Abstract
Solid organ transplant recipients have an elevated incidence of thyroid cancer. We evaluated a wide range of potential risk factors in a cohort of 229,300 U.S. solid organ transplant recipients linked with 15 stage/regional cancer registries (1987–2012). Incidence rate ratios (IRRs) were adjusted for age, sex, race/ethnicity, transplanted organ, year of transplant, and time since transplant. Hazards ratios (HRs) for death and/or graft failure were adjusted for age, sex, race/ethnicity, transplanted organ, and year of transplant. Following transplant, 356 thyroid cancers were diagnosed. Thyroid cancer incidence was 2.50-fold higher in transplant recipients than the general population (95%CI 2.25–2.77). Among recipients of different organs, kidney recipients had the highest incidence of thyroid cancer (IRR=1.26, 95%CI 1.03–1.53). Elevated thyroid cancer incidence was associated with cholestatic liver disease/cirrhosis as an indication for liver transplant (IRR=1.69, 95%CI 1.09–2.63), hypertensive nephrosclerosis as an indication for kidney transplant (IRR=1.41, 95%CI 1.03–1.94), and longer prior dialysis among kidney recipients (5+ versus <1 year, IRR=1.92, 95%CI 1.32–2.80; P-trend<0.01). Post-transplant diagnosis of thyroid cancer was associated with modestly increased risk of death (HR=1.33, 95%CI 1.02–1.73). Overall, our results suggest that end-stage organ disease and longer duration of dialysis may contribute to higher thyroid cancer incidence in transplant recipients.
INTRODUCTION
Thyroid cancer incidence has increased in many parts of the world over the past several decades (1). This trend is largely attributable to a rise in papillary thyroid cancer (PTC), the most common histologic type. Greater incidental detection of small (<1 cm), indolent PTCs through more widespread use of ultrasonography and other imaging modalities may account for much of this increase (2). However, the incidence of large (>5 cm) PTCs has increased at nearly the same rate, suggesting that there also is a true increase in thyroid cancer due to greater exposure to environmental risk factors (3). Nonetheless, the etiology of thyroid cancer remains poorly understood, and few risk factors have been identified apart from female sex, exposure to ionizing radiation, particularly at young ages, and excess body weight (4–6).
Solid organ transplant recipients have an increased incidence of thyroid cancer compared to the general population, according to most (7–9), but not all (10), studies on the topic, but the reasons for any excess have not been evaluated. Among transplant recipients, the higher incidence of some cancers, particularly those that are virus-related, is attributable to immune suppression that results from medications used to prevent graft rejection. However, immune suppression does not play an obvious role in thyroid cancer development. For instance, thyroid cancer does not arise in excess in people with human immunodeficiency virus (HIV) infection, another immunosuppressed population (11).
The increased thyroid cancer incidence in transplant recipients could be at least partially attributable to heightened medical surveillance (7–9). Compared to the general population, transplant recipients have much greater engagement with the medical care system, including frequent clinical evaluations that could involve thyroid palpation or diagnostic imaging of the head, neck, and chest. Evaluation of endocrine abnormalities in transplant recipients could also lead to incidental detection of thyroid cancer. Over-diagnosis might be expected to manifest as increased thyroid cancer incidence in the few months following organ transplantation, when transplant recipients are under closest medical evaluation, but would be less of a factor over the long term. A longer-term increase in incidence following organ transplantation could, on the other hand, result from factors that promote thyroid cancer development and progression. Over-diagnosis would also be associated with elevated incidence of early-stage thyroid cancers but could not explain any excess in metastatic cases. Exposures that are unique to the transplant population include the use of immunosuppressant medications, which could have direct carcinogenic effects (12) or could indirectly, via immunosuppression, promote the development of undiagnosed thyroid cancer at transplant. In addition, certain underlying medical conditions, are more prevalent among transplant recipients and may also contribute to an increased risk of thyroid cancer. End-stage renal disease (ESRD), present in all kidney recipients, has been linked to an elevated risk of thyroid cancer (13–15). However, to date, the evidence regarding a possible association of these factors with thyroid cancer risk in transplant recipients is limited.
An understanding of the epidemiology of thyroid cancer among transplant recipients will help clarify the etiology of this malignancy and provide information to help clinicians improve outcomes in this high-risk population. Using registry data from a large U.S. population of solid organ transplant recipients linked with multiple cancer registries, we assessed thyroid cancer incidence in transplant recipients and evaluated a wide range of potential risk factors, including demographic factors, transplant characteristics, immunosuppressant medications, medical indication for organ transplantation, and, among kidney recipients, duration of prior dialysis.
METHODS
The Transplant Cancer Match Study links the Scientific Registry of Transplant Recipients (SRTR), a nationwide registry of all U.S. transplants, with 15 population-based cancer registries (7). The resulting cohort includes 45% of the U.S. transplant population between 1987 and 2012, specifically transplant recipients in California (years of cancer registry coverage: 1988–2012), Colorado (1988–2009), Connecticut (1987–2009), Florida (1987–2009), Georgia (1995–2010), Hawaii (1987–2007), Illinois (1987–2007), Iowa (1987–2009), Kentucky (1995–2011), Michigan (1987–2009), New Jersey (1987–2010), New York (1987–2010), North Carolina (1990–2010), Texas (1995–2010), Utah (1987–2008), and the metropolitan area of Seattle, Washington (1987–2008). We restricted the study to individuals of the major race/ethnicity groups (non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander) to allow comparisons with general population cancer rates. The Transplant Cancer Match Study was approved by human subjects’ research review committees at the National Cancer Institute and, as required, participating cancer registries.
Follow-up for transplant recipients began at transplantation or the start of cancer registry coverage, whichever came later, and ended at thyroid cancer diagnosis, death, transplant failure, retransplantation, loss to follow-up, or end of cancer registry coverage, whichever came first. Person-time was apportioned separately for each transplant for people who received multiple successive transplants.
Thyroid cancers (International Classification of Diseases for Oncology ([ICD-O], version 3 topography code C73) were classified according to histologic type (16): papillary (ICD-O-3 histology codes 8050, 8260, 8340–8344, 8350, 8450–8460), follicular (8290, 8330–8335), medullary (8345, 8510–8513), anaplastic (8020–8035), and other/unspecified histology. Surveillance, Epidemiology, and End Results (SEER) summary stage was used to classify thyroid cancers according to stage at diagnosis (local, regional, distant).
We obtained information on potential thyroid cancer risk factors from the SRTR. This included demographic characteristics (age at transplant, sex, race/ethnicity), transplant characteristics (transplanted organ), medical conditions that are the indication for transplant, induction and initial maintenance immunosuppressant medications (prescribed at the time of transplant and initial hospital discharge, respectively), and among kidney recipients, duration of dialysis prior to transplant.
