Abstract
Accumulating mental health research encourages a shift in focus towards transdiagnostic dimensional features that are shared across categorical disorders. In support of this shift, recent studies have identified a general liability factor for psychopathology – sometimes called the ‘p factor’ – that underlies shared risk for a wide range of mental disorders. Identifying neural correlates of this general liability would substantiate its importance in characterizing the shared origins of mental disorders and help us begin to understand the mechanisms through which the ‘p factor’ contributes to risk. Here we first replicate the ‘p factor’ using cross-sectional data from a volunteer sample of 1,246 university students, and then, using high-resolution multimodal structural neuroimaging, demonstrate that individuals with higher ‘p factor’ scores show reduced structural integrity of white matter pathways, as indexed by lower fractional anisotropy values, uniquely within the pons. Whole-brain analyses further revealed that higher ‘p factor’ scores are associated with reduced gray matter volume in the occipital lobe and left cerebellar lobule VIIb, which is functionally connected with prefrontal regions supporting cognitive control. Consistent with the preponderance of cerebellar afferents within the pons, we observed a significant positive correlation between the white matter integrity of the pons and cerebellar gray matter volume associated with higher ‘p factor’ scores. The results of our analyses provide initial evidence that structural alterations in cortico-cerebellar circuitry supporting core functions related to the basic integration, coordination, and monitoring of information may contribute to a general liability for common mental disorders.
Introduction
Comorbidity — the co-occurrence of multiple health conditions — is one of the most vexing clinical and etiologic challenges in mental-health practice and research. Comorbidity rates are very high among mental disorders with approximately half of individuals who meet diagnostic criteria for one disorder meeting diagnostic criteria for a second disorder at the same time (e.g., 1). Clinically, comorbidity is associated with greater severity of impairment and complexity in treatment planning, compliance, and coordination of services (2). Comorbidity also is an etiologic challenge because it makes it very difficult to find causes, biomarkers, and treatments with specificity to individual disorders. For example, behavioral genetics studies show that many different disorders share a common genetic etiology (3,4,5,6), and treatment studies show that targeting a specific brain chemistry or cognitive process is often as effective in treating one disorder as another (7,8).
The high rates of comorbidity observed among mental disorders suggest that there may be a more parsimonious structure to psychopathology than implied by current psychiatric nosologies, such as DSM-5 or ICD-10, which identify as many as 541 separate and distinct disorders. In fact, research on the structure of mental disorders and comorbidity indicates that many different disorders may be manifestations of a smaller number of transdiagnostic latent factors (9,10,11). Factor-analytic studies of multiple symptoms and diagnoses suggest that the structure of mental disorders can be summarized by three such factors: an “internalizing” liability to depression and anxiety disorders, an “externalizing” liability to antisocial and substance-use disorders, and a “thought disorder” liability to schizophrenia, bipolar disorder, and obsessive-compulsive disorder. These observations have led some to argue that studying the mechanisms underlying transdiagnostic latent factors should be the focus of etiological research and applied practice (e.g., 12).
The empirical observation that even these three transdiagnostic latent factors are positively correlated (13) has given rise to a more radical hypothesis, which is that people may differ from each other in a generalized propensity to experience all forms of common mental disorders (14). The work that first confirmed this hypothesis reported a single factor in psychopathology data (14), which has been subsequently replicated and labeled the ‘p factor’ because it is thought to parallel the ‘g factor’ that emerges in studies of the latent structure of cognitive abilities (15). Although cognitive abilities are dissociable into separate components, such as verbal skills, visuospatial skills, working memory, or processing speed, the ‘g factor’ summarizes the observation that individuals who do well on one type of cognitive test tend to do well on all other types of cognitive tests (16,17,18). Thus, the ‘g factor’ accounts for the positive correlation among all cognitive test scores, suggesting that there may be a common etiology that influences or contributes in some way to all cognitive abilities. Analogously, the ‘p factor’ suggests that there may be a general factor of psychopathology that accounts for the positive correlation (or comorbidity) among psychiatric symptoms (and disorders). As the ‘g’ dimension reflects low-to-high mental ability, the ‘p’ dimension represents low-to-high liability to develop any common mental disorder. Multiple studies in different parts of the world, in different age groups, using different assessment instruments, have now replicated this ‘p factor’ (e.g., 19,20,21,22,23,24,25).
