina, USA).
RESULTS
Study participants
In total, 79 consecutive patients with apparent RHTN referred to the UAB Hypertension Clinic were included the analysis. Tables 1–3 show baseline characteristics, comorbidities, and baseline laboratory measures, respectively of all analyzed patients and compares those patients with a favorable BP response (yes) vs. patients with an unfavorable BP (no) response to spironolactone.
TABLE 1.
Baseline characteristics and the results of bivariate analysis for favorable SBP reduction (≥ 10 mmHg) of patients treated with spironolactone
| Parameters | Favorable SBP Reduction | |||
|---|---|---|---|---|
| All (n = 79) | Yes (n = 54) | No (n = 25) | P value | |
| Age (years), mean ± SD | 56.2 ± 12.3 | 57.7 ± 11.6 | 53.0 ± 13.5 | 0.12 |
| Women, n (%) | 42.0 (53.2%) | 30.0 (55.6%) | 7.0 (28.0%) | 0.02 |
| African-Americans, n (%) | 43.0 (55.8%) | 26.0 (50.0%) | 17.0 (68.0%) | 0.14 |
| BMI (kg/m2), mean ± SD | 34.5 (7.0%) | 34.8 (7.2%) | 33.9 (6.9%) | 0.60 |
| Obesity (BMI > 30Kg/m2), n (%) | 57.0 (76.0%) | 40.0 (76.9%) | 17.0 (73.9%) | 0.78 |
| SBP (mmHg), mean ± SD | 155.0 ± 23.5 | 157.7 ± 23.6 | 149.0 ± 22.5 | 0.13 |
| DBP (mmHg), mean ± SD | 85.2 ± 14.9 | 84.2 ± 14.3 | 87.4 ± 16.3 | 0.38 |
| Year(s) of hypertension, mean ± SD | 17.4 ± 10.7 | 17.7 ± 10.1 | 16.6 ± 12.0 | 0.68 |
| Number of antihypertensive medications, mean ± SD | 4.1 ± 1.0 | 4.1 ± 1.1 | 3.9 ± 1.0 | 0.50 |
| SBP at follow-up (mmHg), mean ± SD | 139.5 ± 24.9 | 132.7 ± 21.1 | 154.1 ± 26.2 | NA |
| Mean SBP reduction at Follow-up (mmHg), mean ± SD | 15.5 ± 20.7 | −25.1 ± 15.8 | 5.08 ± 14.1 | NA |
NA, not assessed.
TABLE 3.
Baseline laboratory measures and the results of bivariate analysis for favorable SBP reduction (≥10mmHg) of patients treated with spironolactone
| Laboratory measures | Favorable SBP reduction | |||
|---|---|---|---|---|
| All (n = 79) | Yes (n = 54) | No (n = 25) | P value | |
| Serum creatinine (mg/dl), mean ± SD | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.79 |
| Serum potassium (mEq/24h), mean ± SD | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.4 | 0.38 |
| Glomerular filtration rate (ml/min/1.73m2), mean ± SD | 77.6 ± 21.7 | 75.4 ± 19.4 | 82.3 ± 25.2 | 0.19 |
| Serum aldosterone concentration (ng/dl), mean ± SD | 14.3 ± 10.1 | 15.3 ± 11.0 | 12.2 ± 7.7 | 0.20 |
| Plasma renin activity (ng/ml/h), mean ± SD | 1.7 ± 3.5 | 1.6 ± 3.8 | 1.9 ± 3.0 | 0.73 |
| Aldosterone-to-renin ratio, mean ± SD | 22.3 ± 21.7 | 25.7 ± 24.1 | 15.0 ± 13.1 | 0.016 |
| Urinary aldosterone (μg/24 h), mean ± SD | 17.2 ± 13.0 | 18.2 ± 12.7 | 15.2 ± 13.6 | 0.35 |
| Urinary sodium (mEq/24h), mean ± SD | 200.0 ± 97.4 | 214.7 ± 101.2 | 170.2 ± 81.9 | 0.04 |
| Urinary potassium (mEq/24h), mean ± SD | 71.0 ± 36.5 | 76.5 ± 33.8 | 58.8 ± 40.1 | 0.01 |
| Urinary cortisol (μg/24h), mean ± SD | 80.3 ± 90.3 | 102.4 ± 94.7 | 34.3 ± 59.0 | 0.0003 |
| Urinary creatinine (mg/24h), mean ± SD | 1729.8 ± 646.8 | 1833.7 ± 667.6 | 1505.4 ± 546.5 | 0.04 |
Demographics
Patients were on average 56.2 ± 12.3 years old with slightly more women (53.2%) and African-Americans (55.8%). Patients had a mean baseline SBP of 155 ± 23.5 mmHg.