We calculated standardized incidence ratios (SIRs) to compare thyroid cancer incidence in transplant recipients with incidence in the general U.S. population. SIRs were calculated as the number of observed thyroid cancer cases divided by the number expected based on general population rates specific to registry, five-year age group, sex, race/ethnicity, and calendar period. We observed an especially high SIR in the first year after transplantation; in a sensitivity analysis, this period was excluded from SIR calculations and all internal risk comparisons to evaluate the potential impact of surveillance bias around the time of organ transplantation. We then estimated SIRs overall, according to histology or stage at diagnosis, and separately by sex, race/ethnicity, age at transplantation, calendar year of transplant, and transplanted organ.
For internal comparisons among transplant recipients, we used Poisson regression to calculate incidence rate ratios (IRRs). We examined associations for thyroid cancer overall, PTC, local stage thyroid cancer, and regional/distant stage thyroid cancer. Attained age, sex, race/ethnicity, transplanted organ, time since transplantation, and year of transplantation were assessed in mutually-adjusted models. These six factors were also included as adjustment factors in multivariable models that assessed associations of individual induction and maintenance medications prescribed at transplant and initial hospital discharge, respectively, and, among patients receiving a particular organ, grouped medical conditions that were indications for transplantation. Associations with race/ethnicity, transplanted organ, and medical conditions were modeled using effect parameterization, which compares each category to the overall average. We also evaluated thyroid cancer incidence in relation to duration of dialysis prior to transplant among kidney recipients. Other factors potentially related to immunosuppression in this population that were evaluated included pre-transplant history of diabetes (available for transplants from 1995-onward), body mass index, human leukocyte antigen (HLA) mismatch, and, among kidney recipients, panel reactive antibody (PRA).
As a sensitivity analysis, we restricted the analysis of immunosuppressant medications to transplants occurring between 1998 and 2002, a transition period when most of these medications were overlapping in use. Because SIRs were highest in the first year after transplantation (see Results), which could reflect surveillance bias or delayed diagnosis of prevalent cases around the time of organ transplantation, in another sensitivity analysis we excluded the first year of follow-up.
Finally, we evaluated the association between thyroid cancer diagnosis following organ transplantation and subsequent risk of death and/or graft failure or retransplantation. For this analysis, we used Cox proportional hazards regression models adjusted for age at transplantation, sex, race/ethnicity, transplanted organ, and year of transplantation. Diagnosis of thyroid cancer was treated as a time-dependent risk factor. Follow-up started on the date of transplantation or start of cancer registry coverage, whichever came later, and ended on the date of death, graft failure, retransplantation, loss to follow-up, or end of cancer registry coverage, whichever came first.
RESULTS
We studied 229,300 individuals who received a total of 248,136 transplants. Median follow-up time was 3.9 years (interquartile range 1.4–7.4 years). Most transplants occurred in patients who were male (61%), non-Hispanic white (61%), and aged 35–64 years (68%) (Table 1). Kidney transplants were the most common (58%), followed by liver (22%), heart (10%), lung (4%), and other or multiple organs (6%). Most transplants (91%) were first transplants. Transplants that were excluded from the study population, largely because the recipient’s state of residence did not participate in the linkage with the transplant registry or the transplant did not overlap with years of cancer registry coverage, were similar to those that were included by sex, age at transplant, transplanted organ, and transplant number. Transplants that were excluded were more likely to have occurred in non-Hispanic whites (69% versus 61%), less likely to have occurred in Hispanics (7% versus 16%) and Asian/Pacific Islanders (3% versus 6%), and more likely to have occurred in the earliest and most recent calendar year periods (23% versus 16% in 1987–1994, 22% versus 7% in 2010–2012).
Table 1.
Characteristics of 248,136 solid organ transplants included and 304,959 solid organ transplants not included in the U.S. Transplant Cancer Match Study.
| Characteristic | No. of transplants included (% total) | No. of transplants excluded (% of total) |
|---|---|---|
| Sex | ||
| Male | 152,059 (61) | 188,266 (62) |
| Female | 96,077 (39) | 166,693 (38) |
| Race/ethnicity | ||
| White, Non-Hispanic | 150,854 (61) | 211,391 (69) |
| Black, Non-Hispanic | 42,588 (17) | 58,713 (19) |
| Hispanic | 40,357 (16) | 21,324 (7) |
| Asian/Pacific Islander | 14,337 (6) | 7,927 (3) |
| Other/unknown | 0a | 5,604 (2) |
| Age at transplantation, years | ||
| 0–19 | 21,438 (9) | 24,457 (8) |
| 20–34 | 36,257 (15) | 45,140 (15) |
| 35–49 | 75,298 (30) | 92,740 (30) |
| 50–64 | 92,254 (37) | 114,104 (37) |
| 65+ | 22,889 (9) | 28,517 (9) |
| Unknown | 0 | 1 (0) |
| Year of transplantation | ||
| 1987–1994 | 39,388 (16) | 70,072 (23) |
| 1995–1999 | 53,884 (22) | 47,276 (16) |
| 2000–2004 | 66,345 (27) | 55,762 (18) |
| 2005–2009 | 71,978 (29) | 65,557 (22) |
| 2010–2012 | 16,541 (7) | 66,292 (22) |
| Transplanted organ | ||
| Kidney | 144,276 (58) | 184,171 (60.4) |
| Liver | 54,105 (22) | 57,754 (18.9) |
| Heart | 24,154 (10) | 29,122 (10) |
| Lung | 10,837 (4) | 14,009 (5) |
| Other or Multiple | 14,764 (6) | 19,903 (7) |
| Transplant number | ||
| First | 225,804 (91) | 276,259 (91) |
| Second | 20,347 (8) | 25,804 (8) |
| Third or Higher | 1,985 (1) | 2,896 (1) |
Recipients with other/unknown race/ethnicity were excluded from the study population to allow comparisons with general population cancer rates.
Thyroid cancer was diagnosed in 356 transplant recipients (incidence rate 29.2 per 100,000 person-years). The vast majority were PTCs (91%), while 5% were follicular, 2% were medullary, 1% were anaplastic, and 2% were other/unspecified types.
Thyroid cancer incidence was elevated 2.50-fold in these transplant recipients compared with the general population (95%CI 2.25–2.77) (Table 2). SIRs were significantly elevated over the entire follow-up period, with the highest elevation occurring in the first year after transplant (SIR for 0–0.49 years=4.31, 95%CI 3.17–5.74; SIR for 0.5–0.99 years=4.10, 95%CI 2.96–5.54), a nadir around 2.0–4.9 years after transplant (SIR=1.83, 95%CI 1.45–2.27), and a subsequent increase over time (5–9 years, SIR=2.29, 95%CI 1.85–2.81; 10+ years, SIR=2.43, 95%CI 1.76–3.27). A similar pattern was observed for PTC, local stage thyroid cancer, and regional/distant stage thyroid cancer (Figure 1). SIRs were significantly elevated in men and women and for all racial/ethnic groups. The highest SIRs were observed in recipients who underwent transplantation before age 20 (11 cases, SIR=6.14, 95%CI 3.07–10.99), and SIRs declined with increasingly older age groups. By transplanted organ, the highest elevations were observed in kidney recipients and the lowest in liver recipients. By histology, incidence was significantly elevated for PTC and other/unspecified types but not for follicular, medullary, or anaplastic types. Incidence was elevated for local, regional, and unstaged cancer; the SIR was not increased for distant stage cancer (8 cases), but the CI was wide.
