Abstract
Target identification and contact selection are known contributors to variability in efficacy across different clinical indications of deep brain stimulation surgery. A retrospective analysis of responders to subcallosal cingulate deep brain stimulation (SCC DBS) for depression demonstrated the common impact of the electrical stimulation on a stereotypic connectome of converging white matter bundles (forceps minor, uncinate fasciculus, cingulum and fronto-striatal fibers). To test the utility of a prospective connectomic approach for SCC DBS surgery, this pilot study used the four-bundle tractography “connectome blueprint” to plan surgical targeting in eleven participants with treatment-resistant depression. Before surgery, targets were selected individually using deterministic tractography. Selection of contacts for chronic stimulation was made by matching the postoperative probabilistic tractography map to the presurgical deterministic tractography map for each subject. Intraoperative behavioral responses were used as a secondary verification of location. A probabilistic tract map of all participants demonstrated inclusion of the four bundles as intended, matching the connectome blueprint previously defined. Eight of 11 patients (72.7%) were responders and 5 were remitters after 6 months of open-label stimulation. At one year, nine of 11 patients (81.8%) were responders, with six of them in remission. These results support the utility of a group probabilistic tractography map as a connectome blueprint for individualized, patient-specific, deterministic tractography targeting, confirming retrospective findings previously published. This new method represents a connectomic approach to guide future SCC DBS studies.
Keywords: Depression, Deep Brain Stimulation, Connectomics, Tractography, Subcallosal Cingulate
Introduction
Deep brain stimulation (DBS) is a developing experimental therapy for treatment resistant depression (TRD), and a range of grey and white matter targets are being studied, including the subcallosal cingulate (SCC), the ventral capsule/ventral striatum (VC/VS), the nucleus accumbens, the lateral habenula, the inferior thalamic peduncle, and the medial forebrain bundle (1–8). The SCC white matter target has been investigated most extensively with published data now reported for 77 patients implanted at eight separate centers (9). The response rate at 6 months in these open-label reports range between 33 and 87.5%. Combined results of all open-label studies (n=77) demonstrate a response rate of 53% at 6 months and 47% at 12 months, while remission rates are 27% at 6 months and 30% at 12 months (1, 2, 10–15). While generally positive, the results from open-label DBS studies in depression have not been replicated in large scale randomized controlled studies. A double-blind, randomized clinical trial failed to show efficacy in the VC/VS target and a similar one in the SCC was halted (16, 17). While sources of trial failure are likely multifactorial, the precision of surgical targeting is a critical and modifiable variable. Target identification and contact selection are known contributors to variability in efficacy across different clinical indications of DBS surgery. For example, even after 25 years of clinical experience the optimal imaging method to localize the subthalamic DBS target to treat Parkinson’s disease remains a focus of ongoing refinement (18, 19). In comparison, the nuances of surgical targeting for DBS to treat depression are only beginning to be addressed. The accuracy ensures as well that contact selection is limited within certain anatomical boundaries, therefore considerably limiting all the possible combination of contacts to be selected for stimulation, and making procedural steps simpler.
Targeting of the SCC for DBS in the initial proof of principle study was guided by identifying the anatomical location of metabolic changes seen on PET scans with successful antidepressant response to other treatments (1). As the intended goal of DBS at this site was to modulate this ‘SCC hub’ as well as remote cortical and subcortical areas connected to it, the surgical target included both grey and white matter (1). All subsequent published studies studies (as well as the industry-sponsored SCC DBS trial) have followed this basic anatomical approach. However, this is a region of high anatomical variability without clear anatomical landmarks identifiable with routine in vivo imaging. As such, attempts to better define the ’optimal’ implant location using standard anatomical coordinates have not proven useful (20, 21). Further, methods to confirm optimized lead implantation using microelectrode recordings are not yet reliable in this target, most likely because subsequent analyses have determined that therapeutic stimulation is primarily delivered in white matter (10).
Antidepressant effects of chronic high-frequency DBS likely involve modulation of a distributed, multi-region network in addition to local changes in the SCC region (22). Our group described in a retrospective study that clinical response to SCC DBS is mediated by direct impact on a combination of fiber bundles passing through the SCC region. These fiber bundles included bilateral forceps minor of the anterior corpus callosum connecting the right and left medial frontal cortices, the bilateral cingulum bundles connecting ipsilateral subcallosal cingulate to rostral, dorsal anterior, and midcingulate cortices; and medial branch of the uncinate fasciculus bilaterally connecting subcallosal cingulate and medial frontal cortex rostrally and subcallosal cingulate to the nucleus accumbens, anterior thalamus, and other subcortical regions caudally. All responders to SCC DBS, regardless of the time point chosen (6 months or 2 years) shared this common map (23). With this in mind, DBS targeting might now be optimized using a formalized “connectomics” approach (24, 25).