Although the possibility of a general risk factor for mental disorders that cuts across diagnostic categories is in keeping with new transdiagnostic perspectives on psychopathology (26,27), it is not clear how to interpret the meaning of such a general liability. Some commentators have wondered whether such a general factor is real and meaningful or merely factor-analytic foolery (28). Others have suggested that a general factor of psychopathology may be a measurement artifact reflecting nothing more than a biased response style in which people systematically endorse (or deny) all symptoms (22). As has been true in studies of the ‘g factor’ (29), examining neural and genetic correlates of the ‘p factor’ may help to clarify the meaning of a general liability for common mental disorders. For example, if the ‘p factor’ is just a measurement artifact then a meaningful neural correlate is unlikely to exist. In contrast, the presence of neural correlates not only would suggest that the ‘p factor’ is measuring meaningful variance, but also would point to possible mechanisms underlying a general liability for mental disorders.
In the current study, we use multimodal neuroimaging to study the structural neural correlates of the ‘p factor’. We opted to focus on brain structure rather than function because it allows us to explore the neural correlates of general psychopathology without having to test specific hypotheses about regions of interest, which functional neuroimaging analyses typically require. We conducted these analyses in the following step-wise fashion. First, we estimated ‘p factor’ scores in a large sample of young adult university students. Second, having replicated the ‘p factor’ in our study sample, we conducted exploratory whole-brain analyses of the structural integrity of white matter pathways and regional gray matter volume correlates of ‘p factor’ scores identified from diffusion tensor and high-resolution structural imaging data, respectively. Third, we examined relationships between white matter and gray matter correlates of the ‘p factor’ emerging from these exploratory analyses in an effort to better understand the possible neural mechanisms for a general liability for common mental disorders.
Materials and Methods
Participants
Data were available from 1,246 undergraduate students (727 women; mean age: 19.69 +/− 1.26; 72.4% of participants’ parents ≥ Bachelor’s degree) who had successfully completed the ongoing Duke Neurogenetics Study. All participants provided informed consent in accordance with Duke University Medical Center Institutional Review Board guidelines prior to participation. All participants were in good general health and free of the following study conditions: 1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
Measurement of Psychiatric Symptoms
The electronic Mini International Neuropsychiatric Interview (30) and multiple self-report mental health questionnaires were used to assess: 1) internalizing symptoms of depression, generalized anxiety, and fears/phobias; 2) externalizing symptoms of antisocial personality/psychopathy, delinquency, and alcohol, cannabis, and other drug abuse/dependence; and 3) thought disorder symptoms of obsessive-compulsive behavior, mania, and psychosis (see Supplemental Information).
MRI Acquisition and Preprocessing
High-resolution diffusion tensor and structural MRI data were acquired using an eight-channel head coil for parallel imaging on one of two identical research-dedicated GE MR750 3T scanners at the Duke-UNC Brain Imaging and Analysis Center. Diffusion tensor imaging was used to assess regional fractional anisotropy (FA) correlates as a metric of the structural integrity of white matter pathways and optimized voxel-based morphometry was used to assess regional gray matter volume (GMV) correlates of ‘p factor’ scores. Out of the 1,246 participants included in the study, diffusion tensor imaging analyses were available for 951 participants and voxel-based morphometry analyses were available for 1200 participants with overlapping structural MRI and clinical symptom data surviving our stringent, multilevel quality control procedures (see Supplemental Information).
Results
Replication of the ‘p factor’
We measured a variety of psychiatric symptoms in our sample, and as in previous studies, observed that correlations between different psychiatric symptoms were positive, with a few exceptions, indicating that individuals who experienced one set of symptoms (e.g., depression) also were likely to experience other sets of symptoms (e.g., anxiety, alcohol abuse, etc.; Supplemental Table 1). We used the program MPlus (31) to compare different structural models of the psychiatric symptoms in our data. Our results indicated that a bi-factor model that includes one general factor accounting for shared variance common to all symptoms and two specific factors accounting for shared variance unique to internalizing and externalizing symptoms fit the data well. The general factor replicated the presence of a shared liability for common mental illness, which we also label the ‘p factor’ in our data (Supplemental Table 2). This alone is important as it further suggests the ‘p factor’ is relatively robust to different samples and measures of psychiatric symptoms. Subsequently, ‘p factor’ scores were extracted using the standard regression method for structural neuroimaging analyses. We standardized the ‘p factor’ to a mean of 100 (SD=15), with higher scores indicating a greater propensity to experience all forms of psychiatric symptoms (sample range=75–205).
Exploratory analyses of structural neural correlates of the ‘p factor’
To identify structural neural correlates of the ‘p factor,’ we conducted exploratory whole-brain analyses of white matter integrity and GMV using voxel-based estimates of FA and optimized voxel-based morphometry, respectively. Specifically, we conducted linear regressions with ‘p factor’ scores predicting differences in FA and GMV controlling for age, sex, and average whole-brain FA values for the diffusion tensor imaging and total intracranial volume for the voxel-based morphometry analyses. All analyses were conducted using Monte Carlo simulation-derived whole-brain corrected thresholds with an overall family-wise error rate of α<.05. Furthermore, we verified that resulting associations were not confounded by parental socioeconomic status or unduly affected by extreme ‘p factor’ scores by controlling for parental education level (Supplemental Information) and conducting sensitivity analyses wherein outlier values (defined as values > ±3 standard deviations from the mean) were winsorized before testing (32).