Table 2.
Standardized incidence ratios for thyroid cancer in transplant recipients
| Cases | SIR (95%CI) | |
|---|---|---|
| Overall | 356 | 2.50 (2.25–2.77) |
| Sex | ||
| Male | 150 | 2.77 (2.35–3.25) |
| Female | 206 | 2.33 (2.02–2.67) |
| Race/ethnicity | ||
| White, non-Hispanic | 231 | 2.38 (2.08–2.71) |
| Black, non-Hispanic | 38 | 2.69 (1.90–3.69) |
| Hispanic | 59 | 2.76 (2.10–3.55) |
| Asian/Pacific Islander | 28 | 2.84 (1.89–4.10) |
| Age at transplantation, years | ||
| 0–19 | 11 | 6.14 (3.07–10.99) |
| 20–34 | 48 | 2.96 (2.18–3.92) |
| 35–49 | 133 | 2.75 (2.30–3.26) |
| 50–64 | 142 | 2.28 (1.92–2.68) |
| 65+ | 22 | 1.61 (1.01–2.43) |
| Year of transplantation | ||
| 1987–1994 | 75 | 3.08 (2.42–3.86) |
| 1995–1999 | 100 | 2.53 (2.06–3.08) |
| 2000–2004 | 104 | 2.24 (1.83–2.71) |
| 2005–2012 | 77 | 2.40 (1.89–3.00) |
| Time since transplantation, years | ||
| 0.0–0.4 | 47 | 4.31 (3.17–5.74) |
| 0.5–0.9 | 42 | 4.10 (2.96–5.54) |
| 1.0–1.9 | 50 | 2.67 (1.98–3.52) |
| 2.0–4.9 | 81 | 1.83 (1.45–2.27) |
| 5.0–9.9 | 93 | 2.29 (1.85–2.81) |
| 10.0+ | 43 | 2.43 (1.76–3.27) |
| Transplanted organ | ||
| Kidney | 237 | 2.87 (2.52–3.26) |
| Liver | 53 | 1.63 (1.22–2.13) |
| Heart | 34 | 2.39 (1.65–3.34) |
| Lung | 13 | 2.34 (1.25–4.00) |
| Other or multiple | 19 | 2.49 (1.50–3.89) |
| Thyroid cancer histology | ||
| Papillary | 323 | 2.68 (2.39–2.99) |
| Follicular | 17 | 1.21 (0.70–1.93) |
| Medullary | 6 | 1.75 (0.64–3.82) |
| Anaplastic | 3 | 1.85 (0.38–5.41) |
| Other/unspecifieda | 7 | 2.60 (1.04–5.35) |
| Thyroid cancer stage at diagnosis | ||
| Local | 241 | 2.53 (2.22–2.87) |
| Regional | 91 | 2.65 (2.13–3.25) |
| Distant | 8 | 1.03 (0.45–2.03) |
| Unstaged | 16 | 3.31 (1.89–5.37) |
Abbreviations: SIR, standardized incidence ratio; CI, confidence interval.
This category includes 3 cases of unspecified carcinoma (International Classification of Diseases for Oncology version 3 morphology code 8010), 1 mixed adenocarcinoma (8255), 1 papillotubular adenocarcinoma (8263), 1 insular carcinoma (8337), and 1 mucoepidermoid carcinoma (8430).
Figure 1.
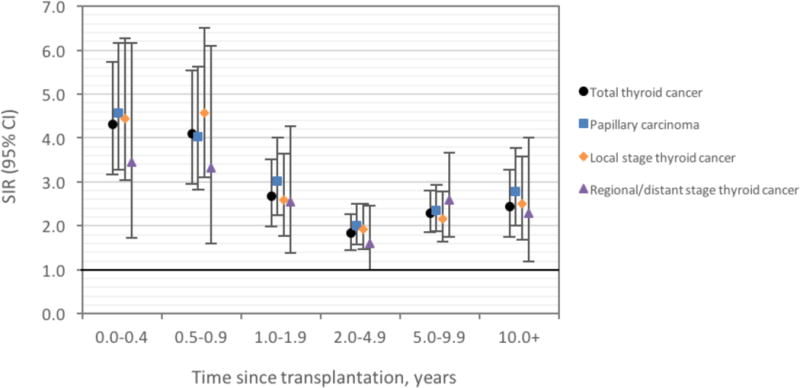
Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for thyroid cancer among U.S. transplant recipients, by time since transplantation.
Among transplant recipients, overall thyroid cancer incidence increased significantly with greater attained age and was higher in women than men (Table 3). Compared with the average, non-Hispanic blacks had a lower incidence (IRR=0.68, 95%CI 0.52–0.88), and no significant differences were observed for non-Hispanic whites, Hispanics, and Asian/Pacific Islanders. Compared with the average, kidney recipients had significantly higher thyroid cancer incidence (IRR=1.26, 95%CI 1.03–1.53), liver recipients had lower incidence (IRR=0.68, 95%CI 0.52–0.89), but no significant differences in incidence were observed for heart, lung, or other/multiple organ recipients. Thyroid cancer incidence was modestly increased with more recent calendar year of transplantation. Results according to age, sex, and organ type were similar after we restricted analysis to PTC or local stage thyroid cancers. For regional/distant (but not local) stage cancers, non-Hispanic blacks had lower incidence while Hispanics and Asian/Pacific Islanders had higher incidence compared to the average.
Table 3.