This pilot study tests the potential utility of using an individualized tractography map that is based on a group “connectome blueprint” of past responders to prospectively identify the SCC DBS surgical target. The hypothesis is that this method can guide surgical implantation and contact selection for chronic stimulation, thus reducing one of the key degrees of freedom impacting variability in DBS for TRD.
Methods
Participants and clinical protocol
Eleven consecutive patients with severe, chronic TRD were enrolled in a research protocol at Emory University testing safety and efficacy of SCC DBS for TRD (clinicaltrials.gov NCT00367003). Participants provided written informed consent to participate. The protocol was approved by the Emory University Institutional Review Board and the US Food and Drug Administration under an Investigational Device Exemption (G060028 held by H.S.M.) and is monitored by the Emory University Department of Psychiatry and Behavioral Sciences Data and Safety Monitoring Board.
The inclusion and exclusion criteria were essentially identical to those previously published by Holtzheimer et al. describing the first seventeen subjects implanted at Emory University (15). Key inclusion criteria consisted of: 18 to 70 years-old with a diagnosis of major depressive disorder (confirmed with the Structured Clinical Interview for DSM-IV and by the study psychiatrists (PRP, SJG, PEH, ALC)); a current depressive episode of at least 12 months without significant response to at least 4 adequate antidepressant treatments (scoring 3 or higher on the Antidepressant Treatment History Form and verified through medical records); lifetime failure or intolerance of electroconvulsive therapy or inability to receive electroconvulsive therapy; average score ≥ 20 on the 17-item Hamilton Depression Rating Scale (HDRS-17) averaged over the four weeks prior to surgery; Global Assessment of Functioning (GAF) of 50 or less; capacity to provide informed consent; and being able to relocate to the Atlanta area for 7 months (26–29).
Key exclusion criteria were defined as: clinically significant medical or psychiatric comorbidity (including personality disorders as determined by a review of medical records, the Structured Clinical Interview-II, and clinical examination); active substance use disorder; active suicidal ideation with plan or intent, a suicide attempt within the past 6 months, or more than 2 suicide attempts within the past 2 years; pregnancy or planning to become pregnant during the study; or contraindication for DBS surgery or chronic stimulation.
The preoperative evaluation lasted a minimum of 4 weeks. Chronic stimulation was initiated 4 weeks following surgery. Outcome was assessed weekly for 24 weeks of active stimulation using the HDRS-17. Response to treatment was defined as at least a 50% decrease and remission as 7 points or less on the HDRS-17 at 24 weeks. For any missing timepoint the previous observation was carried forward to the next timepoint. Medication changes were not allowed during the preoperative evaluation phase or the initial 24 weeks of chronic DBS. All patients received cognitive behavioral therapy. Patients were aware that they were receiving active stimulation, but were not privy to the criteria for target identification, contact selection or dose increases. All procedures were carried out at Emory University.
Procedure for subcallosal cingulate cortex deep brain stimulation