Comorbidities are shown in Table 2. More than two-thirds of the study participants were obese (mean BMI 34.5 ± 7 kg/m2). The prevalence of diabetes was 32.9 and 30.4% had been diagnosed with obstructive sleep apnea. History of coronary artery disease (5.1%), heart failure (10.1%), prior stroke (2.5%), and atrial fibrillation (6.3%) was uncommon. However, 45.6% had dyslipidemia and 46.2% had primary aldosteronism.
TABLE 2.
Characteristics and the results of bivariate analysis for favorable SBP reduction (≥10mmHg) of patients treated with spironolactone
| Characteristics | Favorable SBP Reduction | |||
|---|---|---|---|---|
| All (n = 79) | Yes (n = 54) | No (n = 25) | P value | |
| Diabetes mellitus, n (%) | 26 (32.9%) | 19 (73.1%) | 7 (28%) | 0.53 |
| Obstructive sleep apnea, n (%) | 24 (30.4%) | 16 (29.6%) | 8 (32%) | 0.83 |
| Coronary artery disease, n (%) | 4 (5.1%) | 3 (5.6%) | 1 (4%) | 1.00 |
| Heart failure, n (%) | 8 (10.1%) | 5 (9.3%) | 3 (12%) | 0.7 |
| Dyslipidemia, n (%) | 36 (45.6%) | 31 (57.4%) | 12 (48%) | 0.43 |
| Atrial fibrillation, n (%) | 5 (6.3%) | 3 (5.6%) | 2 (8%) | 0.65 |
| Stroke, n (%) | 2 (2.5%) | 1 (1.9%) | 1 (4%) | 0.54 |
| Primary aldosteronism | 36 (46.2%) | 30 (56.6%) | 6 (24%) | 0.007 |
Primary aldosteronism: plasma renin activity less than 1ng/ml/h and urinary aldosterone more than 12ug/24 h.
Mean biochemical values are shown in Table 1. Mean serum potassium was 3.7 ± 0.4mEq/dl and the mean GFR of 77.6 ± 21.7 ml/min per 1.73 m2. Almost half (46.2%) of the patients had parameters consistent with biochemical primary aldosteronism (Table 1). The mean U-Na+ was 200 ± 97.4mEq/24h during normal dietary intake.
Effects of spironolactone on blood pressure
After the addition of spironolactone to the treatment regimen, the mean reduction in SBP and DBP at 4–6 weeks follow-up was 15.5 ± 20.7mmHg and 4.5 ± 12.1 mmHg, respectively (Table 1). There was no significant difference in BP response to spironolactone whether patients were on 12.5 (n = 7/79) or 25 mg (n = 72/79) of spironolactone.
Bivariate analysis indicated that sex, primary aldosteronism, ARR, U-Na+, U-K+, U-C, and U-Cr were associated with reduction in SBP (P < 0.05). In particular, a significant difference in SBP reduction was observed between patients with at least 200 and less than 200 mEq/24 h (22.2 vs. 9.9 mmHg, respectively; P = 0.008, Fig. 1). A multivariable analysis confirmed that U-Na+ and baseline SBP were independent predictors of favorable SBP response after adjusting for potential confounding variables, including primary aldosteronism (Table 4). The U-Na+ yielded an odds ratio of 1.27 (P = 0.021), implying that for every 20 meq/24h increase in U-Na+, there is a 27% increase in the likelihood of favorable SBP response.