Associations of thyroid cancer with demographic and transplant characteristics among solid organ transplant recipientsa
| Total thyroid cancer | Papillary carcinoma | Local | Regional/distant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Cases | IRR (95%CI) | Phet | Cases | IRR (95%CI) | Phet | Cases | IRR (95%CI) | Phet | Cases | IRR (95%CI) | Phet |
|
|
|
|
|
|||||||||
| Attained age, per categoryb | 356 | 1.14 (1.03–1.27) | 323 | 1.13 (1.01–1.25) | 241 | 1.15 (1.02–1.30) | 99 | 1.06 (0.88–1.27) | ||||
| Sex | ||||||||||||
| Female | 206 | 2.14 (1.73–2.65) | 188 | 2.17 (1.73–2.71) | 149 | 2.52 (1.93–3.27) | 48 | 1.46 (0.98–2.17) | ||||
| Male | 150 | 1.00 (Ref) | 135 | 1.00 (Ref) | 92 | 1.00 (Ref) | 51 | 1.00 (Ref) | ||||
| Race/ethnicityc | 0.02 | <0.01 | 0.60 | <0.01 | ||||||||
| White, non–Hispanic | 231 | 1.09 (0.92–1.30) | 213 | 1.12 (0.94–1.35) | 152 | 1.03 (0.84–1.27) | 65 | 1.37 (0.92–2.04) | ||||
| Black, non–Hispanic | 38 | 0.68 (0.52–0.88) | 33 | 0.65 (0.49–0.87) | 34 | 0.86 (0.64–1.14) | 3 | 0.24 (0.10–0.57) | ||||
| Hispanic | 59 | 1.06 (0.85–1.34) | 50 | 1.00 (0.78–1.28) | 36 | 0.93 (0.70–1.24) | 22 | 1.69 (1.07–2.67) | ||||
| Asian/Pacific Islander | 28 | 1.27 (0.95–1.72) | 27 | 1.37 (1.01–1.86) | 19 | 1.22 (0.85–1.75) | 9 | 1.81 (1.01–3.23) | ||||
| Transplanted organc | <0.01 | <0.01 | <0.01 | 0.38 | ||||||||
| Kidney | 237 | 1.26 (1.03–1.53) | 215 | 1.27 (1.03–1.56) | 163 | 1.26 (0.69–1.48) | 65 | 1.41 (0.93–2.15) | ||||
| Liver | 53 | 0.68 (0.52–0.89) | 47 | 0.67 (0.50–0.89) | 33 | 0.63 (0.45–0.89) | 18 | 0.91 (0.54–1.52) | ||||
| Heart | 34 | 0.99 (0.72–1.35) | 31 | 0.99 (0.71–1.38) | 23 | 1.01 (0.69–1.48) | 9 | 1.00 (0.53–1.89) | ||||
| Lung | 13 | 1.06 (0.67–1.68) | 11 | 0.98 (0.60–1.62) | 10 | 1.19 (0.71–2.01) | 2 | 0.69 (0.22–2.16) | ||||
| Other or multiple | 19 | 1.11 (0.75–1.63) | 19 | 1.21 (0.82–1.80) | 12 | 1.04 (0.64–1.69) | 5 | 1.13 (0.52–2.45) | ||||
| Time since transplantation | <0.01 | <0.01 | <0.01 | 0.15 | ||||||||
| 0.0–0.4 years | 47 | 1.00 (Ref) | 42 | 1.00 (Ref) | 32 | 1.00 (Ref) | 11 | 1.00 (Ref) | ||||
| 0.5–0.9 years | 42 | 0.98 (0.64–1.48) | 35 | 0.91 (0.58–1.43) | 31 | 1.06 (0.65–1.74) | 10 | 0.99 (0.42–2.34) | ||||
| 1.0–1.9 years | 50 | 0.65 (0.44–0.97) | 48 | 0.70 (0.46–1.06) | 32 | 0.62 (0.38–1.01) | 14 | 0.78 (0.35–1.72) | ||||
| 2.0–4.9 years | 81 | 0.49 (0.34–0.70) | 75 | 0.51 (0.35–0.74) | 57 | 0.51 (0.33–0.80) | 21 | 0.54 (0.26–1.14) | ||||
| 5.0–9.9 years | 93 | 0.71 (0.50–1.03) | 81 | 0.71 (0.48–1.04) | 59 | 0.69 (0.44–1.08) | 31 | 1.06 (0.52–2.18) | ||||
| 10.0+ years | 43 | 0.98 (0.63–1.53) | 42 | 1.09 (0.68–1.73) | 30 | 1.03 (0.60–1.77) | 12 | 1.29 (0.53–3.12) | ||||
| Ptrend= 0.11 | Ptrend= 0.26 | Ptrend= 0.16 | Ptrend= 0.74 | |||||||||
| Year of transplantation | 0.38 | 0.30 | 0.37 | 0.36 | ||||||||
| 1987–1994 | 75 | 1.00 (Ref) | 69 | 1.00 (Ref) | 52 | 1.00 (Ref) | 18 | 1.00 (Ref) | ||||
| 1995–1999 | 100 | 1.04 (0.77–1.41) | 90 | 1.04 (0.75–1.43) | 66 | 0.99 (0.69–1.43) | 29 | 1.31 (0.72–2.37) | ||||
| 2000–2004 | 104 | 1.17 (0.86–1.61) | 93 | 1.18 (0.85–1.65) | 67 | 1.09 (0.74–1.61) | 31 | 1.58 (0.85–2.93) | ||||
| 2005–2012 | 77 | 1.33 (0.93–1.91) | 71 | 1.39 (0.96–2.02) | 56 | 1.38 (0.90–2.12) | 21 | 1.82 (0.89–3.70) | ||||
Abbreviations: CI, confidence interval; IRR, incidence rate ratio
The P-value for heterogeneity was calculated using the likelihood ratio test for variables with more than two levels; the P-value for trend was calculated using the Wald test for categorical variables modeled ordinally
IRRs for attained age, sex, race/ethnicity, transplanted organ, time since transplantation, year of transplantation were mutually-adjusted for each other.
The categories of attained age were 0–19, 20–34, 35–49, 50–64, 65+ years.
These variables were modeled using effect parameterization, which compares each category to the average of all of the categories.
We next evaluated associations with specific immunosuppressant medications (Table 4). Compared with initial maintenance therapy with tacrolimus and mycophenolate mofetil, cyclosporine and azathioprine use was associated with significantly lower thyroid cancer incidence (IRR=0.61, 95%CI 0.40–0.93). Likewise, initial maintenance therapy with cyclosporine (compared to no use of this drug) (IRR=0.79, 95%CI 0.60–1.02) and maintenance therapy with azathiopronine (compared to no use of this drug) (IRR=0.72, 95%CI 0.52–0.99) were each, separately, associated with reduced thyroid cancer incidence. Other maintenance and induction medications were not associated with incidence. When we restricted the analysis to transplants occurring in 1998–2002, a transition period during which all medications of interest were overlapping in use, the number of cases in this analysis dropped substantially, but the interpretation for most of the results stayed the same (data not shown in tables). However, a positive association emerged for mycophenolate mofetil (IRR=2.35, 95%CI 1.33–4.16, based on 82 exposed cases), which was particularly strong after restricting the outcome to regional/distant thyroid cancer (IRR=8.60, 95%CI 1.15–64.17, based on 25 exposed cases).
Table 4.