Figure 1 illustrates the procedure for subcallosal cingulate deep brain stimulation including pre-surgical planning, surgical procedure, and post-surgical analysis.
Figure 1.
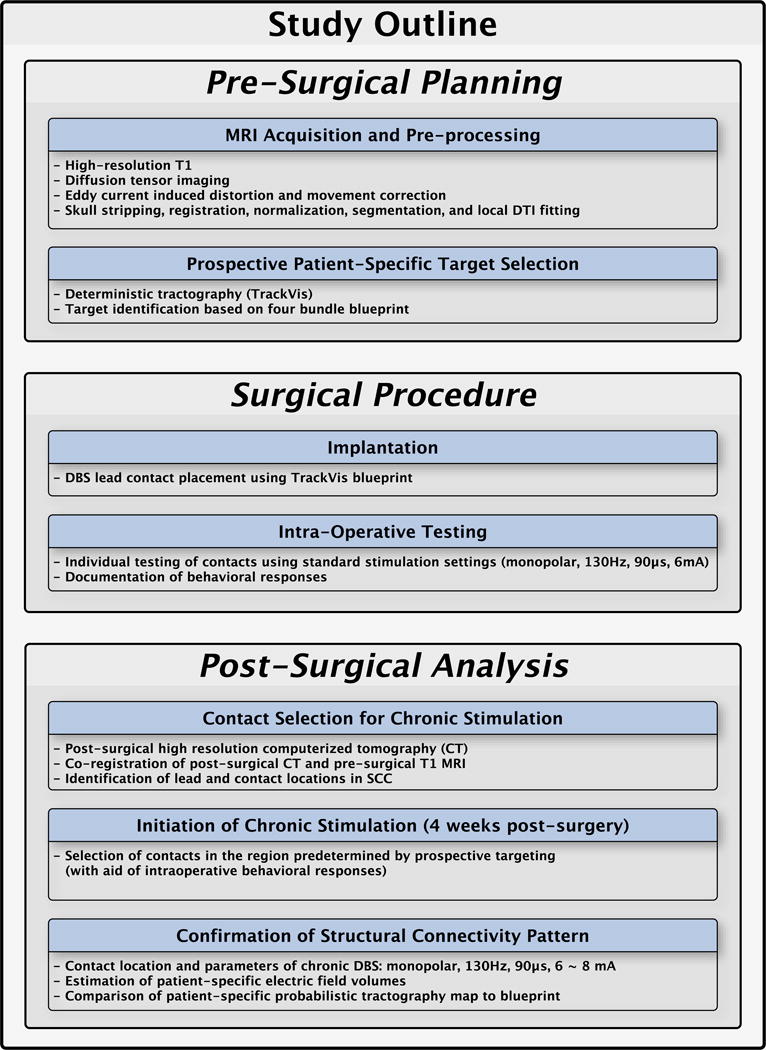
Flowchart of pre-surgical, surgery, and post-surgical analysis
A. Pre-surgical planning
Magnetic resonance image acquisition and pre-processing
High resolution T1 and diffusion-weighted images were collected within a single session for each subject on a research-dedicated Siemens 3T Tim-Trio scanner (Siemens Medical Solution, Malvern, PA, USA). All image data were preprocessed using tools from FSL software (FMRIB software Library, http://www.fmrib.ox.ac.uk/fsl)(30, 31). High-resolution T1 images underwent skull stripping (32), image registration and normalization to an MNI152 template (MNI152 standard-space T1-weighted average structural temple image, FSL, FMRIB), and image segmentation into into grey matter (GM)/white matter (WM)/cerebrospinal fluid (CSF)(30, 33). DWI data underwent the simultaneous eddy current and distortion correction with rotation of b-vector, image registration to T1, and local tensor fitting (34). Detailed image acquisition parameters and pre-processing steps are described in Supplement 1.
Prospective patient-specific target selection
Structural connectivity-based target selection was performed in each patient using deterministic tractography (TrackVis, TrackVis.org) in the week before surgery (35). The target was defined on each patient’s own MRI-DTI scan to impact the predefined 4-bundle white matter blueprint: uncinate fasciculus, forceps minor, cingulum bundle, and fronto-striatal fibers (Figure 2 – left panel). The ‘blueprint’ was used as a archetype or reference template to prospective identify the optimal ‘target’ on the actual deterministic tractography scan. A 3 mm radius spherical region of interest (e.g., ‘seed’) was placed in the SCC corresponding to the estimated volume of tissue activated by standard DBS parameters. Volumes were defined using the predictive algorithm described in Chaturvedi et al. (36)(for details, see supplement 1). Tracks from the seed were generated in each hemisphere. A dynamic evaluation of small modifications in the seed was performed independently by three investigators (HSM, PRP, KSC) to define the target location that best visually matched the reference ‘blueprint’. A consensus target and lead trajectory was finalized with the neurosurgeon (REG) in the operating room (Figure 2 – right panel), who planned the insertion of the leads avoiding cerebral vasculature and choosing the point of entry. Once the target was determined, a verification with tractography was confirmed, so it did not deviate from the presurgically defined plan.
Figure 2.
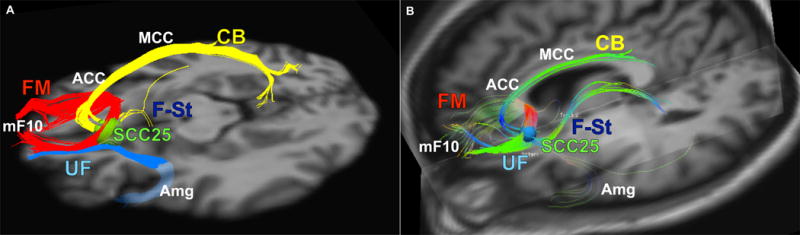
structural connectivity based target selection for one subject
B. Surgical procedure
Implantation surgery
The surgical procedure for DBS lead and pulse generator implantation followed published methods (15). The combined tractography and anatomical images guided standard localization of the DBS lead tip and trajectory using a surgical planning workstation (StealthStation S7, Medtronic Inc, Louisville, CO). Bilateral DBS leads (Libra system, St Jude Medical, Plano, TX), each with 4 contacts (1.5 mm inter-contact spacing) were inserted and secured and with the patient awake and alert for testing initiated thereafter. Placement of the implantable pulse generator (IPG) and extension cables was performed under general anesthesia following completion of intra-operative testing.