Whole-brain analyses of FA revealed that individuals with higher ‘p factor’ scores had significantly decreased white matter integrity exclusively within the right and left pons as indexed by lower FA values (Figure 1A, Table 1). Whole-brain analyses of GMV revealed that individuals with higher ‘p factor’ scores had significantly less volume within the right and left lingual gyrus and right intracalcarine cortex of the occipital lobes (Figure 1B) as well as the left posterior cerebellum (Figure 1C) (Table 1). There were no significant positive associations between ‘p factor’ scores and FA or GMV. Interestingly, white matter pathways encompassing afferent connections between the cerebrum and cerebellum pass through the pons (33). Thus, the decreased FA observed within the pons among participants with higher ‘p factor’ scores suggests that individuals with higher liability exhibit decreased structural integrity and, possibly, impaired functional communication between structures within the cerebrum and the cerebellum. In addition, Internalizing, Externalizing, and Thought Disorder factors from the correlated factor model showed similar associations with pons FA and cerebellar and occipital GMV as did the ‘p factor’ (Supplemental Table 5). In general, this pattern of associations is consistent with the hypothesis that ‘p factor’ scores reflect what is shared across liabilities to Internalizing, Externalizing, and Thought Disorder symptoms.
Figure 1.
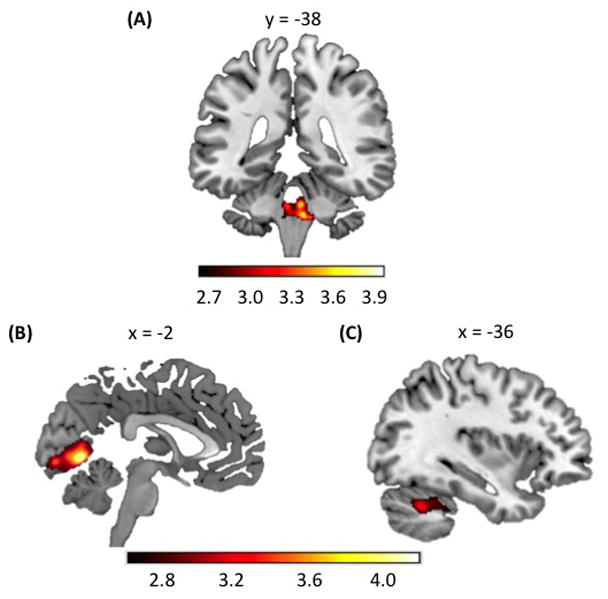
Statistical parametric maps from whole-brain exploratory analyses are shown to illustrate voxels exhibiting a significant negative correlation with ‘p factor’ scores controlling for age, sex, and average whole-brain FA and intracranial volume for the diffusion tensor and voxel-based morphometry analyses, respectively. (A) Diffusion tensor imaging analyses show poorer FA in the bilateral pons. Voxel-based morphometry analyses show GMV deficits in (B) bilateral occipital lobe and (C) left posterior cerebellum. Colorbars reflect t scores.
Table 1.
Differences in Fractional Anisotropy and Gray Matter Volume Associated with ‘p Factor’ Scores.