FIGURE 1.
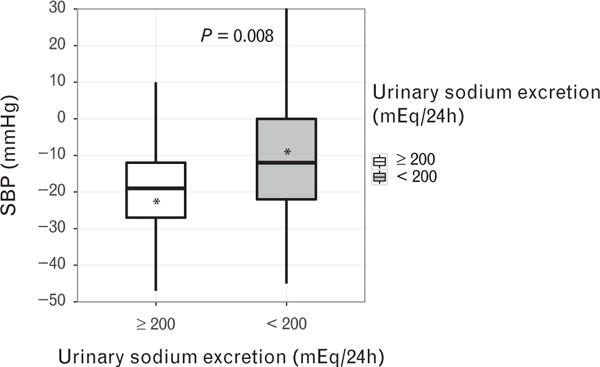
Box plot showing SBP reduction in resistant hypertension patients with urinary sodium excretion at least 200 and less than 200 mEq/24 h. The asterisk denotes the mean; the line inside the box depicts the median; the upper and lower hinges represent 25th and 75th percentile, respectively.
TABLE 4.
Results of multivariable logistic regression for favorable SBP reduction of at least 10mmHg
| Parameters | Favorable SBP reduction
|
||
|---|---|---|---|
| Adjusted odds ratio | 95% confidence interval | P value | |
| Age (increasing years) | 1.08 | (0.99, 1.17) | 0.09 |
|
| |||
| Sex (women vs. men) | 0.23 | (0.04, 1.47) | 0.12 |
|
| |||
| Race (African-American vs. White) | 4.76 | (0.58, 39.1) | 0.15 |
|
| |||
| SBP (mmHg) | 1.06 | (1.02, 1.11) | 0.003 |
|
| |||
| Comorbidities | |||
| Primary aldosteronism (Yes vs. No) | 1.85 | (0.25, 13.6) | 0.55 |
|
| |||
| Laboratory measures | |||
| Glomerular filtration rate (ml/min per 1.73 m2) | 1.01 | (0.98, 1.05) | 0.54 |
| Aldosterone-to-renin ratio | 1.04 | (0.97, 1.12) | 0.26 |
| Urinary sodium (20 mEq/24 h) | 1.27 | (1.04, 1.55) | 0.021 |
| Urinary potassium (mEq/24 h) | 1.01 | (0.98, 1.03) | 0.68 |
| Urinary cortisol (μg/24 h) | 1.02 | (0.99, 1.04) | 0.05 |
DISCUSSION
The current findings indicate that patients with uncontrolled RHTN ingesting a high-sodium diet, as indicated by 24 h urinary sodium excretion, have a significantly greater BP response to spironolactone, irrespective of their aldosterone status, compared with patients ingesting a normal sodium diet. To our knowledge, this is the first study to show that dietary sodium intake mediates the BP response to an MRA independent of aldosterone levels.