Intraoperative testing
Individual contacts on both leads (4 per side) with 4 interspersed sham stimulation conditions were tested in random order, with the clinical rater and patient blinded to the conditions. Stimulation parameters were the same as used for initial chronic stimulation (6mA, 130 Hz, 90 μsec). Stimulation of contacts in the presurgically-defined optimal target areas elicited distinct intraoperative responses (37). Each stimulation condition lasted 3 minutes, during which the patient was asked to report any behavioral changes. Contacts that generated a salient response were noted. These behaviorally salient responses usually have a change in patient’s relationship with the external world (i.e. desire to be with one’s partner doing something enjoyable) as well as an interoceptive change (i.e. “feeling lighter” or “warmer”, a release of the heaviness of depression).
C. Post-surgical analysis
Contact Selection for chronic stimulation
Final location of the DBS leads and respective optimal contact in each hemisphere for each subject were identified in native space based on post-surgical high-resolution computerized tomography (CT) images acquired on a LightSpeed16 (GE Medical System; resolution 0.46×0.46×0.65 mm3) and then transferred to T1 MRI space by linear transformation (FLIRT toolbox, FSL). The CT scan was performed about three weeks after surgery to allow for resolution of brain edema or electrode shift post-implantation. The post-surgical CT, preoperative MRI and preoperative DWI were merged. This hybrid 3D image was used to generate probabilistic tractography maps for each of the eight contacts using Fdt toolkit in FSL. Details of this probabilistic tractography method is described in supplement 1.
Initiation of chronic stimulation
Final selection of a single right and left contact for chronic stimulation in each subject was made by comparing the postoperative probabilistic tractography map to the presurgical deterministic tractography map and confirming a match in the intended inclusion of the 4-white matter bundles. Intraoperative responses from the blinded acute stimulation were used as a secondary verification of a match between the intended and final contact location. In cases when two contacts had similar tractography patterns on the post-op probabilistic maps, the selection of one contact over the other was determined by the presence and quality of evoked intraoperative behavior.
Stimulation was initiated in all patients four weeks after surgery and continued for the 24 weeks open-label phase using standard stimulation settings (monopolar stimulation, current = 6 mA, frequency = 130 Hz, pulse width = 91 microseconds). In this pilot study of a refined targeting method, contact changes prior to 6 months were not made unless the participant had a deterioration in mood. Parameters remained stable, and adjustments were only made in current (6 to 8 mA) if HDRS-17 scores plateaued or worsened over a four-week interval. No changes in pulse width or frequency were made.
Post-treatment/Post-surgical confirmation of structural connectivity pattern in responders
A common probabilistic structural connectivity map was created for responders at 24 weeks using methods previously developed (23). Whole brain tractography was calculated using bilateral stimulation volumes as seeds with the results binarized with a threshold value of 0.1%. A common population map of voxels shared by all subjects was generated (e.g., all subjects have these tracts) (see supplement 1 for detailed methods).
Stimulation contact refinements after 6 months in nonresponders
As the purpose of this study was to evaluate the utility of tractography-based targeting, alternative contacts were considered in patients who did not meet response criteria after 6 months of chronic stimulation. At this point, an evaluation of the individual probabilistic tractography maps for the remaining 6 contacts was made in order to search of an option that had an equivalent or better pattern of connectivity with respect to the first contact selection. If no contact met these criteria, parameters were not changed regardless of non-response status. No other parameter changes, such as frequency or pulse width, were employed, and no patients received stimulation on more than 1 contact per hemisphere. The maximum current allowed was 8 mA.
Statistical analysis
Characteristics of the study sample were summarized using mean and standard deviation for continuous variables, and percentage for categorical variables. Clinical response rates were calculated using HDRS-17 scores at 24 weeks and one year relative to the preoperative baseline. Probabilistic group tract maps were compared to the previously published tractography ‘blueprint’ using qualitative assessments of 3D common maps for both groups.
Results
Clinical outcomes
All 11 patients completed the protocol. Eight of 11 patients (72.7%) were responders and 6 were in remission after the initial 24 weeks (six months) of stimulation (Table 2, Figure 3). At one year, nine of 11 patients (81.8%) were responders and 6 were in remission. One participant did not have the 12-month evaluation but was considered a responder because the 9 and 18-month evaluations indicated a 50% or greater response in HDRS-17. One of the three six-month nonresponders became a responder at one year. Two of the 11 patients never met response criteria, one with minimal variation in the depression scores, the other having a 40% decline at 12 months (participants 5 and 6 in Table 2).