| Cluster Size (k) | Peak Region | MNI Coordinates | T score | R2 (p Factor) | ||
|---|---|---|---|---|---|---|
| x | y | Z | ||||
| Diffusion Tensor Imaging Analysis | ||||||
|
| ||||||
| 272 | Right pons | 12 | −39 | −42 | 3.96 | 0.016 |
| Right pons | 5 | −37 | −33 | 3.62 | 0.014 | |
| Left pons | −5 | −37 | −35 | 3.30 | 0.011 | |
|
| ||||||
| Voxel-Based Morphometry Analysis | ||||||
|
| ||||||
| 2,353 | Left lingual gyrus | −2 | −69 | −2 | 4.28 | 0.015 |
| Right intracalcarine cortex | 11 | −74 | 17 | 4.06 | 0.014 | |
| Left lingual gyrus | −2 | −89 | −5 | 3.46 | 0.010 | |
| 710 | Left posterior cerebellum | −36 | −57 | −33 | 3.26 | 0.009 |
| Left posterior cerebellum | −41 | −41 | −35 | 3.15 | 0.008 | |
Associations between white matter integrity and gray matter volume
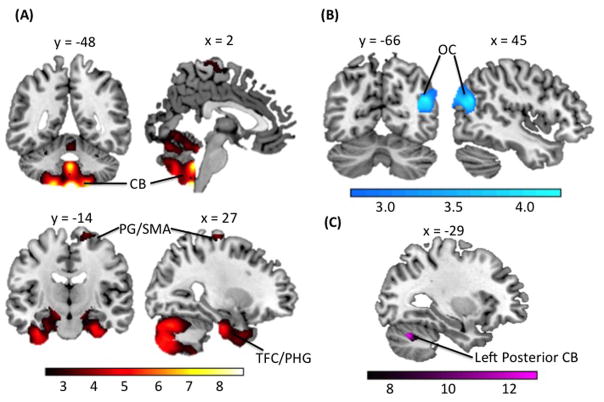
Given that the pons acts as a structural bridge between the cerebrum and cerebellum (33), we hypothesized that pons FA would be associated with cerebellar GMV. To test this hypothesis, we used linear regression to examine whether differences in FA of white matter tracts in the pons predict differences in cerebellar GMV, effectively linking the two structural neuroimaging modalities. As such, we extracted FA cluster-wise values from the bilateral pons found to be associated with ‘p factor’ scores to predict differences in GMV using whole-brain voxel-based morphometry (N = 951) controlling for age, sex, average whole-brain FA, and total intracranial volume. We found that pons FA was associated with increased GMV in large portions of the bilateral cerebellum and fusiform cortex, as well as right precentral gyrus/supplementary motor area (Figure 2A, Table 2). Pons FA also was associated with reduced GMV in the right occipital cortex (Figure 2B, Table 2). Importantly, we identified a 203-voxel cluster in left posterior cerebellum (x=−29, y=−66, z=−30; T=5.44; R2=0.03) wherein GMV correlated with both pons FA and ‘p factor’ scores (Figure 2C).
Figure 2.
Statistical parametric maps of differences in GMV associated with pons FA. (A) shows increases in GMV associated with pons FA in the bilateral cerebellum (CB), temporal fusiform cortex (TFC)/parahippocampal gyrus (PHG), and precentral gyrus (PG)/supplementary motor area (SMA). (B) shows decreases in GMV associated with pons FA in the occipital cortex. (C) depicts the 203-voxel overlapping gray matter cluster in the left posterior cerebellum (in violet) found to be associated both with ‘p factor’ scores and pons FA. Colorbars reflect t scores.
Table 2.
Differences in Gray Matter Volume Associated with Pons Fractional Anisotropy.
| Cluster Size (k) | Peak Region | MNI Coordinates | T score | R2 (Pons FA) | ||
|---|---|---|---|---|---|---|
| X | y | z | ||||
| Increased Gray Matter Volume | ||||||
|
| ||||||
| 30,591 | Right brainstem | 2 | −48 | −62 | 8.40 | 0.069 |
| Vermis X | 0 | −47 | −41 | 7.61 | 0.058 | |
| Right lobule VIIIb | 20 | −51 | −63 | 7.52 | 0.056 | |
| 5,983 | Right temporal fusiform cortex (posterior division) | 39 | −23 | −33 | 5.31 | 0.029 |
| Right parahippocampal gyrus (anterior division) | 27 | −14 | −30 | 5.22 | 0.028 | |
| Right temporal fusiform cortex (posterior division) | 18 | −5 | −41 | 4.37 | 0.020 | |
| 6,241 | Left temporal fusiform cortex (posterior division) | −35 | −15 | −36 | 5.31 | 0.029 |
| Left temporal fusiform cortex (anterior division) | −35 | −3 | −47 | 5.30 | 0.029 | |
| Left temporal pole | −30 | 8 | −38 | 5.15 | 0.027 | |
| 842 | Right precentral gyrus | 29 | −20 | 72 | 3.81 | 0.015 |
| Right precentral gyrus/supplementary motor area | 11 | −18 | 68 | 3.74 | 0.015 | |
| Right precentral gyrus/superior frontal gyrus | 18 | −14 | 72 | 3.63 | 0.014 | |
|
| ||||||
| Decreased Gray Matter Volume | ||||||
|
| ||||||
| 2,351 | Right lateral occipital cortex | 45 | −66 | 24 | 4.42 | 0.020 |
| Right occipital pole | 35 | −93 | 14 | 3.84 | 0.015 | |
| Right occipital pole | 26 | −92 | 14 | 3.80 | 0.015 | |
In addition to showing a statistical association between pons FA and cerebellar GMV, we also investigated whether the structural integrity of specific white matter tracts within the pons and cerebellum was associated with ‘p factor’ scores. To do this, we extracted average FA values from white matter tracts in the right and left medial lemniscus, right and left inferior cerebellar peduncles, and right and left superior cerebellar peduncles (see Supplemental Information). We were unable to measure FA within the middle cerebellar peduncles, which are the afferent white matter tracts that connect the pons and cerebellum, as they typically fall out of the field of view and thus are difficult to reliably image (34).