The effect of dietary sodium on BP has been evaluated in a large number of observational and clinical trials, including both hypertensive and normotensive individuals, and in a limited number of studies of patients with RHTN. A recent Cochrane review comparing effect of low (< 120 mmol) and high-sodium (≥ 150 mmol) diet on BP showed that in hypertensive whites short-term sodium reduction resulted in a 5.48mmHg reduction in BP. However, in whites with normal BP, a low-sodium diet decreased SBP by 1.27mmHg. In Asians and African-Americans, sodium restriction has resulted in greater BP reduction, but there were fewer studies that included these ethnic and racial groups [16]. A recently updated meta-analysis that included randomized clinical trials with modest reductions in sodium for an average of 4 weeks indicated that in both hypertensive and normotensive patients dietary sodium reduction decreased SBP and DBP irrespective of ethnicity and race. Moreover, a significant correlation between SBP reduction and 24 h urinary sodium levels was found, that is, the greater the reduction in urinary sodium excretion the greater the decrease in SBP [17]. In a prospective, randomized study, Pimenta et al. evaluated the effects of dietary sodium reduction on BP in patients with RHTN. In a crossover evaluation, they compared the effects low (46.1 ± 26.8 mmol/24h) vs. High-sodium diet (252.2 ± 64.6 mmol/24h). The mean office BP was significantly reduced by 22.7 and 9.1 mmHg, respectively, in this difficult to treat cohort [18]. In conclusion, a low-sodium diet causes a significant reduction in SBP especially in RHTN patients and less so in normotensive patients.
To stratify the BP response in relation to dietary sodium intake, a bivariate analysis comparing patients with U-Na+ less than or at least 200mEq/24h was done (Fig. 1). The findings demonstrated that patients with U-Na+ at least 200 mEq/24 h (n = 37) had a greater SBP mean reduction of 22.2 mmHg [95% confidence interval (CI): −29.2, − 15.3 mmHg], whereas those U-Na+ less than 200 mEq/24 h (n = 41) had a mean SBP reduction of 9.8mmHg (95% CI: −15.9, −3.8mmHg) (P= 0.008). Therefore, patients with RHTN ingesting a high-sodium diet (≥ 200 mEq/24 h) were more likely to have a favorable SBP response (≥ 10 mmHg reduction).
A multivariate analysis confirmed that U-Na+ serves as an independent predictor of SBP response to spironolactone after adjusting for confounding variables, that is, the greater the sodium intake the more likely patients with RHTN will have a favorable SBP response to spironolactone (Table 4). Age, sex, and race did not affect the SBP response to spironolactone. Although, aldosterone excess is strongly associated with RHTN [5,18–22] and likely contributes importantly to sodium and fluid retention; the sodium effect on SBP response was independent of primary aldosteronism, SAC, U-Aldo, and ARR. Therefore, independent of aldosterone status, a favorable SBP response to spironolactone in RHTN is observed with greater sodium intake.
Studies of hypertensive populations suggest that serum potassium levels influence the response to spironolactone. Sharabi et al. evaluated patients with essential hypertension on at least two antihypertensive agents and elevated BP (SBP > 140 mmHg) and without hyperaldosteronism. He compared BP reduction when spironolactone was added vs. addition of other antihypertensive agents. The mean reduction in SBP was 23.2 with spironolactone and 7.6 mmHg with other antihypertesives addition, respectively. The BP reduction in patients receiving spironolactone was significantly greater when initial serum potassium was less than 4mmol/l (28 ± 17 mmHg) compared with at least 4mmol/l (14 ± 13mmHg), P = 0.04 [23]. A recent study identified predictors of a satisfactory BP response defined as more than 10% reduction in SBP after beginning spironolactone in 48 patients with RHTN. Serum potassium less than 4.5mmol/l was associated with a satisfactory BP response and every 1 mEq/l lower level in serum potassium was independently associated with a five-fold increased likelihood of a satisfactory response [24]. In our study, the mean baseline serum potassium was 3.7 ± 0.4 mEq/l (mmol/l) and unlike the above studies, serum potassium level was not shown to be a predictor of BP response to spironolactone.