Table 2.
(HDRS-17 monthly)
| Patient | Baseline | Stim On | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24.25 | 27 | 21 | 22 | 15 | 21 | 17 | 13 | 11 | 9 |
| 2 | 27.5 | 12 | 14 | 9 | 17 | 7 | 9 | 6 | 12 | 10 |
| 3 | 23.75 | 21 | 19 | 19 | 25 | 15 | 11 | 9 | 10 | 5 |
| 4 | 22 | 15 | 16 | 13 | 13 | 16 | 10 | 6 | 10 | 9 |
| 5 | 22.75 | 22 | 19 | 18 | 19 | 16 | 17 | 16 | 23 | 19 |
| 6 | 20.25 | 21 | 19 | 19 | 16 | 16 | 18 | 17 | 11 | 12 |
| 7 | 26.25 | 20 | 18 | 19 | 26 | 17 | 11 | 12 | 8 | 6 |
| 8 | 19.5 | 7 | 10 | 12 | 5 | 3 | 2 | 7 | 4 | 1 |
| 9 | 25.25 | 18 | 8 | 6 | 6 | 6 | 7 | 6 | 10 | 10* |
| 10 | 21 | 17 | 6 | 5 | 4 | 9 | 3 | 6 | 1 | 2 |
| 11 | 21.5 | 15 | 9 | 8 | 15 | 10 | 9 | 6 | 8 | 5 |
| Mean (Std Dev) |
23.09 (2.55) |
17.73 (5.42) |
14.45 (5.32) |
13.64 (6.04) |
14.64 (7.39) |
12.36 (5.63) |
10.36 (5.35) |
9.45 (4.3) |
9.82 (5.47) |
8 (5.04) |
Patient missed 1 year appointment (returned for 18 month appointment). This is the 9 month score
Figure 3.
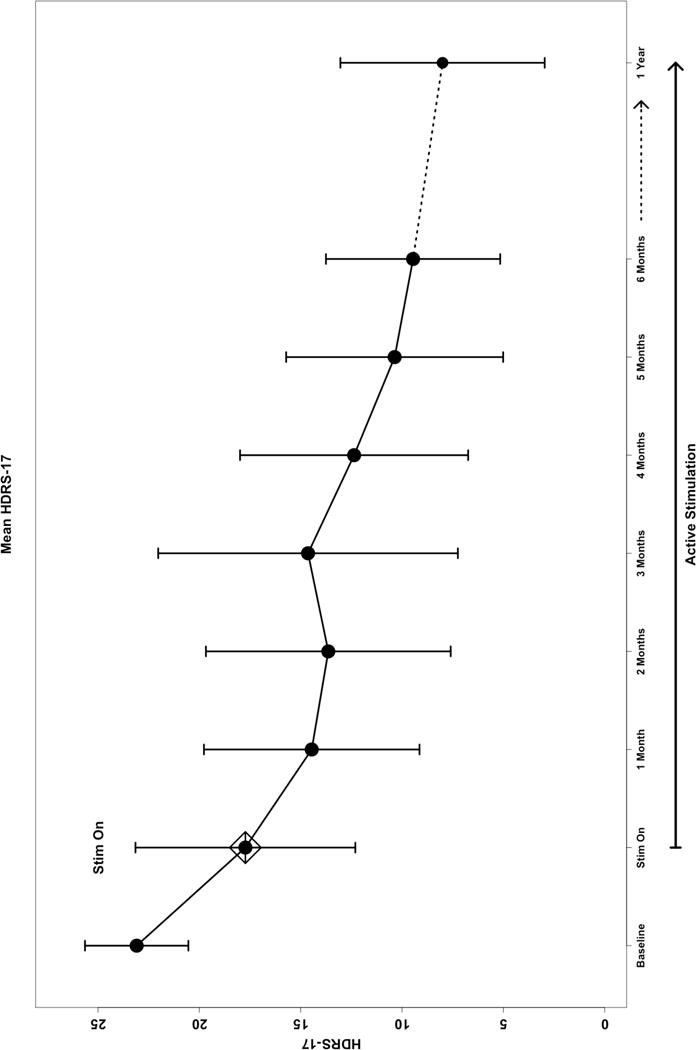
(HDRS-17 graph)
Impact of Tractography-guided Targeting on Contact and Parameter Selection
All patients had bilateral contacts that matched the four-bundle blueprint. Of the 22 individual contacts (2/patient) used for chronic DBS, only two (one contact in two patients) were changed within the first year of stimulation. One contact was changed in the first week of active stimulation due to deterioration and an atypical transient response (dysphoria and emotional lability without mania during the first minute of stimulation). Symptoms resolved with this contact change, and response was achieved and maintained without further changes. Both contacts selected matched the four-bundle blueprint. Eight of the 11 subjects had an increase in current from 6 to 8 mA within the first six months. No changes were made in pulse width or frequency.