We conducted linear regressions of ‘p factor’ scores predicting average FA within these six white matter tracts controlling for age, sex, and average whole-brain FA. We found that higher ‘p factor’ scores were associated with lower integrity of the right (r=−.116) and left medial lemniscus (r=−.120, both p’s<.001), as well as the left superior cerebellar peduncle (r=−.070, p<.05; Supplemental Table 3). In contrast, ‘p factor’ scores were unrelated to the bilateral inferior (right: p=.368; left: p=.211) and right superior (p=.130) cerebellar peduncles.
Cerebellar-specific differences in gray matter volume associated with ‘p factor’ scores
Given the above convergent evidence from our exploratory analyses that ‘p factor’ scores are associated with alterations in cerebellar circuitry, we employed follow-up analyses of GMV within the cerebellum to clarify the nature of the associations. Namely, we used the Spatially Unbiased Atlas Template (SUIT) of the cerebellum and brainstem (35) to improve the anatomical localization of gray matter correlates of ‘p factor’ scores (see Supplemental Information). As with the whole-brain analyses, we conducted a linear regression with ‘p factor’ scores predicting differences in cerebellar GMV controlling for age, sex, and intracranial volume. We found that ‘p factor’ scores were associated with GMV deficits within the left lobule VIIb (Figure 3; Supplemental Figure 1 and Table 4), a cerebellar region that has been found to be functionally connected with orbitofrontal, dorsolateral, and medial prefrontal cortex regions supporting cognitive control (36,37). As with the more general correlation between cerebellar GMV and pons FA, this left lobule VIIb GMV found to be associated with ‘p factor’ scores, also was correlated with pons FA (standardized B=.105, p<.01) controlling for age, sex, average whole-brain FA, and intracranial volume.
Figure 3.
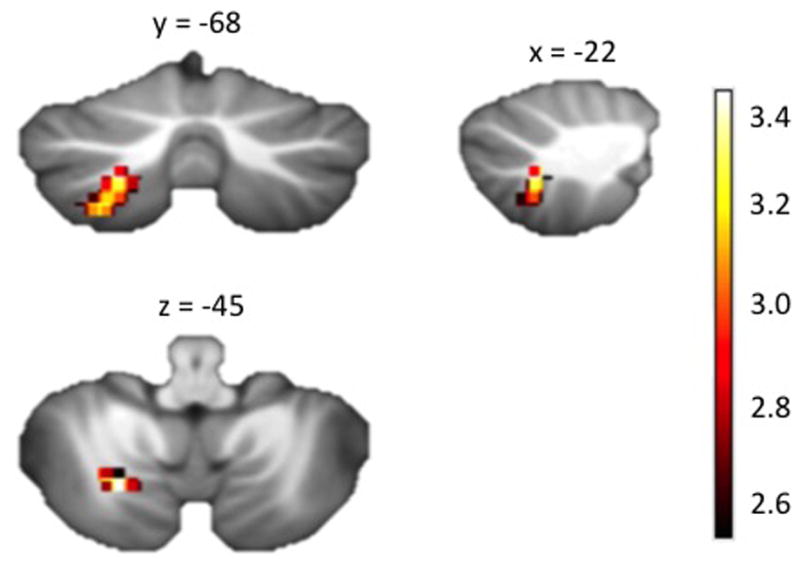
A statistical parametric map from the SUIT cerebellar-specific, voxel-based morphometry analysis illustrating voxels exhibiting a significant negative correlation with ‘p factor’ scores within the left cerebellar lobule VIIb (x = −22 y = −68 z = −45). This association was independent of age, sex, and intracranial volume. Colorbar reflects t scores.
Discussion
Some commentators have questioned the utility of a general factor that underlies shared risk for a wide range of common mental disorders by suggesting that it is analogous to arguing that all forms of physical illness can be represented as a general state of being “unwell” (28). But this analogy ignores the fact that all mental disorders are expressed through dysfunction of the same organ whereas physical diseases such as atherosclerosis, emphysema, and diabetes are manifest through dysfunction of different organ systems. Collectively, our current findings provide initial evidence that the ‘p factor’ may capture a neural mechanism underlying a general liability for mental disorders.