Associations of thyroid cancer with immunosuppressive medication use among solid organ transplant recipients
| Total thyroid cancer | Papillary carcinoma | Local | Regional/distant | |||||
|---|---|---|---|---|---|---|---|---|
| Medication | Cases | IRR (95%CI)a | Cases | IRR (95%CI)a | Cases | IRR (95%CI)a | Cases | IRR (95%CI)a |
| Maintenance medications at hospital discharge | ||||||||
| Combined regimen | ||||||||
| Tacrolimus and mycophenolate mofetil | 130 | 1.00 (Ref) | 120 | 1.00 (Ref) | 89 | 1.00 (Ref) | 37 | 1.00 (Ref) |
| Cyclosporine and azathioprine | 69 | 0.61 (0.40–0.93) | 63 | 0.59 (0.38–0.92) | 49 | 0.65 (0.39–1.08) | 17 | 0.61 (0.27–1.36) |
| Other | 157 | 0.82 (0.62–1.07) | 140 | 0.78 (0.59–1.05) | 103 | 0.81 (0.58–1.13) | 45 | 0.81 (0.49–1.35) |
| Tacrolimus | 163 | 1.08 (0.82–1.41) | 151 | 1.15 (0.86–1.54) | 111 | 1.08 (0.82–1.41) | 43 | 0.83 (0.50–1.37) |
| Cyclosporine | 154 | 0.79 (0.60–1.02) | 137 | 0.74 (0.56–0.98) | 101 | 0.79 (0.60–1.02) | 47 | 1.14 (0.69–1.88) |
| Mycophenolate mofetil | 204 | 1.27 (0.96–1.69) | 183 | 1.21 (0.90–1.63) | 138 | 1.26 (0.89–1.79) | 61 | 1.55 (0.90–2.67) |
| Azathioprine | 80 | 0.72 (0.52–0.99) | 73 | 0.72 (0.51–1.01) | 59 | 0.84 (0.57–1.23) | 17 | 0.53 (0.28–1.03) |
| mTOR inhibitors | 13 | 0.65 (0.37–1.15) | 11 | 0.61 (0.33–1.12) | 9 | 0.68 (0.35–1.35) | 2 | 0.33 (0.08–1.35) |
| Corticosteroids | 305 | 0.77 (0.57–1.04) | 276 | 0.76 (0.55–1.05) | 206 | 0.77 (0.57–1.04) | 86 | 0.87 (0.48–1.58) |
| Induction medications at transplant | ||||||||
| Any induction therapy | 140 | 0.81 (0.64–1.03) | 129 | 0.83 (0.65–1.06) | 96 | 0.80 (0.60–1.07) | 39 | 0.85 (0.54–1.33) |
| Polyclonal antibody | 58 | 0.77 (0.58–1.03) | 52 | 0.75 (0.55–1.02) | 41 | 0.78 (0.55–1.11) | 16 | 0.82 (0.47–1.44) |
| Monoclonal antibody | 16 | 0.71 (0.43–1.20) | 15 | 0.73 (0.43–1.25) | 9 | 0.59 (0.30–1.16) | 5 | 0.85 (0.34–2.16) |
| IL2 receptor antagonists | 66 | 1.10 (0.83–1.47) | 62 | 1.17 (0.86–1.57) | 46 | 1.15 (0.81–1.63) | 18 | 1.03 (0.59–1.78) |
| Alemtuzumab | 8 | 1.18 (0.58–2.43) | 8 | 1.30 (0.63–2.68) | 6 | 1.24 (0.54–2.85) | 2 | 1.13 (0.27–4.73) |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio
Incidence rate ratios were adjusted for attained age, sex, race/ethnicity, transplanted organ, time since transplant, and year of transplantation.
We also evaluated thyroid cancer incidence by medical indication for organ transplantation (Table 5). Among kidney recipients, hypertensive nephrosclerosis as the indication for transplant was associated with elevated thyroid cancer risk compared to the average (IRR=1.41, 95%CI 1.03–1.94), while diabetes mellitus was associated with reduced risk (IRR=0.78, 95%CI 0.56–1.09), particularly after restricting the outcome to PTC (IRR=0.65, 95%CI 0.44–0.94). Among liver recipients, cholestatic liver disease/cirrhosis was associated with increased risk compared to the average (IRR=1.69, 95%CI 1.09–2.63). The higher risk associated with cholestatic liver disease did not differ substantially when we separately considered the two major disease entities, primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC). Specifically, compared with liver recipients without either condition, increased thyroid cancer risk was observed in those with PSC (IRR=1.74, 95%CI 0.61–4.92, based on n=4 cases) or PBC (IRR=2.62, 95%CI 1.22–5.64, n=10). There were too few cases to evaluate medical indications for transplant among heart and lung recipients.
Table 5.
Associations of thyroid cancer with underlying medical indication for transplant among kidney and liver recipients
| Total thyroid cancer | Papillary carcinoma | Local | Regional/distant | |||||
|---|---|---|---|---|---|---|---|---|
| Indication for transplant | Cases | IRR (95%CI)a | Cases | IRR (95%CI)a | Cases | IRR (95%CI)a | Cases | IRR (95%CI)a |
| Kidney recipients | ||||||||
| Glomerular diseases | 73 | 0.97 (0.75–1.27) | 67 | 0.97 (0.74–1.28) | 56 | 1.13 (0.83–1.55) | 16 | 0.74 (0.44–1.25) |
| Diabetes mellitus | 36 | 0.78 (0.56–1.09) | 27 | 0.65 (0.44–0.94) | 23 | 0.76 (0.50–1.16) | 11 | 0.80 (0.44–1.47) |
| Hypertensive nephrosclerosis | 45 | 1.41 (1.03–1.94) | 43 | 1.50 (1.08–2.07) | 30 | 1.38 (0.93–2.05) | 12 | 1.39 (0.77–2.54) |
| Polycystic kidney disease | 21 | 0.78 (0.51–1.17) | 20 | 0.81 (0.53–1.24) | 13 | 0.74 (0.44–1.25) | 6 | 0.77 (0.36–1.67) |
| Tubular and interstitial diseases | 16 | 1.00 (0.63–1.58) | 16 | 1.08 (0.68–1.72) | 12 | 1.13 (0.66–1.94) | 4 | 0.89 (0.36–2.19) |
| Vascular diseases | 11 | 1.37 (0.80–2.36) | 9 | 1.25 (0.69–2.27) | 9 | 1.69 (0.91–3.11) | 2 | 0.90 (0.26–3.13) |
| Congenital/familial/metabolic disorders | 6 | 0.83 (0.40–1.73) | 6 | 0.90 (0.43–1.87) | 3 | 0.62 (0.22–1.73) | 3 | 1.47 (0.50–4.29) |
| Other/unknown conditions | 29 | 1.06 (0.74–1.51) | 27 | 1.08 (0.74–1.56) | 17 | 0.95 (0.60–1.52) | 11 | 1.35 (0.74–2.46) |
| Liver recipients | ||||||||
| Cholestatic liver disease/cirrhosis | 14 | 1.69 (1.09–2.63) | 12 | 1.58 (0.99–2.54) | 9 | 1.66 (0.95–2.88) | 4 | 1.73 (0.77–3.89) |
| Noncholestatic cirrhosis | 26 | 0.81 (0.56–1.18) | 23 | 0.81 (0.55–1.21) | 16 | 0.79 (0.50–1.27) | 10 | 1.04 (0.54–2.03) |
| Other/unknown conditions | 13 | 0.73 (0.46–1.15) | 12 | 0.78 (0.48–1.25) | 8 | 0.76 (0.43–1.35) | 4 | 0.55 (0.24–1.29) |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio
IRRs were adjusted for attained age, sex, race/ethnicity, transplanted organ, time since transplant, and year of transplantation and were modeled using effect parameterization, which compares each category to the average of all of the categories.