In our analysis, SAC, ARR, nor U-K+ predicted BP response to spironolactone even after multivariable adjustment. Our results were consistent with other studies, including the Addition of Spironolactone in Patients with Resistant Arterial Hypertension trial. This trial included patients who were on at least three antihypertensive agents, including a diuretic, and with a serum aldosterone/PRA ratio (ARR) more than 17 and baseline potassium of 4.1 mmol/l or less had a larger BP reduction in response to spironolactone compared with receiving placebo [25]. In a prospective study performed on patients with true RHTN based on 24 h ambulatory BP monitor, 175 Brazilian patients received spironolactone in doses of 25–100 mg daily. Neither plasma aldosterone or the ARR predicted a better BP response. In that study after a median of 7 months the mean ambulatory BP reductions were 16 and 9 mmHg, respectively in the group receiving 25 and 100 mg of spironolactone (95% CIs: 13–18 and 7–10mmHg; P < 0.001). Office SBP decreased by 14 mmHg as well and controlled BP was reached in 48% of patients. Factors associated with better response were higher waist circumference, lower aortic wave velocity, and lower serum potassium, the latter being in contrast with our results [26]. In another study conducted by Parathasarathy et al. similar observations were made in mild-to-moderate hypertensive patients, that is, ARR did not predict the BP response to spironolactone [27].
Overall, prior studies are consistent in reporting limited value of aldosterone levels or ARR to predict BP response to MRAs, whereas the role of potassium status demonstrates conflicting results. In the current study, we did not observe serum or urinary potassium levels to be predictive of a favorable BP response to spironolactone. The current study, however, investigated the role of dietary sodium for the first time and found that high dietary sodium intake quantified by urinary sodium excretion was an independent predictor of SBP response to spironolactone.
Strengths of the current analysis include evaluation of a diverse, well characterized cohort, including rigorous assessment of aldosterone status by measurement of serum aldosterone, PRA, and 24 h U-Aldo excretion. Study limitations include its retrospective design and lack of ambulatory BP monitoring to exclude patients with white coat RHTN and lack of rigorous assessment of medication adherence.
In conclusion, the antihypertensive benefit of spironolactone is positively related to high dietary sodium intake independent of aldosterone levels. If extrapolated to general hypertensive cohorts, these findings suggest that the use of MRAs may be of preferential benefit in counteracting the effects of high dietary sodium intake [28]. Prospective studies are needed to test this for this hypothesized benefit.
Reviewers’ Summary Evaluations.
Referee 1
The goal of the study was to identify patient characteristics, hemodynamics, and biochemical parameters that predict a favorable blood pressure response to addition of spirono-lactone to existing multi-drug treatment regimen in patients with resistant hypertension. Using multivariable analysis, urinary sodium excretion was identified as a significant predictor of favorable systolic blood pressure response after controlling for potential confounders including primary aldosteronism. These findings suggest that miner-alocorticoid receptor antagonists may be of benefit in counteracting blood pressure effect of high dietary sodium. They warrant further large-scale clinical studies of the impact of aldosterone on increased urinary sodium excretion.
Referee 2
The strengths of this study are the novelty of the observation and the potential practical use of the study. Weaknesses include: the relatively small number of patients included; this is not a prospective interventional study and potential biases cannot be excluded; the inclusion of patients with primary aldosteronism; the non-generalizability of the data to other patient populations.
Acknowledgments
American College of Cardiology, Alabama Chapter Winter Meeting – American Society of Hypertension 2015 Young Investigator-in-Training Abstract Competition: Oral presentation.
This research was supported by the National Institutes of Health (NIH 1R01 HL113004).