Our results implicate alterations in the structure of cerebellar circuitry as a transdiagnostic biomarker of a general liability for mental disorders in the form of reduced white matter integrity of pontine pathways encompassing cerebellar afferents and reduced cerebellar GMV. Although the cerebellum is most widely known as a region involved in basic motor processing and coordination, it has been long implicated in higher-order cognitive and emotional processes through its structural and functional connectivity with various cerebral structures (38,39,40). This dynamic communication occurs through what are known as cerebello-thalamo-cerebro-cortical circuits (CTCCs) (39,41). The cerebellum as part of these CTCCs has been found to be activated during a number of complex cognitive and affective tasks, such as working memory, set-shifting, associative fear learning, and recognizing emotional facial expressions (42,43,44,45). More generally, investigators have theorized that the cerebellum functions as a general “forward controller” creating internal models of how a given output will fit with contextual information (46,47). As such, the cerebellum may compute models that provide a representation of future action plans, thoughts, and emotions and subsequently modify these models based on external feedback; in effect, comparing intention with execution (39,48). Thus, reduced cerebellar GMV and pontine white matter integrity associated with higher ‘p factor’ scores may reflect impaired processing and communication of information, respectively, necessary to guide behavior.
Not surprisingly, there is evidence of cerebellar dysfunction in specific categorical disorders including major depressive disorder, mania, obsessive-compulsive disorder, autism spectrum disorders, and schizophrenia (e.g., 49,50,51,52). In patients with psychosis, for example, studies have found global cerebellar atrophy (39), reduced FA in the left cerebellar peduncle (53), and a recent meta-analysis showed relatively decreased activation of the cerebellum during a variety of cognitive and affective tasks (54). Interestingly, in cerebellar cognitive affective syndrome, which results from damage to the cerebellum, patients experience symptoms across cognitive and affective domains including executive function impairment, difficulties with spatial cognition, personality change (i.e., blunting of affect and/or disinhibited and inappropriate behavior), and language deficits (55,56). These symptoms, typically referred to as “dysmetria of thought,” bear resemblance to the symptoms of schizophrenia and other thought disorders (57). Although cerebellar dysfunction has been reported in association with several specific mental disorder categories, this study provides initial evidence that abnormal cerebellar structure and potential dysfunctional communication with the cerebrum through the pons may underlie a general liability for psychopathology more broadly.
Of particular interest, the region of the cerebellum in which we find the strongest GMV association with ‘p factor’ scores (lobule VIIb) is part of a cognitive control network including orbitofrontal, dorsolateral, and medial prefrontal cortex (36,37). Similarly, meta-analyses of fMRI studies show that lobule VIIb is activated during cognitive control tasks (e.g., 58,59), which is consistent with our hypothesis that poorer cortico-cerebellar communication may manifest behaviorally as reduced ability to regulate bottom-up drives. Furthermore, we also found that lower FA of the left superior cerebellar peduncle was associated with higher ‘p factor’ scores. Anatomically, the superior cerebellar peduncles are efferent white matter tracts that send information to the cerebrum through the thalamus, whereas the inferior cerebellar peduncles are primarily afferent tracts that receive proprioceptive and motor information from the spinal cord and medulla oblongata (60). These results are consistent with our hypothesis that individuals with higher ‘p factor’ scores may show poorer communication between the cerebellum and cerebral cortex. Whether the deficits are specific to efferent pathways to the cerebrum through the thalamus or afferent pathways from the cerebrum to the pons, or some combination of both, remains to be determined.
In addition to our findings of impairments in cortico-cerebellar circuitry as a potential biomarker of general psychopathology, we also found reduced GMV within regions of the occipital lobes in individuals with high ‘p factor’ scores. We found the strongest association with ‘p factor’ scores in the lingual gyrus, a region supporting visual attention and memory processing (61) with direct connections to the corticolimbic circuit (62). Volumetric studies have shown that depressed individuals exhibit relatively decreased GMV in the lingual gyrus (63,64). Another study found that reduced volume of the lingual gyrus was associated with poorer response to antidepressants and poorer cognitive functioning in depression (65). One hypothesis is that the lingual gyrus may be involved in top-down attentional control of frontoparietal and corticolimbic circuits (66,65), such that reduced GMV in the lingual gryus may manifest as dysregulated cognition and emotion contributing to risk for a wide range of disorders.
Of course, our study is not without limitations. First, as the initial exploratory findings were not hypothesized in advance, replication in other cohorts is needed. Second, the cross-sectional design of our study precludes establishing temporal order among the observed links between ‘p factor’ scores, pons white matter integrity, and regional GMV. Future longitudinal research should examine this question. Third, we conducted our neuroimaging study using a large volunteer sample of university students. The fact that our sample was untreated with psychiatric medications is an advantage for imaging research, but it may be a disadvantage for clinicians needing an evidence base that generalizes to patients. Fourth, our effect sizes were small; however, it is somewhat remarkable that we were able to detect any differences in brain structure associated with the ‘p factor’ in such a relatively high-functioning sample. Fifth, because our measurement of psychiatric symptoms was not exhaustive, we have an incomplete view of people’s liability to psychiatric disorders and the findings need to be replicated with a wider age range of participants and an even more comprehensive assessment of psychiatric symptoms. Sixth, ideally, we would have been able to examine the integrity of the middle cerebellar peduncles that directly connect the pons and cerebellum; however, this was not possible because of signal drop out (34). Higher-resolution diffusion tensor imaging may better assess these peduncles. Finally, the extracted factor scores for ‘p’ undoubtedly contain error, which is not systematically modeled in subsequent regression analyses, possibly resulting in biased standard errors. As the aim of this research was to identify regions of interest for future studies, replication, including in studies using latent modeling, is imperative.