Among kidney recipients, greater duration of dialysis prior to transplant was associated with increased thyroid cancer incidence (5+ versus 0–<1 years, IRR=1.92, 95%CI 1.32–2.80; P-trend<0.01; Table 6). Results were more pronounced when we restricted the outcome to regional/distant stage cancer (IRR=4.01, 95%CI 1.98–8.11; P-trend<0.01).
Table 6.
Associations of thyroid cancer with duration on dialysis prior to transplantation among kidney recipients
| Total thyroid cancer | Papillary carcinoma | Local | Regional/distant | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | IRR (95%CI)a | xxx Cases | IRR (95%CI)a | txxx Cases | IRR (95%CI)a | xxx Cases | IRR (95%CI)a | |
| Duration on dialysis | ||||||||
| 0–<1 years | 62 | 1.00 (Ref) | 58 | 1.00 (Ref) | 45 | 1.00 (Ref) | 13 | 1.00 (Ref) |
| 1–<3 years | 79 | 1.31 (0.94–1.84) | 67 | 1.21 (0.84–1.72) | 56 | 1.28 (0.86–1.90) | 22 | 1.74 (0.87–3.48) |
| 3–<5 years | 39 | 1.44 (0.95–2.16) | 38 | 1.52 (1.00–2.31) | 32 | 1.59 (0.99–2.53) | 6 | 1.08 (0.41–2.89) |
| 5+ years | 54 | 1.92 (1.32–2.80) | 49 | 1.88 (1.27–2.79) | 29 | 1.36 (0.84–2.20) | 22 | 4.01 (1.98–8.11) |
| Unknown | 3 | 0.85 (0.26–2.72) | 3 | 0.90 (0.28–2.89) | 1 | 0.39 (0.05–2.82) | 2 | 2.79 (0.62–12.60) |
| Ptrend<0.01 | Ptrend<0.01 | Ptrend=0.10 | Ptrend<0.01 | |||||
Abbreviations: CI, confidence interval; IRR, incidence rate ratio
The P-value for trend was calculated using the Wald test for categorical variables modeled ordinally and excluded unknown values.
IRRs were adjusted for attained age, sex, race/ethnicity, transplanted organ, time since transplant, and year of transplantation.
Pre-transplant history of diabetes, body mass index, HLA mismatch, and, among kidney recipients, PRA were not clearly associated with thyroid cancer incidence (Table S1).
In a sensitivity analysis that excluded the first year of follow-up (Tables S2–S6), the main difference was the slightly attenuated SIRs for thyroid cancer overall (SIR=2.20, 95%CI 1.94–2.48), by histology and stage at diagnosis, and for population subgroups (Table S2).
There were 51,667 deaths and 43,004 graft failures or retransplantations during follow-up. A diagnosis of thyroid cancer following organ transplantation was associated with modestly increased risks of death (hazard ratio [HR]=1.33, 95% CI 1.02–1.73), graft failure or retransplantation (HR=1.18, 95% CI 0.89–1.57), and the combined outcome of death, graft failure, or retransplantation (HR=1.23, 95% CI 1.01–1.49).
DISCUSSION
Solid organ transplant recipients are a high-risk population for thyroid cancer. As we observed previously in an overview of cancer risk in this cohort (7), incidence of thyroid cancer, including the most common histologic type (PTC), is elevated more than two-fold among U.S. solid organ transplant recipients compared with the general population. In the present study, we evaluated a wide range of factors that could potentially explain this increase, including heightened medical surveillance, immunosuppressant medications, medical indications for transplant, and, for kidney recipients, duration of prior dialysis.
Due to their engagement with the medical care system (8), we expected that transplant recipients would be more likely than the general population to undergo evaluation for thyroid nodules, which could lead to detection of thyroid cancer, particularly in the time immediately prior to and soon after the date of organ transplantation. Consistent with some degree of over-diagnosis, we observed much higher incidence of thyroid cancer in the first year of follow-up, particularly for local stage thyroid cancer, and the incidence declined thereafter. Nonetheless, thyroid cancer incidence rose again subsequently and remained significantly elevated above general population rates for more than 10 years after transplant, and the pattern was similar for regional/distant thyroid cancer, indicating that over-diagnosis was not the entire explanation. Furthermore, the distribution of local, regional, distant, and unstaged thyroid cancers (68%, 26%, 3%, and 4%, respectively) in this patient population was similar to that of the general U.S. population (2006–2012) (4). Alternatively, the high incidence of thyroid cancer post-transplantation, particularly in the first year, may be attributable to a promotional influence of immunosuppression on thyroid cancers that were undiagnosed at the time of transplantation.
We hypothesized that etiologic factors would be more strongly associated with regional/distant stage thyroid cancer whereas surveillance-related factors would be associated with local stage thyroid cancers, which are generally smaller than more advanced tumors and, thus, more likely to be identified incidentally. The associations of regional/distant stage thyroid cancer with race/ethnicity and duration of dialysis (among kidney recipients) suggest that these factors were unlikely to be explained by increased surveillance. Although the SIR for thyroid cancer was especially elevated in young transplant recipients, these cancers were rare, and thyroid cancer incidence increased with older age. Indeed, the associations with older age and female sex were present only for local stage cancer, perhaps mainly reflecting opportunity for early detection of thyroid cancer.
Kidney recipients had the highest incidence of thyroid cancer among recipients of different organs. Of note, the highest risk was observed for patients whose indication for kidney transplant was hypertensive nephrosclerosis, which is a leading cause of ESRD. Other studies have demonstrated an elevated risk of thyroid cancer among ESRD patients on dialysis (13–15). ESRD has been hypothesized to influence thyroid cancer development due to impaired DNA repair, reduced antioxidant defense, and accumulation of carcinogenic compounds resulting from reduced renal elimination (13). Reduced kidney function and chronic kidney disease are also commonly associated with thyroid dysfunction, goiter, and nodules (17–19), which have been hypothesized to play a direct role in thyroid cancer development (20–27). We cannot rule out the possibility that diagnostic work-up of conditions that are prevalent in ESRD patients, including benign thyroid conditions and secondary hyperparathyroidism, could lead to incidental detection of thyroid cancer, but such work-up would not be expected to lead to an association with regional/distant stage thyroid cancer. We previously reported that, among ESRD patients in the Transplant Cancer Match Study, a higher incidence of thyroid cancer was present during periods of dialysis than during transplant intervals (when patients were receiving immunosuppressant medications) (28). Similar findings were observed in a cohort of kidney transplant recipients in Australia and New Zealand (15,29). Here we noted that risk of thyroid cancer, particularly regional/distant stage cancer, was highest among kidney recipients with the greatest duration of dialysis prior to transplant (5+ years). Thus, ESRD and factors associated with greater duration of dialysis appear to contribute to the increased risk of thyroid cancer among kidney recipients.