Abbreviations
- ARR
aldosterone-to-renin ratio
- BP
blood pressure
- GFR
glomerular filtration rate
- MRAs
mineralocorticoid receptor antagonists
- PRA
plasma renin activity
- RHTN
resistant hypertension
- SAC
serum aldosterone concentration
- U-Aldo
urinary aldosterone
- U-C
urinary cortisol
- U-Cr
urinary creatinine
- U-K+
urinary potassium
- U-Na+
urinary sodium
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation. 2008;117(25):e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control. 2013;6:139–151. doi: 10.2147/IBPC.S33984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdam-Marx, Ye X, Sung JC, Brixner DI, Khaler KH. Results of a retrospective, observational pilot study using electronic medical records to assess the prevalence and characteristics of patients with resistant hypertension in the ambulatory care setting. Clin Ther. 2009;31:1116–1123. doi: 10.1016/j.clinthera.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Garg JP, Elliot WJ, Folker A, et al. Resistant hypertension revisited: a comparison of two university-based cohorts. Am J Hypertens. 2005;18:619–626. doi: 10.1016/j.amjhyper.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Shimosawa T. Salt, the renin-angiotensin-aldosterone system and resistant hypertension. Hypertens Res. 2013;36:657–660. doi: 10.1038/hr.2013.69. [DOI] [PubMed] [Google Scholar]
- 6.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 7.Acelajado MC, Pisoni R, Dudenbostel T, et al. Refractory hypertension: definition, prevalence and patient characteristics. J Clin Hypertens (Greenwich) 2012;14:7–12. doi: 10.1111/j.1751-7176.2011.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Keyzer W, Huybrechts I, Dekkers AL, Geelen A, Crispim S, Hulshof PJ, et al. Predicting urinary creatinine excretion and its usefulness to identify incomplete 24 h urine collections. Br J Nutr. 2012;108:1118–1125. doi: 10.1017/S0007114511006295. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 12.Allison PD. Logistic regression using SAS®: theory and application. Second. Cary, North Carolina: SAS Institute Inc; 2012. [Google Scholar]
- 13.Farrahi J, Nakhaee N, Sheibani V, et al. Psychometric properties of the Persian version of the Pittsburgh Sleep Quality Index addendum for PTSD (PSQI-A) Sleep Breath. 2009;13:259–262. doi: 10.1007/s11325-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 14.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. New York: McGraw–Hill/Irwin; 2004. [Google Scholar]
- 15.O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- 16.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD004022.pub3. ArtNo: CD004022. [DOI] [PubMed] [Google Scholar]
- 17.He FJ, Li J, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD004937.pub2. Art No: CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S, Calhoun DA. Resistant hypertension characterized by increased aldosterone levels and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159–1164. doi: 10.1001/archinte.168.11.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79:1585–1592. doi: 10.1016/j.lfs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Blaustein MP, Zhang L, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol. 2006;290:R514–R523. doi: 10.1152/ajpregu.00819.2005. [DOI] [PubMed] [Google Scholar]
- 22.Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–247. doi: 10.1146/annurev-med-042711-135929. [DOI] [PubMed] [Google Scholar]
- 23.Sharabi Y, Adler E, Shamis A, et al. Efficacy of add-on aldosterone receptor in uncontrolled hypertension. Am J Hypertens. 2006;19:750–755. doi: 10.1016/j.amjhyper.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Shomai G, Sella T, Sharabi Y, et al. Serum potassium levels predict blood pressure response to aldosterone antagonists in resistant hypertension. Hypertens Res. 2014;37:1037–1041. doi: 10.1038/hr.2014.77. [DOI] [PubMed] [Google Scholar]
- 25.Vaclavik J, Sedlak R, Jarkovsky J, et al. Effect of spironolactone in resistant arterial hypertension A Randomized, Double-Blind, Placebo-Controlled Trial (ASPIRANT-EXT) Medicine. 2014;93:e162. doi: 10.1097/MD.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988. [DOI] [PubMed] [Google Scholar]
- 27.Parathasarathy HK, Alhashmi K, McMahon AD, et al. Does the ratio of serum aldosterone to plasma renin activity predict the efficacy of diuretics in hypertension? Results of RENALDO. J Hypertens. 2010;28:170–177. doi: 10.1097/HJH.0b013e328332b79b. [DOI] [PubMed] [Google Scholar]
- 28.Nishizaka MK, Pratt-Ubunama M, Zaman MA, et al. Validity of plasm aldosterone-to-renin ratio activity in African American and white subjects with resistant hypertension. Am J Hypertens. 2005;18:805–812. doi: 10.1016/j.amjhyper.2005.01.002. [DOI] [PubMed] [Google Scholar]