The tractography maps in the three six-month nonresponders were reevaluated and only one participant warranted a contact change based on the preestablished criteria. In this case the new contact better matched the blueprint with more symmetric involvement of tracts in the subcortical fibers (Supplementary Figure 1). This patient improved clinically but did not yet meet response criteria at one year; but became a sustained responder at a later timepoint.
The two remaining six-month nonresponders did not have alternative contacts that yielded an improvement over the initial selection, thus neither was changed. One subject was nonetheless a responder at 1 year; the other remained a long-term nonresponder (Supplementary figure 2).
Reproducibility of the deterministic tractography method for targeting
The common probabilistic tract map generated for all subjects at six months (both responders and non responders) demonstrates inclusion of forceps minor, uncinate fasciculus, fronto-striatal fibers and cingulum bundle as intended. This common response map matches the connectome blueprint defined by the responders in the initial cohort (Figure 4).
Figure 4.
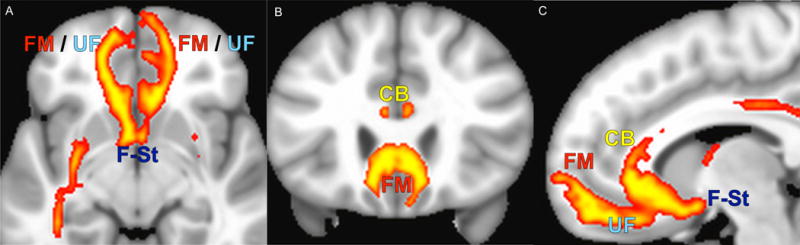
(Common map)
Discussion
This pilot study tested the implementation of a modified method for SCC DBS targeting using prospective tractography. The four-bundle white matter blueprint was reliably defined and precisely implanted in each of the 11 subjects. Stimulation maintained at these targets resulted in good clinical outcomes. These results support the utility of a group probabilistic tractography blueprint (Figure 2A) for individualized, patient-specific, deterministic tractography targeting (Figure 2B), confirming retrospective findings previously published (23).
While this open-label study was not designed or powered to directly compare the efficacy of DBS to our previous cohort (15) or any other SCC DBS targeting method or cohort (2, 10–14), the 73% response rates at 6 months and 82% at 1 year are notable, with stable and even increasing response rates up to the 1 year endpoint. For practical consideration in the planning and implementing any future SCC DBS study, reliance on the tractography-defined optimal location resulted in few contact changes and current adjustments within and across subjects over the duration of the protocol.
We can conclude that based on the analysis of this cohort, deterministic tractography can be reliably performed in an individual subject to choose the implantation target in the SCC, augmenting the standard approach that relies solely on anatomical landmarks or stereotactic coordinates (21).
One of the most important benefits of our tractography-guided methodology was the simplified programming in the active stimulation phase during the clinical study. Establishing that stimulation through the appropriately selected contact in the patient matched the predefined tractography blueprint gave reassurance to the team that the response is not likely to depend on further interrogation of the parameter space. Instead, a focus on other contributors to nonresponse - unrelated to stimulation - could be pursued, as is true for pharmacological antidepressant interventions. Such factors include more systematic attention to variability in clinical or demographic characteristics of TRD subjects who meet the already restricted inclusion/exclusion criteria as well as examination of potential imaging, genetic or other biomarkers that might further define this SCC-responsive TRD subtype (38). Given the few number of non-responders in this small cohort, these types of analyses will require larger samples.
An unresolved issue is which combination of the 4 bundles within our tractography blueprint is sufficient to achieve antidepressant response to SCC DBS. The independent contribution of individual bundles or the relative importance of left versus right sided stimulation is currently unknown. Post-hoc analysis of the relative contribution of the right sided contacts to left sided contacts demonstrates symmetric contribution to the forceps minor from each hemisphere in all subjects (data not shown). However, limitations of this generation of deterministic and probabilistic tractography methods are that they cannot disambiguate direct connections between the SCC and the frontal cortex from fibers of passage in and around the stimulated zone. Similarly, the lack of precise resolution within the stimulated zone cannot separate forceps minor from uncinate fasciculus. Next generation scanners and diffusion sequences do have potential to allow more detailed studies of the critical pathways mediating the SCC DBS antidepressant effects (39–41). Concurrent physiological recordings and/or functional imaging will also be required to further address mechanisms mediating DBS antidepressant effects facilitated by targeting these pathways (42).