Nonetheless, it remains unclear whether transplant recipients would benefit from screening to allow for early detection of thyroid cancer. Thyroid cancer is typically associated with an excellent prognosis unless diagnosed at an advanced stage (4), and risks of thyroid cancer recurrence and thyroid cancer mortality (which reflects both cancer incidence and survival following diagnosis) have not appeared to be increased among solid organ transplant recipients (30–34). There are substantial concerns about over-diagnosis and over-treatment of small thyroid cancers in the general population, including well-documented health and financial implications for affected patients (35, 36). We note that a diagnosis of thyroid cancer may delay listing for patients with end-stage organ disease, or lead to their removal from the wait list (37). Additionally, a post-transplantation diagnosis of thyroid cancer may change the course of immunosuppressant therapy (38). Our results showing slightly increased risks of death and graft failure/retransplantation following a post-transplant diagnosis of thyroid cancer could reflect effects of the cancer itself, or may be primarily due to the adverse impact of a reduction in immunosuppression. Thus, a more comprehensive analysis weighing the risks and benefits of thyroid cancer screening among transplant recipients is warranted.
We did not see strong associations with immunosuppressant medications, as we have observed previously for other cancers (39–41). Among maintenance medications prescribed at discharge, use of cyclosporine and azathioprine was associated with lower risk of thyroid cancer than use of tacrolimus and mycophenolate mofetil. Although there have been temporal changes in the use of these medications, our analyses adjusted for calendar year of transplant. When we restricted to transplants occurring between 1998 and 2002, a period when all medications of interest were in use, most of the results did not change importantly; however, initial use of mycophenolate mofetil (versus no use of this medication) during this time was associated with a 2.35-fold increased risk of overall thyroid cancer and an 8.60-fold increased risk of regional/distant thyroid cancer. The reason for this association is unclear. We were unable to confirm our previous finding of a greater than three-fold higher risk of thyroid cancer following induction immunosuppression with alemtuzumab (42), either overall or among kidney recipients (data not shown). However, our earlier finding was based on shorter follow-up and a smaller number of cases.
Finally, individuals whose medical indication for liver transplantation was cholestatic liver disease/cirrhosis had a 69% increased incidence of thyroid cancer compared to the average; the risk was similarly elevated for PSC and PBC. This finding is unexplained as, to our knowledge, liver diseases are not a well-recognized risk factor for thyroid cancer. Thyroid cancer incidence was not especially increased among heart or lung recipients, who typically receive the most intensive immunosuppressive therapy. This finding, along with the absence of increased incidence among HIV-infected individuals (11), further supports that immunosuppression itself is not a strong risk factor for thyroid cancer.
Strengths of our study include the representative sample of transplant recipients, selected based on geographic coverage by cancer registries and including approximately 45% of all U.S. transplant recipients. Although there were some differences between transplants that were included versus excluded from the study population (in large part due to the recipient’s state of residence) in terms of race/ethnicity and calendar year of transplantation, these differences would not have affected the results shown here, and the two groups were otherwise very similar. Cancer registry follow-up allowed nearly complete ascertainment of thyroid cancers for individuals who resided in participating states. We also had information on a wide range of demographic characteristics, indications for transplant, and immunosuppressant medications. Information on stage at diagnosis allowed us to distinguish thyroid cancers according to aggressive potential.
Limitations not already discussed include possible under-ascertainment of thyroid cancer cases, particularly for patients who moved out of state following transplantation. However, we expect this bias to be small, considering that only 6% of transplant recipients no longer resided in their initial state or region 10 years after transplantation (7). We lacked information on dose and changes in immunosuppressant medications received by transplantation recipients, which limited our assessment of the effects of these medications on thyroid cancer risk. We note that some subgroups of transplant recipients had few cancer cases, and that wide confidence intervals for some IRRs convey uncertainty regarding the associations. Also, our results were not adjusted for multiple comparisons, and some findings may be due to chance alone.
In summary, our findings indicate that the elevated incidence of thyroid cancer among transplant recipients may not be explained entirely by heightened surveillance and over-diagnosis. We found that hypertensive nephrosclerosis as an indication for kidney transplant and longer duration of prior dialysis were each associated with a higher incidence of thyroid cancer among kidney recipients, suggesting that the metabolic or endocrine effects of kidney disease and factors associated with longer duration on dialysis (5+ years) may contribute to thyroid carcinogenesis. Thyroid cancer incidence was also increased among liver recipients whose indication for transplant was cholestatic liver disease/cirrhosis, which may provide further etiologic clues.
Supplementary Material
Table S1: Associations of thyroid cancer with pre-transplant history of diabetes, body mass index, HLA mismatch, and, among kidney recipients, PRA.