These limitations notwithstanding, our findings both support the significance of the ‘p factor’ as a meaningful dimension of general risk for multiple forms of psychopathology, and provide initial evidence for circumscribed neuroanatomical correlates of this general liability. In particular, our exploratory work suggests that structural alterations in cortico-cerebellar circuitry are associated with a general liability for common mental disorders and sets the stage for future hypothesis-driven, region of interest analyses. Such research also could examine whether greater general psychopathology is associated with poorer cognitive control through dysfunction of cortico-cerebellar circuitry.
Supplementary Material
Acknowledgments
We thank the Duke Neurogenetics Study participants and the staff of the Laboratory of NeuroGenetics. The Duke Neurogenetics Study received support from Duke University as well as US-National Institutes of Health grants R01DA033369 and R01DA031579. ALR was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 1106401. ARK, RH, AC, TEM, and ARH received further support from US-National Institutes of Health grant R01AG049789.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Clark LA, Watson D, Reynolds S. Diagnosis and classification of psychopathology: Challenges to the current system and future directions. Annu Rev Psychol. 1995;46:121–153. doi: 10.1146/annurev.ps.46.020195.001005. [DOI] [PubMed] [Google Scholar]
- 2.Newman DL, Moffitt TE, Caspi A, Silva PA. Comorbid mental disorders: Implications for treatment and sample selection. J Abnorm Psychol. 1998;107:305–311. doi: 10.1037//0021-843x.107.2.305. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS. Major depression and generalised anxiety disorder same genes, (partly) different environments—Revisited. Br J Psychiatry Suppl. 1996;168:68–75. [PubMed] [Google Scholar]
- 4.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettersson E, Larsson H, Lichtenstein P. Common psychiatric disorders share the same genetic origin: A multivariate sibling study of the Swedish population. Mol Psychiatry. 2016;21:717–721. doi: 10.1038/mp.2015.116. [DOI] [PubMed] [Google Scholar]
- 6.Sartor CE, Grant JD, Bucholz KK, Madden PA, Heath AC, Agrawal A, et al. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcohol Clin Exp Res. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlow DH, Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Allen LB, May JT. The unified protocol for transdiagnostic treatment of emotional disorders: Therapist guide. Oxford University Press; New York, NY: 2010. [Google Scholar]
- 8.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 9.Achenbach TM, Edelbrock C. Behavioral problems and competencies reported by parents of normal and disturbed children aged 4 through 16. Monographs Society Res Child Dev. 1981;46:1–82. [PubMed] [Google Scholar]
- 10.Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annu Rev Clin Psychol. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger RF, Markon KE. A dimensional-spectrum model of psychopathology: Progress and opportunities. Arch Gen Psychiatry. 2011;68:10–11. doi: 10.1001/archgenpsychiatry.2010.188. [DOI] [PubMed] [Google Scholar]
- 12.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psych Bull. 2017;143:142–186. doi: 10.1037/bul0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AG, Krueger RF, Hobbs MJ, Markon KE, Eaton NR, Slade T. The structure of psychopathology: Toward an expanded quantitative empirical model. J Abnorm Psychol. 2013;122:281–294. doi: 10.1037/a0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121:971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deary IJ. Intelligence, a very short introduction. Oxford University Press; Oxford, England: 2001. [Google Scholar]
- 17.Jensen AR. The g factor. Praeger; New York, NY: 1998. [Google Scholar]
- 18.Spearman C. “General intelligence,” objectively determined and measured. Am J Psychol. 1904;15:201–292. [Google Scholar]
- 19.Blanco C, Wall MM, He JP, Krueger RF, Olfson M, Jin CJ, et al. The space of common psychiatric disorders in adolescents: Comorbidity structure and individual latent liabilities. J Am Acad Child Adolesc Psychiatry. 2015;54:45–52. doi: 10.1016/j.jaac.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodbeck J, Stulz N, Itten S, Regli D, Znoj H, Caspar F. The structure of psychopathological symptoms and the associations with DSM-diagnoses in treatment-seeking individuals. Compr Psychiatry. 2014;55:714–726. doi: 10.1016/j.comppsych.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Laceulle OM, Vollebergh WAM, Ormel J. The structure of psychopathology in adolescence: Replication of a general psychopathology factor in the TRAILS study. Clin Psychol Sci. 2015;3:850–860. [Google Scholar]