While our results cannot explain the reasons for failed randomized controlled trials of this or other DBS targets for neuropsychiatric disorders, these findings do support the use of tractography-based surgical targeting to reduce variability in the direct effects of stimulation on the patient-specific brain circuitry. In turn, we propose that our new methodology enables more consistent and precise modulation of a predefined collection of axonal pathways in all study subjects. This strategy enables a more controlled analysis of the population and streamlines the stimulation programming. Standardization of targeting and programming algorithms informed by these results may help to refine study design and interpretation of outcomes of future studies.
Supplementary Material
Table 1.
Patient Characteristics
| Characteristics | Mean (SD) or Count |
|---|---|
| Age at Surgery (years) | 48.73 (10.10) |
| Sex | Female (9), Male (2) |
| Employed at Time of Surgery | 2 of 11 |
| Baseline HDRS-17 | 23.09 (2.55) |
| Duration of Current Episode (months) | 36.36 (21.11) |
| Medications in Current Episode | 7.18 (1.83) |
| Age at First Depressive Episode (Years) | 20.45 (11.32) |
| Number of Depressive Episodes | 3.82 (1.47) |
Acknowledgments
Trial registration: clinicaltrials.gov NCT00367003 PI: Helen Mayberg Patricio Riva-Posse and KiSueng Choi share first authorship. They both contributed equally to the design, data analysis, preparation of the manuscript.
Funding sources: Hope for Depression Research Foundation, Dana Foundation, NIH R01MH102238 (PI: Cameron McIntyre). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors would like to thank the collaboration of the research staff: Sinéad Quinn, Kelsey Hagan, Morgan Woody, Lydia Denison, as well as the patients and their families. The authors would also like to acknowledge Mary Kelley, PhD (Rollins School of Public Health, Emory University) for her collaboration and input in data management. We would also like to acknowledge Angela Noecker (Biomedical Engineering, Case Western Reserve University) for assistance in the development of tractography models.
Footnotes
Trial registration: clinicaltrials.gov NCT00367003
Supplementary information: available at Molecular Psychiatry’s website.
Disclosures: Patricio Riva-Posse reported no biomedical financial interests or potential conflicts of interest. Ki Sueng Choi reported no biomedical financial interests or potential conflicts of interest. Paul E. Holtzheimer reported serving as a paid consultant to St Jude Medical Corp. Andrea L. Crowell reported no biomedical financial interests or potential conflicts of interest. Steven J. Garlow reported no biomedical financial interests or potential conflicts of interest. Justin K. Rajendra reported no biomedical financial interests or potential conflicts of interest. Robert E. Gross reported receiving grants from Medtronic Inc., Neuropace and MRI Interventions, honoraria from Medtronic Inc and MRI Interventions; and serving as a paid consultant to St Jude Medical Corp., Medtronic Inc., Neuropace, MRI Intervantions, Neuralstem and SanBio. Cameron C. McIntyre is a paid consultant for Boston Scientific Neuromodulation, and is a shareholder in the following companies: Surgical Information Sciences, Inc.; Autonomic Technologies, Inc.; Cardionomic, Inc.; Enspire DBS, Inc.; Neuros Medical, Inc. Helen S. Mayberg has a consulting agreement with St Jude Medical, Inc., which has licensed her intellectual property to develop SCC DBS for the treatment of severe depression (US 2005/0033379A1). Medtronic and St Jude Medical Corp develop products related to the research described in this article. The terms of these arrangements have been reviewed and approved by Emory University in accordance with their conflict of interest policies. Helen Mayberg had no role in patient selection, clinical ratings or access to raw patient data.