Table S2: Standardized incidence ratios for thyroid cancer in transplant recipients, excluding the first year of follow-up
Table S3: Associations of thyroid cancer with demographic and transplant characteristics among solid organ transplant recipientsa, excluding the first year of follow-up
Table S4: Associations of thyroid cancer with immunosuppressive medication use reported at hospital discharge among solid organ transplant recipients, excluding the first year of follow-up
Table S5: Associations of thyroid cancer with underlying medical indication for transplant among kidney and liver recipients, excluding the first year of follow-up
Table S6: Associations of thyroid cancer with duration on dialysis prior to transplantation among kidney recipients, excluding the first year of follow-up
Acknowledgments
The authors acknowledge the support and assistance provided by individuals the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa (Charles Lynch), Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Sumathy Vasanthan), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021, N01-PC-2013-0021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (5U58/DP003931-02), New York (U58DP003879), North Carolina (U58DP000832), and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
This research was supported by the Intramural Research Program of the National Cancer Institute.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- ICD-O
International Classification of Diseases for Oncology
- IRR
incidence rate ratio
- PTC
papillary thyroid cancer
- SEER
Surveillance, Epidemiology, and End Results
- SIR
standardized incidence ratio
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DR. ELIZABETH YANIK (Orcid ID : 0000-0002-5835-0201)
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 5.Ron E, Lubin JH, Shore R, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation-a pooled analysis of 7 studies. Radiat Res. 1995;141(3):259–277. [PubMed] [Google Scholar]
- 6.Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016;26:306–18. doi: 10.1089/thy.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tessari G, Naldi L, Boschiero L, Minetti E, Sandrini S, Nacchia F, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. Am J Transplant. 2013;13(1):214–221. doi: 10.1111/j.1600-6143.2012.04294.x. [DOI] [PubMed] [Google Scholar]
- 9.Karamchandani D, Arias-Amaya R, Donaldson N, Gilbert J, Schulte KM. Thyroid cancer and renal transplantation: a meta-analysis. Endocrine-Related Cancer. 2010;17(1):159–167. doi: 10.1677/ERC-09-0191. [DOI] [PubMed] [Google Scholar]
- 10.Kluijfhout WP, Drake FT, Pasternak JD, Beninato T, Mitmaker EJ, Gosnell JE, et al. De novo thyroid cancer following solid organ transplantation—a 25-year experience at a high-volume institution with a review of the literature. J Surg Oncol. 2017;115(2):105–108. doi: 10.1002/jso.24495. [DOI] [PubMed] [Google Scholar]
- 11.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 12.Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77(12):1777–1782. doi: 10.1097/01.tp.0000120181.89206.54. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved. Am J Nephrol. 1998;18(2):89–95. doi: 10.1159/000013314. [DOI] [PubMed] [Google Scholar]
- 14.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354(9173):93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 15.Vajdic CM, McDonald SP, McCredie MRE, Van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 16.Egevad L, Heanue M, Berney D, Fleming K, Ferlay J. In: Cancer Incidence in Five Continents. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, editors. IX. International Agency for Research on Cancer; Lyon: 2007. pp. 61–66. (IARC Scientific Publications No. 160). Chapter 4. [Google Scholar]
- 17.Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev. 1996;17(1):45–63. doi: 10.1210/edrv-17-1-45. [DOI] [PubMed] [Google Scholar]
- 18.Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney International. 2005;67(3):1047–1052. doi: 10.1111/j.1523-1755.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chang Y, Ryu S, Cho J, Lee WY, Rhee EJ, et al. Thyroid hormone levels and incidence chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. Int J Epidemiol. 2014;43(5):1624–1632. doi: 10.1093/ije/dyu126. [DOI] [PubMed] [Google Scholar]
- 20.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91(11):4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 21.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93(3):809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20(1):75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldi S, Plummer M, Biessy C, Tsilidis KK, Ostergaard JN, Overvad K, et al. Thyroid stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J Natl Cancer Inst. 2014;106(6):dju097. doi: 10.1093/jnci/dju097. [DOI] [PubMed] [Google Scholar]
- 24.Balasubramaniam S, Ron E, Gridley G, Schneider AB, Brenner AV. Association between benign thyroid and endocrine disorders and subsequent risk of thyroid cancer among 4.5 million U.S. male veterans. J Clin Endocrinol Metab. 2012;97(8):2661–2669. doi: 10.1210/jc.2011-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellemgaard A, From G, Jørgensen T, Johansen C, Olsen JH, Perrild H. Cancer risk in individuals with benign thyroid disorders. Thyroid. 1998;8(9):751–754. doi: 10.1089/thy.1998.8.751. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi S, Preston-Martin S, Dal Maso L, Negri E, La Vecchia C, Mack WJ, et al. A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control. 1999;10(6):583–595. doi: 10.1023/a:1008907227706. [DOI] [PubMed] [Google Scholar]
- 27.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, et al. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol. 2010;171(2):242–252. doi: 10.1093/aje/kwp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanik EL, Clarke CA, Snyder JJ, Pfeiffer RM, Engels EA. Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J Am Soc Nephrol. 2016;27(5):1495–1504. doi: 10.1681/ASN.2015040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Leeuwen MT, Webster AC, McCredie MRE, Stewart JH, McDonald SP, Amin J, et al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ. 2010;340:c570. doi: 10.1136/bmj.c570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluijfhout WP, Drake FT, Pasternak JD, Beninato T, Mitmaker EJ, Gosnell JE, et al. De novo thyroid cancer following solid organ transplantation—a 25-year experience at a high-volume institution with a review of the literature. J Surg Oncol. 2017;115(2):105–108. doi: 10.1002/jso.24495. [DOI] [PubMed] [Google Scholar]
- 31.Acuna SA, Fernandes KA, Daly C, Hicks LK, Sutradhar R, Kim J, et al. Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol. 2016;2(4):463–469. doi: 10.1001/jamaoncol.2015.5137. [DOI] [PubMed] [Google Scholar]
- 32.Farrugia D, Mahboob S, Cheshire J, Begaj I, Khosla S, Ray D, et al. Malignancy-related mortality following kidney transplantation is common. Kidney International. 2014;85(6):1395–1403. doi: 10.1038/ki.2013.458. [DOI] [PubMed] [Google Scholar]
- 33.Kiberd BA, Rose C, Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. 2009;9(8):1868–1875. doi: 10.1111/j.1600-6143.2009.02728.x. [DOI] [PubMed] [Google Scholar]
- 34.Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am J Transplant. 2013;13(1):1296–1304. doi: 10.1111/ajt.12192. [DOI] [PubMed] [Google Scholar]
- 35.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brattström C, Granath F, Edgren G, Smedby KE, Wilczek HE. Overall and cause-specific mortality in transplant recipients with a pretransplantation cancer history. Transplantation. 2013;96(3):297–305. doi: 10.1097/TP.0b013e31829854b7. [DOI] [PubMed] [Google Scholar]
- 38.Ajithkumar TV, Parkinson CA, Butler A, Hatcher HM. Management of solid tumours in organ-transplant recipients. Lancet Oncol. 2007;8(10):921–932. doi: 10.1016/S1470-2045(07)70315-7. [DOI] [PubMed] [Google Scholar]
- 39.Safaeian M, Robbins HA, Berndt SI, Lynch CF, Fraumeni JF, Jr, Engels EA. Risk of colorectal cancer after solid organ transplantation in the United States. Am J Transplant. 2016;16(3):960–967. doi: 10.1111/ajt.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015;135(11):2657–2665. doi: 10.1038/jid.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, et al. Risk of merkel cell carcinoma after solid organ transplantion. J Natl Cancer Inst. 2015;107(2) doi: 10.1093/jnci/dju382. pii: dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall EC, Engels EA, Pfeiffer RM, Segev DL. Association of antibody induction immunosuppression with cancer after kidney transplantation. Transplantation. 2015;99(5):1051–1057. doi: 10.1097/TP.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Associations of thyroid cancer with pre-transplant history of diabetes, body mass index, HLA mismatch, and, among kidney recipients, PRA.
Table S2: Standardized incidence ratios for thyroid cancer in transplant recipients, excluding the first year of follow-up
Table S3: Associations of thyroid cancer with demographic and transplant characteristics among solid organ transplant recipientsa, excluding the first year of follow-up
Table S4: Associations of thyroid cancer with immunosuppressive medication use reported at hospital discharge among solid organ transplant recipients, excluding the first year of follow-up
Table S5: Associations of thyroid cancer with underlying medical indication for transplant among kidney and liver recipients, excluding the first year of follow-up
Table S6: Associations of thyroid cancer with duration on dialysis prior to transplantation among kidney recipients, excluding the first year of follow-up