- 22.Lahey BB, Rathouz PJ, Keenan K, Stepp SD, Loeber R, Hipwell AE. Criterion validity of the general factor of psychopathology in a prospective study of girls. J Child Psychol Psychiatry. 2015;56:415–422. doi: 10.1111/jcpp.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olino TM, Dougherty LR, Bufferd SJ, Carlson GA, Klein DN. Testing models of psychopathology in preschool-aged children using a structured interview-based assessment. J Abnorm Child Psychol. 2014;42:1201–1211. doi: 10.1007/s10802-014-9865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patalay P, Fonagy P, Deighton J, Belsky J, Vostanis P, Wolpert M. A general psychopathology factor in early adolescence. Br J Psychiatry. 2015;1:15–22. doi: 10.1192/bjp.bp.114.149591. [DOI] [PubMed] [Google Scholar]
- 25.Neumann A, Pappa I, Lahey BB, Verhulst F, Medina-Gomez C, Jaddoe V, et al. Single nucleotide polymorphism heritability of a general psychopathology factor in children. J Am Acad Child Adolesc Psychiatry. 2016;55:1038–1045. doi: 10.1016/j.jaac.2016.09.498. [DOI] [PubMed] [Google Scholar]
- 26.Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM-IV anxiety and mood disorders: Implications for assessment and treatment. Psychol Assess. 2009;21:256–271. doi: 10.1037/a0016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuthbert BN. The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNally R, Robinaugh DJ, Wu GW, Wang L, Deserno MK, Borsboom D. Mental disorders as causal systems: A network approach to posttraumatic stress disorder. Clin Psychol Sci. 2015;3:836–849. [Google Scholar]
- 29.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I. N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 31.Muthén LK, Muthén BO. MPlus user’s guide. 7. Muthén & Muthén; Los Angeles, CA: 1998–2013. [Google Scholar]
- 32.Wilcox RR. Wiley StatsRef: Statistics References Online. 2005. Winsorized robust measures. [Google Scholar]
- 33.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 36.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front Neural Circuits. 2013;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keren-Happuch E, Chen SH, Ho MH, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 2014;35:593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 42.Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, et al. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: A positron emission tomography study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 43.Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working memory and finger tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 45.Sacchetti B, Scelfo B, Strata P. The cerebellum: Synaptic changes and fear conditioning. Neuroscientist. 2005;11:217–227. doi: 10.1177/1073858405276428. [DOI] [PubMed] [Google Scholar]
- 46.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 47.Ito M. Movement and thought: Identical control mechanisms by the cerebellum. Trends Neurosci. 1993;16:448–450. doi: 10.1016/0166-2236(93)90073-u. [DOI] [PubMed] [Google Scholar]
- 48.Ghez C. The cerebellum. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. Appleton & Lange; Norwalk, CT: 1991. pp. 626–646. [Google Scholar]
- 49.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Lazaro L, Calvo A, Ortiz AG, Ortiz AE, Morer A, Moreno E, et al. Microstructural brain abnormalities and symptom dimensions in child and adolescent patients with obsessive-compulsive disorder: A diffusion tensor imaging study. Depress Anxiety. 2014;31:1007–1017. doi: 10.1002/da.22330. [DOI] [PubMed] [Google Scholar]
- 51.Mills NP, DelBello MP, Adler CM, Strakowski SM. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry. 2005;162:1530–1532. doi: 10.1176/appi.ajp.162.8.1530. [DOI] [PubMed] [Google Scholar]
- 52.Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, Li K. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol. 2011;80:395–399. doi: 10.1016/j.ejrad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: A functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3:545–566. doi: 10.1177/2167702614542463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 56.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 57.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 58.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 59.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: A clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50:607–614. doi: 10.1136/jnnp.50.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conrad CD, Stumpf WE. Direct visual input to the limbic system: Crossed retinal projections to the nucleus anterodorsalis thalami in the tree shrew. Exp Brain Res. 1975;23:141–149. doi: 10.1007/BF00235456. [DOI] [PubMed] [Google Scholar]
- 62.Du MY, Wu QZ, Yue Q, Li J, Liao Y, Kuang WH, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:11–16. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 63.Yang X, Ma X, Huang B, Sun G, Zhao L, Lin D, et al. Gray matter volume abnormalities were associated with sustained attention in unmedicated major depression. Compr Psychiatry. 2015;63:71–79. doi: 10.1016/j.comppsych.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Jung J, Kang J, Won E, Nam K, Lee MS, Tae WS, et al. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: A voxel-based morphometry study. J Affect Disord. 2014;169:179–187. doi: 10.1016/j.jad.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.