References
- 1.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological psychiatry. 2008;64(6):461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biological psychiatry. 2009;65(4):267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Medical hypotheses. 2007;69(6):1305–8. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585–93. doi: 10.1227/01.neu.0000170434.44335.19. discussion -93. [DOI] [PubMed] [Google Scholar]
- 7.Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biological psychiatry. 2013;73(12):1204–12. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhe HG, Notten P, van Laarhoven J, et al. Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA psychiatry. 2016;73(5):456–64. doi: 10.1001/jamapsychiatry.2016.0152. [DOI] [PubMed] [Google Scholar]
- 9.Crowell AL, Riva-Posse P, Garlow SJ, Mayberg H. Toward an Understanding of the Neural Circuitry of Major Depressive Disorder Through the Clinical Response to Deep Brain Stimulation of Different Anatomical Targets. Current Behavioral Neuroscience Reports. 2014;1(2):55–63. [Google Scholar]
- 10.Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, et al. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. Journal of neurosurgery. 2012;116(2):315–22. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 11.Puigdemont D, Perez-Egea R, Portella MJ, Molet J, de Diego-Adelino J, Gironell A, et al. Deep brain stimulation of the subcallosal cingulate gyrus: further evidence in treatment-resistant major depression. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–13. doi: 10.1017/S1461145711001088. [DOI] [PubMed] [Google Scholar]
- 12.Merkl A, Schneider GH, Schonecker T, Aust S, Kuhl KP, Kupsch A, et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Experimental neurology. 2013;249:160–8. doi: 10.1016/j.expneurol.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Ramasubbu R, Anderson S, Haffenden A, Chavda S, Kiss ZH. Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. Journal of psychiatry & neuroscience. 2013;38(3):120–60. doi: 10.1503/jpn.120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guinjoan SM, Mayberg HS, Costanzo EY, Fahrer RD, Tenca E, Antico J, et al. Asymmetrical contribution of brain structures to treatment-resistant depression as illustrated by effects of right subgenual cingulum stimulation. The Journal of neuropsychiatry and clinical neurosciences. 2010;22(3):265–77. doi: 10.1176/jnp.2010.22.3.265. [DOI] [PubMed] [Google Scholar]
- 15.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Archives of general psychiatry. 2012;69(2):150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biological psychiatry. 2015;78(4):240–8. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Schlaepfer TE. Deep Brain Stimulation for Major Depression—Steps on a Long and Winding Road. Biological Psychiatry. 2015;78(4):218–9. doi: 10.1016/j.biopsych.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Welter ML, Schupbach M, Czernecki V, Karachi C, Fernandez-Vidal S, Golmard JL, et al. Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology. 2014;82(15):1352–61. doi: 10.1212/WNL.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenstein SA, Koller JM, Black KD, Campbell MC, Lugar HM, Ushe M, et al. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Annals of neurology. 2014;76(2):279–95. doi: 10.1002/ana.24204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dombrowski SM, Hilgetag CC, Barbas H. Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cerebral cortex. 2001;11(10):975–88. doi: 10.1093/cercor/11.10.975. [DOI] [PubMed] [Google Scholar]
- 21.Hamani C, Mayberg H, Snyder B, Giacobbe P, Kennedy S, Lozano AM. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. Journal of neurosurgery. 2009;111(6):1209–15. doi: 10.3171/2008.10.JNS08763. [DOI] [PubMed] [Google Scholar]
- 22.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. The Journal of clinical investigation. 2009;119(4):717–25. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining Critical White Matter Pathways Mediating Successful Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression. Biological psychiatry. 2014;76(12):963–9. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson JM. “Connectomic surgery”: diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Frontiers in integrative neuroscience. 2012;6:15. doi: 10.3389/fnint.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coenen VA, McIntyre CC. Letter to the Editor: Correlation of diffusion tensor imaging and intraoperative macrostimulation. Journal of neurosurgery. 2015;123(1):291–2. doi: 10.3171/2014.12.JNS142690. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Disorders—Research Version (SCID-II, Version 2.0) New York: Biometrics Research Dept, New York State Psychiatric Institute; 1994. [Google Scholar]
- 28.Association AP, editor. Diagnostic and statistical manual of mental disorders: DSM-IV-TR Revised. 4. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 29.Sackeim HA. The definition and meaning of treatment-resistant depression. The Journal of clinical psychiatry. 2001;62(Suppl 16):10–7. [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 34.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2003;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Li Z, Wu Z, Chen J, Wang Z, Peng D, et al. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology. 2014;231(4):685–93. doi: 10.1007/s00213-013-3297-0. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi A, Lujan JL, McIntyre CC. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. Journal of neural engineering. 2013;10(5):056023. doi: 10.1088/1741-2560/10/5/056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi KS, Riva-Posse P, Gross RE, Mayberg HS. Mapping the “Depression Switch” During Intraoperative Testing of Subcallosal Cingulate Deep Brain Stimulation. JAMA neurology. 2015;72(11):1252–60. doi: 10.1001/jamaneurol.2015.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrath CL, Kelley ME, Dunlop BW, Holtzheimer PE, III, Craighead WE, Mayberg HS. Pretreatment brain States identify likely nonresponse to standard treatments for depression. Biological psychiatry. 2014;76(7):527–35. doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(28):10392–402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilbronner SR, Haber SN. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(30):10041–54. doi: 10.1523/JNEUROSCI.5459-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage. 2013;80:220–33. doi: 10.1016/j.neuroimage.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smart OL, Tiruvadi VR, Mayberg HS. Multimodal approaches to define network oscillations in depression. Biological psychiatry. 2015;77(12):1061–70. doi: 10.1016/j.biopsych.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


